Current Advances of Nanomaterial-Based Oral Drug Delivery for Colorectal Cancer Treatment
Abstract
1. Introduction
2. Physiological Environmental Factors and Major Challenges for CRC Therapy
2.1. Intestinal pH
2.2. Transit Time
2.3. Colonic Fluid
2.4. Enzymatic Content
2.5. Colonic Microbiota
2.6. Mucus
2.7. The Gut-Associated Lymphoid Tissue (GALT)
3. Need and Design Strategies for Novel OCDDS
Systemic Issues with DDS
4. Controlled-Release Oral Nano-Formulations
4.1. Multilayered Types of Controlled Release Nano-Formulations
4.2. Porous Types of Nanomaterials
5. Microenvironment-Responsive Oral Nanomaterials
5.1. pH-Responsive Oral Nano-Formulations
5.2. Enzyme-Responsive Oral Nano-Formulations
5.3. ROS-Responsive Oral Nano-Formulations
5.4. Photo-Responsive Oral Nano-Formulations
6. Magnetic Oral Nano-Formulations
6.1. Magnetically Targeted Oral Nano-Formulations
6.2. Magneto-Thermal Responsive Oral Nano-Formulations
6.3. Magnetically Controlled Release Oral Nano-Formulations
6.4. Oral Nano-Formulations Utilizing Magnetic Resonance Techniques
7. Targeted Oral Nano-Formulations
7.1. Targeting Tumor Cell Membrane Receptors
7.2. Targeting Intestinal Epithelial Cells
7.3. Targeting the Intestinal Mucosa
8. Other Types of Nano-Formulations
8.1. Oral Nano-Formulations for Overcoming Intestinal Barriers
8.2. Oral Nano-Formulations with Bioadhesive Properties
8.3. Intestinal Microbiome Recognition and Responsive Oral Nano-Formulations
9. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- de la Rica, R.; Aili, D.; Stevens, M.M. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv. Drug Deliv. Rev. 2012, 64, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak, Z.; Szostak-Paluch, K.; Przybyło, M.; Langner, M.; Witkiewicz, W.; Jędruchniewicz, N.; Dąbrowska, K. Endocytosis in cellular uptake of drug delivery vectors: Molecular aspects in drug development. Bioorganic Med. Chem. 2020, 28, 115556. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Jiang, G.; Yu, W.; Liu, D.; Liu, Y.; Kong, X.; Yao, J. Preparation of poly(lactic-co-glycolic acid) and chitosan composite nanocarriers via electrostatic self assembly for oral delivery of insulin. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 420–428. [Google Scholar] [CrossRef]
- Elbassiouni, F.E.; El-Kholy, W.M.; Elhabibi, E.-S.M.; Albogami, S.; Fayad, E. Comparative Study between Curcumin and Nanocurcumin Loaded PLGA on Colon Carcinogenesis Induced Mice. Nanomaterials 2022, 12, 324. [Google Scholar] [CrossRef]
- Md, S.; Abdullah, S.; Awan, Z.A.; Alhakamy, N.A. Smart Oral pH-Responsive Dual Layer Nano-Hydrogel for Dissolution Enhancement and Targeted Delivery of Naringenin Using Protein-Polysaccharides Complexation Against Colorectal Cancer. J. Pharm. Sci. 2022, 111, 3155–3164. [Google Scholar] [CrossRef] [PubMed]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1117–1132. [Google Scholar] [CrossRef]
- Kotla, N.G.; Rana, S.; Sivaraman, G.; Sunnapu, O.; Vemula, P.K.; Pandit, A.; Rochev, Y. Bioresponsive drug delivery systems in intestinal inflammation: State-of-the-art and future perspectives. Adv. Drug Deliv. Rev. 2019, 146, 248–266. [Google Scholar] [CrossRef]
- Zeeshan, M.; Ali, H.; Khan, S.; Khan, S.A.; Weigmann, B. Advances in orally-delivered pH-sensitive nanocarrier systems; an optimistic approach for the treatment of inflammatory bowel disease. Int. J. Pharm. 2019, 558, 201–214. [Google Scholar] [CrossRef]
- Schiller, C.; Fröhlich, C.-P.; Giessmann, T.; Siegmund, W.; Mönnikes, H.; Hosten, N.; Weitschies, W. Intestinal fluid volumes and transit of dosage forms as assessed by magnetic resonance imaging. Aliment. Pharmacol. Ther. 2005, 22, 971–979. [Google Scholar] [CrossRef]
- Feng, Z.; Hom, M.E.; Bearrood, T.E.; Rosenthal, Z.C.; Fernández, D.; Ondrus, A.E.; Gu, Y.; McCormick, A.K.; Tomaske, M.G.; Marshall, C.R.; et al. Targeting colorectal cancer with small-molecule inhibitors of ALDH1B1. Nat. Chem. Biol. 2022, 18, 1065–1075. [Google Scholar] [CrossRef]
- Murdocca, M.; Capuano, R.; Pucci, S.; Cicconi, R.; Polidoro, C.; Catini, A.; Martinelli, E.; Paolesse, R.; Orlandi, A.; Mango, R.; et al. Targeting LOX-1 Inhibits Colorectal Cancer Metastasis in an Animal Model. Front. Oncol. 2019, 9, 927. [Google Scholar] [CrossRef]
- Na, Y.J.; Kim, B.R.; Kim, J.L.; Kang, S.; Jeong, Y.A.; Park, S.H.; Jo, M.J.; Kim, J.-Y.; Kim, H.J.; Oh, S.C.; et al. Deficiency of 15-LOX-1 Induces Radioresistance through Downregulation of MacroH2A2 in Colorectal Cancer. Cancers 2019, 11, 1776. [Google Scholar] [CrossRef] [PubMed]
- Murdocca, M.; De Masi, C.; Pucci, S.; Mango, R.; Novelli, G.; Di Natale, C.; Sangiuolo, F. LOX-1 and cancer: An indissoluble liaison. Cancer Gene Ther. 2021, 28, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.E.; Moore, L.H. Intestinal floras of populations that have a high risk of colon cancer. Appl. Environ. Microbiol. 1995, 61, 3202–3207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-J.; Zhao, D.-D.; Liu, H.; Chen, H.-T.; Li, J.-J.; Mu, X.-Q.; Liu, Z.; Li, X.; Tang, L.; Zhao, Z.-Y.; et al. Cancer killers in the human gut microbiota: Diverse phylogeny and broad spectra. Oncotarget 2017, 8, 49574–49591. [Google Scholar] [CrossRef] [PubMed]
- Gulbake, A.; Jain, S.K. Chitosan: A potential polymer for colon-specific drug delivery system. Expert Opin. Drug Deliv. 2012, 9, 713–729. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-N.; Yu, T.-C.; Zhao, H.-J.; Sun, T.-T.; Chen, H.-M.; Chen, H.-Y.; An, H.-F.; Weng, Y.-R.; Yu, J.; Li, M.; et al. Berberine may rescue Fusobacterium nucleatum-induced colorectal tumorigenesis by modulating the tumor microenvironment. Oncotarget 2015, 6, 32013–32026. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Hendriks, A.J.; Nolte, T.M. A generic model based on the properties of nanoparticles and cells for predicting cellular uptake. Colloids Surf. B Biointerfaces 2022, 209, 112155. [Google Scholar] [CrossRef]
- Loktionov, A. Colon mucus in colorectal neoplasia and beyond. World J. Gastroenterol. 2022, 28, 4475–4492. [Google Scholar] [CrossRef] [PubMed]
- Juge, N. Relationship between mucosa-associated gut microbiota and human diseases. Biochem. Soc. Trans. 2022, 50, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zhou, X.; Jin, C.; Wu, J.; Xia, Y.; Lu, M.; Yang, Y.; Jin, X.; Ji, F.; Wang, B. Acid-Base Transformative HADLA Micelles Alleviate Colitis by Restoring Adaptive Immunity and Gut Microbiome. J. Control Release 2023, 364, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Japiassu, K.B.; Fay, F.; Marengo, A.; Louaguenouni, Y.; Cailleau, C.; Denis, S.; Chapron, D.; Tsapis, N.; Nascimento, T.L.; Lima, E.M.; et al. Interplay between mucus mobility and alveolar macrophage targeting of surface-modified liposomes. J. Control. Release 2022, 352, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Nie, X.; Lu, W.; Zhang, Q.; Fang, W.; Gao, S.; Chen, S.; Hu, R. Mucus-Penetrating Alginate-Chitosan Nanoparticles Loaded with Berberine Hydrochloride for Oral Delivery to the Inflammation Site of Ulcerative Colitis. AAPS PharmSciTech 2022, 23, 179. [Google Scholar] [CrossRef]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Mörbe, U.M.; Jørgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Sang, R.; Stratton, B.; Engel, A.; Deng, W. Liposome technologies towards colorectal cancer therapeutics. Acta Biomater. 2021, 127, 24–40. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, W.; Wang, Z.; Lu, J. Lipid-Based Nanotechnology: Liposome. Pharmaceutics 2023, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Lin, Y.; Zhang, X.; Feng, C.; Lu, Y.; Gao, Y.; Dong, C. Cyclic RGD peptide-modified liposomal drug delivery system for targeted oral apatinib administration: Enhanced cellular uptake and improved therapeutic effects. Int. J. Nanomed. 2017, 12, 1941–1958. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wu, C.; Zhu, C.; He, Y.; Yang, D.; Cheng, Y.; Gao, X. Oral Administration of Liposome-Apatinib and Locally Delivery of Docetaxel/MPEG-PCL by Fibrin Glue Synergistically Improve Therapeutic Effect in Colorectal Cancer. J. Biomed. Nanotechnol. 2018, 14, 2077–2091. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Yao, Y.; Yang, H.; Fan, Y.; Nomura, N.; Zhou, W.; Ni, D.; Li, X.; Jiang, W.; Qiu, P.; et al. Incorporating Cobalt Nanoparticles in Nitrogen-Doped Mesoporous Carbon Spheres through Composite Micelle Assembly for High-Performance Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2021, 13, 38604–38612. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Warczinski, L.; Li, X.; Lu, M.; Bitzer, J.; Heidelmann, M.; Eckhard, T.; Fu, Q.; Schulwitz, J.; Merko, M.; et al. Formic Acid-Assisted Selective Hydrogenolysis of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran over Bifunctional Pd Nanoparticles Supported on N-Doped Mesoporous Carbon. Angew. Chem. Int. Ed. 2021, 60, 6807–6815. [Google Scholar] [CrossRef] [PubMed]
- Karimi Bavandpour, A.; Bakhshi, B.; Najar-Peerayeh, S. The roles of mesoporous silica and carbon nanoparticles in antigen stability and intensity of immune response against recombinant subunit B of cholera toxin in a rabbit animal model. Int. J. Pharm. 2020, 573, 118868. [Google Scholar] [CrossRef]
- Lu, H.; Li, S.; Dai, D.; Zhang, Q.; Min, Z.; Yang, C.; Sun, S.; Ye, L.; Teng, C.; Cao, X.; et al. Enhanced treatment of cerebral ischemia-Reperfusion injury by intelligent nanocarriers through the regulation of neurovascular units. Acta Biomater. 2022, 147, 314–326. [Google Scholar] [CrossRef]
- Rawal, S.; Patel, M.M. Threatening cancer with nanoparticle aided combination oncotherapy. J. Control. Release 2019, 301, 76–109. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Xin, H.; Guo, Y.; Zhu, B.; Su, L.; Wang, S.; Zeng, J.; Chen, Q.; Deng, R.; et al. Mitochondria-targeting folic acid-modified nanoplatform based on mesoporous carbon and a bioactive peptide for improved colorectal cancer treatment. Acta Biomater. 2022, 152, 453–472. [Google Scholar] [CrossRef]
- Słota, D.; Piętak, K.; Florkiewicz, W.; Jampilek, J.; Tomala, A.; Urbaniak, M.M.; Tomaszewska, A.; Rudnicka, K.; Sobczak-Kupiec, A. Clindamycin-Loaded Nanosized Calcium Phosphates Powders as a Carrier of Active Substances. Nanomaterials 2023, 13, 1469. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Maroni, A.; Zema, L.; Loreti, G.; Palugan, L.; Gazzaniga, A. Film coatings for oral pulsatile release. Int. J. Pharm. 2013, 457, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Sohail, M.; Minhas, M.U.; Mahmood, A.; Shah, S.A.; Munir, A.; Kashif, M.-U.-R. Folic acid-decorated alginate nanoparticles loaded hydrogel for the oral delivery of diferourylmethane in colorectal cancer. Int. J. Biol. Macromol. 2023, 233, 123585. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Wu, X.; Peng, L.; Yu, N.; Gou, G.; Zuo, W.; Yang, J. pH-responsive bufadienolides nanocrystals decorated by chitosan quaternary ammonium salt for treating colon cancer. Int. J. Biol. Macromol. 2023, 242, 124819. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.D.; Barros, I.; Brandão, P.; Lacerda, L. Amino Acid Profiles in the Biological Fluids and Tumor Tissue of CRC Patients. Cancers 2023, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Majidpoor, J.; Mortezaee, K. Liquid biopsy in colorectal cancer. Clin. Chim. Acta 2024, 553, 117674. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N.; Safaei, M. Application of Various Types of Liposomes in Drug Delivery Systems. Adv. Pharm. Bull. 2017, 7, 3–9. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Song, Q.; Jia, J.; Niu, X.; Zheng, C.; Zhao, H.; Sun, L.; Zhang, H.; Wang, L.; Zhang, Z.; Zhang, Y. An oral drug delivery system with programmed drug release and imaging properties for orthotopic colon cancer therapy. Nanoscale 2019, 11, 15958–15970. [Google Scholar] [CrossRef] [PubMed]
- Kos, J. Proteases: Role and Function in Cancer. Int. J. Mol. Sci. 2022, 23, 4632. [Google Scholar] [CrossRef]
- Nguyen, M.M.; Carlini, A.S.; Chien, M.-P.; Sonnenberg, S.; Luo, C.; Braden, R.L.; Osborn, K.G.; Li, Y.; Gianneschi, N.C.; Christman, K.L. Enzyme-Responsive Nanoparticles for Targeted Accumulation and Prolonged Retention in Heart Tissue after Myocardial Infarction. Adv. Mater. 2015, 27, 5547–5552. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, T.; Perche, F.; Taigind, A.; Torchilin, V.P. Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety. Proc. Natl. Acad. Sci. USA 2013, 110, 17047–17052. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Liu, X.; Yang, K.; Li, Z.; Liu, J. MMP2 Polymorphisms and Colorectal Cancer Susceptibility in a Chinese Han Population. Int. J. Gen. Med. 2022, 15, 6009–6019. [Google Scholar] [CrossRef]
- Rashidzadeh, H.; Ramazani, A.; Tabatabaei Rezaei, S.J.; Danafar, H.; Rahmani, S.; Veisi, H.; Rajaeinejad, M.; Jamalpoor, Z.; Hami, Z. Targeted co-delivery of methotrexate and chloroquine via a pH/enzyme-responsive biocompatible polymeric nanohydrogel for colorectal cancer treatment. J. Biomater. Sci. Polym. Ed. 2023, 34, 1824–1842. [Google Scholar] [CrossRef]
- Li, S.; Jin, M.; Wu, Y.; Jung, S.; Li, D.; He, N.; Lee, M.-S. An efficient enzyme-triggered controlled release system for colon-targeted oral delivery to combat dextran sodium sulfate (DSS)-induced colitis in mice. Drug Deliv. 2021, 28, 1120–1131. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Fang, Y.; Yan, C.; Zhao, Q.; Xu, J.; Liu, Z.; Gao, J.; Zhu, H.; Dai, Z.; Wang, D.; Tang, D. The roles of microbial products in the development of colorectal cancer: A review. Bioengineered 2021, 12, 720–735. [Google Scholar] [CrossRef]
- Lin, S.; Li, Y.; Zamyatnin, A.A.; Werner, J.; Bazhin, A.V. Reactive oxygen species and colorectal cancer. J. Cell. Physiol. 2018, 233, 5119–5132. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, Y.; Xu, Y.; Zhu, C.; Liu, T.; Wang, K. Chitosan nanoparticles for oral photothermally enhanced photodynamic therapy of colon cancer. Int. J. Pharm. 2020, 589, 119763. [Google Scholar] [CrossRef]
- Xiang, J.; Tong, X.; Shi, F.; Yan, Q.; Yu, B.; Zhao, Y. Near-infrared light-triggered drug release from UV-responsive diblock copolymer-coated upconversion nanoparticles with high monodispersity. J. Mater. Chem. B 2018, 6, 3531–3540. [Google Scholar] [CrossRef]
- Chen, G.; Wang, K.; Zhou, Y.; Ding, L.; Ullah, A.; Hu, Q.; Sun, M.; Oupický, D. Oral Nanostructured Lipid Carriers Loaded with Near-Infrared Dye for Image-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2016, 8, 25087–25095. [Google Scholar] [CrossRef]
- Zahin, N.; Anwar, R.; Tewari, D.; Kabir, M.T.; Sajid, A.; Mathew, B.; Uddin, M.S.; Aleya, L.; Abdel-Daim, M.M. Nanoparticles and its biomedical applications in health and diseases: Special focus on drug delivery. Environ. Sci. Pollut. Res. Int. 2020, 27, 19151–19168. [Google Scholar] [CrossRef]
- Darroudi, M.; Gholami, M.; Rezayi, M.; Khazaei, M. An overview and bibliometric analysis on the colorectal cancer therapy by magnetic functionalized nanoparticles for the responsive and targeted drug delivery. J. Nanobiotechnology 2021, 19, 399. [Google Scholar] [CrossRef]
- Lu, I.-L.; Yu, T.-W.; Liu, T.-I.; Chen, H.-H.; Yang, Y.-C.; Lo, C.-L.; Wang, C.-Y.; Chiu, H.-C. Microfluidized Dextran Microgels Loaded with Cisplatin/SPION Lipid Nanotherapeutics for Local Colon Cancer Treatment via Oral Administration. Adv. Healthc. Mater. 2022, 11, e2201140. [Google Scholar] [CrossRef]
- Shen, M.-Y.; Liu, T.-I.; Yu, T.-W.; Kv, R.; Chiang, W.-H.; Tsai, Y.-C.; Chen, H.-H.; Lin, S.-C.; Chiu, H.-C. Hierarchically targetable polysaccharide-coated solid lipid nanoparticles as an oral chemo/thermotherapy delivery system for local treatment of colon cancer. Biomaterials 2019, 197, 86–100. [Google Scholar] [CrossRef]
- Calero, M.; Gutiérrez, L.; Salas, G.; Luengo, Y.; Lázaro, A.; Acedo, P.; Morales, M.P.; Miranda, R.; Villanueva, A. Efficient and safe internalization of magnetic iron oxide nanoparticles: Two fundamental requirements for biomedical applications. Nanomedicine 2014, 10, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, L.; Kou, F.; Zhao, J.; Lei, J.; He, J. Targeted therapeutic effects of oral magnetically driven pectin nanoparticles containing chlorogenic acid on colon cancer. Particuology 2024, 84, 53–59. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Zhu, Y.; Zhang, M.; Tang, S.; Li, J.; Pei, W.; Huang, B.; Niu, C. Triple-Modal Imaging-Guided Chemo-Photothermal Synergistic Therapy for Breast Cancer with Magnetically Targeted Phase-Shifted Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 42102–42114. [Google Scholar] [CrossRef]
- Liao, J.; Wei, X.; Ran, B.; Peng, J.; Qu, Y.; Qian, Z. Polymer hybrid magnetic nanocapsules encapsulating IR820 and PTX for external magnetic field-guided tumor targeting and multifunctional theranostics. Nanoscale 2017, 9, 2479–2491. [Google Scholar] [CrossRef] [PubMed]
- Kijima, S.; Sasaki, T.; Nagata, K.; Utano, K.; Lefor, A.T.; Sugimoto, H. Preoperative Evaluation of Colorectal Cancer Using CT Colonography, MRI, and PET/CT. World J. Gastroenterol. 2014, 20, 16964. [Google Scholar] [CrossRef] [PubMed]
- Medema, J.P. Cancer stem cells: The challenges ahead. Nat. Cell Biol. 2013, 15, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Huber, J.; Hadaschik, B.; Siegers, G.M.; Fiebig, H.-H.; Schüler, J. Characterization of colon cancer cells: A functional approach characterizing CD133 as a potential stem cell marker. BMC Cancer 2012, 12, 96. [Google Scholar] [CrossRef]
- Yang, Z.-L.; Zheng, Q.; Yan, J.; Pan, Y.; Wang, Z.-G. Upregulated CD133 expression in tumorigenesis of colon cancer cells. World J. Gastroenterol. 2011, 17, 932–937. [Google Scholar] [CrossRef]
- Galizia, G.; Gemei, M.; Del Vecchio, L.; Zamboli, A.; Di Noto, R.; Mirabelli, P.; Salvatore, F.; Castellano, P.; Orditura, M.; De Vita, F.; et al. Combined CD133/CD44 expression as a prognostic indicator of disease-free survival in patients with colorectal cancer. Arch. Surg. 2012, 147, 18–24. [Google Scholar] [CrossRef]
- Zahiri, M.; Babaei, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Hybrid Nanoreservoirs Based on Dextran-Capped Dendritic Mesoporous Silica Nanoparticles for CD133-Targeted Drug Delivery. J. Cell. Physiol. 2020, 235, 1036–1050. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, C.; Cui, B.; Wang, Y.; Lim, K.; Li, K.; Thiery, J.P.; Chen, J.; Ho, C.L. Targeted EpCAM-binding for the development of potent and effective anticancer proteins. Biomed. Pharmacother. 2023, 161, 114443. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Huang, J.; Chen, J.; Yang, M.; Wang, H.; Qiao, H.; Chen, Z.; Hu, L.; Di, L.; Li, J. Enhanced anti-colon cancer efficacy of 5-fluorouracil by epigallocatechin-3-gallate co-loaded in wheat germ agglutinin-conjugated nanoparticles. Nanomedicine 2019, 21, 102068. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Belogurova, N.G.; Poddubnaya, I.V.; Kudryashova, E.V. Mucosal Adhesive Chitosan Nanogel Formulations of Antibiotics and Adjuvants (Terpenoids, Flavonoids, etc.) and Their Potential for the Treatment of Infectious Diseases of the Gastrointestinal Tract. Pharmaceutics 2023, 15, 2353. [Google Scholar] [CrossRef] [PubMed]
- Choukaife, H.; Seyam, S.; Alallam, B.; Doolaanea, A.A.; Alfatama, M. Current Advances in Chitosan Nanoparticles Based Oral Drug Delivery for Colorectal Cancer Treatment. Int. J. Nanomed. 2022, 17, 3933–3966. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Chen, K.; Jin, M.; Vu, S.H.; Jung, S.; He, N.; Zheng, Z.; Lee, M.-S. Application of chitosan/alginate nanoparticle in oral drug delivery systems: Prospects and challenges. Drug Deliv. 2022, 29, 1142–1149. [Google Scholar] [CrossRef]
- Rajpoot, K.; Jain, S.K. Oral delivery of pH-responsive alginate microbeads incorporating folic acid-grafted solid lipid nanoparticles exhibits enhanced targeting effect against colorectal cancer: A dual-targeted approach. Int. J. Biol. Macromol. 2020, 151, 830–844. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Z.; He, S.; Du, W.; Yang, X.; Kou, B.; Jiang, Y.; Bian, P.; Yin, L. Hyaluronic acid oligosaccharide-modified zeolitic imidazolate framework-8 nanoparticles loaded with oxaliplatin as a targeted drug-delivery system for colorectal cancer therapy. Nanomedicine 2023, 18, 891–905. [Google Scholar] [CrossRef]
- Liu, L.; Yang, S.; Chen, F.; Cheng, K.W. Hyaluronic Acid–Zein Core-Shell Nanoparticles Improve the Anticancer Effect of Curcumin Alone or in Combination with Oxaliplatin against Colorectal Cancer via CD44-Mediated Cellular Uptake. Molecules 2022, 27, 1498. [Google Scholar] [CrossRef]
- Carvalho, M.R.; Reis, R.L.; Oliveira, J.M. Dendrimer nanoparticles for colorectal cancer applications. J. Mater. Chem. B 2020, 8, 1128–1138. [Google Scholar] [CrossRef]
- Pishavar, E.; Ramezani, M.; Hashemi, M. Co-delivery of doxorubicin and TRAIL plasmid by modified PAMAM dendrimer in colon cancer cells, in vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2019, 45, 1931–1939. [Google Scholar] [CrossRef]
- Peterson, C.G.B.; Eklund, E.; Taha, Y.; Raab, Y.; Carlson, M. A new method for the quantification of neutrophil and eosinophil cationic proteins in feces: Establishment of normal levels and clinical application in patients with inflammatory bowel disease. Am. J. Gastroenterol. 2002, 97, 1755–1762. [Google Scholar] [CrossRef]
- Han, H.-K.; Shin, H.-J.; Ha, D.H. Improved oral bioavailability of alendronate via the mucoadhesive liposomal delivery system. Eur. J. Pharm. Sci. 2012, 46, 500–507. [Google Scholar] [CrossRef]
- Coco, R.; Plapied, L.; Pourcelle, V.; Jérôme, C.; Brayden, D.J.; Schneider, Y.J.; Préat, V. Drug Delivery to Inflamed Colon by Nanoparticles: Comparison of Different Strategies. Int. J. Pharm. 2013, 440, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Parikh, K.; Antanaviciute, A.; Fawkner-Corbett, D.; Jagielowicz, M.; Aulicino, A.; Lagerholm, C.; Davis, S.; Kinchen, J.; Chen, H.H.; Alham, N.K.; et al. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature 2019, 567, 49–55. [Google Scholar] [CrossRef]
- Berardi, A.; Bisharat, L.; AlKhatib, H.S.; Cespi, M. Zein as a Pharmaceutical Excipient in Oral Solid Dosage Forms: State of the Art and Future Perspectives. AAPS PharmSciTech 2018, 19, 2009–2022. [Google Scholar] [CrossRef]
- Karakas, C.Y.; Ordu, H.R.; Bozkurt, F.; Karadag, A. Electrosprayed chitosan-coated alginate-pectin beads as potential system for colon-targeted delivery of ellagic acid. J. Sci. Food Agric. 2022, 102, 965–975. [Google Scholar] [CrossRef]
- Zhao, R.; Du, S.; Liu, Y.; Lv, C.; Song, Y.; Chen, X.; Zhang, B.; Li, D.; Gao, S.; Cui, W.; et al. Mucoadhesive-to-penetrating controllable peptosomes-in-microspheres co-loaded with anti-miR-31 oligonucleotide and Curcumin for targeted colorectal cancer therapy. Theranostics 2020, 10, 3594–3611. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J.; et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Li, S.; Chen, W.; Han, Y.; Yao, Y.; Yang, L.; Li, Q.; Xiao, Q.; Wei, J.; Liu, Z.; et al. Postoperative Probiotics Administration Attenuates Gastrointestinal Complications and Gut Microbiota Dysbiosis Caused by Chemotherapy in Colorectal Cancer Patients. Nutrients 2023, 15, 356. [Google Scholar] [CrossRef]
- Darbandi, A.; Mirshekar, M.; Shariati, A.; Moghadam, M.T.; Lohrasbi, V.; Asadolahi, P.; Talebi, M. The effects of probiotics on reducing the colorectal cancer surgery complications: A periodic review during 2007–2017. Clin. Nutr. 2020, 39, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Asghar, S.; Jin, X.; Hu, Z.; Ping, Q.; Chen, Z.; Shao, F.; Xiao, Y. The enhancing effect of N-acetylcysteine modified hyaluronic acid-octadecylamine micelles on the oral absorption of paclitaxel. Int. J. Biol. Macromol. 2019, 138, 636–647. [Google Scholar] [CrossRef]
- Wan, X.; Guo, H.; Liang, Y.; Zhou, C.; Liu, Z.; Li, K.; Niu, F.; Zhai, X.; Wang, L. The physiological functions and pharmaceutical applications of inulin: A review. Carbohydr. Polym. 2020, 246, 116589. [Google Scholar] [CrossRef] [PubMed]
- Hellwege, E.M.; Czapla, S.; Jahnke, A.; Willmitzer, L.; Heyer, A.G. Transgenic Potato (Solanum tuberosum) Tubers Synthesize the Full Spectrum of Inulin Molecules Naturally Occurring in Globe Artichoke (Cynara scolymus) roots. Proc. Natl. Acad. Sci. USA 2000, 97, 8699–8704. [Google Scholar] [CrossRef]
- Hou, Y.; Jin, J.; Duan, H.; Liu, C.; Chen, L.; Huang, W.; Gao, Z.; Jin, M. Targeted therapeutic effects of oral inulin-modified double-layered nanoparticles containing chemotherapeutics on orthotopic colon cancer. Biomaterials 2022, 283, 121440. [Google Scholar] [CrossRef]
- Lang, T.; Zhu, R.; Zhu, X.; Yan, W.; Li, Y.; Zhai, Y.; Wu, T.; Huang, X.; Yin, Q.; Li, Y. Combining gut microbiota modulation and chemotherapy by capecitabine-loaded prebiotic nanoparticle improves colorectal cancer therapy. Nat. Commun. 2023, 14, 4746. [Google Scholar] [CrossRef]
- Zheng, D.-W.; Dong, X.; Pan, P.; Chen, K.-W.; Fan, J.-X.; Cheng, S.-X.; Zhang, X.-Z. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat. Biomed. Eng. 2019, 3, 717–728. [Google Scholar] [CrossRef]
- Chionh, F.; Lau, D.; Yeung, Y.; Price, T.; Tebbutt, N. Oral Versus Intravenous Fluoropyrimidines for Colorectal Cancer. Cochrane Database Syst. Rev. 2017, 7, CD008398. [Google Scholar] [CrossRef]
- Gibson, S.A.; McFarlan, C.; Hay, S.; MacFarlane, G.T. Significance of Microflora in Proteolysis in the Colon. Appl. Environ. Microbiol. 1989, 55, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.R. Factors affecting metabolic activity of the intestinal microflora. Drug Metab. Rev. 1988, 19, 243–261. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.T.; Stevens, S.E.; Cerniglia, C.E. The reduction of azo dyes by the intestinal microflora. Crit. Rev. Microbiol. 1992, 18, 175–190. [Google Scholar] [CrossRef]
- Hu, S.; Li, W.; Cai, Z.; Tang, C.; Li, B.; Liu, S.; Li, Y. Research progress on chitin/chitosan-based emulsion delivery systems and their application in lipid digestion regulation. Crit. Rev. Food Sci. Nutr. 2023, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Khanal, T.; Kim, H.G.; Lee, D.H.; Yeo, H.K.; Lee, Y.S.; Ahn, Y.T.; Kim, D.H.; Jeong, H.G.; Jeong, T.C. Role of metabolism by human intestinal microflora in geniposide-induced toxicity in HepG2 cells. Arch. Pharm. Res. 2012, 35, 733–738. [Google Scholar] [CrossRef] [PubMed]

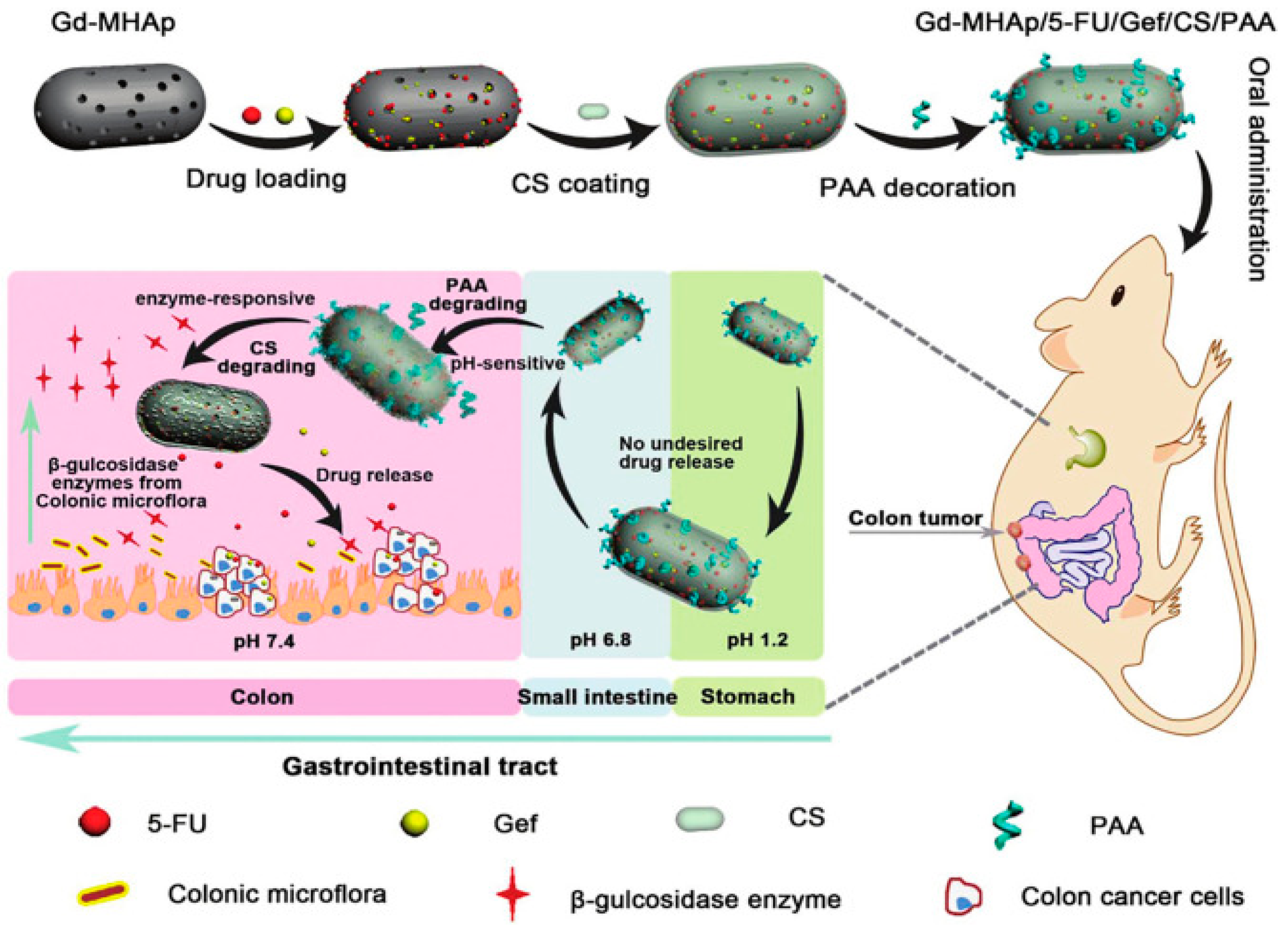

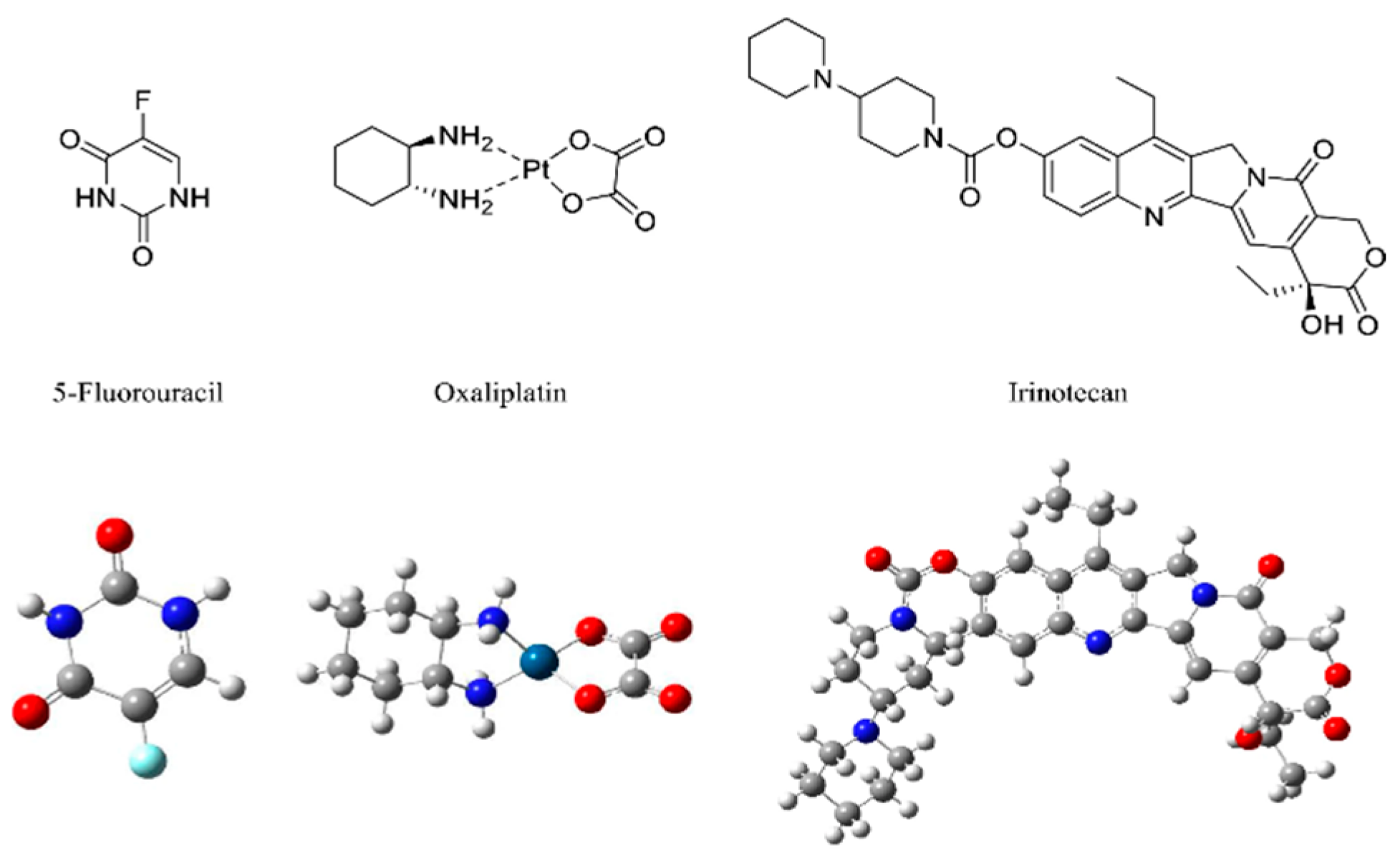
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Chen, L.; Huang, W.; Gao, Z.; Jin, M. Current Advances of Nanomaterial-Based Oral Drug Delivery for Colorectal Cancer Treatment. Nanomaterials 2024, 14, 557. https://doi.org/10.3390/nano14070557
Wang N, Chen L, Huang W, Gao Z, Jin M. Current Advances of Nanomaterial-Based Oral Drug Delivery for Colorectal Cancer Treatment. Nanomaterials. 2024; 14(7):557. https://doi.org/10.3390/nano14070557
Chicago/Turabian StyleWang, Nuoya, Liqing Chen, Wei Huang, Zhonggao Gao, and Mingji Jin. 2024. "Current Advances of Nanomaterial-Based Oral Drug Delivery for Colorectal Cancer Treatment" Nanomaterials 14, no. 7: 557. https://doi.org/10.3390/nano14070557
APA StyleWang, N., Chen, L., Huang, W., Gao, Z., & Jin, M. (2024). Current Advances of Nanomaterial-Based Oral Drug Delivery for Colorectal Cancer Treatment. Nanomaterials, 14(7), 557. https://doi.org/10.3390/nano14070557







