Lithiophilic Interlayer with Electrolyte-Reservoir and Dendrite-Buffer for High-Performance Lithium Metal Batteries
Abstract
1. Introduction
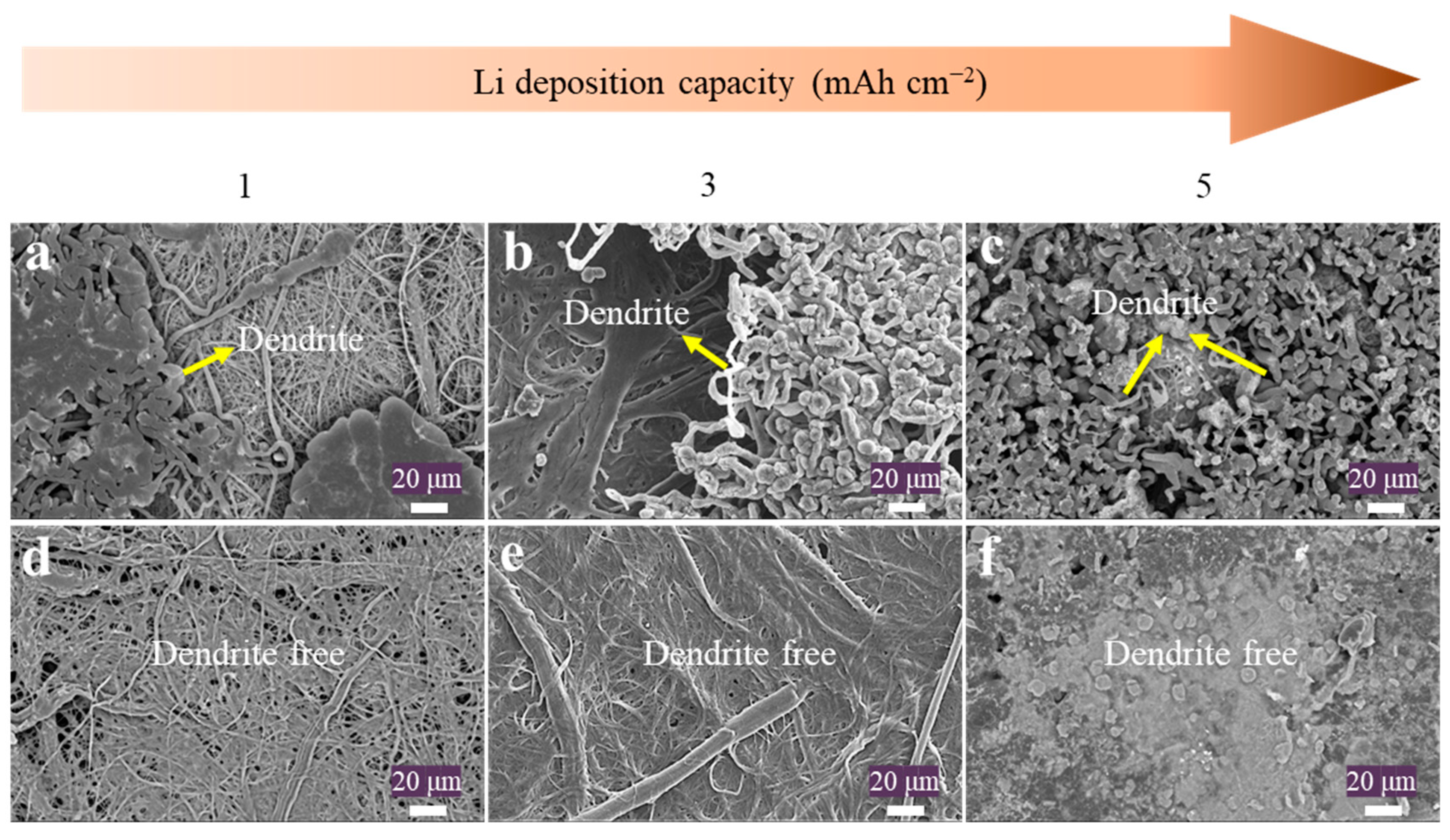
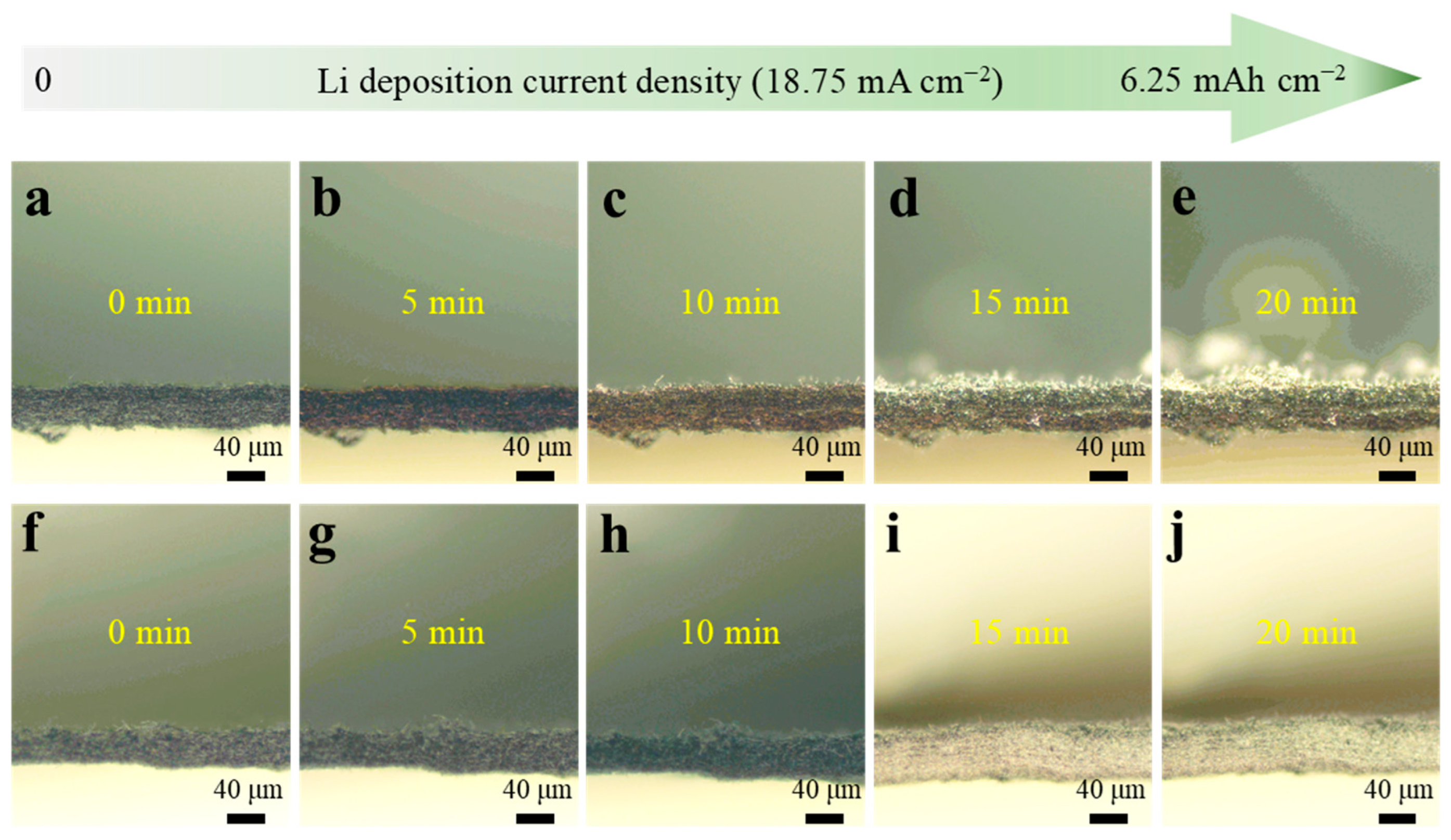
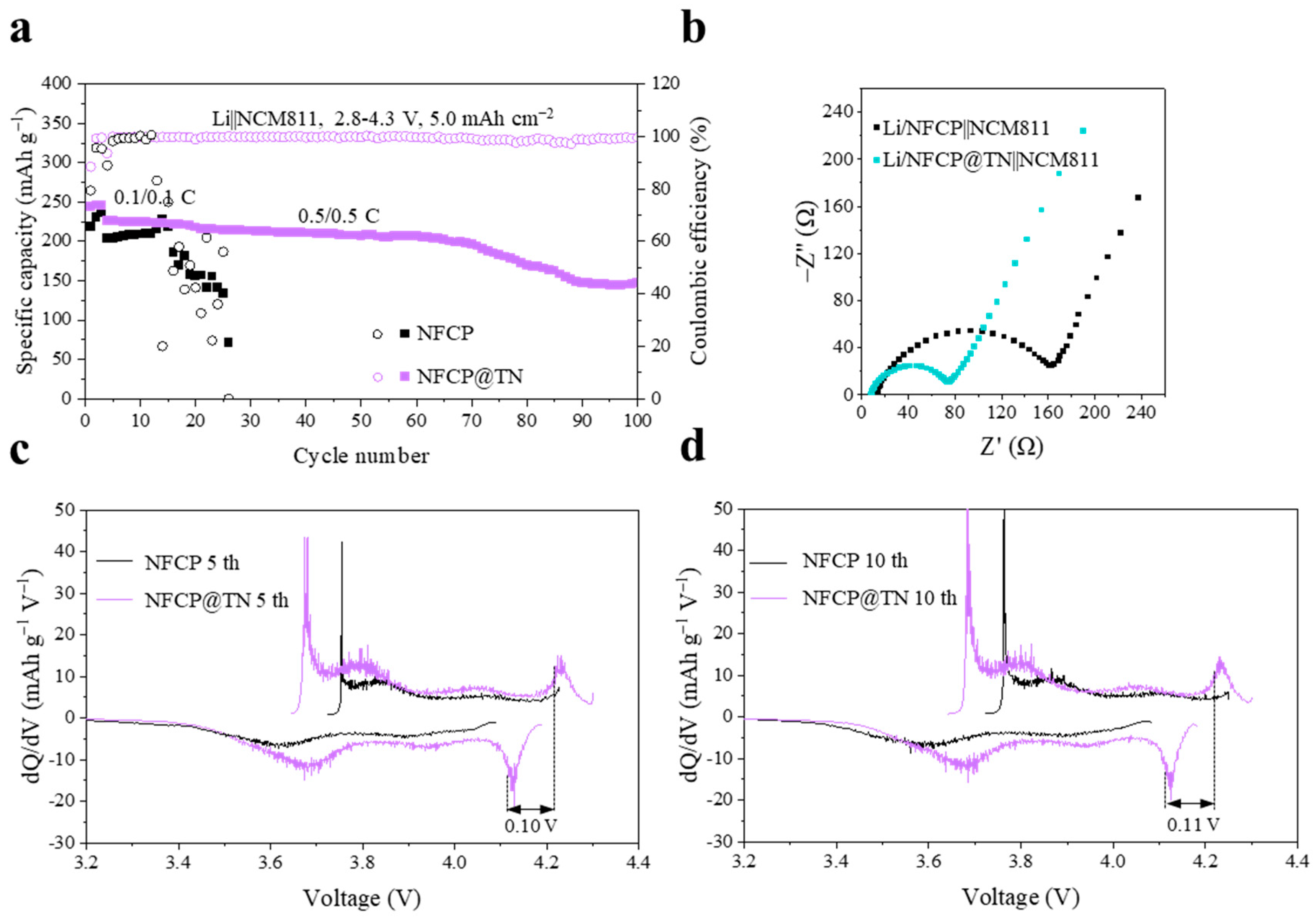
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du, M.; He, Z.; Zhang, Y.; Cai, Y.P.; Zheng, Q. Sustainable Release of LiNO3 from a Fluorine-Decorated Metal–Organic Framework Separator to Enable High-Performance Li-Metal Batteries in Carbonate Electrolytes. Adv. Energy Mater. 2025, 2403674. [Google Scholar] [CrossRef]
- Jiang, J.; Li, M.; Liu, X.; Yi, J.; Jiang, Y.; Wu, C.; Liu, H.; Zhao, B.; Li, W.; Sun, X.; et al. Multifunctional Additives to Realize Dendrite-Free Lithium Deposition in Carbonate Electrolytes toward Low-Temperature Li Metal Batteries. Adv. Energy Mater. 2024, 14, 2400365. [Google Scholar] [CrossRef]
- Ming, J.; Cao, Z.; Wahyudi, W.; Li, M.; Kumar, P.; Wu, Y.; Hwang, J.-Y.; Hedhili, M.N.; Cavallo, L.; Sun, Y.-K.; et al. New insights on graphite anode stability in rechargeable batteries: Li ion coordination structures prevail over solid electrolyte interphases. ACS Energy Lett. 2018, 3, 335–340. [Google Scholar] [CrossRef]
- Sun, D.; Zhao, L.; Xiao, Z.; Zhao, K.; Lin, R.; Song, H.; Zhang, X.; Ma, X.; Peng, C.; Huang, X.; et al. Boosting of reversible capacity delivered at a low voltage below 0.5 V in mildly expanded graphitized needle coke anode for a high-energy lithium ion battery. J. Energy Chem. 2022, 74, 100–110. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as anode materials: Fundamental mechanism, recent progress and advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Chae, S.-U.; Yi, S.; Yoon, J.; Hyun, J.C.; Doo, S.; Lee, S.; Lee, J.; Kim, S.J.; Yun, Y.S.; Lee, J.-H.; et al. Highly defective Ti3CNTx-MXene-based fiber membrane anode for lithium metal batteries. Energy Storage Mater. 2022, 52, 76–84. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, H.; Zhang, Y.; Du, Y.; Wang, Z.; Zhang, J. 3D lithiophilic and conductive N-CNT@Cu2O@Cu framework for a dendrite-free lithium metal battery. Chem. Mater. 2020, 32, 9656–9663. [Google Scholar] [CrossRef]
- Lin, L.; Zheng, H.; Luo, Q.; Lin, J.; Wang, L.; Xie, Q.; Peng, D.L.; Lu, J. Regulating lithium nucleation at the electrolyte/electrode interface in lithium metal batteries. Adv. Funct. Mater. 2024, 34, 2315201. [Google Scholar] [CrossRef]
- Pal, U.; Rakov, D.; Lu, B.; Sayahpour, B.; Chen, F.; Roy, B.; MacFarlane, D.R.; Armand, M.; Howlett, P.C.; Meng, Y.S.; et al. Interphase control for high performance lithium metal batteries using ether aided ionic liquid electrolyte. Energy Environ. Sci. 2022, 15, 1907–1919. [Google Scholar] [CrossRef]
- Weng, C.; Ma, L.; Wang, B.; Meng, F.; Yang, J.; Ji, Y.; Liu, B.; Mai, W.; Huang, S.; Pan, L.; et al. Single-solvent ionic liquid strategy achieving wide-temperature and ultra-high cut-off voltage for lithium metal batteries. Energy Storage Mater. 2024, 71, 103584. [Google Scholar] [CrossRef]
- Tang, W.; Zhao, T.; Wang, K.; Yu, T.; Lv, R.; Li, L.; Wu, F.; Chen, R. Dendrite-free lithium metal batteries enabled by coordination chemistry in polymer-ceramic modified separators. Adv. Funct. Mater. 2024, 34, 2314045. [Google Scholar] [CrossRef]
- Liu, X.; Tan, H.; Li, Y.; Wu, H.; Wu, S.; Yang, L.; Liang, Y.; Xu, G.; Huang, J.; Wang, G.; et al. Constructing Fast Ion/Electron Conducting Pathway within 3D Stable Scaffold for Dendrite-Free Lithium Metal Anode. Adv. Funct. Mater. 2024, 35, 2420382. [Google Scholar] [CrossRef]
- Tan, L.; Wei, C.; Zhang, Y.; Xiong, S.; Li, H.; Feng, J. Self-assembled, highly-lithiophilic and well-aligned biomass engineered MXene paper enables dendrite-free lithium metal anode in carbonate-based electrolyte. J. Energy Chem. 2022, 69, 221–230. [Google Scholar] [CrossRef]
- Zhang, D.; Dai, A.; Wu, M.; Shen, K.; Xiao, T.; Hou, G.; Lu, J.; Tang, Y. Lithiophilic 3D porous CuZn current collector for stable lithium metal batteries. ACS Energy Lett. 2019, 5, 180–186. [Google Scholar] [CrossRef]
- Wan, M.; Duan, X.; Cui, H.; Du, J.; Fu, L.; Chen, Z.; Lu, Z.; Li, G.; Li, Y.; Mao, E.; et al. Stabilized Li metal anode with robust C-Li3N interphase for high energy density batteries. Energy Storage Mater. 2022, 46, 563–569. [Google Scholar] [CrossRef]
- Wang, M.; Ren, Z.; Chen, Z.; Lin, H.; Yan, J.; Lu, Z.; Chen, S.; Li, H.; Shen, Y.; Xie, M.; et al. A multifunctional subassembly of carbon nanotube paper for stable lithium metal anodes. Mater. Today Energy 2022, 29, 101134. [Google Scholar] [CrossRef]
- Zhang, T.; Ran, F. Design strategies of 3D carbon-based electrodes for charge/ion transport in lithium ion battery and sodium ion battery. Adv. Funct. Mater. 2021, 31, 2010041. [Google Scholar] [CrossRef]
- Huang, A.; Wu, Y.; Huang, H.; Li, C.; Sun, Y.; Li, L.; Peng, S. Lithiophilic Mo2C Clusters-Embedded Carbon Nanofibers for High Energy Density Lithium Metal Batteries. Adv. Funct. Mater. 2023, 33, 2303111. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Wu, G.; Ma, T.; Li, M.; Tian, Y.; Chen, S.; Cai, S.; Li, Z. Lithiophilic Interlayer with Electrolyte-Reservoir and Dendrite-Buffer for High-Performance Lithium Metal Batteries. Nanomaterials 2025, 15, 710. https://doi.org/10.3390/nano15100710
Shen H, Wu G, Ma T, Li M, Tian Y, Chen S, Cai S, Li Z. Lithiophilic Interlayer with Electrolyte-Reservoir and Dendrite-Buffer for High-Performance Lithium Metal Batteries. Nanomaterials. 2025; 15(10):710. https://doi.org/10.3390/nano15100710
Chicago/Turabian StyleShen, Huasen, Guoning Wu, Tingting Ma, Mengjun Li, Yunan Tian, Si Chen, Shaojun Cai, and Zhaohuai Li. 2025. "Lithiophilic Interlayer with Electrolyte-Reservoir and Dendrite-Buffer for High-Performance Lithium Metal Batteries" Nanomaterials 15, no. 10: 710. https://doi.org/10.3390/nano15100710
APA StyleShen, H., Wu, G., Ma, T., Li, M., Tian, Y., Chen, S., Cai, S., & Li, Z. (2025). Lithiophilic Interlayer with Electrolyte-Reservoir and Dendrite-Buffer for High-Performance Lithium Metal Batteries. Nanomaterials, 15(10), 710. https://doi.org/10.3390/nano15100710





