Electrospun Bis(2,4,4-trimethylpentyl) Tetradecyltrihexylphosphinate/Polyacrylonitrile Nanofiber Membranes with Enhanced Benzene, Toluene, and Xylene Adsorption Performance and Regenerability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of [P(14)666]TMPP/PAN Composite Membrane via Electrospinning
2.3. Characterisation
2.4. VOCs Adsorption Experiment
- (1)
- Sample Preparation: Each membrane specimen was sectioned into dimensions of approximately 3 cm × 5 cm, weighed (designated as M0), and positioned within a tiny glass container.
- (2)
- Vapour Generation: 1 g each of benzene, toluene, and xylene was introduced into a 100 mL sealed reagent container, permitting the liquids to volatilise and form a saturated vapour phase of BTX.
- (3)
- Exposure Setup: The glass vial containing the membrane sample was positioned within the BTX-saturated bottle, guaranteeing that the membrane was solely exposed to the vapour phase, avoiding direct contact with the liquids.
- (4)
- Adsorption Duration: The system was sustained at 30 °C for 24 h to facilitate adequate adsorption.
- (5)
- Post-adsorption: The sample was extracted, and its ultimate weight (M₁) was documented.
- (6)
- Calculation of Adsorption Capacity: The specific adsorption capacity (C, in mg/g) was determined using the subsequent equation, with each test conducted in triplicate:
2.5. Regeneration of TMPP/P Membrane for Reuse
2.6. Adsorption Kinetics Models
3. Results and Discussion
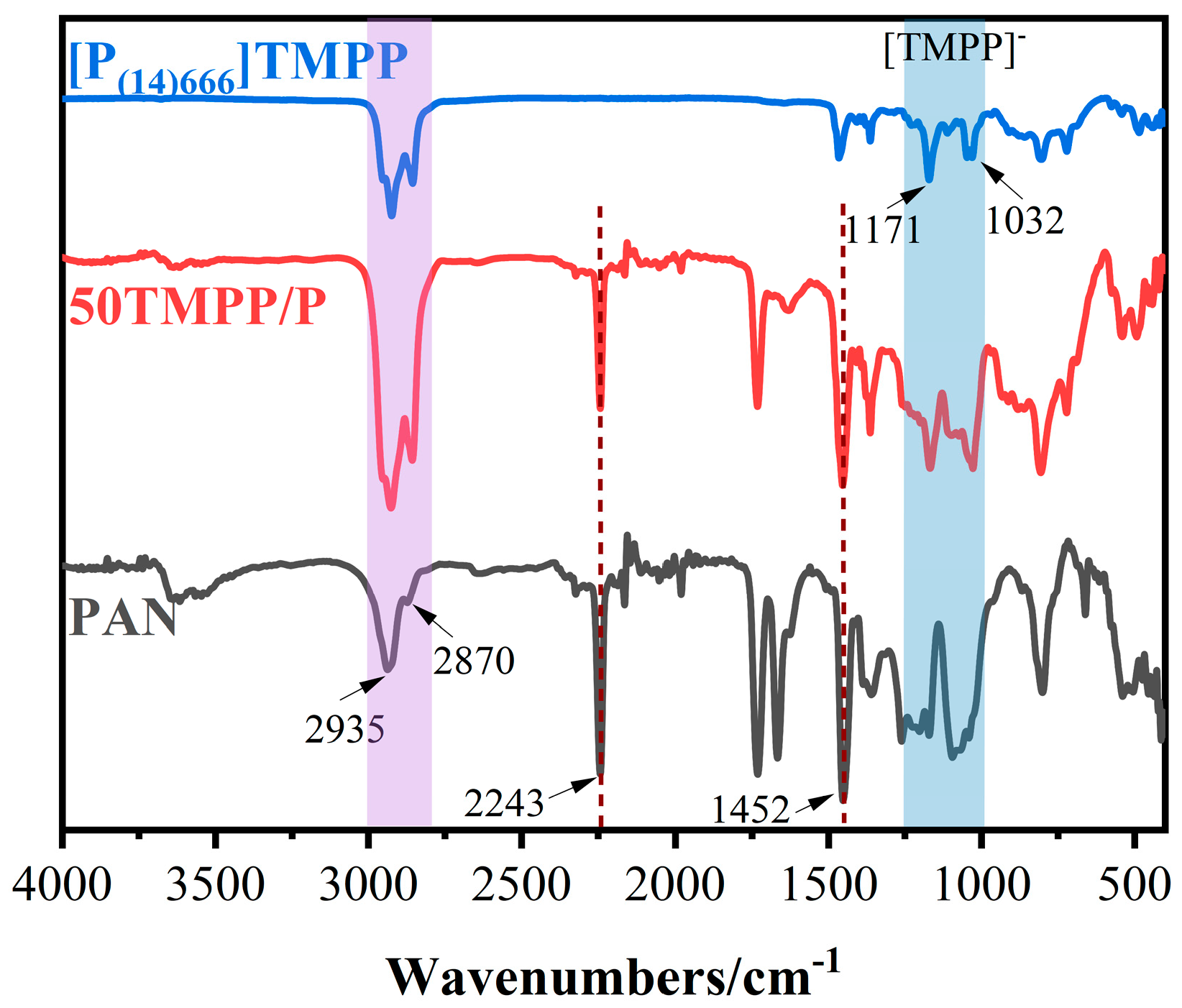
3.1. Characterisation of [P(14)666]TMPP and TMPP/P
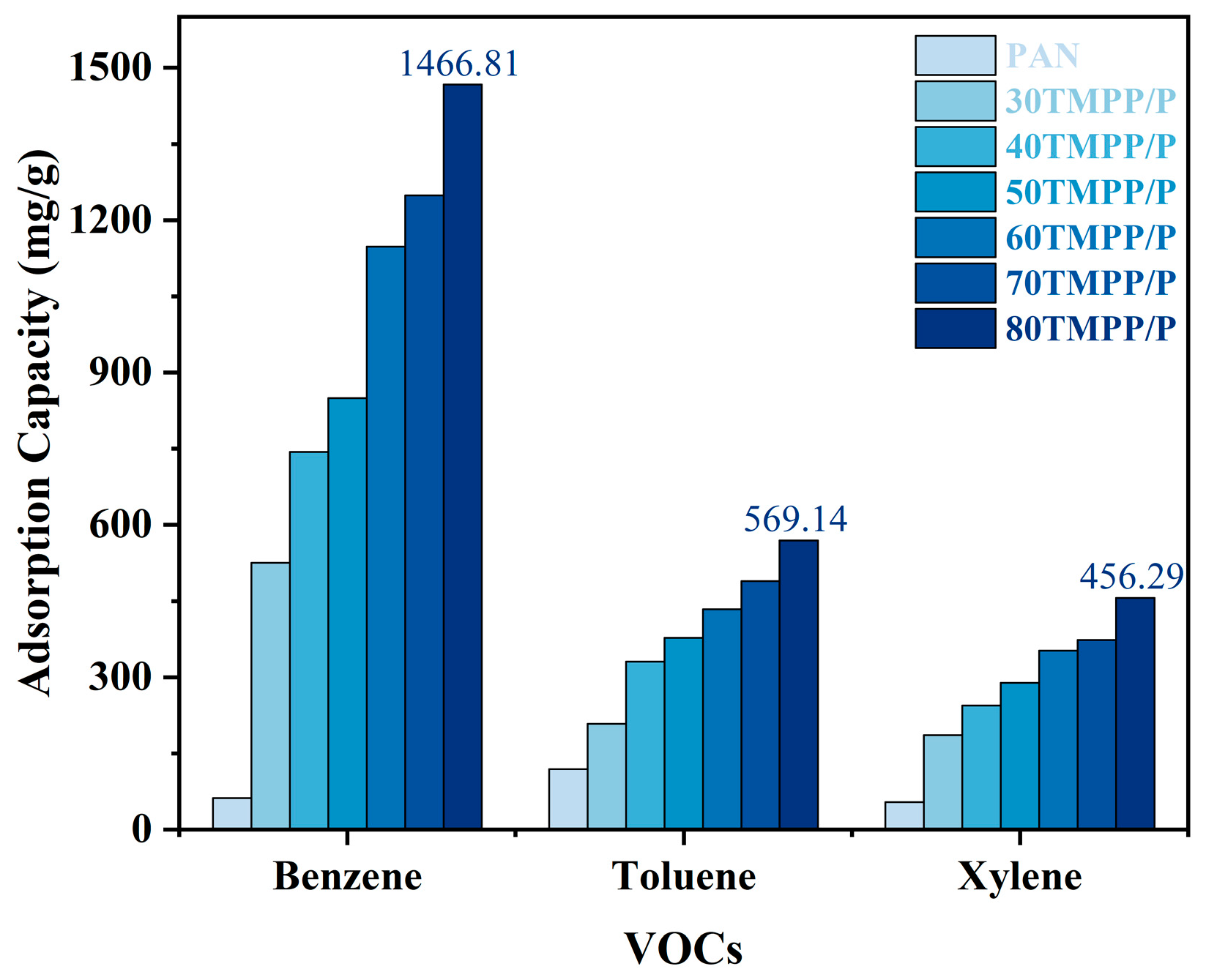
3.2. VOC Absorption Performance of TMPP/P Membranes
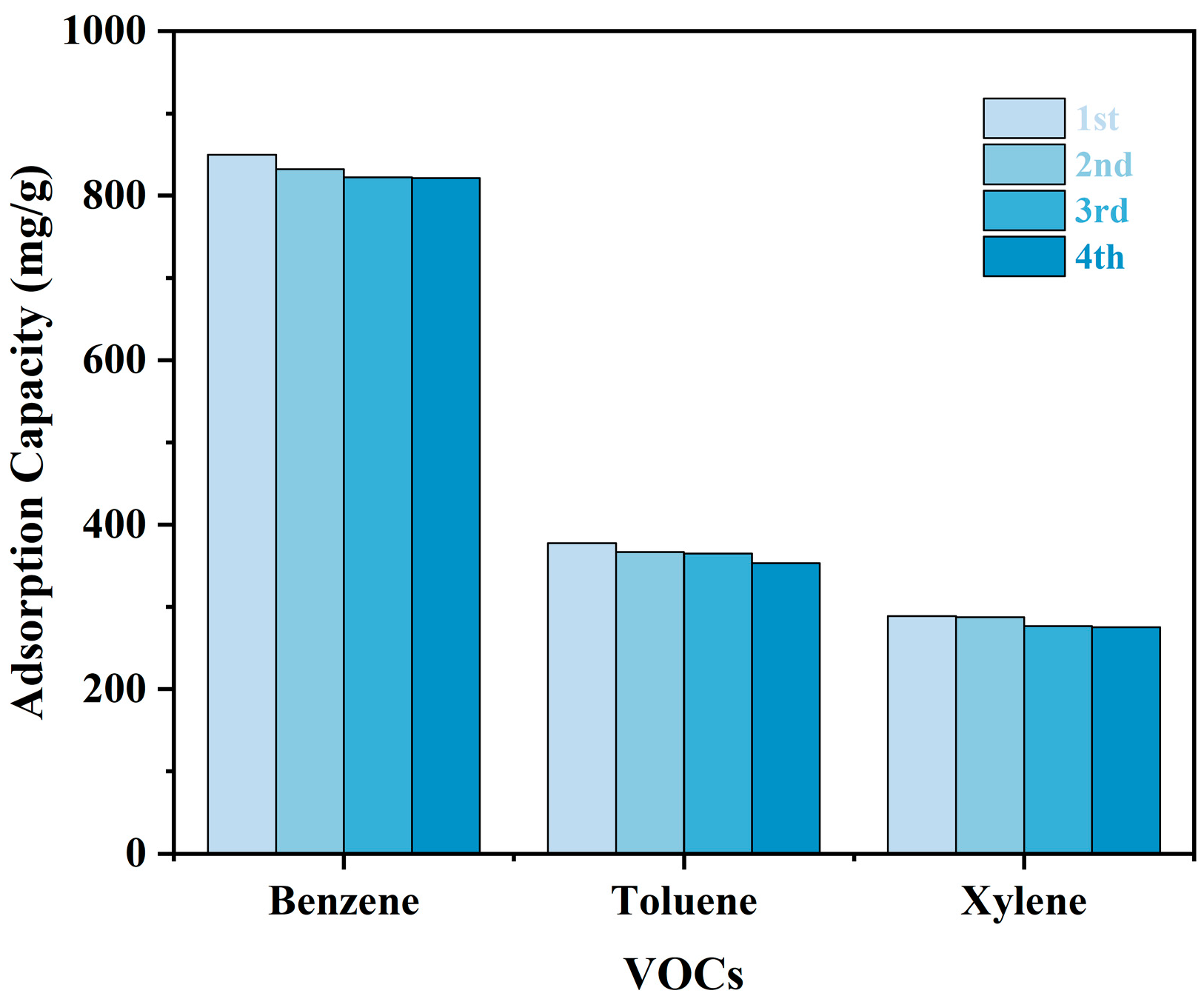
3.3. Regeneration of TMPP/P Membranes for Reuse
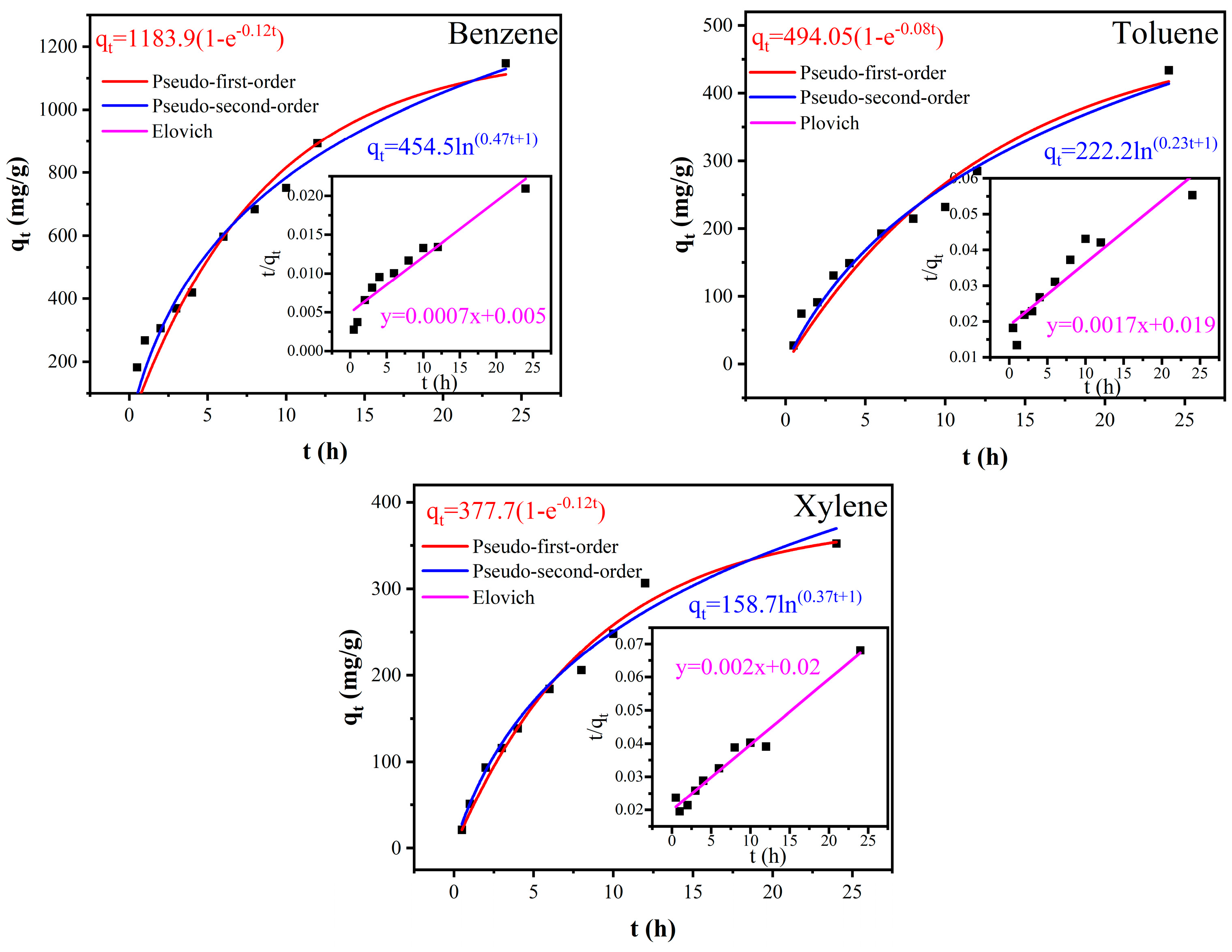
3.4. Kinetic Models Fitting
3.5. Comparison of the Adsorption Performance of TMPP/P Membranes with Other Adsorbents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ge, J.C.; Choi, N.J. Fabrication of functional polyurethane/rare earth nanocomposite membranes by electrospinning and its VOCs absorption capacity from air. Nanomaterials 2017, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, M.; Wysocki, C.J.; Leyden, J.J.; Spielman, A.; Sun, X.; Preti, G. Analyses of volatile organic compounds from human skin. Br. J. Dermatol. 2008, 159, 780–791. [Google Scholar] [CrossRef]
- Dobslaw, D.; Schulz, A.; Helbich, S.; Dobslaw, C.; Engesser, K.-H. VOC removal and odor abatement by a low-cost plasma enhanced biotrickling filter process. J. Environ. Chem. Eng. 2017, 5, 5501–5511. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ling, Z.H.; Cheng, H.R.; Simpson, I.J.; Lyu, X.P.; Wang, X.M.; Shao, M.; Lu, H.X.; Ayoko, G.; Zhang, Y.L.; et al. Tropospheric volatile organic compounds in China. Sci. Total Environ. 2017, 574, 1021–1043. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.P.; Chen, N.; Guo, H.; Zhang, W.H.; Wang, N.; Wang, Y.; Liu, M. Ambient volatile organic compounds and their effect on ozone production in Wuhan, central China. Sci. Total Environ. 2016, 541, 200–209. [Google Scholar] [CrossRef]
- Topuz, F.; Abdulhamid, M.A.; Hardian, R.; Holtzl, T.; Szekely, G. Nanofibrous membranes comprising intrinsically microporous polyimides with embedded metal–organic frameworks for capturing volatile organic compounds. J. Hazard. Mater. 2022, 424, 127347. [Google Scholar] [CrossRef]
- Hamad, A.; Fayed, M.E. Simulation-aided optimization of volatile organic compounds recovery using condensation. Chem. Eng. Res. Des. 2004, 82, 895–906. [Google Scholar] [CrossRef]
- Khan, F.I.; Ghoshal, A.K. Removal of volatile organic compounds from polluted air. J. Loss Prev. Process. 2000, 13, 527–545. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Petrusová, Z.; Machanová, K.; Stanovský, P.; Izák, P. Separation of organic compounds from gaseous mixtures by vapor permeation. Sep. Purif. Technol. 2019, 217, 95–107. [Google Scholar] [CrossRef]
- Cheng, Y.; He, H.; Yang, C.; Zeng, G.; Li, X.; Chen, H.; Yu, G. Challenges and solutions for biofiltration of hydrophobic volatile organic compounds. Biotechnol. Adv. 2016, 34, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Sadegh, F.; Sadegh, N.; Wongniramaikul, W.; Apiratikul, R.; Choodum, A. Adsorption of volatile organic compounds on biochar: A review. Process Saf. Environ. 2024, 182, 559–578. [Google Scholar] [CrossRef]
- Camper, D.; Becker, C.; Koval, C.; Noble, R. Diffusion and solubility measurements in room temperature ionic liquids. Ind. Eng. Chem. Res. 2006, 45, 445–450. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Gong, J.; Liang, C.; Majeed, Z.; Tian, M.; Zhao, C.; Luo, M.; Li, C. Advances of imidazolium ionic liquids for the extraction of phytochemicals from plants. Separations 2023, 10, 151. [Google Scholar] [CrossRef]
- Jacquemin, J.; Gomes, M.F.C.; Husson, P.; Majer, V. Solubility of carbon dioxide, ethane, methane, oxygen, nitrogen, hydrogen, argon, and carbon monoxide in 1-butyl-3-methylimidazolium tetrafluoroborate between temperatures 283 K and 343 K and at pressures close to atmospheric. J. Chem. Thermodyn. 2006, 38, 490–502. [Google Scholar] [CrossRef]
- Chakraborty, M.; Dobaria, D.; Parikh, P.A. The separation of aromatic hydrocarbons through a supported ionic liquid membrane. Pet. Sci. Technol. 2012, 30, 2504–2516. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Majumdar, S.; Bhaumik, D.; Sirkar, K.K. Performance of commercial-size plasmapolymerized PDMS-coated hollow fiber modules in removing VOCs from N2/air. J. Membr. Sci. 2003, 214, 323–330. [Google Scholar] [CrossRef]
- Cichowska-Kopczyńska, I.; Aranowski, R. Use of pyridinium and pyrrolidinium ionic liquids for removal of toluene from gas streams. J. Mol. Liq. 2020, 298, 112091. [Google Scholar] [CrossRef]
- Yan, X.; Anguille, S.; Bendahan, M.; Moulin, P. Ionic liquid membrane process for removal of volatile organic compounds from lab to industrial scale. Chem. Eng. Technol. 2021, 44, 2159–2163. [Google Scholar] [CrossRef]
- Xu, Y.; Bao, J.; Zhang, X.; Li, W.; Xie, Y.; Sun, S.; Zhao, W.; Zhao, C. Functionalized polyethersulfone nanofibrous membranes with ultra-high adsorption capacity for organic dyes by one-step electrospinning. J. Colloid Interface Sci. 2019, 533, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ma, Q.; Zhang, J.-B.; Liu, G.-B. Electrospun SiO2 aerogel/polyacrylonitrile composited nanofibers with enhanced adsorption performance of volatile organic compounds. Appl. Surf. Sci. 2020, 512, 145697. [Google Scholar] [CrossRef]
- Ding, L.; Gao, H.; Xie, F.; Li, W.; Bai, H.; Li, L. Porosity-enhanced polymers from hyper-cross-linked polymer precursors. Macromolecules 2017, 50, 956–962. [Google Scholar] [CrossRef]
- Wei, Z.; Gong, A.; Su, Q.; Wu, Y.; Zhu, C.; Long, S.; Yang, J.; Yang, J.; Wang, X. In-situ grown UiO-66-NH2 nanofibrous membrane for highly effective capture PM2.5 and VOCs. Ind. Eng. Chem. Res. 2023, 62, 10133–10143. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Behaviour of activated carbons with different pore size distributions and surface oxygen groups for benzene and toluene adsorption at low concentrations. Carbon 2005, 43, 1758–1767. [Google Scholar] [CrossRef]
- Salar-García, M.J.; Ortiz-Martínez, V.M.; Hernández-Fernández, F.J.; de los Ríos, A.P.; Quesada-Medina, J. Ionic liquid technology to recover volatile organic compounds (VOCs). J. Hazard. Mater. 2017, 321, 484–499. [Google Scholar] [CrossRef]
- Tan, L.; Zhu, J.; He, X.; Zhou, M.; Zhang, S. The mechanism of toluene absorption by phosphonium ionic liquids with multiple sites. J. Mol. Liq. 2021, 331, 115501. [Google Scholar] [CrossRef]
- Kang, J.; Lu, S.; Zhu, J.; Shi, L.; Liu, B. Absorption performance and mechanism of volatile organic compounds by phosphonium ionic liquids with different anions. J. Chem. Technol. Biotechnol. 2024, 99, 1541–1552. [Google Scholar] [CrossRef]
- Liu, H.B.; Yang, B.; Xue, N.D. Enhanced adsorption of benzene vapor on granular activated carbon under humid conditions due to shifts in hydrophobicity and total micropore volume. J. Hazard. Mater. 2016, 318, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wu, M.; Liu, S.; Huang, J.; Sun, B.; Zhou, Y.; Zhu, Q.; Lu, H. Concentration control of volatile organic compounds by ionic liquid absorption and desorption. Chin. J. Chem. Eng. 2019, 27, 2383–2389. [Google Scholar] [CrossRef]
- Baytar, O.; Şahin, Ö.; Horoz, S.; Kutluay, S. High-performance gas-phase adsorption of benzene and toluene on activated carbon: Response surface optimization, reusability, equilibrium, kinetic, and competitive adsorption studies. Environ. Sci. Pollut. Res. 2020, 27, 26191–26210. [Google Scholar] [CrossRef]
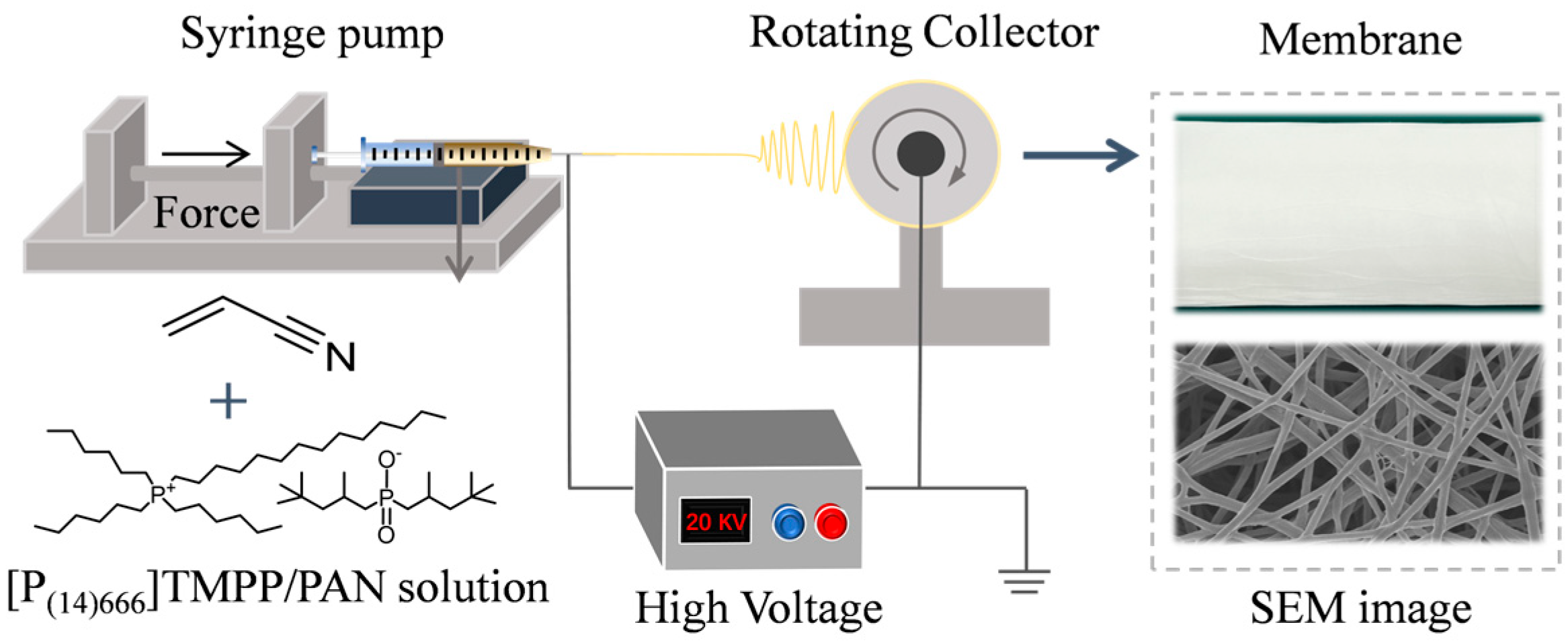
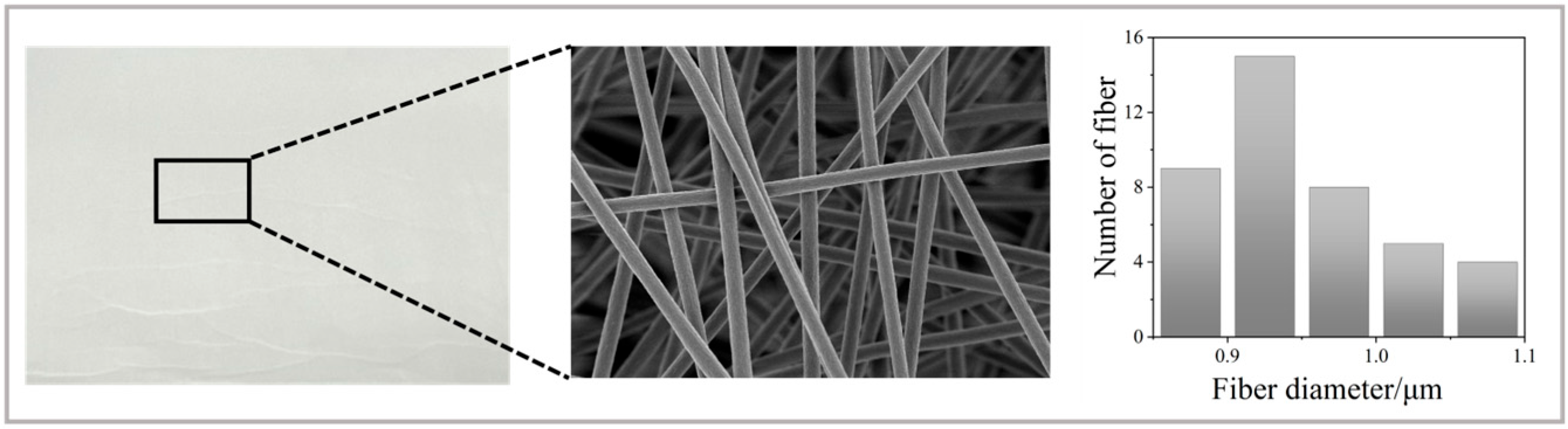

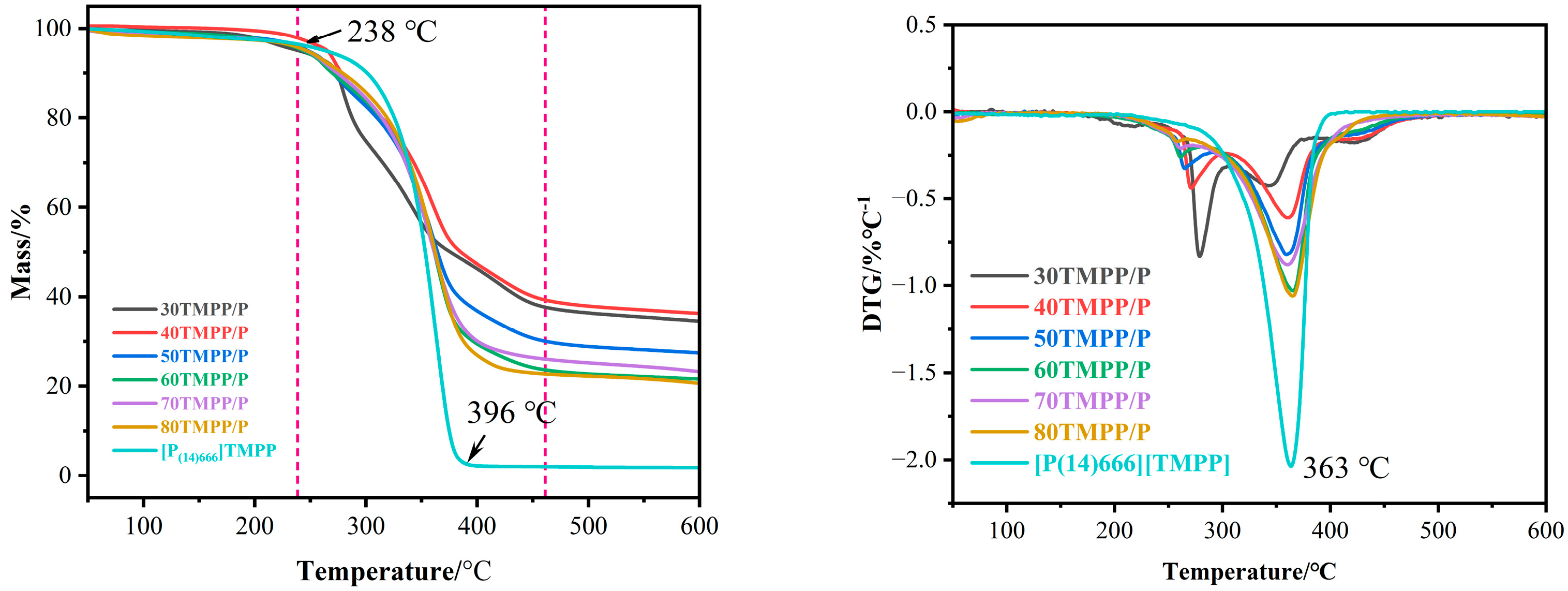
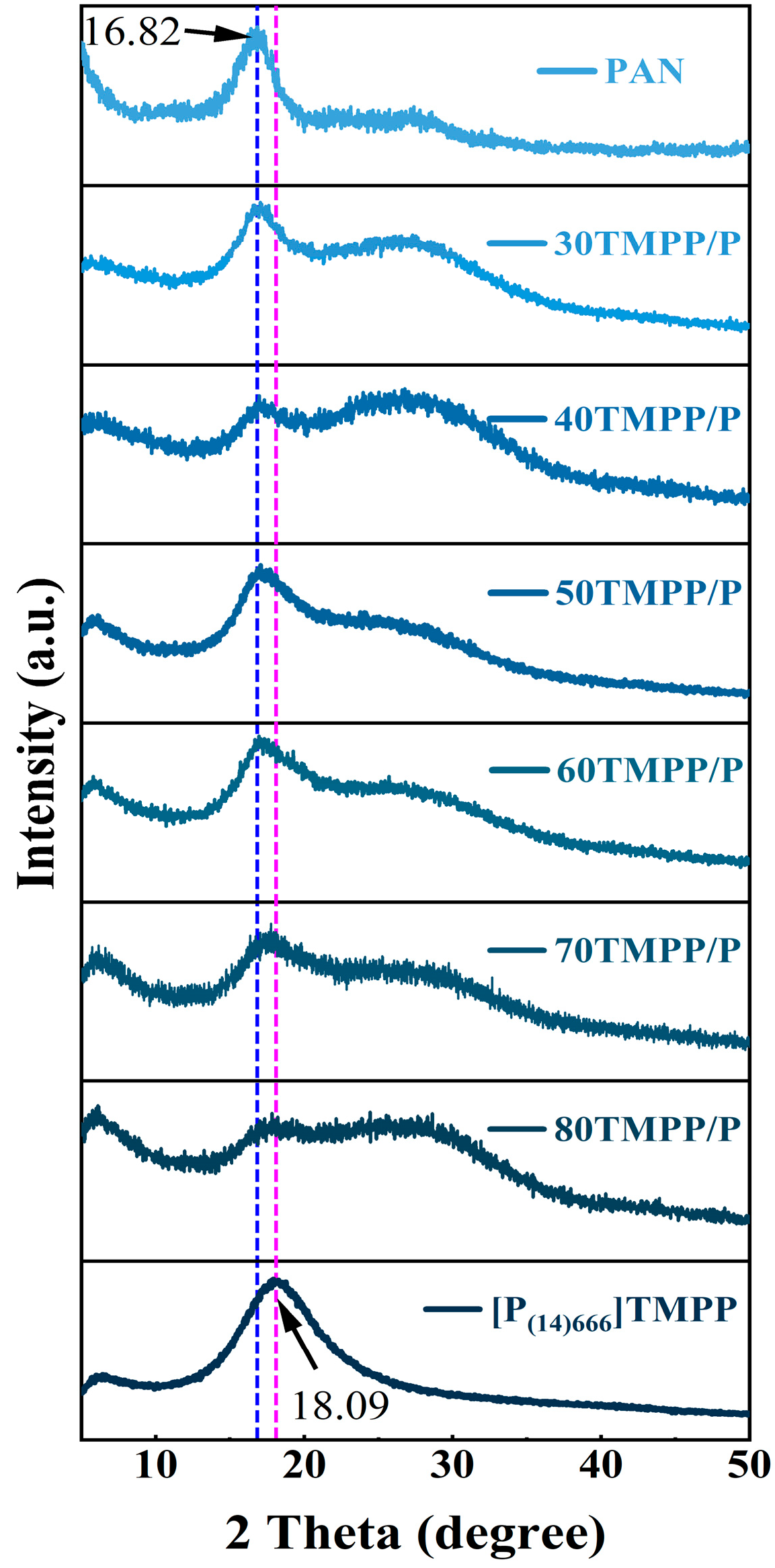

| IL/PAN Name Abbreviation | Content of PAN (g) | Content of IL (g) | IL Ratio in Electrospinning Solution (wt%) |
|---|---|---|---|
| PAN | 1.0 | 0 | 0 |
| 30 TMPP/P | 0.7 | 0.3 | 30 |
| 40 TMPP/P | 0.6 | 0.4 | 40 |
| 50 TMPP/P | 0.5 | 0.5 | 50 |
| 60 TMPP/P | 0.4 | 0.6 | 60 |
| 70 TMPP/P | 0.3 | 0.7 | 70 |
| 80 TMPP/P | 0.2 | 0.8 | 80 |
| Kinetic Models | Benzene | Toluene | Xylene |
|---|---|---|---|
| Pseudo-first-order kinetics model | |||
| qe | 1183.94 | 494.07 | 377.70 |
| k1 | 0.11679 | 0.0776 | 0.11509 |
| R2 | 0.95009 | 0.96036 | 0.98555 |
| Pseudo-second-order kinetics model | |||
| qe | 1394.16 | 574.71 | 505.05 |
| k2 | 0.0001039 | 0.00016463 | 0.00019691 |
| R2 | 0.92471 | 0.89581 | 0.97145 |
| Elovich model | |||
| α | 212.18 | 50.10 | 59.29 |
| β | 0.0022 | 0.0045 | 0.0063 |
| R2 | 0.9721 | 0.9772 | 0.9926 |
| Adsorption Materials | Adsorbate | Adsorption Capacity (mg/g) | References |
|---|---|---|---|
| PDMS/AC-150 | Benzene | 360 | (Liu et al. [32]) |
| PDMS/AC-250 | Benzene | 350 | (Liu et al. [32]) |
| AC | Benzene | 336 | (Liu et al. [32]) |
| OPASS/UiO-66-NH2 | Benzene | 20.3 | (Wei et al. [27]) |
| 80 TMPP/P membrane | Benzene | 1466.8 | In current study |
| AC | Toluene | 364.96 | (Liu et al. [32]) |
| OPASS/UiO-66-NH2 | Toluene | 28.7 | (Wei et al. [27]) |
| [Emim]BF4 | Toluene | 4.5 | (Ma et al. [33]) |
| AC | Toluene | 512.03 | (Orhan et al. [34]) |
| 80 TMPP/P | Toluene | 569.1 | In current study |
| [Emim]BF4 | Xylene | 5.5 | (Ma et al. [33]) |
| 80 TMPP/P | Xylene | 456.3 | In current study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Tao, X.; Chu, F. Electrospun Bis(2,4,4-trimethylpentyl) Tetradecyltrihexylphosphinate/Polyacrylonitrile Nanofiber Membranes with Enhanced Benzene, Toluene, and Xylene Adsorption Performance and Regenerability. Nanomaterials 2025, 15, 711. https://doi.org/10.3390/nano15100711
Wang C, Tao X, Chu F. Electrospun Bis(2,4,4-trimethylpentyl) Tetradecyltrihexylphosphinate/Polyacrylonitrile Nanofiber Membranes with Enhanced Benzene, Toluene, and Xylene Adsorption Performance and Regenerability. Nanomaterials. 2025; 15(10):711. https://doi.org/10.3390/nano15100711
Chicago/Turabian StyleWang, Changchang, Xinrong Tao, and Fengjen Chu. 2025. "Electrospun Bis(2,4,4-trimethylpentyl) Tetradecyltrihexylphosphinate/Polyacrylonitrile Nanofiber Membranes with Enhanced Benzene, Toluene, and Xylene Adsorption Performance and Regenerability" Nanomaterials 15, no. 10: 711. https://doi.org/10.3390/nano15100711
APA StyleWang, C., Tao, X., & Chu, F. (2025). Electrospun Bis(2,4,4-trimethylpentyl) Tetradecyltrihexylphosphinate/Polyacrylonitrile Nanofiber Membranes with Enhanced Benzene, Toluene, and Xylene Adsorption Performance and Regenerability. Nanomaterials, 15(10), 711. https://doi.org/10.3390/nano15100711





