Selective Photothermal Therapy Using Antioxidant Nanoparticles Encapsulating Novel Near-Infrared-Absorbing Platinum(II) Complexes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments
2.3. Synthesis of PtL2
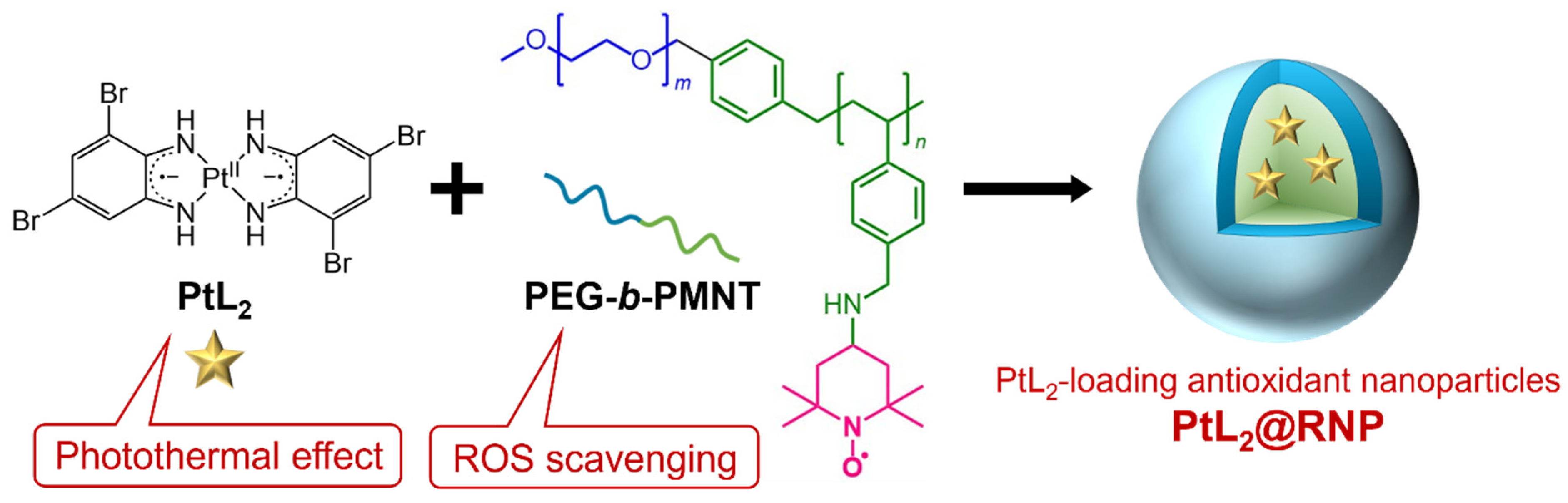
2.4. Preparation of PtL2@RNPs
2.5. Solution Temperature Measurement During NIR Laser Irradiation
2.6. Cell Culture
2.7. Cytotoxicity Assay
2.8. Hyperspectral Imaging of Cells Containing PtL2@RNPs
2.9. Observation for Intracellular Distribution of PtL2@RNP by Spectral Angle Mapper Algorithm
2.10. Quantitative Analysis of the Intracellular Amount of PtII Ions
2.11. Time-Lapse Observation of Cell-Killing by the Photothermal Conversion of PtL2@RNPs
3. Results and Discussion
3.1. Characterization of PtL2@RNPs
3.2. Photothermal Conversion Properties of PtL2@RNPs
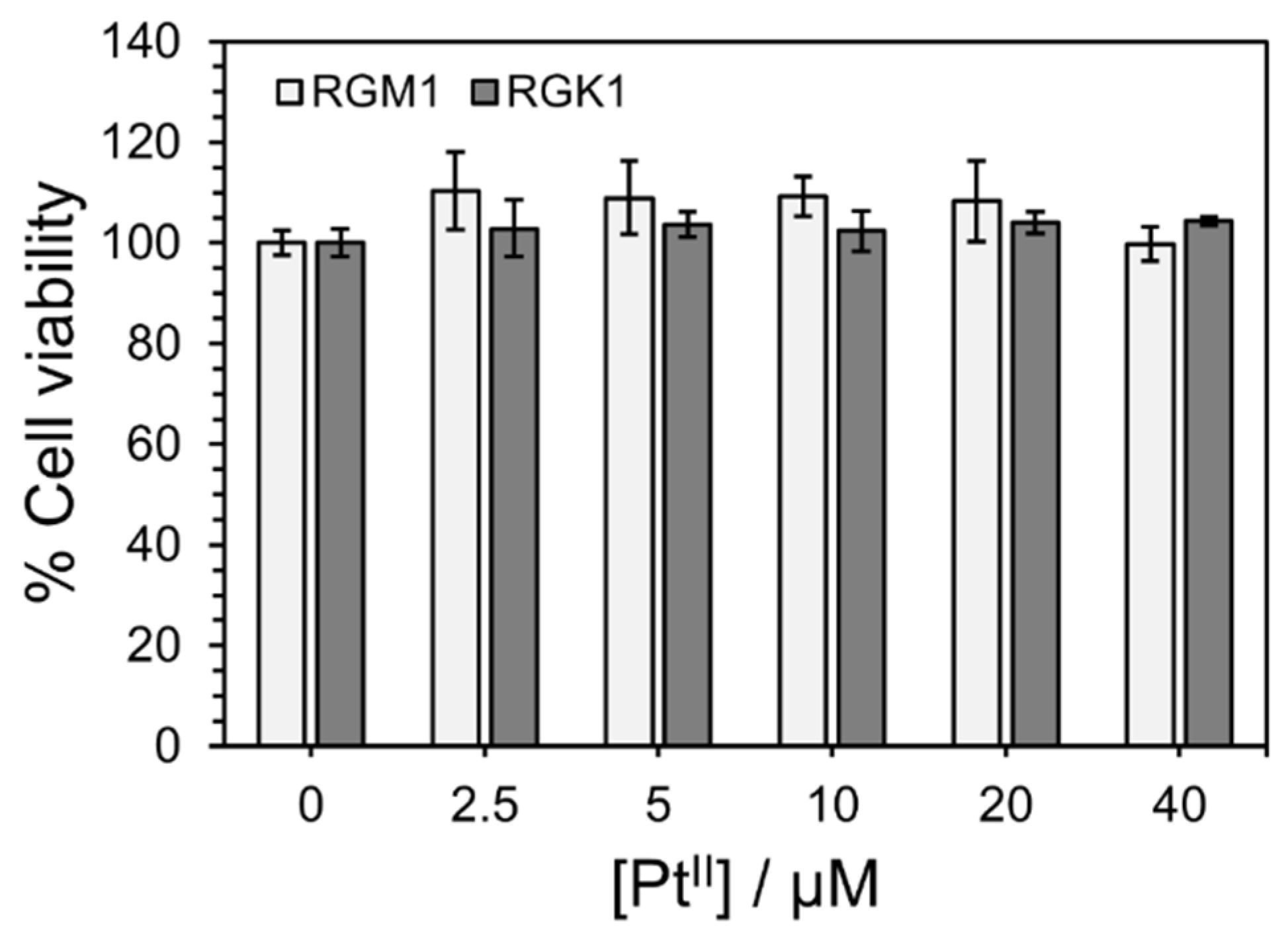
3.3. Cytotoxicity of PtL2@RNPs for RGM1 and RGK1 Cells Under No NIR Irradiation
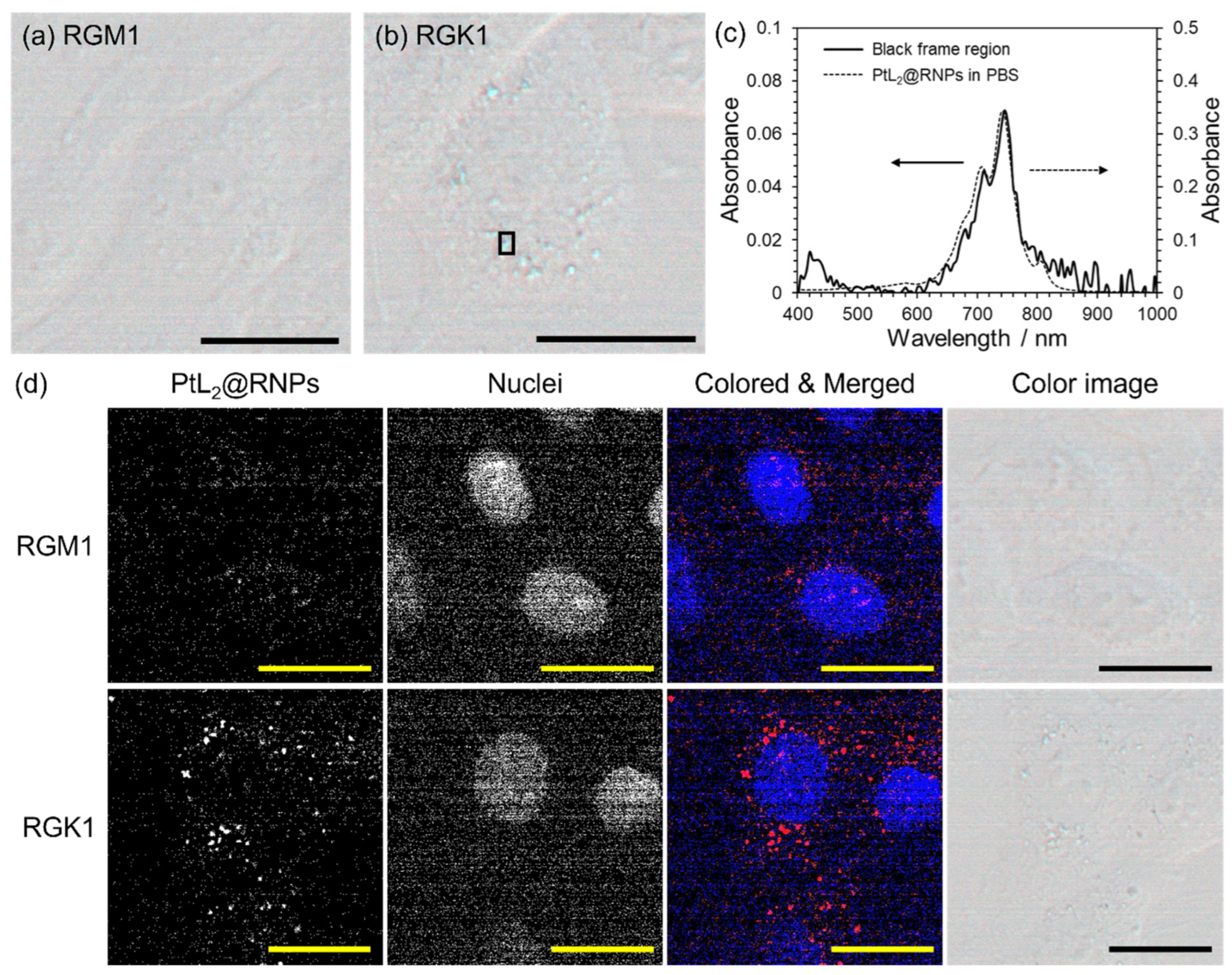
3.4. Difference in the Uptake of PtL2@RNPs for Normal and Cancer Cells
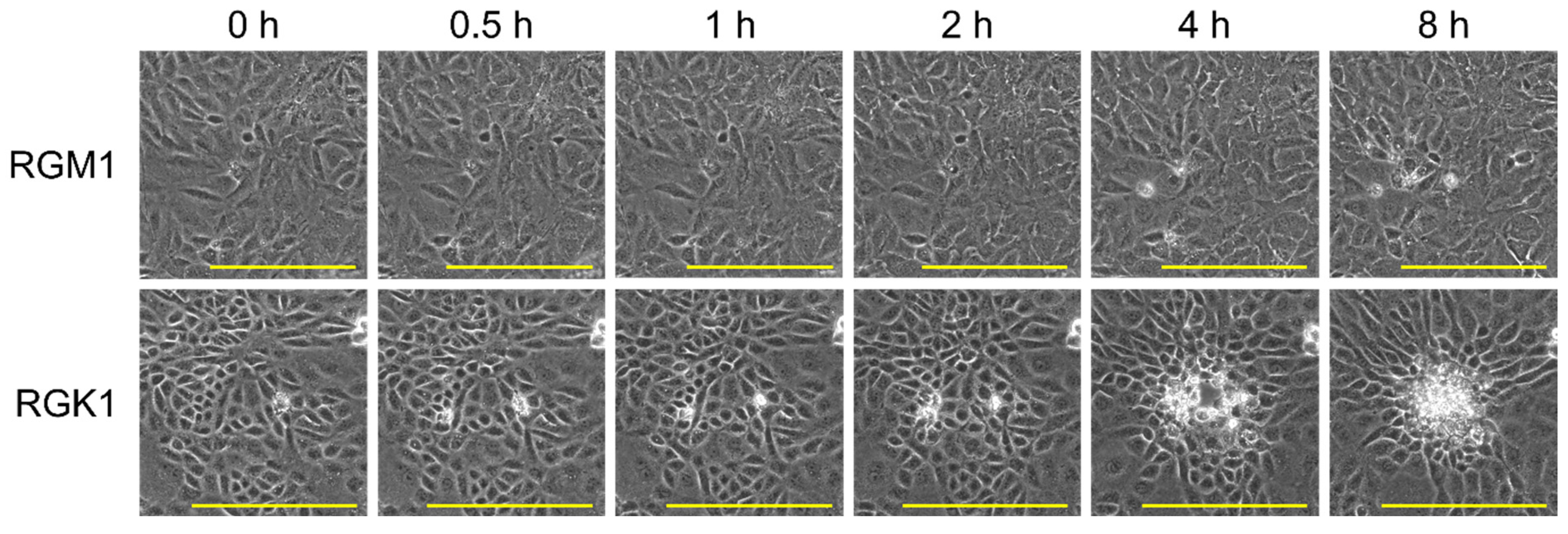
3.5. Cancer Cell-Killing Induced by Photothermal Conversion of PtL2@RNPs Under NIR Irradiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCK-8 | Cell Counting Kit-8 |
| DLS | dynamic light scattering |
| EE | encapsulation efficiency |
| EPR | enhanced permeability and retention |
| FBS | fetal bovine serum |
| ICP-AES | inductively coupled plasma atomic emission spectroscopy |
| LC | loading capacity |
| LLCT | ligand-to-ligand charge transfer |
| NIR | near-infrared |
| NMR | nuclear magnetic resonance |
| PEG | polyethylene glycol |
| PS | penicillin/streptomycin |
| PTAs | photothermal agents |
| PTT | photothermal therapy |
| RGK1 | cancerous mutant cells |
| RGM1 | rat gastric mucosal cells |
| RNPs | redox nanoparticles |
| ROI | region of interest |
| ROS | reactive oxygen species |
| TEMPO | tetramethylpiperidine-N-oxyl |
| UV-Vis | ultraviolet-visible |
References
- Yu, Y.; Wang, A.; Wang, S.; Sun, Y.; Chu, L.; Zhou, L.; Yang, X.; Liu, X.; Sha, C.; Sun, K.; et al. Efficacy of Temozolomide-Conjugated Gold Nanoparticle Photothermal Therapy of Drug-Resistant Glioblastoma and Its Mechanism Study. Mol. Pharm. 2022, 19, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Guo, B.; Wang, W.; Yu, P.; Wu, Z.; Shao, L.; Luo, W. Efficient preparation of nitrogen-doped lignin-based carbon nanotubes and the selectivity of nitrogen speciation for photothermal therapy. Int. J. Biol. Macromol. 2023, 238, 124127. [Google Scholar] [CrossRef] [PubMed]
- Feng, E.; Liu, Y.; Lv, S.; Liu, D.; Huang, S.; Li, Z.; Song, F. Fine-Tuning Cu (II)-Induced Self-Assembly of Hydrophilic Cyanine Dyes for Enhanced Tumor Photothermal Therapy. Adv. Funct. Mater. 2022, 32, 2209258. [Google Scholar] [CrossRef]
- Weber, J.; Beard, P.C.; Bohndiek, S.E. Contrast agents for molecular photoacoustic imaging. Nat. Methods 2016, 13, 639–650. [Google Scholar] [CrossRef]
- Nomura, S.; Morimoto, Y.; Tsujimoto, H.; Arake, M.; Harada, M.; Saitoh, D.; Hara, I.; Ozeki, E.; Satoh, A.; Takayama, E.; et al. Highly reliable, targeted photothermal cancer therapy combined with thermal dosimetry using a near-infrared absorbent. Sci. Rep. 2020, 10, 9765. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, X.; Xiong, J.; Peng, S.; Huang, W.; Joshi, R.; Cai, Y.; Liu, Y.; Li, R.; Yuan, K.; et al. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci. Rep. 2018, 8, 8720. [Google Scholar] [CrossRef]
- Taylor, M.L.; Wilson, R.E., Jr.; Amrhein, K.D.; Huang, X. Gold Nanorod-Assisted Photothermal Therapy and Improvement Strategies. Bioengineering 2022, 9, 200. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Ming, T.; Sun, Z.; Zhao, C.; Yang, B.; Wang, J. Understanding the Photothermal Conversion Efficiency of Gold Nanocrystals. Small 2010, 6, 2272–2280. [Google Scholar] [CrossRef]
- Oh, Y.; Lin, J.-O.; Oh, J. Photothermal-triggered control of sub-cellular drug accumulation using doxorubicin-loaded single-walled carbon nanotubes for the effective killing of human breast cancer cells. Nanotechnology 2017, 28, 125101. [Google Scholar] [CrossRef]
- Cheon, Y.A.; Bae, J.H.; Chung, B.G. Reduced Graphene Oxide Nanosheet for Chemo-photothermal Therapy. Langmuir 2016, 32, 2731–2736. [Google Scholar] [CrossRef]
- Manivasagan, P.; Hoang, G.; Moorthy, M.S.; Mondal, S.; Doan, V.H.M.; Kim, H.; Phan, T.T.V.; Nguyen, T.P.; Oh, J. Chitosan/fucoidan multilayer coating of gold nanorods as highly efficient near-infrared photothermal agents for cancer therapy. Carbohydr. Polym. 2019, 211, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-T.; Liu, C.-H.; Chin, Y.; Chen, S.-Y.; Liu, S.H.; Hsu, Y.-C.; Wu, K.C.-W. Biocompatible and multifunctional gold nanorods for effective photothermal therapy of oral squamous cell carcinoma. J. Mater. Chem. B 2019, 7, 4451–4460. [Google Scholar] [CrossRef]
- Yoo, S.; Hou, J.; Yi, W.; Li, Y.; Chen, W.; Meng, L.; Si, J.; Hou, X. Enhanced Response of Metformin towards the Cancer Cells due to Synergism with Multi-walled Carbon Nanotubes in Photothermal Therapy. Sci. Rep. 2017, 7, 1071. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zang, Y.; Qu, J.; Tang, M.; Zhang, T. The Toxicity Of Metallic Nanoparticles On Liver: The Subcellular Damages, Mechanisms, And Outcomes. Int. J. Nanomed. 2019, 14, 8787–8804. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, W.; Chen, X. Near-Infrared Fluorescence Imaging of Tumor Integrin αvβ3 Expression with Cy7-Labeled RGD Multimers. Mol. Imaging Biol. 2006, 8, 226–236. [Google Scholar] [CrossRef]
- Li, S.; Johnson, J.; Peck, A.; Xie, Q. Near infrared fluorescent imaging of brain tumor with IR780 dye incorporated phospholipid nanoparticles. J. Transl. Med. 2017, 15, 18. [Google Scholar] [CrossRef]
- Pan, G.-Y.; Jia, H.-R.; Zhu, Y.-X.; Wu, F.-G. Turning double hydrophilic into amphiphilic: IR825-conjugated polymeric nanomicelles for near-infrared fluorescence imaging-guided photothermal cancer therapy. Nanoscale 2018, 10, 2115–2127. [Google Scholar] [CrossRef]
- Tian, Y.; Younis, M.R.; Tang, Y.; Liao, X.; He, G.; Wang, S.; Teng, Z.; Huang, P.; Zhang, L.; Lu, G. Dye-loaded mesoporous polydopamine nanoparticles for multimodal tumor theranostics with enhanced immunogenic cell death. J. Nanobiotechnol. 2021, 19, 365. [Google Scholar] [CrossRef]
- Guo, B.; Huang, Z.; Shi, Q.; Middha, E.; Xu, S.; Li, L.; Wu, M.; Jiang, J.; Hu, Q.; Fu, Z.; et al. Organic Small Molecule Based Photothermal Agents with Molecular Rotors for Malignant Breast Cancer Therapy. Adv. Funct. Mater. 2020, 30, 1907093. [Google Scholar] [CrossRef]
- Zhao, Y.; He, Z.; Zhang, Q.; Wang, J.; Jia, W.; Jin, L.; Zhao, L.; Lu, Y. 880 nm NIR-Triggered Organic Small Molecular-Based Nanoparticles for Photothermal Therapy of Tumor. Nanomaterials 2021, 11, 773. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Cheng, Q.; Tian, Y.; Dang, H.; Teng, C.; Yan, L. An anti-aggregation NIR-II heptamethine-cyanine dye with a stereo-specific cyanine for imaging-guided photothermal therapy. J. Mater. Chem. B 2021, 9, 2688–2696. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Herebian, D.; Bothe, E.; Neese, F.; Weyhermüller, T.; Wieghardt, K. Molecular and Electronic Structures of Bis-(o-diiminobenzosemiquinonato)metal(II) Complexes (Ni, Pd, Pt), Their Monocations and -Anions, and of Dimeric Dications Containing Weak Metal-Metal Bonds. J. Am. Chem. Soc. 2003, 125, 9116–9128. [Google Scholar] [CrossRef]
- Sawamura, R.; Sato, M.; Masuya-Suzuki, A.; Iki, N. Photostable near-infrared-absorbing diradical-platinum(ii) complex solubilized by albumin toward a cancer photothermal therapy agent. RSC Adv. 2020, 10, 6460–6463. [Google Scholar] [CrossRef]
- Sawamura, R.; Masuya-Suzuki, A.; Iki, N. Study on cellular uptake of a hydrophobic near-infrared-absorbing diradical-platinum(II) complex solubilized by albumin using hyperspectral imaging, spectrophotometry, and spectrofluorimetry. Anal. Sci. 2024, 40, 1857–1865. [Google Scholar] [CrossRef]
- Yoshitomi, T.; Miyamoto, D.; Nagasaki, Y. Design of Core−Shell-Type Nanoparticles Carrying Stable Radicals in the Core. Biomacromolecules 2009, 10, 596–601. [Google Scholar] [CrossRef]
- Yoshitomi, T.; Hirayama, A.; Nagasaki, Y. The ROS scavenging and renal protective effects of pH-responsive nitroxide radical-containing nanoparticles. Biomaterials 2011, 32, 8021–8026. [Google Scholar] [CrossRef]
- Kim, A.; Yonemoto, C.; Feliciano, C.P.; Shashni, B.; Nagasaki, Y. Antioxidant Nanomedicine Significantly Enhances the Survival Benefit of Radiation Cancer Therapy by Mitigating Oxidative Stress-Induced Side Effects. Small 2021, 17, 2008210. [Google Scholar] [CrossRef]
- Kobayashi, I.; Kawano, S.; Tsuji, S.; Matsui, H.; Nakama, A.; Sawaoka, H.; Masuda, E.; Takei, Y.; Nagano, K.; Fusamoto, H.; et al. RGM1, a cell line derived from normal gastric mucosa of rat. Vitr. Cell. Dev. Biol. Anim. 1996, 32, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, O.; Matsui, H.; Nagano, Y.; Kaneko, T.; Shibahara, T.; Nakahara, A.; Hyodo, I.; Yanaka, A.; Majima, H.J.; Nakamura, Y.; et al. Neoplastic transformation and induction of H+,K+-adenosine triphosphatase by N-methyl-N’-nitro-N-nitrosoguanidine in the gastric epithelial RGM-1 cell line. Vitr. Cell. Dev. Biol. Anim. 2008, 44, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Konno, Y.; Matsushita, N. Crystalline Polymorphism of Bis[o-semibenzoquinonediimine(1-)]platinum(II). Bull. Chem. Soc. Jpn. 2006, 79, 1237–1239. [Google Scholar] [CrossRef]
- Kruse, F.A.; Lefkoff, A.B.; Boardman, J.W.; Heidebrecht, K.B.; Shapiro, A.T.; Barloon, P.J.; Goetz, A.F.H. The spectral image processing system (SIPS)—Interactive visualization and analysis of imaging spectrometer data. Remote Sens. Environ. 1993, 44, 145–163. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, W. Enhanced drug loading capacity of 10-hydroxycamptothecin-loaded nanoparticles prepared by two-step nanoprecipitation method. J. Drug Deliv. Sci. Tech. 2016, 36, 183–191. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Zhou, T.; Zhang, J.; Xia, H.; Li, H.; He, J.; He, S.; Wang, L. Positively charged micelles based on a triblock copolymer demonstrate enhanced corneal penetration. Int. J. Nanomed. 2015, 10, 6027–6037. [Google Scholar] [CrossRef]
- Tamura, K.; Masuya, A.; Hoshino, H.; Iki, N. A molecular probe for recognizing the size of hydrophobic cavities based on near-infrared absorbing diradical–PtII complexes. Chem. Commun. 2013, 49, 4812–4814. [Google Scholar] [CrossRef]
- Masuya-Suzuki, A.; Hayashi, T.; Tamura, K.; Iki, N. Capillary electrophoretic separation of cis/trans isomers of bis(o-diiminobenzoquinonato)platinum(II) complexes using β-cyclodextrins as the selector. New J. Chem. 2017, 41, 7605–7612. [Google Scholar] [CrossRef]
- Li, F.; Li, T.; Zhi, D.; Xu, P.; Wang, W.; Hu, Y.; Zhang, Y.; Wang, S.; Thomas, J.M.; Norman, J.B.; et al. Novel ultrasmall multifunctional nanodots for dual-modal MR/NIR-II imaging-guided photothermal therapy. Biomaterials 2020, 256, 120219. [Google Scholar] [CrossRef]
- Kurokawa, H.; Ito, H.; Terasaki, M.; Matsui, H. Hyperthermia enhances photodynamic therapy by regulation of HCP1 and ABCG2 expressions via high level ROS generation. Sci. Rep. 2019, 9, 1638. [Google Scholar] [CrossRef]
- Imashiro, C.; Takeshita, H.; Morikura, T.; Miyata, S.; Takemura, K.; Komotori, J. Development of accurate temperature regulation culture system with metallic culture vessel demonstrates different thermal cytotoxicity in cancer and normal cells. Sci. Rep. 2021, 11, 21466. [Google Scholar] [CrossRef]
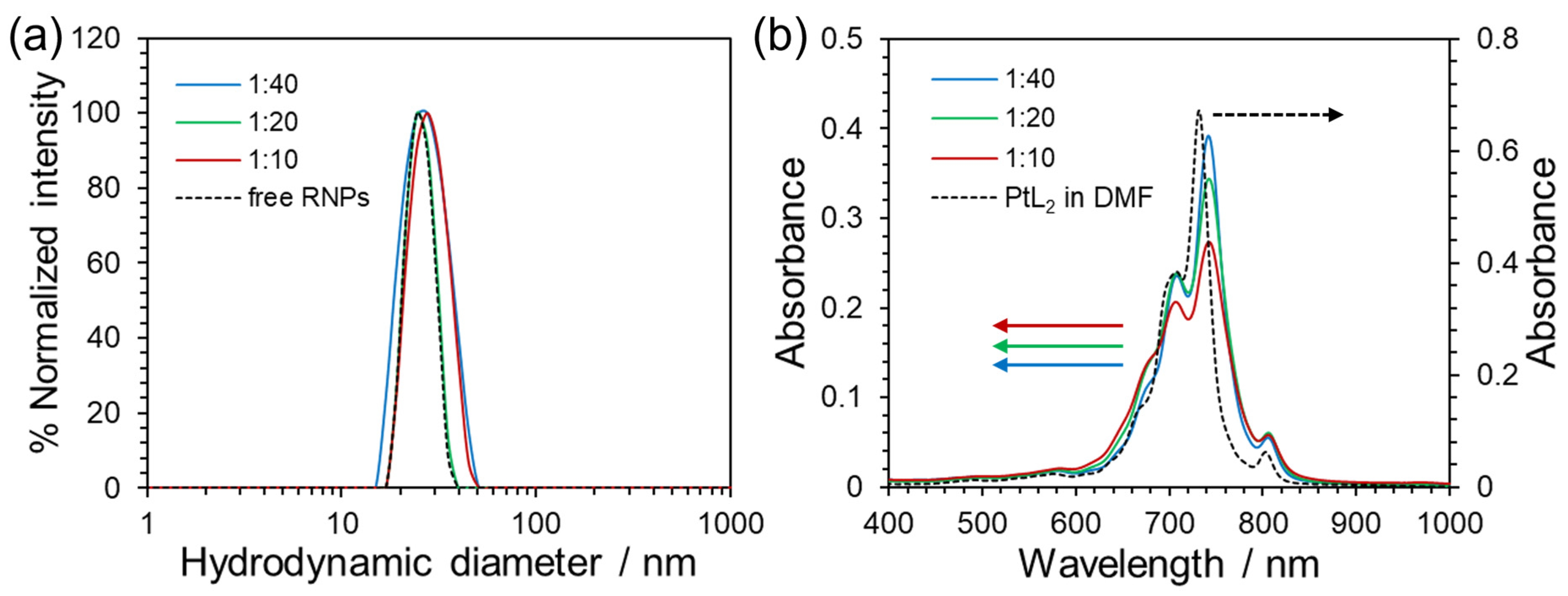

| Mass Ratio | Mean Hydrodynamic Diameter [nm] | Polydispersity Index | Zeta Potential [mV] | EE [%] | LC [%] |
|---|---|---|---|---|---|
| 1:40 | 25.9 | 0.072 | −0.40 | 99 | 3.4 |
| 1:20 | 25.8 | 0.103 | 0.60 | 92 | 5.3 |
| 1:10 | 26.7 | 0.039 | −0.16 | 91 | 12 |
| free RNPs | 24.9 | 0.103 | −0.94 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawamura, R.; Kurokawa, H.; Taninaka, A.; Toriumi, T.; Nagasaki, Y.; Shigekawa, H.; Matsui, H.; Iki, N. Selective Photothermal Therapy Using Antioxidant Nanoparticles Encapsulating Novel Near-Infrared-Absorbing Platinum(II) Complexes. Nanomaterials 2025, 15, 796. https://doi.org/10.3390/nano15110796
Sawamura R, Kurokawa H, Taninaka A, Toriumi T, Nagasaki Y, Shigekawa H, Matsui H, Iki N. Selective Photothermal Therapy Using Antioxidant Nanoparticles Encapsulating Novel Near-Infrared-Absorbing Platinum(II) Complexes. Nanomaterials. 2025; 15(11):796. https://doi.org/10.3390/nano15110796
Chicago/Turabian StyleSawamura, Ryota, Hiromi Kurokawa, Atsushi Taninaka, Takuto Toriumi, Yukio Nagasaki, Hidemi Shigekawa, Hirofumi Matsui, and Nobuhiko Iki. 2025. "Selective Photothermal Therapy Using Antioxidant Nanoparticles Encapsulating Novel Near-Infrared-Absorbing Platinum(II) Complexes" Nanomaterials 15, no. 11: 796. https://doi.org/10.3390/nano15110796
APA StyleSawamura, R., Kurokawa, H., Taninaka, A., Toriumi, T., Nagasaki, Y., Shigekawa, H., Matsui, H., & Iki, N. (2025). Selective Photothermal Therapy Using Antioxidant Nanoparticles Encapsulating Novel Near-Infrared-Absorbing Platinum(II) Complexes. Nanomaterials, 15(11), 796. https://doi.org/10.3390/nano15110796