Novel Characterization Techniques for Multifunctional Plasmonic–Magnetic Nanoparticles in Biomedical Applications
Abstract
:1. Introduction
2. Experimental Section
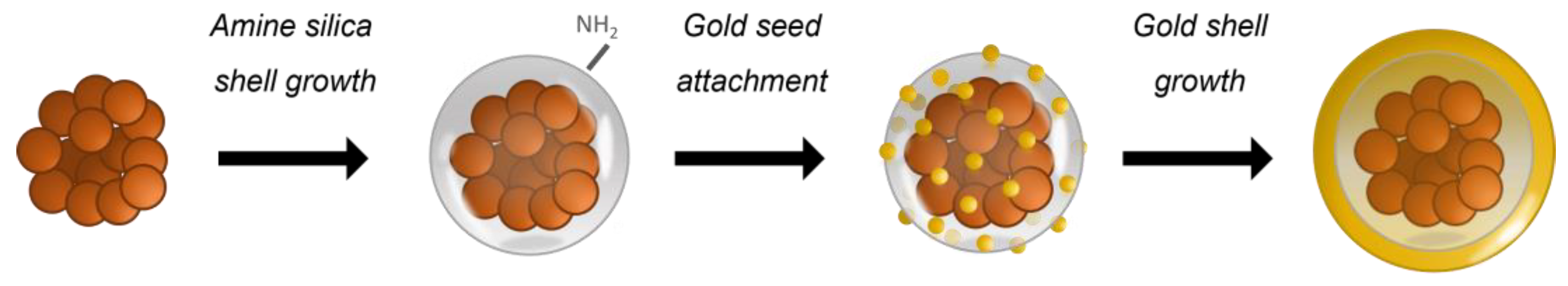
2.1. Materials and Synthesis
2.2. Instruments and Methods
3. Results
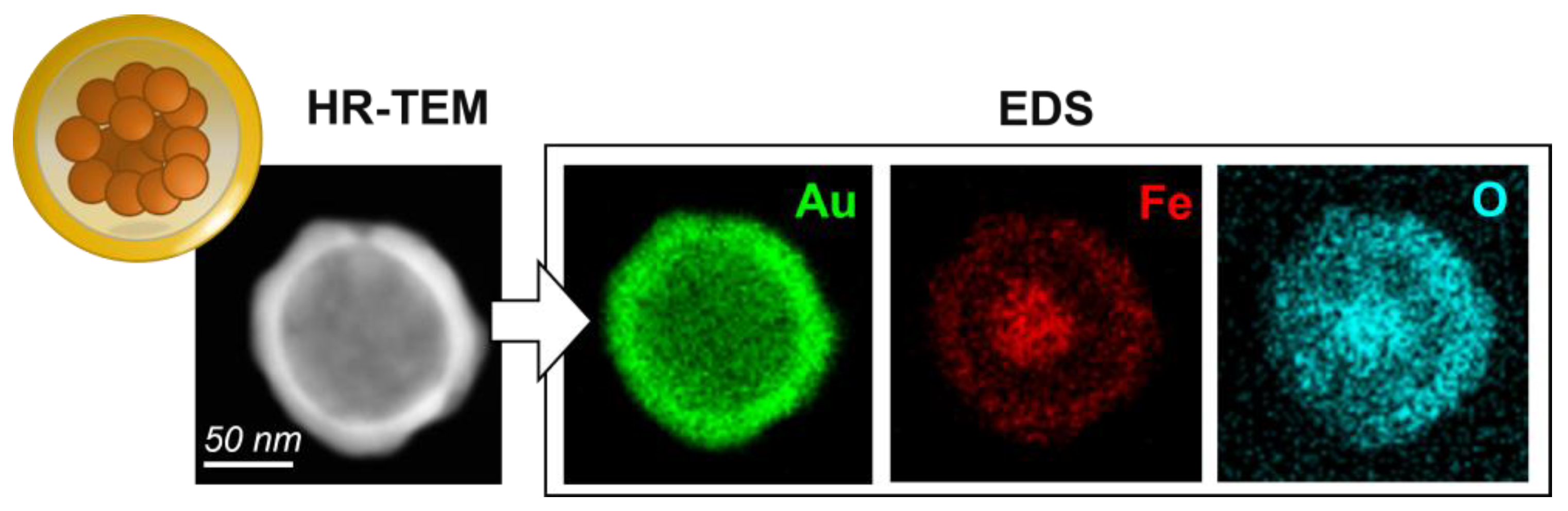
3.1. Morphological and Compositional Analysis
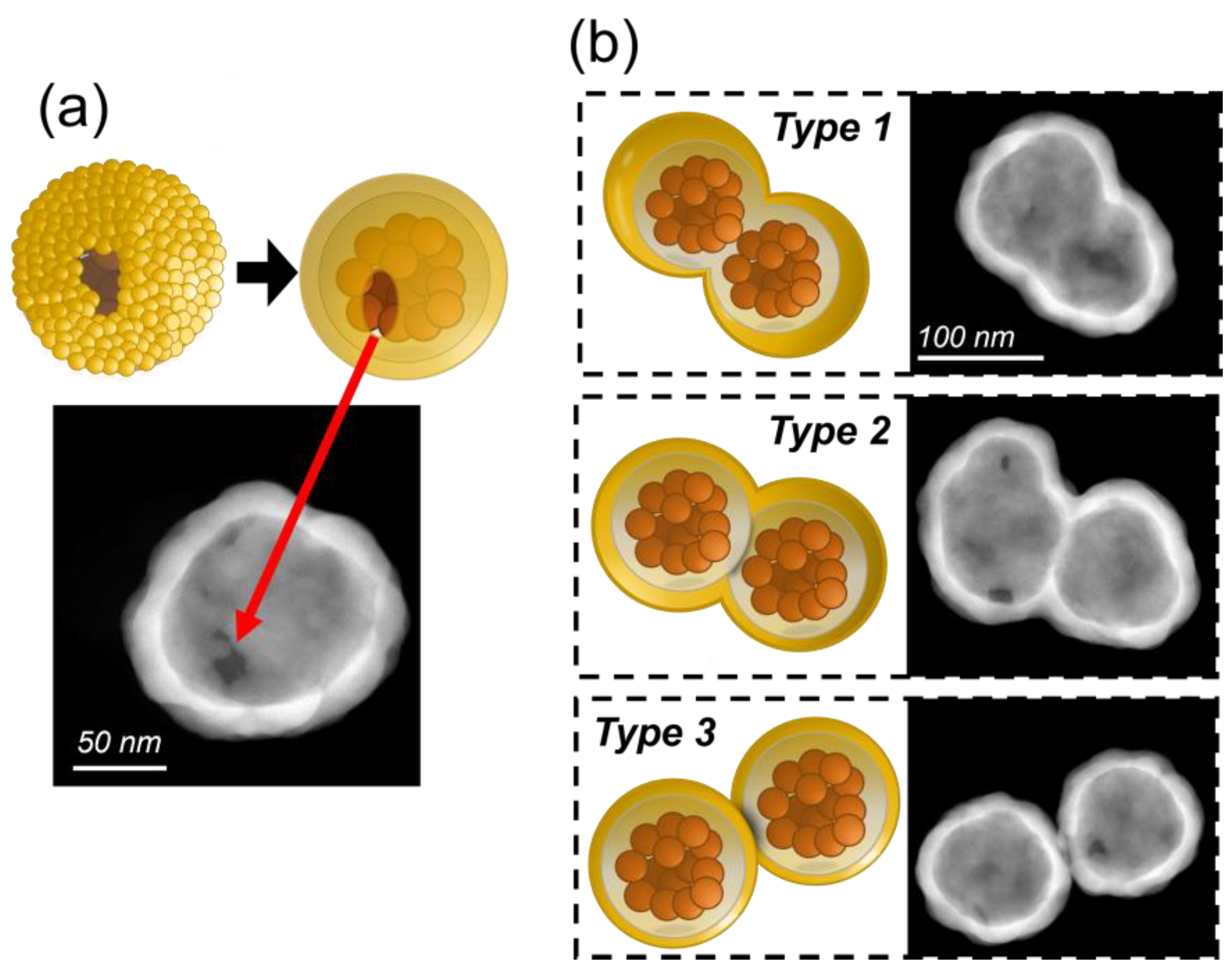
3.2. Further Morphological Analysis: Inhomogeneities and Impurities
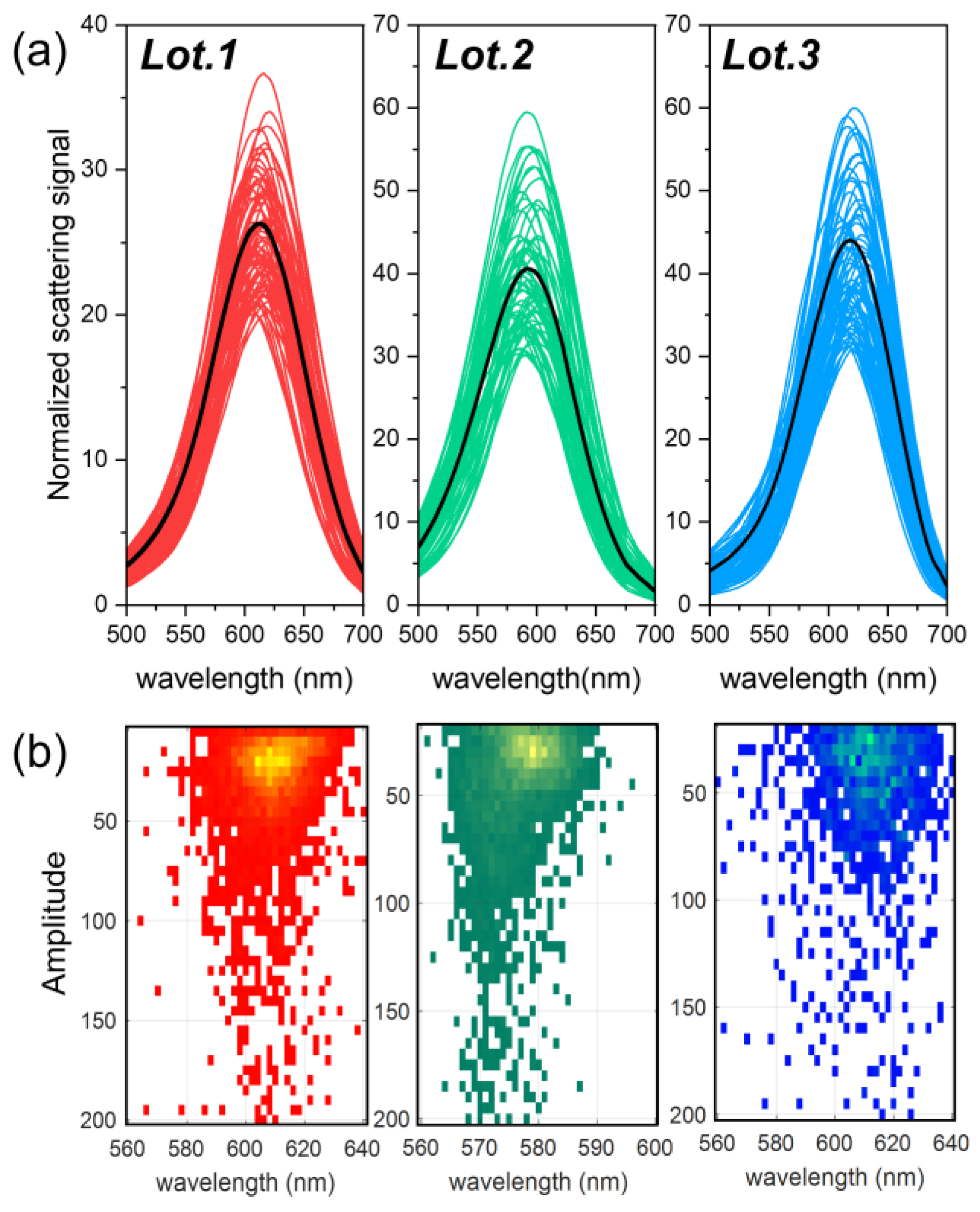
3.3. Optical and Spectral Characterization: Dark-Field Single-Particle Spectrophotometry
3.4. Magnetic Characterization
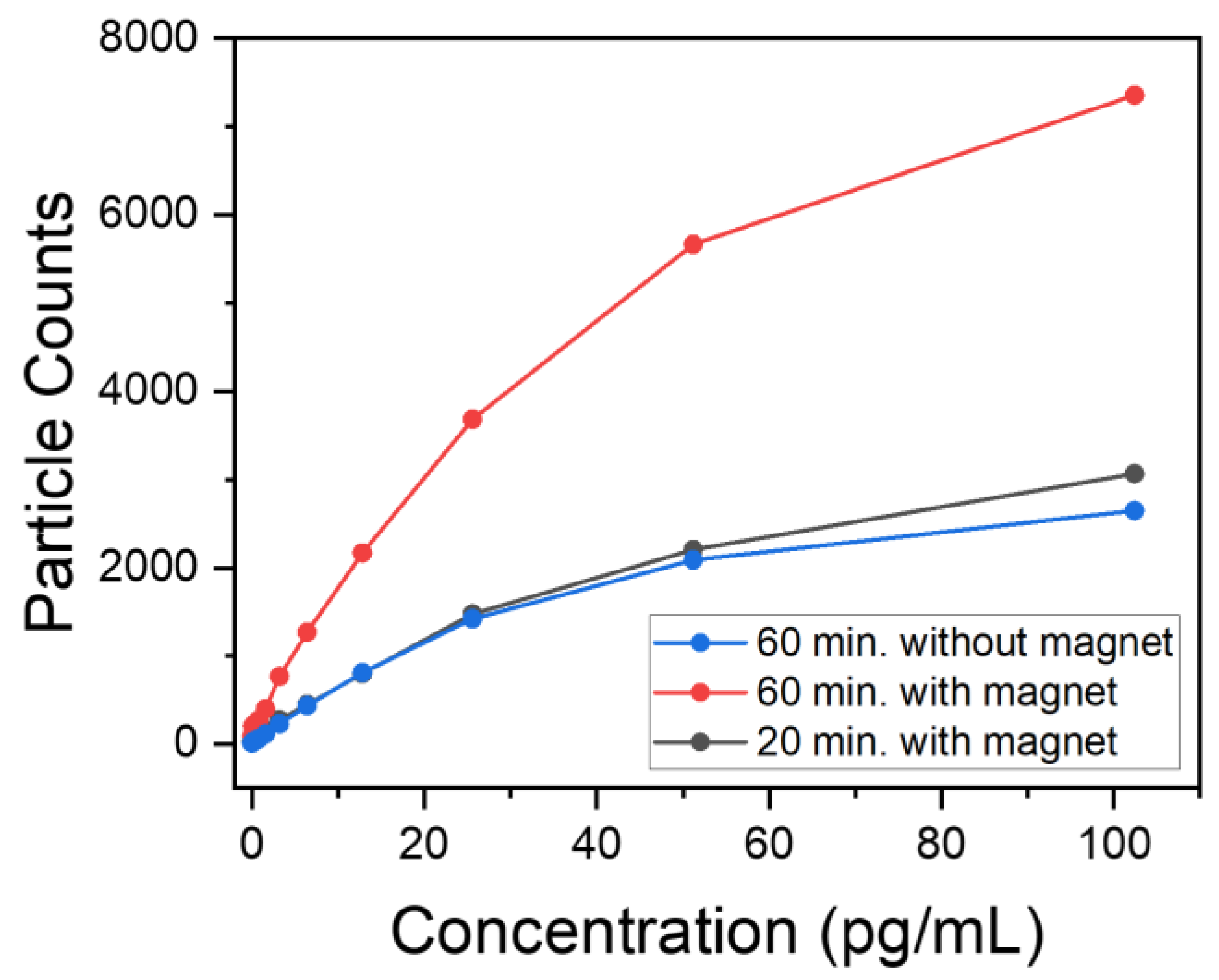
3.5. Biological Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarhadi, V.K.; Armengol, G. Molecular Biomarkers in Cancer. Biomolecules 2022, 12, 1021. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Wang, K.; Oppong-Gyebi, A.; Hu, J. Application of Nanotechnology in Cancer Diagnosis and Therapy—A Mini-Review. Int. J. Med. Sci. 2020, 17, 2964–2973. [Google Scholar] [CrossRef] [PubMed]
- Tempany, C.; Jayender, J.; Kapur, T.; Bueno, R.; Golby, A.; Agar, N.; Jolesz, F. Multimodal Imaging for Improved Diagnosis and Treatment of Cancers. Cancer 2015, 121, 817–827. [Google Scholar] [CrossRef] [PubMed]
- de la Encarnación, C.; Jimenez de Aberasturi, D.; Liz-Marzán, L.M. Multifunctional plasmonic-magnetic nanoparticles for bioimaging and hyperthermia. Adv. Drug Deliv. Rev. 2022, 189, 114484. [Google Scholar] [CrossRef]
- Billen, A.; de Cattelle, A.; Jochum, J.K.; Van Bael, M.J.; Billen, J.; Seo, J.W.; Brullot, W.; Koeckelberghs, G.; Verbiest, T. Novel synthesis of superparamagnetic plasmonic core-shell iron oxide-gold nanoparticles. Phys. B Condens. Matter 2019, 560, 85–90. [Google Scholar] [CrossRef]
- García-Figueiras, R.; Baleato-González, S.; Padhani, A.R.; Luna-Alcalá, A.; Vallejo-Casas, J.; Sala, E.; Vilanova, J.C.; Koh, D.; Herranz-Carnero, M.; Vargas, H.A. How clinical imaging can assess cancer biology. Insights Into Imaging 2019, 10, 28. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- d’Amora, M.; Raffa, V.; De Angelis, F.; Tantussi, F. Toxicological Profile of Plasmonic Nanoparticles in Zebrafish Model. Int. J. Mol. Sci. 2021, 22, 6372. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, S.; Yue, S. Raman Spectroscopy and Imaging for Cancer Diagnosis. J. Heal. Eng. 2018, 2018, 8619342. [Google Scholar] [CrossRef]
- Hanna, K.; Krzoska, E.; Shaaban, A.M.; Muirhead, D.; Abu-Eid, R.; Speirs, V. Raman spectroscopy: Current applications in breast cancer diagnosis, challenges and future prospects. Br. J. Cancer 2022, 126, 1125–1139. [Google Scholar] [CrossRef]
- Jimenez de Aberasturi, D.; Serrano-Montes, A.B.; Langer, J.; Henriksen-Lacey, M.; Parak, W.J.; Liz-Marzán, L.M. Surface Enhanced Raman Scattering Encoded Gold Nanostars for Multiplexed Cell Discrimination. Chem. Mater 2016, 28, 6779–6790. [Google Scholar] [CrossRef]
- Tu, Y.; Cheng, K.; Shen, B.; Cheng, Z. 7—Near-infrared fluorescence nanoparticle-based probes: Application to in vivo imaging of cancer. In Applications of Nanoscience in Photomedicine; Hamblin, M.R., Avci, P., Eds.; Chandos Publishing: Oxford, UK, 2015; pp. 131–151. [Google Scholar]
- Xi, D.; Dong, S.; Meng, X.; Lu, Q.; Meng, L.; Ye, J. Gold nanoparticles as computerized tomography (CT) contrast agents. RSC Adv. 2012, 2, 12515–12524. [Google Scholar] [CrossRef]
- Dong, Y.C.; Hajfathalian, M.; Maidment, P.S.N.; Hsu, J.C.; Naha, P.C.; Si-Mohamed, S.; Breuilly, M.; Kim, J.; Chhour, P.; Douek, P.; et al. Effect of Gold Nanoparticle Size on Their Properties as Contrast Agents for Computed Tomography. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.S.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic nanoparticles in biomedical applications: A review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Shen, Z.; Wu, A.; Chen, X. Iron Oxide Nanoparticle Based Contrast Agents for Magnetic Resonance Imaging. Mol. Pharm. 2017, 14, 1352–1364. [Google Scholar] [CrossRef]
- Gavilán, H.; Avugadda, S.K.; Fernández-Cabada, T.; Soni, N.; Cassani, M.; Mai, B.T.; Chantrell, R.; Pellegrino, T. Magnetic nanoparticles and clusters for magnetic hyperthermia: Optimizing their heat performance and developing combinatorial therapies to tackle cancer. Chem. Soc. Rev. 2021, 50, 11614–11667. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Gorgoń, S.; Radoń, A.; Bajdak-Rusinek, K. Magnetite Nanoparticles in Magnetic Hyperthermia and Cancer Therapies: Challenges and Perspectives. Nanomaterials 2022, 12, 1807. [Google Scholar] [CrossRef] [PubMed]
- Reguera, J.; Aberasturi, D.J.d.; Henriksen-Lacey, M.; Langer, J.; Espinosa, A.; Szczupak, B.; Wilhelm, C.; Liz-Marzán, L.M. Janus plasmonic–magnetic gold–iron oxide nanoparticles as contrast agents for multimodal imaging. Nanoscale 2017, 9, 9467–9480. [Google Scholar] [CrossRef] [PubMed]
- León Félix, L.; Sanz, B.; Sebastián, V.; Torres, T.E.; Sousa, M.H.; Coaquira, J.a.H.; Ibarra, M.R.; Goya, G.F. Gold-decorated magnetic nanoparticles design for hyperthermia applications and as a potential platform for their surface-functionalization. Sci. Rep. 2019, 9, 4185. [Google Scholar] [CrossRef]
- Veiseh, O.; Gunn, J.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef]
- Smith, M.; McKeague, M.; DeRosa, M.C. Synthesis, transfer, and characterization of core-shell gold-coated magnetic nanoparticles. MethodsX 2019, 6, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Moraes Silva, S.; Tavallaie, R.; Sandiford, L.; Tilley, R.D.; Gooding, J.J. Gold coated magnetic nanoparticles: From preparation to surface modification for analytical and biomedical applications. Chem. Commun. 2016, 52, 7528–7540. [Google Scholar] [CrossRef] [PubMed]
- Salgueiriño-Maceira, V.; Correa-Duarte, M.A.; Farle, M.; López-Quintela, A.; Sieradzki, K.; Diaz, R. Bifunctional gold-coated magnetic silica spheres. Chem. Mater. 2006, 18, 2701–2706. [Google Scholar] [CrossRef]
- Lim, J.; Tilton, R.D.; Eggeman, A.; Majetich, S.A. Design and synthesis of plasmonic magnetic nanoparticles. J. Magn. Magn. Mater. 2007, 311, 78–83. [Google Scholar] [CrossRef]
- Yang, D.; Pang, X.; He, Y.; Wang, Y.; Chen, G.; Wang, W.; Lin, Z. Precisely Size-Tunable Magnetic/Plasmonic Core/Shell Nanoparticles with Controlled Optical Properties. Angew. Chem. Int. Ed. 2015, 54, 12091–12096. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H. Size-Controlled Synthesis of Magnetite Nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Synthesis and Characterization of Magnetic-Optical Co–Au Core–Shell Nanoparticles. Available online: https://pubs.acs.org/doi/pdf/10.1021/jp066871y (accessed on 4 August 2023).
- Elbialy, N.S.; Fathy, M.M.; Khalil, W.M. Preparation and characterization of magnetic gold nanoparticles to be used as doxorubicin nanocarriers. Phys. Medica 2014, 30, 843–848. [Google Scholar] [CrossRef]
- Ge, J.; Hu, Y.; Biasini, M.; Beyermann, W.; Yin, Y. Superparamagnetic Magnetite Colloidal Nanocrystal Clusters. Angew. Chem. Int. Ed. 2007, 46, 4342–4345. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Z.; Sang, T.; Cheng, X.; Li, M.; Chen, L.; Wang, Z. Preparation of spherical silica particles by Stöber process with high concentration of tetra-ethyl-orthosilicate. J. Colloid Interface Sci. 2010, 341, 23–29. [Google Scholar] [CrossRef]
- Ruvalcaba-Ontiveros, R.I.; Murillo-Ramírez, J.G.; Medina-Vázquez, J.A.; Carrasco-Hernández, A.R.; Duarte-Möller, J.A.; Esparza-Ponce, H.E. Synthesis of gold decorated silica nanoparticles and their photothermal properties. Micron 2023, 166, 103415. [Google Scholar] [CrossRef]
- Oldenburg, S.J.; Averitt, R.D.; Westcott, S.L.; Halas, N.J. Nanoengineering of optical resonances. Chem. Phys. Lett. 1998, 288, 243–247. [Google Scholar] [CrossRef]
- Hueso, J.L.; Sebastián, V.; Mayoral, Á.; Usón, L.; Arruebo, M.; Santamaría, J. Beyond gold: Rediscovering tetrakis-(hydroxymethyl)-phosphonium chloride (THPC) as an effective agent for the synthesis of ultra-small noble metal nanoparticles and Pt-containing nanoalloys. RSC Adv. 2013, 3, 10427–10433. [Google Scholar] [CrossRef]
- Choma, J.; Dziura, A.; Jamioła, D.; Nyga, P.; Jaroniec, M. Preparation and properties of silica–gold core–shell particles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 373, 167–171. [Google Scholar] [CrossRef]
- Lermusiaux, L.; Plissonneau, M.; Bertry, L.; Drisko, G.L.; Buissette, V.; Le Mercier, T.; Duguet, E.; Tréguer-Delapierre, M. Seeded growth of ultrathin gold nanoshells using polymer additives and microwave radiation. Sci. Rep. 2021, 11, 17831. [Google Scholar] [CrossRef] [PubMed]
- Thon, A.; Pini, V.; Salvador-Matar Renteria, A.; Cebrián Hernando, V.; García Aguado, C.; Ahumada Heredero, O. Method for Optically Detecting Biomarkers. U.S. Patent US11519856B2, 6 December 2022. [Google Scholar]
- Pini, V.; Thon, A.; Salvador-Matar Renteria, A.; Cebrián Hernando, V.; García Aguado, C.; Ahumada Heredero, O. Biosensor Platform and Method for the Simultaneous, Multiplexed, Ultra-Sensitive and High Throughput Optical Detection of Biomarkers. U.S. Patent US11519843B2, 6 December 2022. [Google Scholar]
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape- and Size-Dependent Refractive Index Sensitivity of Gold Nanoparticles. Langmuir 2008, 24, 5233–5237. [Google Scholar] [CrossRef] [PubMed]
- Robinson, I.; Tung, L.D.; Maenosono, S.; Wälti, C.; Thanh, N.T.K. Synthesis of core-shell gold coated magnetic nanoparticles and their interaction with thiolated DNA. Nanoscale 2010, 2, 2624. [Google Scholar] [CrossRef] [PubMed]
- Anjum, D.H. Characterization of nanomaterials with transmission electron microscopy. IOP Conf. Ser. Mater. Sci. Eng. 2016, 146, 012001. [Google Scholar] [CrossRef]
- Yuen, H.; Princen, J.; Illingworth, J.; Kittler, J. A Comparative Study of Hough Transform Methods for Circle Finding. in Procedings of the Alvey Vision Conference. Alvey Vis. Club 1990, 8, 71–77. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, X. Electron microscopic methods (TEM, SEM and energy dispersal spectroscopy). In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Ulloa, J.A.; Lorusso, G.; Evangelisti, M.; Camón, A.; Barberá, J.; Serrano, J.L. Magnetism of Dendrimer-Coated Gold Nanoparticles: A Size and Functionalization Study. J. Phys. Chem. C 2021, 125, 20482–20487. [Google Scholar] [CrossRef]
- Gutiérrez, L.; de la Cueva, L.; Moros, M.; Mazarío, E.; de Bernardo, S.; de la Fuente, J.M.; Morales, M.P.; Salas, G. Aggregation effects on the magnetic properties of iron oxide colloids. Nanotechnology 2019, 30, 112001. [Google Scholar] [CrossRef] [PubMed]
- Levin, C.S.; Hofmann, C.; Ali, T.A.; Kelly, A.T.; Morosan, E.; Nordlander, P.; Whitmire, K.H.; Halas, N.J. Magnetic−Plasmonic Core−Shell Nanoparticles. ACS Nano 2009, 3, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Myroshnychenko, V.; Rodríguez-Fernández, J.; Pastoriza-Santos, I.; Funston, A.M.; Novo, C.; Mulvaney, P.; Liz-Marzán, L.M.; García de Abajo, F.J. Modelling the optical response of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1792. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Meneghetti, M. Size Evaluation of Gold Nanoparticles by UV−vis Spectroscopy. J. Phys. Chem. C 2009, 113, 4277–4285. [Google Scholar] [CrossRef]
- Zheng, T.; Bott, S.; Huo, Q. Techniques for Accurate Sizing of Gold Nanoparticles Using Dynamic Light Scattering with Particular Application to Chemical and Biological Sensing Based on Aggregate Formation. ACS Appl. Mater. Interfaces 2016, 8, 21585–21594. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV−Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Calvo, R.; Thon, A.; Saad, A.; Salvador-Matar, A.; Manso-Silván, M.; Ahumada, Ó.; Pini, V. Size characterization of plasmonic nanoparticles with dark-field single particle spectrophotometry. Sci. Rep. 2022, 12, 17231. [Google Scholar] [CrossRef]
- Pini, V.; Kosaka, P.M.; Ruz, J.J.; Malvar, O.; Encinar, M.; Tamayo, J.; Calleja, M. Spatially multiplexed dark-field microspectrophotometry for nanoplasmonics. Sci. Rep. 2016, 6, 22836. [Google Scholar] [CrossRef]
- Calvo, R.; Pini, V.; Thon, A.; Saad, A.; Salvador-Matar, A.; Manso Silván, M.; Ahumada, Ó. Amplitude-Resolved Single Particle Spectrophotometry: A Robust Tool for High-Throughput Size Characterization of Plasmonic Nanoparticles. Nanomaterials 2023, 13, 2401. [Google Scholar] [CrossRef]
- Kreibig, U.; Fragstein, C.v. The limitation of electron mean free path in small silver particles. Z. Phys. 1969, 224, 307–323. [Google Scholar] [CrossRef]
- Johnson, P.B.; Christy, R.W. Optical Constants of the Noble Metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]
- Berkowitz, A.E.; Schuele, W.J.; Flanders, P.J. Influence of Crystallite Size on the Magnetic Properties of Acicular γ-Fe2O3 Particles. J. Appl. Phys. 1968, 39, 1261–1263. [Google Scholar] [CrossRef]
- Grubisha, D.S.; Lipert, R.J.; Park, H.; Driskell, J.; Porter, M.D. Femtomolar detection of prostate-specific antigen: An immunoassay based on surface-enhanced Raman scattering and immunogold labels. Anal. Chem. 2003, 75, 5936–5943. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yan, Q.; Liu, H.; Zhou, X.; Xiao, S. Different EDC/NHS Activation Mechanisms between PAA and PMAA Brushes and the Following Amidation Reactions. Langmuir 2011, 27, 12058–12068. [Google Scholar] [CrossRef] [PubMed]
- Bradley, Z.; Coleman, P.A.; Courtney, M.A.; Fishlock, S.; McGrath, J.; Uniacke-Lowe, T.; Bhalla, N.; McLaughlin, J.A.; Hogan, J.; Hanrahan, J.P.; et al. Effect of Selenium Nanoparticle Size on IL-6 Detection Sensitivity in a Lateral Flow Device. ACS Omega 2023, 8, 8407–8414. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yu, X.; Qian, Y.; Chen, W.; Shen, J. Multifunctional magnetic iron oxide nanoparticles: An advanced platform for cancer theranostics. Theranostics 2020, 10, 6278–6309. [Google Scholar] [CrossRef]
- Duraisamy, K.; Gangadharan, A.; Martirosyan, K.S.; Sahu, N.K.; Manogaran, P.; Easwaradas Kreedapathy, G. Fabrication of Multifunctional Drug Loaded Magnetic Phase Supported Calcium Phosphate Nanoparticle for Local Hyperthermia Combined Drug Delivery and Antibacterial Activity. ACS Appl. Bio Mater. 2023, 6, 104–116. [Google Scholar] [CrossRef]

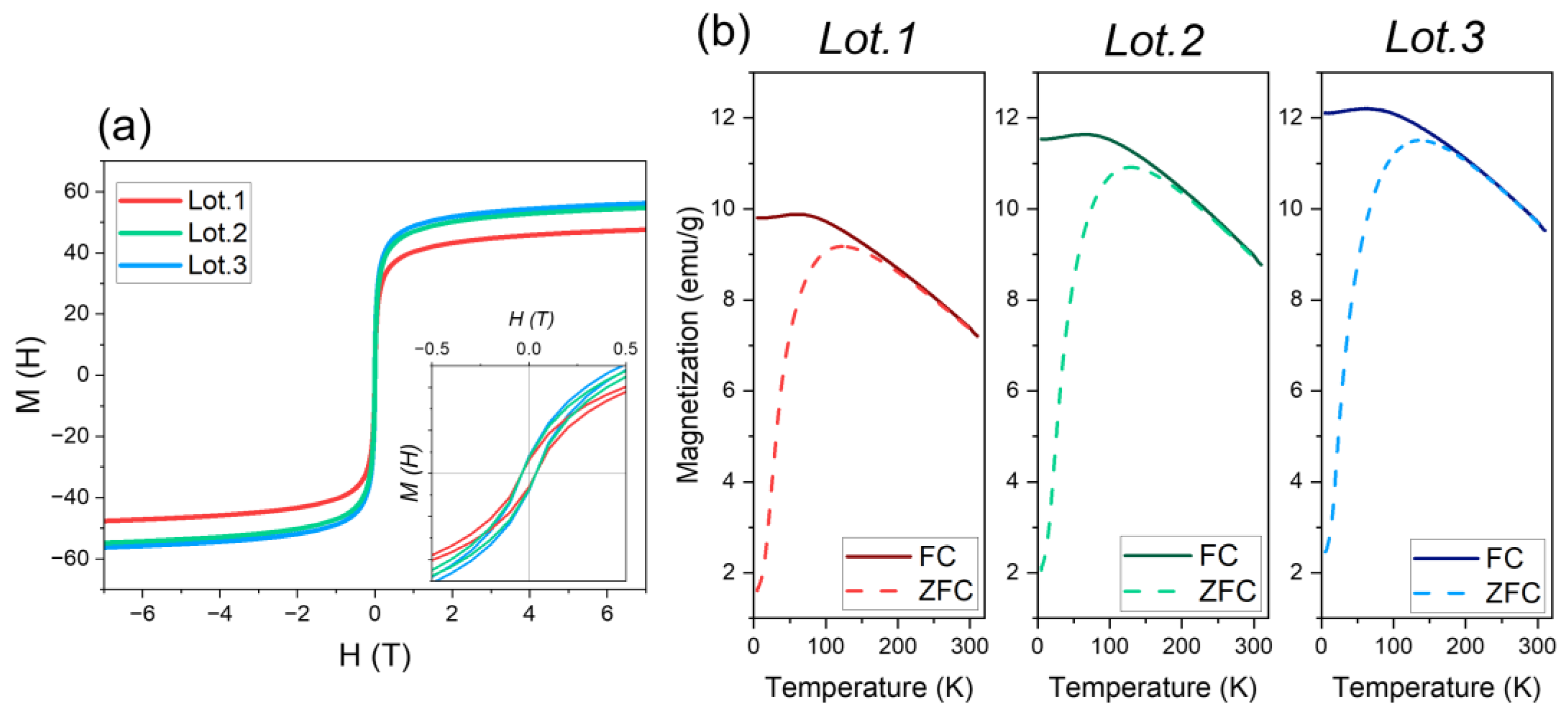
| Lot | TEM Size (nm) | TEM CV % | HR-TEM Core Size (nm) | HR-TEM Shell Size (nm) | Circularity 1 |
|---|---|---|---|---|---|
| Lot 1 | 136 | 12.3 | 95 ± 20 | 21 ± 8 | 0.89 ± 0.05 |
| Lot 2 | 131 | 14.3 | 92 ± 21 | 16 ± 6 | 0.91 ± 0.05 |
| Lot 3 | 131 | 11.9 | 96 ± 12 | 18 ± 8 | 0.91 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo, R.; Rodriguez Mariblanca, I.; Pini, V.; Dias, M.; Cebrian, V.; Thon, A.; Saad, A.; Salvador-Matar, A.; Ahumada, Ó.; Manso Silván, M.; et al. Novel Characterization Techniques for Multifunctional Plasmonic–Magnetic Nanoparticles in Biomedical Applications. Nanomaterials 2023, 13, 2929. https://doi.org/10.3390/nano13222929
Calvo R, Rodriguez Mariblanca I, Pini V, Dias M, Cebrian V, Thon A, Saad A, Salvador-Matar A, Ahumada Ó, Manso Silván M, et al. Novel Characterization Techniques for Multifunctional Plasmonic–Magnetic Nanoparticles in Biomedical Applications. Nanomaterials. 2023; 13(22):2929. https://doi.org/10.3390/nano13222929
Chicago/Turabian StyleCalvo, Rodrigo, Isabel Rodriguez Mariblanca, Valerio Pini, Monica Dias, Virginia Cebrian, Andreas Thon, Asis Saad, Antonio Salvador-Matar, Óscar Ahumada, Miguel Manso Silván, and et al. 2023. "Novel Characterization Techniques for Multifunctional Plasmonic–Magnetic Nanoparticles in Biomedical Applications" Nanomaterials 13, no. 22: 2929. https://doi.org/10.3390/nano13222929
APA StyleCalvo, R., Rodriguez Mariblanca, I., Pini, V., Dias, M., Cebrian, V., Thon, A., Saad, A., Salvador-Matar, A., Ahumada, Ó., Manso Silván, M., Saunders, A. E., Wang, W., & Stassinopoulos, A. (2023). Novel Characterization Techniques for Multifunctional Plasmonic–Magnetic Nanoparticles in Biomedical Applications. Nanomaterials, 13(22), 2929. https://doi.org/10.3390/nano13222929







