In Situ Growth, Etching, and Charging of Nanoscale Water Ice Under Fast Electron Irradiation in Environmental TEM
Abstract
:1. Introduction
2. Materials and Methods
3. Results
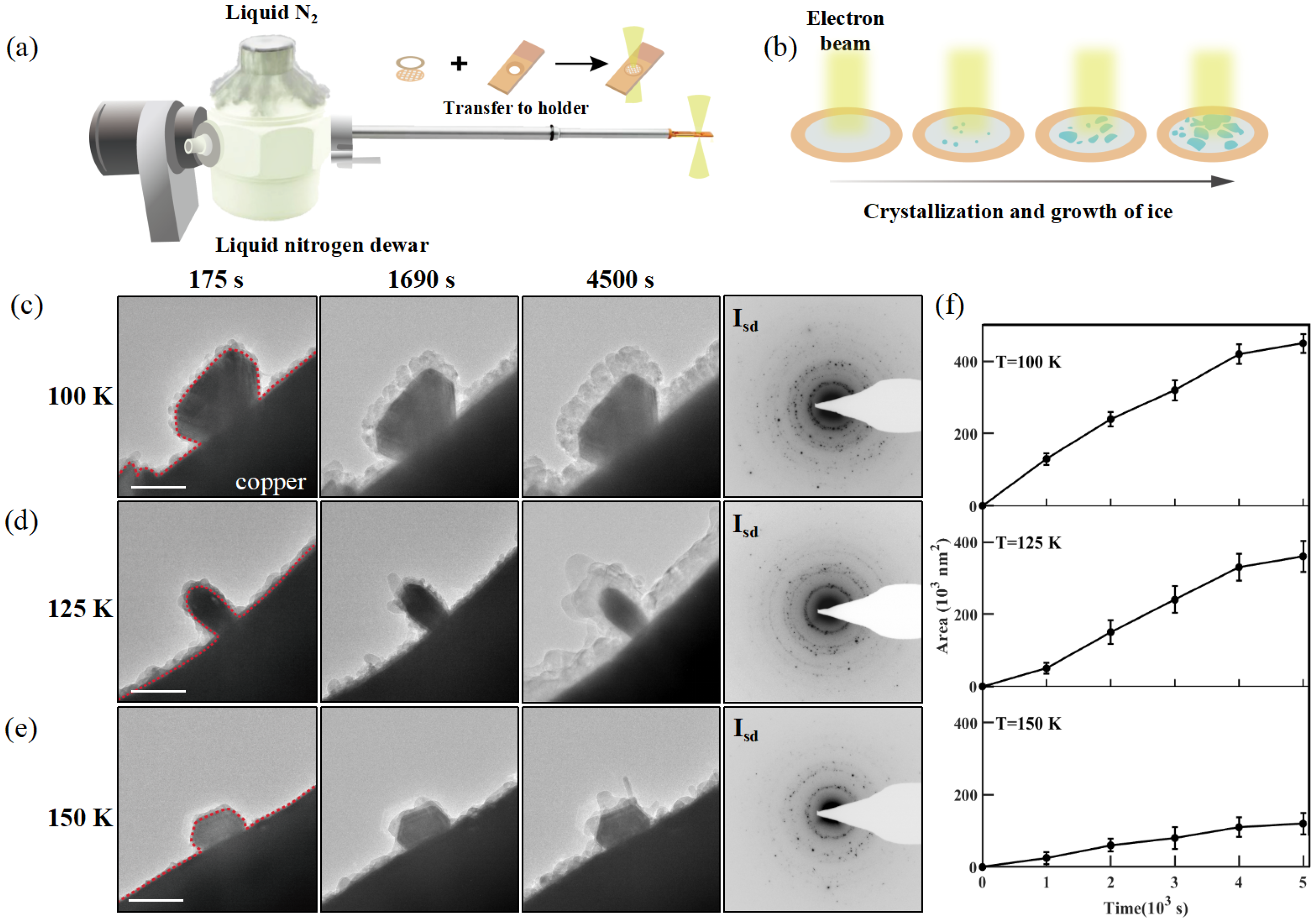
3.1. Growth of Water Ice at Edges of Cu Grid
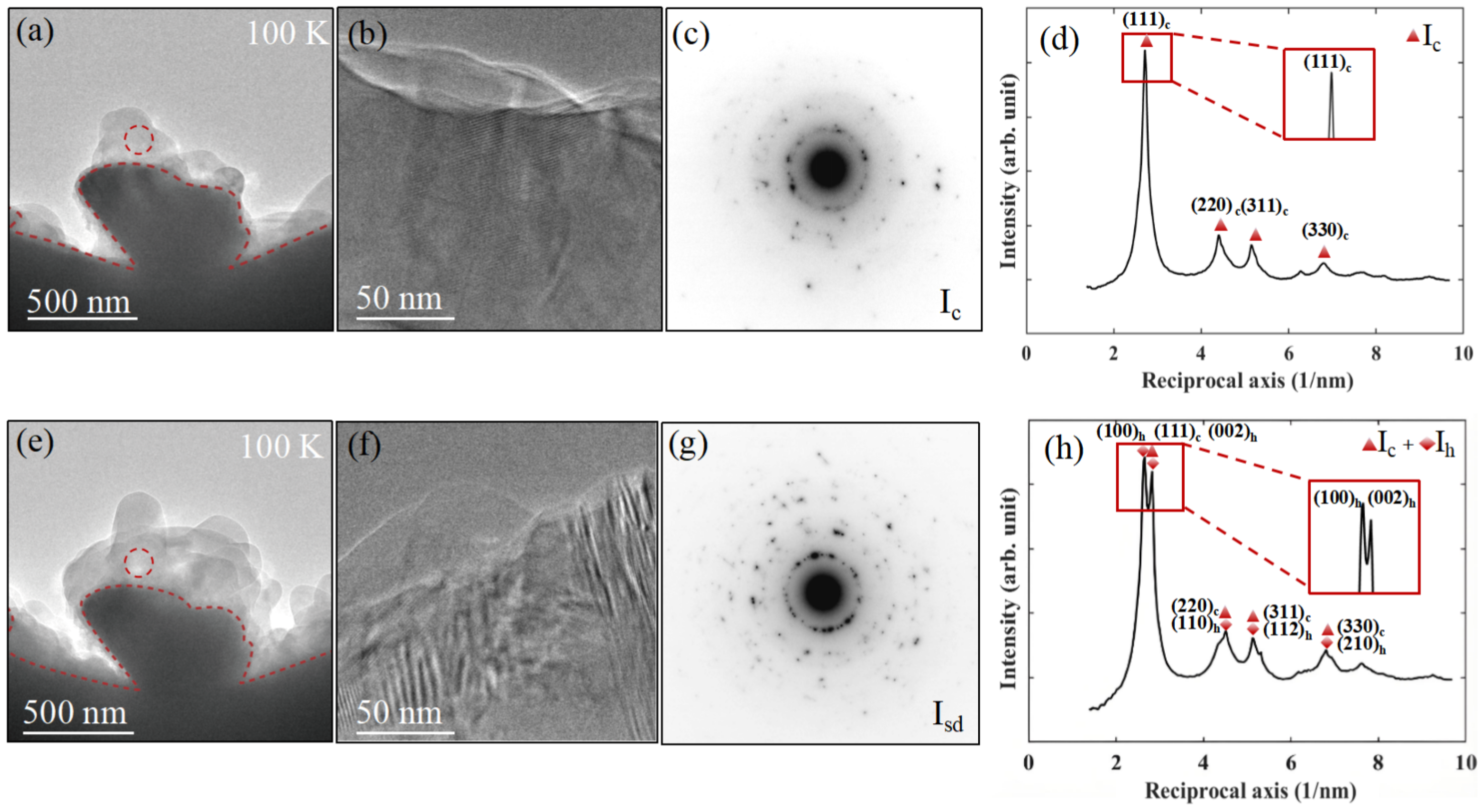
3.2. Determination of Cubic Phase of Water Ice
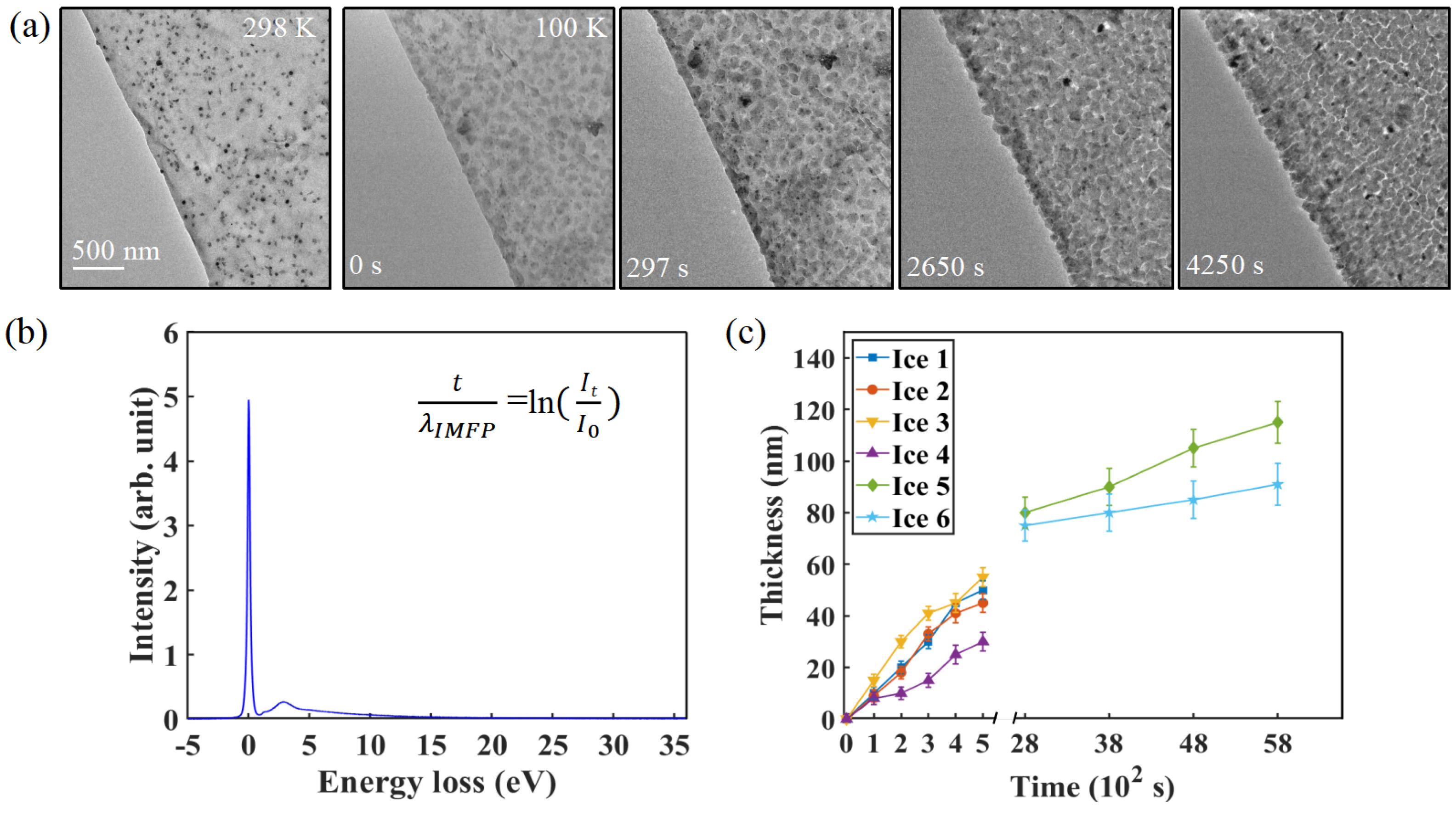
3.3. Time-Dependent Ice Thickness Evolution
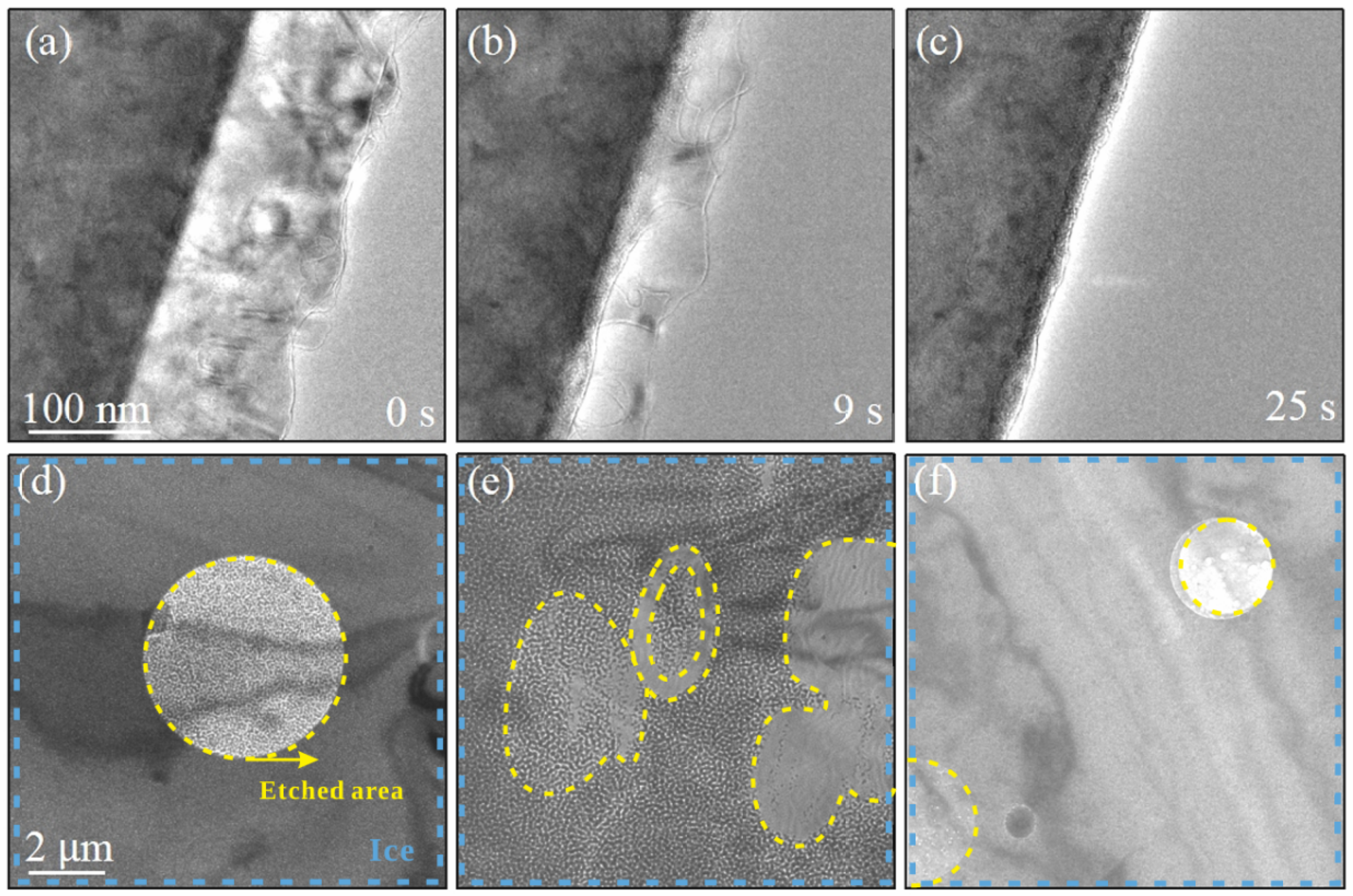
3.4. Etching of Ice via Electron Beam Irradiation
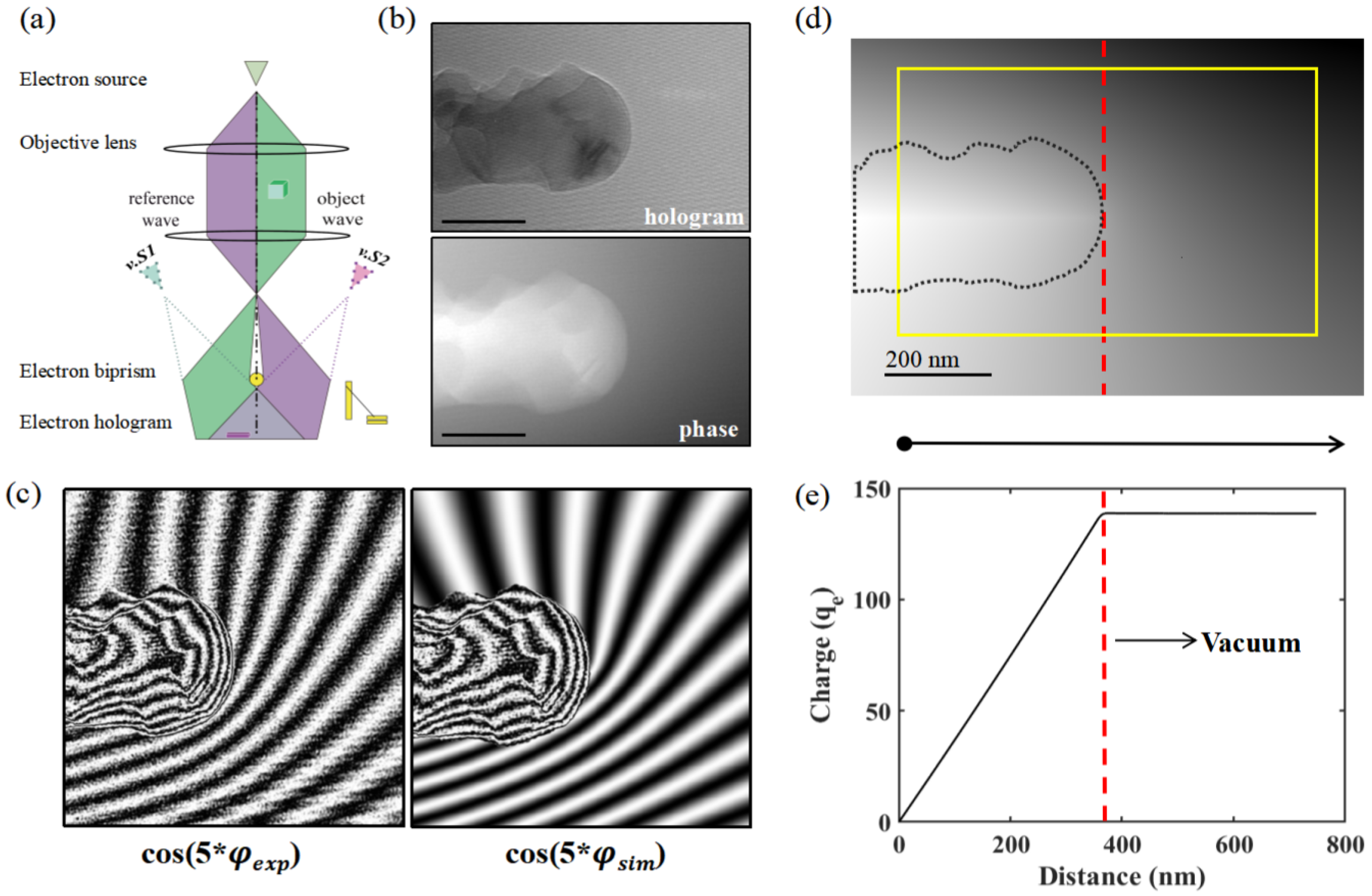
3.5. Charging of Ice via Electron Holography
4. Discussion
4.1. The Growth and Etching and Charging of Water Ice Under Fast Electron Irradiation
4.2. Water Ice Research with Advanced Electron Microscope and Technologies
4.3. Implication and Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TEM | Transmission electron microscopy |
| SAED | Selected area electron diffraction |
| HRTEM | High-resolution TEM |
| ETEM | Environmental transmission electron microscopy |
| ESEM | Environmental scanning electron microscopy |
| EH | Electron holography |
| EELS | Electron energy loss spectroscopy |
| Cubic ice | |
| Hexagonal ice | |
| Stacking-disordered ice |
References
- Pach, E.; Verdaguer, A. Studying ice with environmental scanning electron microscopy. Molecules 2021, 27, 258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fei, L.; Rao, Z.; Liu, D.; Leung, C.; Wang, Y. In situ observation of ice formation from water vapor by environmental SEM. Cryst. Growth Des. 2018, 18, 6602. [Google Scholar] [CrossRef]
- Phakatkar, A.H.; Megaridis, C.M.; Shokuhfar, T.; Shahbazian-Yassar, R. Real-time TEM observations of ice formation in graphene liquid cell. Nanoscale 2023, 15, 7006. [Google Scholar] [CrossRef]
- Ma, R.; Cao, D.; Zhu, C.; Tian, Y.; Peng, J.; Guo, J.; Chen, J.; Li, X.Z.; Francisco, J.S.; Zeng, X.C.; et al. Atomic imaging of the edge structure and growth of a two-dimensional hexagonal ice. Nature 2020, 577, 60. [Google Scholar] [CrossRef]
- Hong, J.; Tian, Y.; Liang, T.; Liu, X.; Song, Y.; Guan, D.; Yan, Z.; Guo, J.; Tang, B.; Cao, D.; et al. Imaging surface structure and premelting of ice Ih with atomic resolution. Nature 2024, 630, 375. [Google Scholar] [CrossRef] [PubMed]
- Teresa, D.; Maria, J. Nanofabrication: Nanolithography Techniques and Their Applications; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Lee, M.; Lee, S.Y.; Kang, M.H.; Won, T.K.; Kang, S.; Kim, J.; Park, J.; Ahn, D.J. Observing growth and interfacial dynamics of nanocrystalline ice in thin amorphous ice films. Nat. Commun. 2024, 15, 908. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Noh, N.; Park, J.; Shim, Y.; Park, S.; Qureshi, Y.; Kang, S.; Huh, Y.; Lee, C.W.; Yuk, J.M. Phase Transition of Cubic Ice to Hexagonal Ice during Growth and Decomposition. Nano Lett. 2024, 24, 11504. [Google Scholar] [CrossRef]
- Huang, X.; Wang, L.; Liu, K.; Liao, L.; Sun, H.; Wang, J.; Tian, X.; Xu, Z.; Wang, W.; Liu, L.; et al. Tracking cubic ice at molecular resolution. Nature 2023, 617, 86. [Google Scholar] [CrossRef]
- Abellan, P.; Gautron, E.; LaVerne, J.A. Radiolysis of Thin Water Ice in Electron Microscopy. J. Phys. Chem. C 2023, 127, 15336. [Google Scholar] [CrossRef]
- Cascajo-Castresana, M.; Morin, S.; Bittner, A.M. The ice–vapour interface during growth and sublimation. Atmos. Chem. Phys. 2021, 21, 18629. [Google Scholar] [CrossRef]
- Yamazaki, T.; Kouchi, A.; Murata, K.i.; Katsuno, H.; Nada, H.; Hama, T.; Kimura, Y. In situ cryogenic transmission electron microscopy observation on the formation of hydrogen-ordered hexagonal ices and its astrophysical implications. Mon. Not. R. Astron. Soc. 2024, 527, 2858. [Google Scholar] [CrossRef]
- Daily, M.I.; Whale, T.F.; Partanen, R.; Harrison, A.D.; Kilbride, P.; Lamb, S.; Morris, G.J.; Picton, H.M.; Murray, B.J. Cryopreservation of primary cultures of mammalian somatic cells in 96-well plates benefits from control of ice nucleation. Cryobiology 2020, 93, 62. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, Y.; Ye, H.; He, Y.; Li, H.; Sun, Y.; Yang, F.; Wang, R. Atomic-scale structure dynamics of nanocrystals revealed by in situ and environmental transmission electron microscopy. Adv. Mater. 2023, 35, 2206911. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bermúdez, I.; Castillo-Suero, A.; Cortés-Inostroza, A.; Jeldrez, C.; Dantas, A.; Hernández, E.; Orellana-Palma, P.; Petzold, G. Observation and measurement of ice morphology in foods: A review. Foods 2023, 12, 3987. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Han, A.; Qiu, M. Ice lithography for 3D nanofabrication. Sci. Bull. 2019, 64, 865. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Chang, B.; Beleggia, M. Electron-beam patterning of vapor-deposited solid anisole. ACS Appl. Mater. Interfaces 2020, 12, 6436. [Google Scholar] [CrossRef]
- Hong, Y.; Zhao, D.; Wang, J.; Lu, J.; Yao, G.; Liu, D.; Luo, H.; Li, Q.; Qiu, M. Solvent-free nanofabrication based on ice-assisted electron-beam lithography. Nano Lett. 2020, 20, 8841. [Google Scholar] [CrossRef]
- Yao, G.; Zhao, D.; Hong, Y.; Zheng, R.; Qiu, M. Ice-assisted electron-beam lithography for MoS2 transistors with extremely low-energy electrons. Nanoscale Adv. 2022, 4, 2479. [Google Scholar] [CrossRef]
- Jin, B.; Lu, Y.; Sun, J.; Sun, X.; Wen, L.; Zhang, Q.; Zhao, D.; Qiu, M. Cryogenic Electron-Beam Writing for Perovskite Metasurface. Nano Lett. 2024, 24, 5610. [Google Scholar] [CrossRef]
- Haque, R.I.; Waafi, A.K.; Jaemin, K.; Briand, D.; Han, A. 80 K cryogenic stage for ice lithography. Micro Nano Eng. 2022, 14, 100101. [Google Scholar] [CrossRef]
- Egerton, R.; Li, P.; Malac, M. Radiation damage in the TEM and SEM. Micron 2004, 35, 399. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Liu, Y.; Ye, X.; An, Q.; Pan, J.; Li, Z.A. A spatial phase-shifting method for real-space wave reconstruction of off-axis electron holograms. Phys. Scr. 2024, 99, 105108. [Google Scholar] [CrossRef]
- Egerton, R. Mechanisms of radiation damage in beam-sensitive specimens, for TEM accelerating voltages between 10 and 300 kV. Microsc. Res. Tech. 2012, 75, 1550. [Google Scholar] [CrossRef] [PubMed]
- Persad, A.H.; Ward, C.A. Expressions for the evaporation and condensation coefficients in the Hertz-Knudsen relation. Chem. Rev. 2016, 116, 7727–7767. [Google Scholar] [CrossRef] [PubMed]
- Libbrecht, K.G. The physics of snow crystals. Rep. Prog. Phys. 2005, 68, 855. [Google Scholar] [CrossRef]
- Libbrecht, K.G. Toward a Comprehensive Model of Snow Crystal Growth: 10. On the Molecular Dynamics of Structure Dependent Attachment Kinetics. arXiv 2020, arXiv:2012.12916. [Google Scholar]
- Egerton, R.F. Electron energy-loss spectroscopy in the TEM. Rep. Prog. Phys. 2008, 72, 016502. [Google Scholar] [CrossRef]
- Malis, T.; Cheng, S.; Egerton, R. EELS log-ratio technique for specimen-thickness measurement in the TEM. J. Electron Microsc. Tech. 1988, 8, 193. [Google Scholar] [CrossRef]
- Egerton, R.; Cheng, S. Measurement of local thickness by electron energy-loss spectroscopy. Ultramicroscopy 1987, 21, 231. [Google Scholar] [CrossRef]
- Tkachenko, K.; Jacobi, H.W. Electrical charging of snow and ice in polar regions and the potential impact on atmospheric chemistry. Environ. Sci. Atmos. 2024, 4, 144. [Google Scholar] [CrossRef]
- Ding, Z.; Li, C.; Da, B.; Liu, J. Charging effect induced by electron beam irradiation: A review. Sci. Technol. Adv. Mater. 2021, 22, 932. [Google Scholar] [CrossRef] [PubMed]
- Lichte, H.; Lehmann, M. Electron holography—basics and applications. Rep. Prog. Phys. 2007, 71, 016102. [Google Scholar] [CrossRef]
- Wagner, T.; Kraft, R.; Nowak, F.; Berger, D.; Günther, C.M.; Çelik, H.; Koch, C.T.; Lehmann, M. The reference window for reduced perturbation of the reference wave in electrical biasing off-axis electron holography. Ultramicroscopy 2024, 267, 114060. [Google Scholar] [CrossRef]
- Matteucci, G.; Missiroli, G.; Nichelatti, E.; Migliori, A.; Vanzi, M.; Pozzi, G. Electron holography of long-range electric and magnetic fields. J. Appl. Phys. 1991, 69, 1835. [Google Scholar] [CrossRef]
- Beleggia, M.; Kasama, T.; Larson, D.J.; Kelly, T.F.; Dunin-Borkowski, R.E.; Pozzi, G. Towards quantitative off-axis electron holographic mapping of the electric field around the tip of a sharp biased metallic needle. J. Appl. Phys. 2014, 116, 024305. [Google Scholar] [CrossRef]
- Gatel, C.; Lubk, A.; Pozzi, G.; Snoeck, E.; Hÿtch, M. Counting elementary charges on nanoparticles by electron holography. Phys. Rev. Lett. 2013, 111, 025501. [Google Scholar] [CrossRef]
- Russo, C.J.; Henderson, R. Charge accumulation in electron cryomicroscopy. Ultramicroscopy 2018, 187, 43. [Google Scholar] [CrossRef]
- Cazaux, J. e-Induced secondary electron emission yield of insulators and charging effects. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2006, 244, 307. [Google Scholar] [CrossRef]
- Cazaux, J. Some physical descriptions of the charging effects in insulators under irradiation. In Ionization of Solids by Heavy Particles; Springer: Greer, SC, USA, 1993; p. 325. [Google Scholar]
- Małolepsza, E.; Keyes, T. Water freezing and ice melting. J. Chem. Theory Comput. 2015, 11, 5613–5623. [Google Scholar] [CrossRef]
- Bi, Y.; Cao, B.; Li, T. Enhanced heterogeneous ice nucleation by special surface geometry. Nat. Commun. 2017, 8, 15372. [Google Scholar] [CrossRef]
- Chen, Y. Nanofabrication by electron beam lithography and its applications: A review. Microelectron. Eng. 2015, 135, 57. [Google Scholar] [CrossRef]
- McCartney, M.R.; Dunin-Borkowski, R.E.; Smith, D.J. Quantitative measurement of nanoscale electrostatic potentials and charges using off-axis electron holography: Developments and opportunities. Ultramicroscopy 2019, 203, 105. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.M.; Koop, T. Review of the vapour pressures of ice and supercooled water for 100 atmospheric applications. Q. J. R. Meteorol. Soc. 2005, 131, 1539. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, H.; An, Q.; Ye, X.; Wu, D.; Liang, B.; Su, J.; Li, Z. In Situ Growth, Etching, and Charging of Nanoscale Water Ice Under Fast Electron Irradiation in Environmental TEM. Nanomaterials 2025, 15, 726. https://doi.org/10.3390/nano15100726
Chu H, An Q, Ye X, Wu D, Liang B, Su J, Li Z. In Situ Growth, Etching, and Charging of Nanoscale Water Ice Under Fast Electron Irradiation in Environmental TEM. Nanomaterials. 2025; 15(10):726. https://doi.org/10.3390/nano15100726
Chicago/Turabian StyleChu, Hongchen, Qianming An, Xianhui Ye, Duanzheng Wu, Binye Liang, Jiaqi Su, and Zian Li. 2025. "In Situ Growth, Etching, and Charging of Nanoscale Water Ice Under Fast Electron Irradiation in Environmental TEM" Nanomaterials 15, no. 10: 726. https://doi.org/10.3390/nano15100726
APA StyleChu, H., An, Q., Ye, X., Wu, D., Liang, B., Su, J., & Li, Z. (2025). In Situ Growth, Etching, and Charging of Nanoscale Water Ice Under Fast Electron Irradiation in Environmental TEM. Nanomaterials, 15(10), 726. https://doi.org/10.3390/nano15100726





