Oxidative Stress and Acrosomal Status of Human Spermatozoa Subjected to Hydrophobic Carbon Soot Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Selection Criteria of the Male Subjects
2.3. Oxidative–Reduction Potential Assays
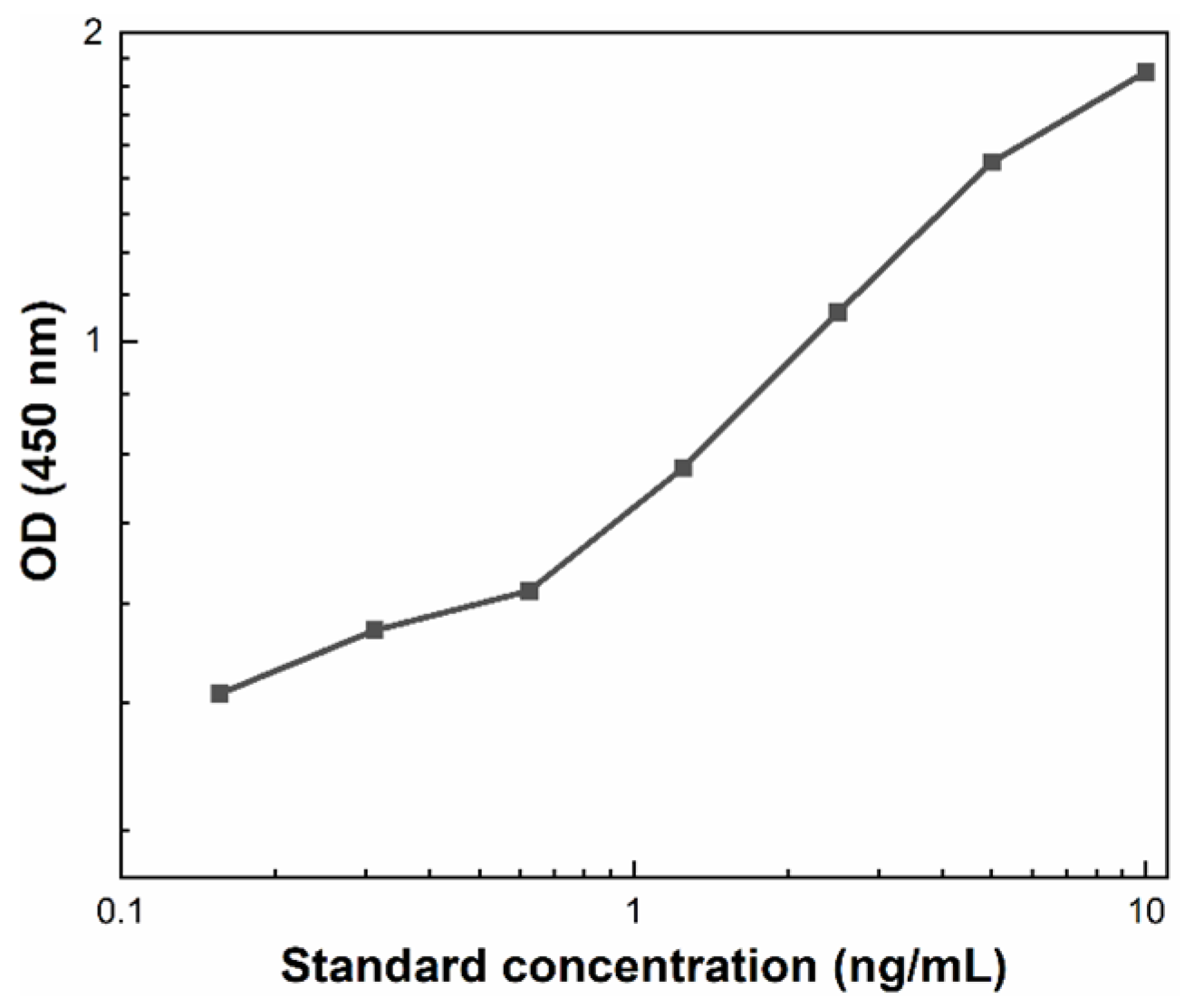
2.4. Evaluation of the Acrosomal Status
2.5. Statistical Analysis
3. Results
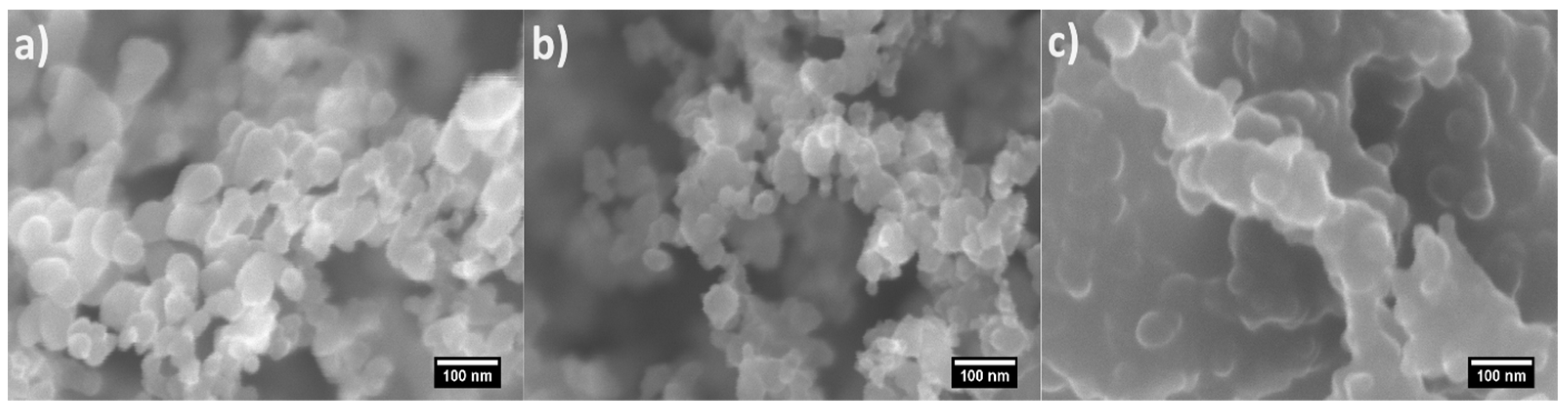
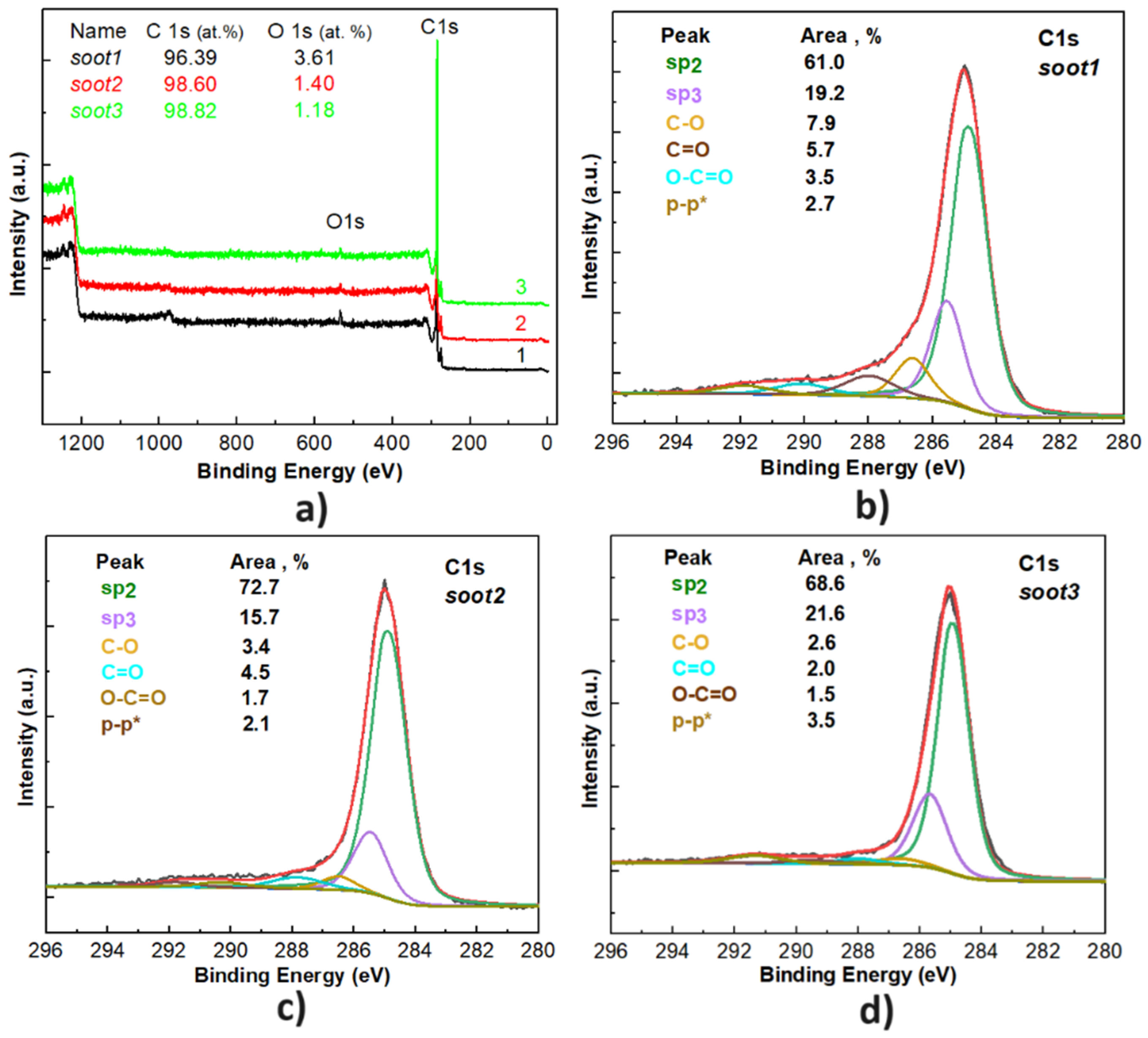
3.1. Morphology and Chemistry of the Soot Patterns
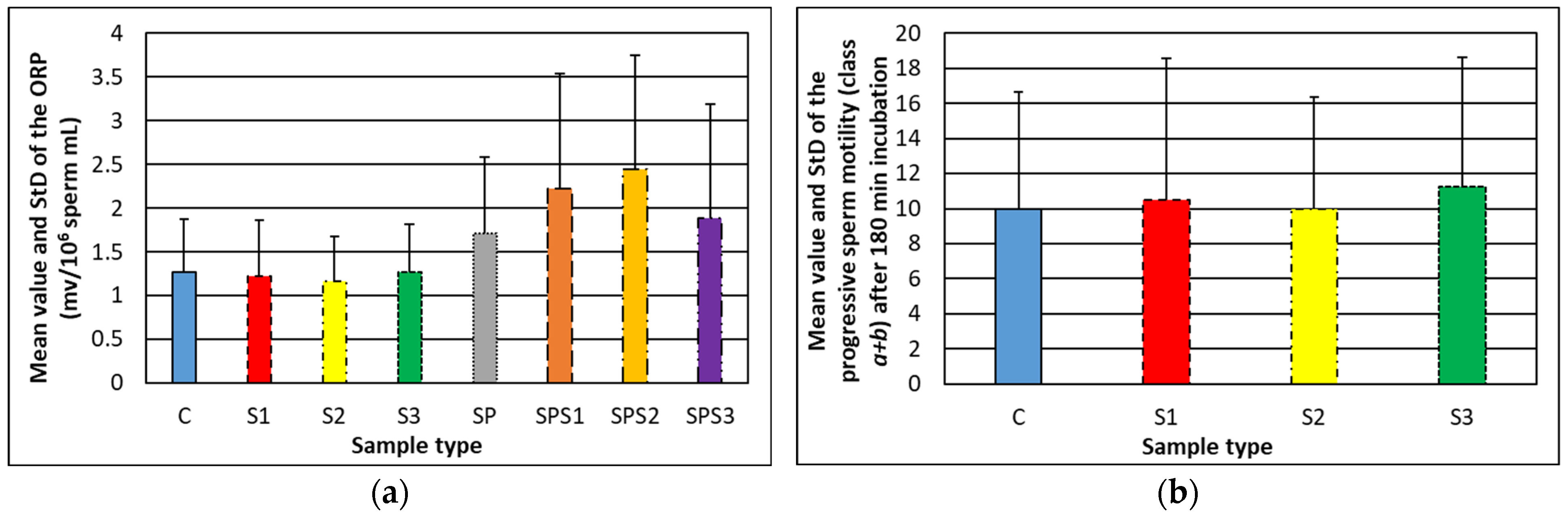
3.2. Relationship between the Oxidative–Reduction Potential of Human Semen and Soot Features
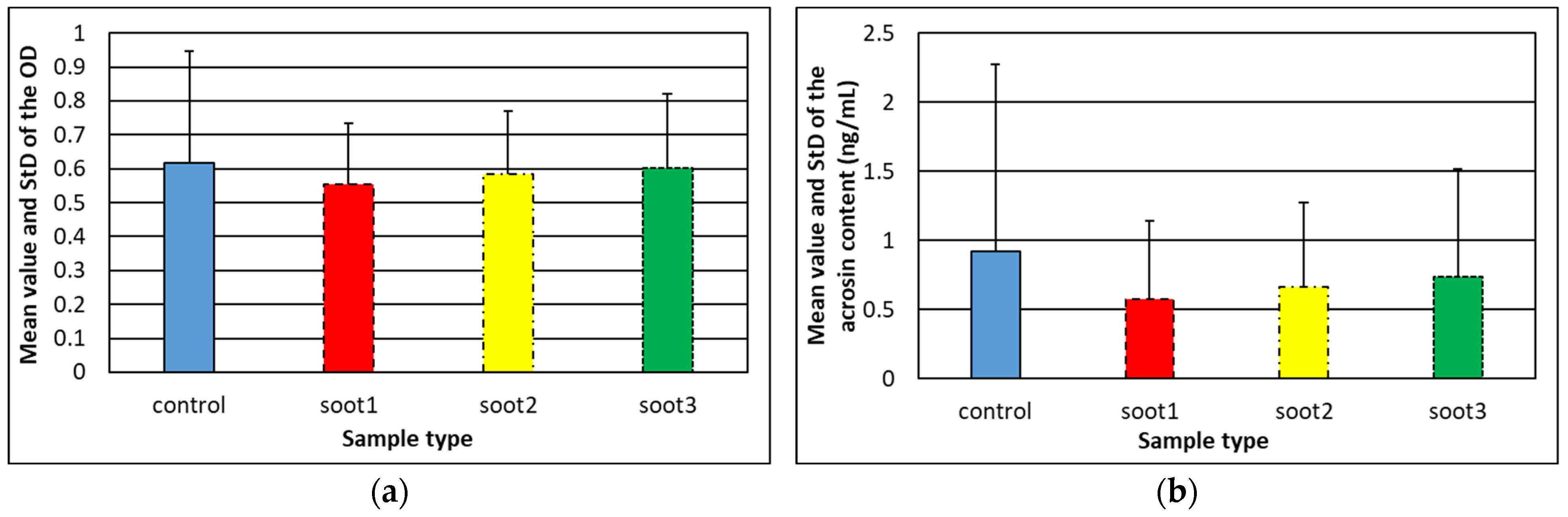
3.3. Effect of Rapeseed Oil Soot on the Acrosome Reaction of Human Spermatozoa
4. Discussion
5. Future Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pierson, R.; Stephanson, R. Imaging reproduction in science and history. J. Med. Humanit. 2010, 31, 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ilacqua, A.; Izzo, G.; Emerenziani, G.P.; Baldari, C.; Aversa, A. Lifestyle and fertility: The influence of stress and quality of life on male fertility. Reprod. Biol. Endocrinol. 2018, 16, 115. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Tada, A.; Kojo, K.; Tsuchiya, H.; Kurobe, M.; Uchida, M.; Yamasaki, K.; Iwamoto, R.; Sato, Y. Analysis of the correlation between gene copy depletion in the AZFc region and male infertility in Japanese men. Reprod. Biol. 2023, 23, 100728. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, U.; Peeters, K.; De Neubourg, D. Sperm nuclear maturity and chromatin stability in subfertile patients: Density gradient centrifugation is fair but not non-discriminative in selecting the right population. Reprod. Biol. 2019, 19, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Serafin, D.; Grabarek, B.O.; Boron, D.; Madej, A.; Cnota, W.; Czuba, B. Evaluation of the risk of birth defects related to the use of assisted reproductive technology: An updated systematic review. Int. J. Environ. Res. Pub. Health 2022, 19, 4914. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, V.Y.; Smith, G.D.; Adashi, E.Y. The future of IVF: The new normal in human reproduction. Reprod. Sci. 2022, 29, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Saha, S. Understanding sperm motility mechanisms and the implication of sperm surface molecules in promoting motility. Mid. East Fert. Soc. J. 2022, 27, 4. [Google Scholar] [CrossRef]
- Hirohashi, N.; Yanagimachi, R. Sperm acrosome reaction: Its site and role in fertilization. Biol. Reprod. 2018, 99, 127–133. [Google Scholar] [CrossRef]
- Tomita, K.; Udayanga, K.G.S.; Satoh, M.; Hashimoto, S.; Morimoto, Y. Relationship between semen oxidative reduction potential in initial semen examination and IVF outcomes. Reprod. Med. Biol. 2023, 22, e12501. [Google Scholar] [CrossRef]
- Ikawa, M.; Inoue, N.; Benham, A.M.; Okabe, M. Fertilization: A sperm’s journey to and interaction with the oocyte. J. Clin. Investig. 2010, 120, 984–994. [Google Scholar] [CrossRef]
- Hussain, T.; Kandeel, M.; Metwally, E.; Murtaza, G.; Kalhoro, D.H.; Yin, Y.; Tan, B.; Chughtai, M.I.; Yaseen, A.; Afzal, A.; et al. Unraveling the harmful effect of oxidative stress on male fertility: A mechanistic insight. Front. Endocrinol. 2023, 14, 1070692. [Google Scholar] [CrossRef]
- Sakkas, D.; Alvarez, J.G. Sperm DNA fragmentation: Mechanisms of origin, impact on reproductive outcome and analysis. Fertil. Steril. 2010, 93, 1027–1036. [Google Scholar] [CrossRef]
- Sedo, C.A.; Bilinski, M.; Lorenzi, D.; Uriondo, H.; Noblia, F.; Longobucco, V.; Lagar, E.V.; Nodar, F. Effect of sperm DNA fragmentation on embryo development: Clinical and biological aspects. JBRA Assist. Reprod. 2017, 21, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Marinaro, J.A.; Schlegel, P.N. Sperm DNA damage and its relevance in fertility treatment: A review of recent literature and current practice guidelines. Int. J. Mol. Sci. 2023, 24, 1446. [Google Scholar] [CrossRef] [PubMed]
- Gual-Frao, J.; Abad, C.; Amengual, M.J.; Hannaoui, N.; Checa, M.A.; Ribas-Maynou, J.; Nikolaou, A.; Benet, J.; Garcia-Piero, A.; Prats, J. Oral antioxidant treatment partly improves integrity of human sperm DNA in infertile grade I varicocele patients. Hum. Fert. 2015, 18, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Soto, J.C.; Domingo, J.C.; Cordobilla, B.; Nicolas, M.; Fernandez, L.; Albero, P.; Gadea, J.; Landeras, J. Dieatry supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Sys. Bio. Rep. Med. 2016, 62, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Noegroho, B.S.; Siregar, S.; Tampubolon, K.A.G. Antioxidant supplementation on sperm DNA fragmentation and sperm paramteres: A systematic review and meta-analysis. Turk. J. Urol. 2022, 48, 336–345. [Google Scholar]
- Silla, A.J.; Keogh, L.M.; Byrne, P.G. Sperm motility activation in the critically endangered booroolong frog: The effect of medium osmolarity and phosphodiesterase inhibitors. Reprod. Fertil. Dev. 2017, 29, 2277–2283. [Google Scholar] [CrossRef]
- Satish, M.; Kumari, S.; Deeksha, W.; Abhishek, S.; Nitin, K.; Adiga, S.K.; Hegde, P.; Dasappa, J.P.; Kalthur, G.; Rajakumara, E. Structure-based redesigning of pentoxifylline analogs against selective phosphodiesterases to modulate sperm functional competence for assisted reproductive technologies. Sci. Rep. 2021, 11, 12293. [Google Scholar] [CrossRef]
- Lu, Y.; Su, H.; Zhang, J.; Wang, Y.; Li, H. Treatment of poor sperm quality and erectile dysfunction with oral pentoxifylline: A systematic review. Front. Pharamacol. 2022, 12, 789787. [Google Scholar] [CrossRef]
- Ayad, B.M.; van der Horst, G.; Du Plessis, S.S. Revisiting the relationship between the ejaculatory abstinence period and semen characteristics. Int. J. Fertil. Steril. 2018, 11, 238–246. [Google Scholar] [PubMed]
- Hanson, B.M.; Aston, K.I.; Jenkins, T.G.; Carrell, D.T.; Hotaling, J.M. The impact of ejaculatory abstinence on semen analysis parameters: A systematic review. J. Assist. Reprod. Gen. 2018, 35, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Anbari, F.; Khalili, M.A.; Ahmed, A.M.S.; Mangoli, E.; Nabi, A.; Dehghanpour, F.; Sabour, M. Microfluidic sperm selection yields higher sperm quality compared to conventional method in ICSI program: A pilot study. Sys. Biol. Reprod. Med. 2021, 67, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Birowo, P.; Wijaya, J.R.; Atmoko, W.; Rasyid, N. The effects of varicoceletomy on the DNA fragmentation index and other sperm parameters: A meta-analysis. Basic Clin. Androl. 2020, 30, 15. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Rangelov, I.; Chaushev, T.A. Hydrophobic soot nanoparticles as a non-cytotoxic motility activator of human spermatozoa. Nanoscale Adv. 2022, 4, 2806–2815. [Google Scholar] [CrossRef] [PubMed]
- Esmeryan, K.D.; Rangelov, I.; Chaushev, T.A. Manipulated sperm motility via soot nanoparticles-induced biochemical alterations in human seminal plasma. Reprod. Biol. 2023, 23, 100793. [Google Scholar] [CrossRef] [PubMed]
- Human Acrosin ELISA Kit, MyBioSource USA. Available online: https://www.biocompare.com/25138-Assay-Kit/9472634-Human-Acrosin-ELISA-Kit/ (accessed on 10 January 2024).
- Kerksick, C.M.; Zuhl, M. Mechanisms of oxidative damage and their impact on contracting muscle. In Antioxidants in Sport Nutrition; Lamprecht, M., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–13. [Google Scholar]
- Aldana, A.; Carneiro, J.; Martinez-Mekler, G.; Darszon, A. Discrete dynamic model of mammalian sperm acrosome reaction: The influence of acrosomal pH and physiological heterogeneity. Front. Physiol. 2021, 12, 682790. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasa, G.; Vineeth, V.S.; Kavitha, P.; Malini, S.S. Evaluation of acrosome intactness status in male infertility in Mysore, South India. Int. J. Appl. Basic Med. Res. 2012, 2, 31–33. [Google Scholar]
- Holder, A.E.; Carter, B.J.; Goldstein, R.G.; Lucas, D.; Koshland, C.P. Increased cytotoxicity of oxidized flame soot. Atmos. Pollut. Res. 2012, 3, 25–31. [Google Scholar] [CrossRef]
- Sabeti, P.; Pourmasumi, S.; Rahiminia, T.; Akyash, F.; Talebi, A.R. Etiologies of sperm oxidative stress. Int. J. Reprod. Biomed. 2016, 14, 231–240. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Castano, C.E.; Gyoshev, S.D.; Lazarov, Y.; Stoimenov, N.I.; Mohammadi, R. On the dynamics of contact line freezing of water droplets on superhydrophobic carbon soot coatings. Curr. Appl. Phys. 2021, 31, 74–86. [Google Scholar] [CrossRef]
- Magdanz, V.; Gebauer, J.; Sharan, P.; Eltoukhy, S.; Voigt, D.; Simmchen, J. Sperm-particle interactions and their prospects for charge mapping. Adv. BioSys. 2019, 3, 1900061. [Google Scholar] [CrossRef]
- Pinto, F.M.; Odriozola, A.; Candenas, L.; Subiran, N. The role of sperm membrane potential and ion channels in regulating sperm function. Int. J. Mol. Sci. 2023, 24, 6995. [Google Scholar] [CrossRef]
- Duan, C.; Goldberg, E. Inhibition of lactate dehydrogenase C4 (LDH-C4) blocks capacitation of mouse sperm in vitro. Cytogenet. Genome Res. 2003, 103, 352–359. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esmeryan, K.D.; Rangelov, I.; Chaushev, T.A. Oxidative Stress and Acrosomal Status of Human Spermatozoa Subjected to Hydrophobic Carbon Soot Treatments. Nanomaterials 2024, 14, 395. https://doi.org/10.3390/nano14050395
Esmeryan KD, Rangelov I, Chaushev TA. Oxidative Stress and Acrosomal Status of Human Spermatozoa Subjected to Hydrophobic Carbon Soot Treatments. Nanomaterials. 2024; 14(5):395. https://doi.org/10.3390/nano14050395
Chicago/Turabian StyleEsmeryan, Karekin D., Ivaylo Rangelov, and Todor A. Chaushev. 2024. "Oxidative Stress and Acrosomal Status of Human Spermatozoa Subjected to Hydrophobic Carbon Soot Treatments" Nanomaterials 14, no. 5: 395. https://doi.org/10.3390/nano14050395
APA StyleEsmeryan, K. D., Rangelov, I., & Chaushev, T. A. (2024). Oxidative Stress and Acrosomal Status of Human Spermatozoa Subjected to Hydrophobic Carbon Soot Treatments. Nanomaterials, 14(5), 395. https://doi.org/10.3390/nano14050395






