The Preparation of High-Performance MoO3 Nanorods for 2.1 V Aqueous Asymmetric Supercapacitor
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
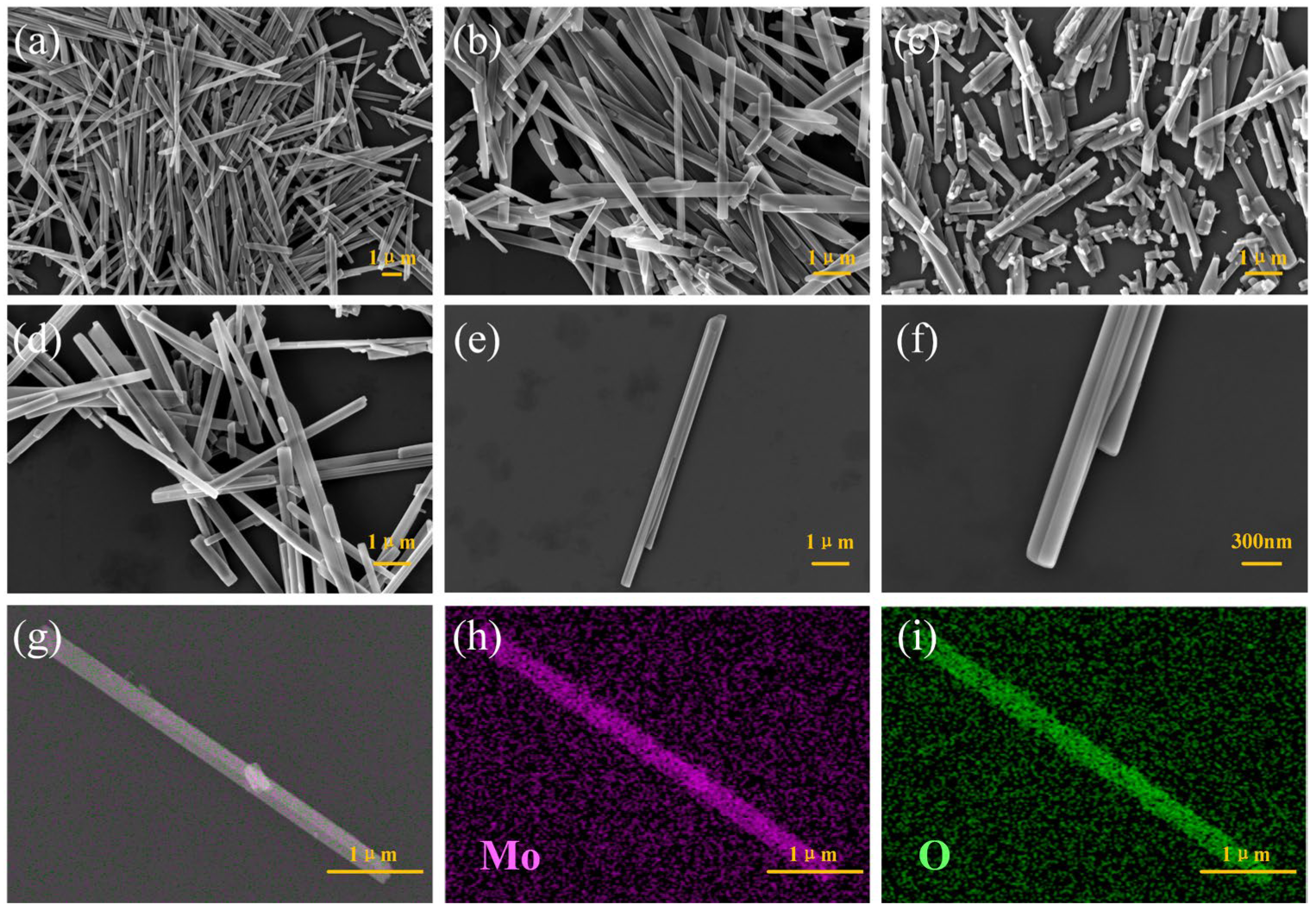
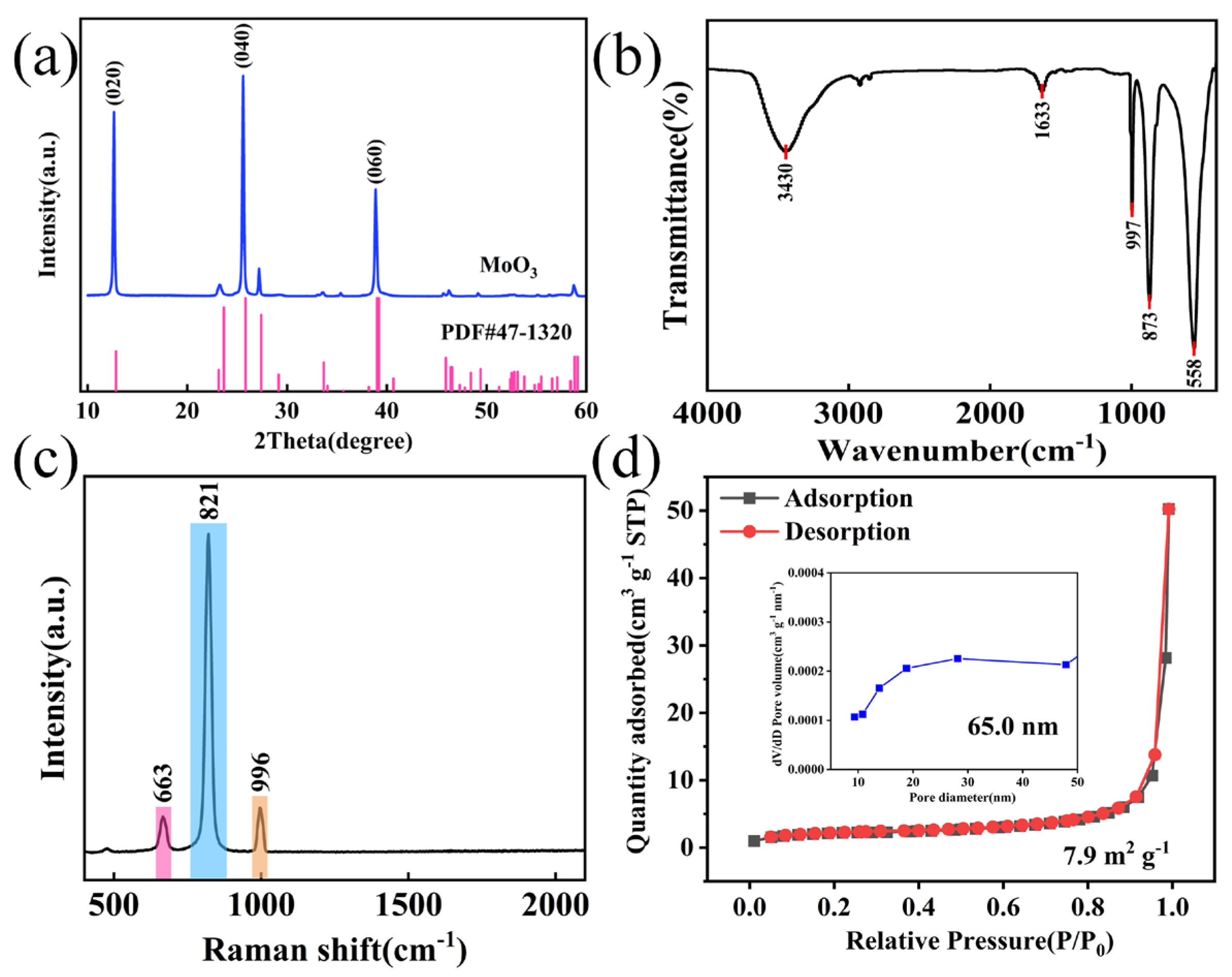
3.1. Structure and Morphology of Materials
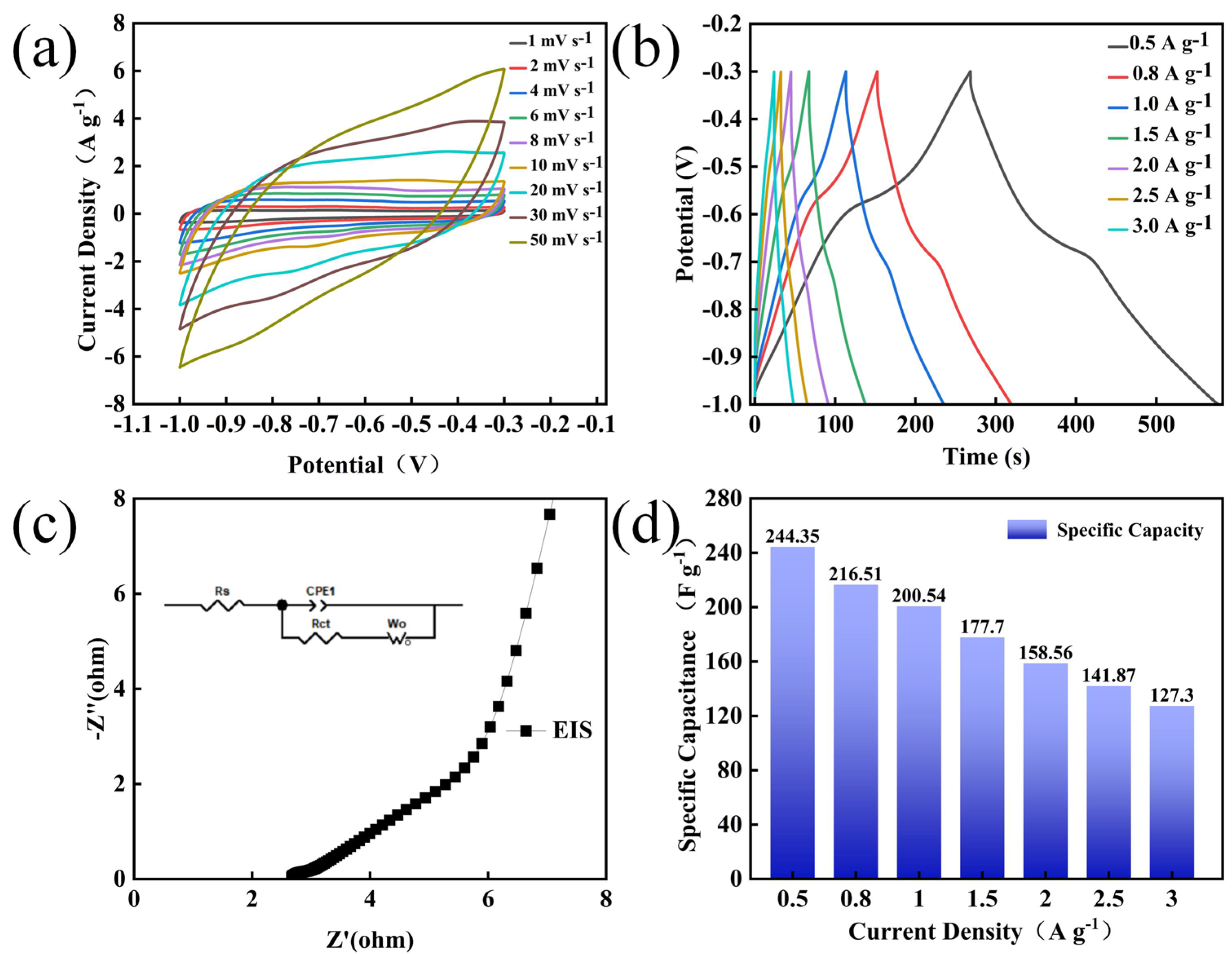
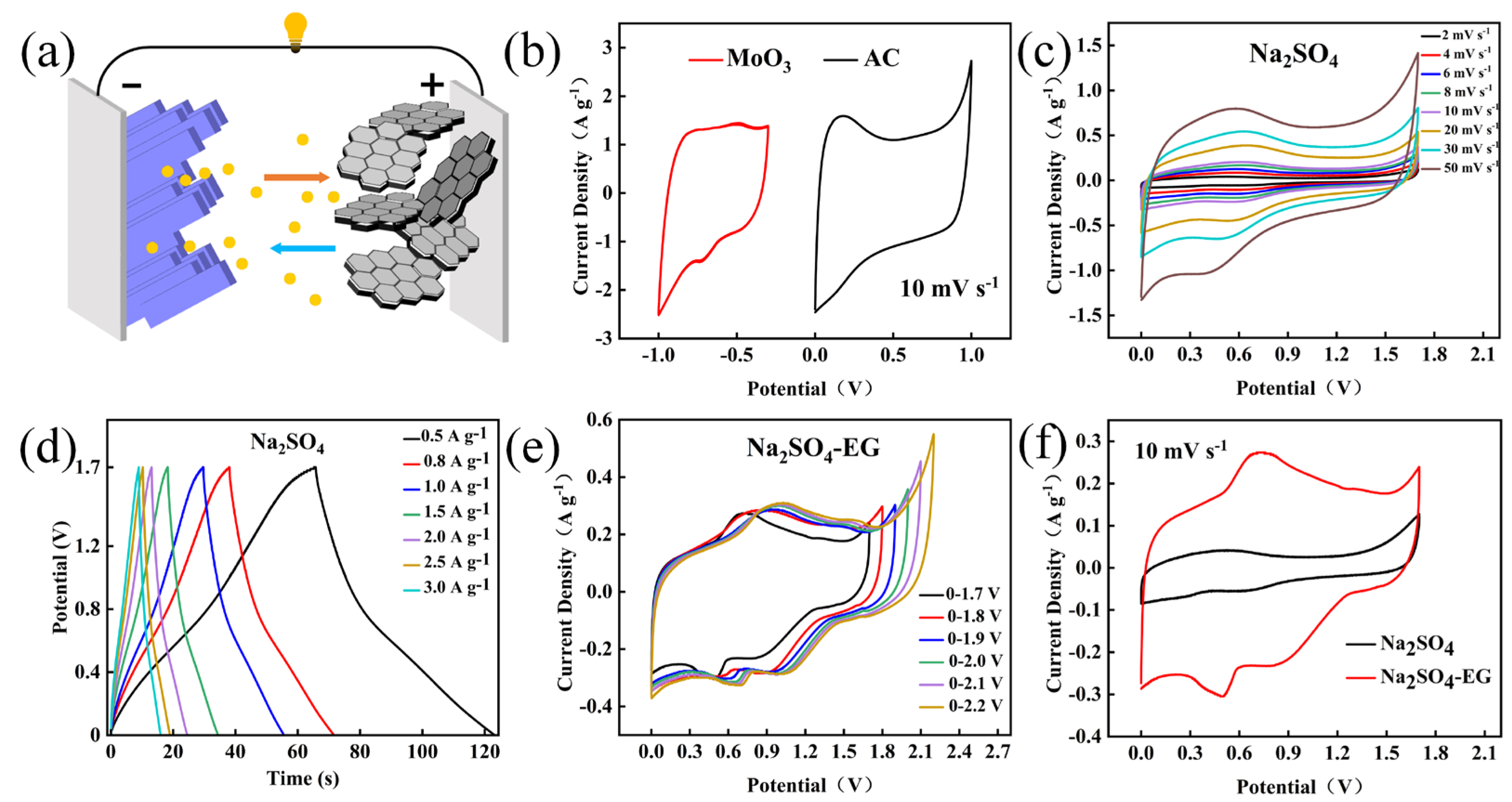
3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, J.L.; Xu, Z.F.; Zhu, H.L.; Liu, R.; Song, X.J.; Song, Q.; Wu, J.; Zhang, C.; Ding, L.; Dong, J.; et al. An ultrafast supercapacitor built by Co3O4 with tertiary hierarchical architecture. Vacuum 2020, 174, 8. [Google Scholar] [CrossRef]
- Guo, R.; Dang, L.Q.; Liu, Z.H.; Lei, Z.B. Incorporation of electroactive NiCo2S4 and Fe2O3 into graphene aerogel for high-energy asymmetric supercapacitor. Colloid. Surf. A Physicochem. Eng. Asp. 2020, 602, 9. [Google Scholar] [CrossRef]
- Libich, J.; Máca, J.; Vondrák, J.; Ech, O.; Sedlaíková, M. Supercapacitors: Properties and applications. J. Energy Storage 2018, 17, 224–227. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, D.Y.; Li, J.T. Supercapacitor performances of MnO2 and MnO2/reduced graphene oxide prepared with various electrodeposition time. Vacuum 2020, 178, 9. [Google Scholar] [CrossRef]
- Mao, X.L.; Liu, H.; Xu, J.H.; Li, M.W.; Yang, W.Y. Surface reconstruction of Co3O4/rGO heterointerface enabling high-performance asymmetric supercapacitors. J. Energy Storage 2024, 102, 114128. [Google Scholar] [CrossRef]
- Pal, S.; Kumar Chattopadhyay, K. Fabrication of Molybdenum Trioxide Nanobelts as High Performance Supercapacitor. Mater. Today Proc. 2018, 5, 9776–9782. [Google Scholar] [CrossRef]
- Wang, L.C.; Gao, L.; Wang, J.; Shen, Y. MoO3 nanobelts for high-performance asymmetric supercapacitor. J. Mater. Sci. 2019, 54, 13685–13693. [Google Scholar] [CrossRef]
- Si, H.; Han, C.; Cui, Y.; Sang, S.; Liu, K.; Liu, H.; Wu, Q. The electrochemical properties of iodine cathode in a novel rechargeable hydrogen ion supercapattery system with molybdenum trioxide as anode. Electrochim. Acta 2021, 399, 139331. [Google Scholar] [CrossRef]
- Lahan, H.; Das, S.K. Reversible Al3+ ion insertion into tungsten trioxide (WO3) for aqueous aluminum-ion batteries. Dalton Trans. 2019, 48, 6337–6340. [Google Scholar] [CrossRef]
- Huang, S.; Li, Z.; Li, P.; Du, X.; Ma, M.; Liang, Z.; Su, Y.; Xiong, L. Ultrahigh-voltage aqueous electrolyte for wide-temperature supercapacitors. J. Mater. Chem. 2023, 11, 15532–15539. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Bellani, S.; Gamberini, A.; Mastronardi, V.; Zappia, M.I.; Abruzzese, M.; Thorat, S.; Calcagno, E.; Bonaccorso, F. Water-based supercapacitors with amino acid electrolytes: A green perspective for capacitance enhancement. Batter. Supercaps 2024, 7, 7. [Google Scholar] [CrossRef]
- Wang, R.Y.; Shi, M.J.; Li, L.Y.; Zhao, Y.; Zhao, L.P.; Yan, C. In-situ investigation and application of cyano-substituted organic electrode for rechargeable aqueous Na-ion batteries. Chem. Eng. J. 2023, 451, 10. [Google Scholar] [CrossRef]
- Liu, S.Q.; Klukas, R.; Porada, T.; Furda, K.; Fernandez, A.M.; Balducci, A. Potassium formate-based electrolytes for high performance aqueous electrochemical capacitors. J. Power Sources 2022, 541, 6. [Google Scholar] [CrossRef]
- Nandagudi, A.; Nagarajarao, S.H.; Santosh, M.S.; Basavaraja, B.M.; Malode, S.J.; Mascarenhas, R.J.; Shetti, N.P. Hydrothermal synthesis of transition metal oxides, transition metal oxide/carbonaceous material nanocomposites for supercapacitor applications. Mater. Today Sustain. 2022, 19, 19. [Google Scholar] [CrossRef]
- Duan, H.; Zhao, Z.; Lu, J.; Hu, W.; Pang, H. When Conductive MOFs Meet MnO2: High Electrochemical Energy Storage Performance in an Aqueous Asymmetric Supercapacitor. ACS Appl. Mater. Intefaces 2021, 13, 33083–33090. [Google Scholar] [CrossRef]
- Wang, T.Y.; Liu, J.; Ma, Y.X.; Han, S.; Gu, C.D.; Lian, J.S. P-N heterojunction NiO/ZnO electrode with high electrochemical performance for supercapacitor applications. Electrochim. Acta 2021, 392, 11. [Google Scholar] [CrossRef]
- Cheng, S.T.; Zhang, Y.X.; Liu, Y.P.; Sun, Z.H.; Cui, P.; Zhang, J.L.; Hua, X.; Su, Q.; Fu, J.; Xie, E. Energizing Fe2O3-based supercapacitors with tunable surface pseudocapacitance via physical spatial-confining strategy. Chem. Eng. J. 2021, 406, 11. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Yan, M.; Yang, Q.J.; Liu, X.Y.; Shi, W.D. Lewis acid etched NixCo1-xSe2 derived from ZIF-L on CoO nanowires for hybrid-supercapacitors. Chem. Eng. J. 2022, 431, 14. [Google Scholar] [CrossRef]
- De Castro, I.A.; Datta, R.S.; Ou, J.Z.; Castellanos-Gomez, A.; Sriram, S.; Daeneke, T.; Kalantar-zadeh, K. Molybdenumoxides-from fundamentals to functionality. Adv. Mater. 2017, 29, 1701619. [Google Scholar] [CrossRef]
- Hu, X.L.; Zhang, W.; Liu, X.X.; Mei, Y.N.; Huang, Y. Nanostructured Mo-based electrode materials for electrochemical energy storage. Chem. Soc. Rev. 2015, 44, 2376–2404. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Wang, S.L.; Liu, H.K.; Li, L. Transition metal based battery-type electrodes in hybrid supercapacitors: A review. ESM 2020, 28, 122–145. [Google Scholar] [CrossRef]
- Pan, Z.H.; Yang, C.H.; Li, Y.; Hu, X.; Ji, X.H. Rational design of A-CNTs/KxMnO2 and Ti3C2Tx/MoO3 free-standing hybrid films for flexible asymmetric supercapacitor. Chem. Eng. J. 2022, 428, 131138. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Zhang, Z.; Qi, X.; Ren, X.H.; Xu, G.H.; Wan, P.B.; Sun, X.M.; Zhang, H. Wall-like hierarchical metal oxide nanosheet arrays grown on carbon cloth for excellent supercapacitor electrodes. Nanoscale 2016, 8, 13273–13279. [Google Scholar] [CrossRef]
- Zhang, S.W.; Yin, B.S.; Liu, C.; Wang, Z.B.; Gu, D.M. Self-assembling hierarchical NiCo2O4/MnO2 nanosheets and MoO3/PPy core-shell heterostructured nanobelts for supercapacitor. Chem. Eng. J. 2017, 312, 296–305. [Google Scholar] [CrossRef]
- Noby, S.Z.; Mohanty, A.; Zirak, P.; Ramadoss, A.; Schmidt-Mende, L. Ramadoss, Hierarchical carbon coated vertically aligned α-MoO3 nanoblades anode materials for supercapacitor application. J. Alloys Compd. 2022, 918, 165530. [Google Scholar] [CrossRef]
- Yu, C.Y.; Xu, H.; Gong, Y.J.; Chen, R.Y.; Hui, Z.Y.; Zhao, X.; Sun, Y.; Chen, Q.; Zhou, J.; Ji, W.; et al. The Jahn-Teller Effect for Amorphization of Molybdenum Trioxide towards High-Performance Fiber Supercapacitor. Research 2021, 2021, 11. [Google Scholar] [CrossRef]
- Mao, X.L.; He, X.; Yang, W.Y.; Liu, H.; Zhou, Y.J.; Xu, J.H.; Yang, Y. Hierarchical holey Co9S8@S-rGO hybrid electrodes for high-performance asymmetric supercapacitors. Electrochim. Acta 2019, 328, 11. [Google Scholar] [CrossRef]
- Hu, H.M.; Deng, C.H.; Xu, J.C.; Zhang, K.H.; Sun, M. Metastable h-MoO3 and stable α-MoO3 microstructures: Controllable synthesis, growth mechanism and their enhanced photocatalytic activity. J. Exp. Nanosci. 2015, 10, 1336–1346. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, X.; Chen, P.; Zhu, K.; Cheng, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J. Creating oxygen-vacancies in MoO3-x nanobelts toward high volumetric energy-density asymmetric supercapacitors with long lifespan. Nano Energy 2019, 58, 455–465. [Google Scholar] [CrossRef]
- Xu, L.M.; Zhou, W.Q.; Chao, S.X.; Liang, Y.M.; Zhao, X.Q.; Liu, C.C.; Xu, J. Advanced Oxygen-Vacancy Ce-Doped MoO3 Ultrathin Nanoflakes Anode Materials Used as Asymmetric Supercapacitors with Ultrahigh Energy Density. Adv. Energy Mater. 2022, 12, 13. [Google Scholar] [CrossRef]
- Saraf, M.; Shuck, C.E.; Norouzi, N.; Matthews, K.; Inman, A.; Zhang, T.; Pomerantseva, E.; Gogotsi, Y. Free-Standing α-MoO3/Ti3C2 MXene Hybrid Electrode in Water-in-Salt Electrolytes. Energy Environ. Mater. 2023, 6, 6–14. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; He, X.; Yang, W.; Yang, Y.; Xu, L.; Zhao, Y.; Zhou, Y. All-solid-state flexible microsupercapacitors based on reduced graphene oxide/multi-walled carbon nanotube composite electrodes. Applied Surface Science. Appl. Surf. Sci. 2018, 435, 1228–1236. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Liu, F.; Song, X.; Cui, H. Fabrication of NiO–ZnO-modified g C3N4 hierarchical composites for high-performance supercapacitors. Vacuum 2020, 178, 109453. [Google Scholar] [CrossRef]
- Yao, D.; Wang, F.L.; Lei, W.; Hua, Y.; Xia, X.F.; Liu, J.P.; Hao, Q. Oxygen vacancies boosting ultra-stability of mesoporous ZnO-CoO@N-doped carbon microspheres for asymmetric supercapacitors. Sci. China-Mater. 2020, 63, 2013–2027. [Google Scholar] [CrossRef]
- Ko, W.Y.; Chen, Y.F.; Lu, K.M.; Lin, K.J. Porous honeycomb structures formed from interconnected MnO2 sheets on CNT-coated substrates for flexible all-solid-state supercapacitors. Sci. Rep. 2016, 6, 18887. [Google Scholar] [CrossRef]
- Tang, C.; Tang, Z.; Gong, H. Hierarchically Porous Ni-Co Oxide for High Reversibility Asymmetric Full-Cell Supercapacitors. J. Electrochem. Soc. 2012, 159, A651. [Google Scholar] [CrossRef]
- Yan, L.; Huang, J.H.; Guo, Z.W.; Dong, X.L.; Wang, Z.; Wang, Y.G. Solid-State Proton Battery Operated at Ultralow Temperature. ACS Energy Lett. 2020, 5, 685–691. [Google Scholar] [CrossRef]
- Xu, T.Z.; Xu, Z.M.; Yao, T.Y.; Zhang, M.; Chen, D.; Zhang, X.; Shen, L. Discovery offast and stableproton storage in bulk hexagonal molybdenum oxide. Nat. Commun. 2023, 14, 8360. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Tan, Y.T.; Li, H. MoO3 nanoplates preparation via self-sacrifice C3N4 for supercapacitors in an acid electrolyte. J. Energy Storage 2023, 60, 106657. [Google Scholar] [CrossRef]
- Iamprasertkun, P.; Hirunpinyopas, W.; Tripathi, A.M.; Bissett, M.A.; Dryfe, R.A.W. Electrochemical intercalation of MoO3-MoS2 composite electrodes: Charge storage mechanism of non-hydrated cations. Electrochim. Acta 2019, 307, 176–187. [Google Scholar] [CrossRef]



| Samples | PH = 0.36 | PH = 0.58 | PH = 0.84 |
|---|---|---|---|
| BET surface area (m2 g−1) | 7.9 | 7.6 | 7.5 |
| BJH average pore size (nm) | 65.0 | 48.7 | 40.0 |
| System | Voltage Window | Number of Cycles | Capacitance Retention Rate (%) | Ref. |
|---|---|---|---|---|
| MnO2 @GF//MoO3 | 0.85–1.55 V | 300 | 81% | [37] |
| I2-NP-CP@Nafion//MoO3 | 0–1.8 V | 500 | 93.2% | [8] |
| Cu0.82Co0.18HCF//h-MoO3 | 0–1.6 V | 10,000 | 83% | [38] |
| YP50//MoO3 | 0–1.4 V | 1500 | 84% | [39] |
| Graphene composite//MoO3−MoS2 | −0.8–0 V | 500 | 50.3% | [40] |
| AC//MoO3 | 0–2.1 V | 5000 | 1092% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, Z.; Mao, X.; Song, Y.; Yao, K.; Zhang, R.; Yan, X.; Li, M. The Preparation of High-Performance MoO3 Nanorods for 2.1 V Aqueous Asymmetric Supercapacitor. Nanomaterials 2024, 14, 2029. https://doi.org/10.3390/nano14242029
Lian Z, Mao X, Song Y, Yao K, Zhang R, Yan X, Li M. The Preparation of High-Performance MoO3 Nanorods for 2.1 V Aqueous Asymmetric Supercapacitor. Nanomaterials. 2024; 14(24):2029. https://doi.org/10.3390/nano14242029
Chicago/Turabian StyleLian, Ziyu, Xiling Mao, Yi Song, Kaihua Yao, Ruifeng Zhang, Xinyu Yan, and Mengwei Li. 2024. "The Preparation of High-Performance MoO3 Nanorods for 2.1 V Aqueous Asymmetric Supercapacitor" Nanomaterials 14, no. 24: 2029. https://doi.org/10.3390/nano14242029
APA StyleLian, Z., Mao, X., Song, Y., Yao, K., Zhang, R., Yan, X., & Li, M. (2024). The Preparation of High-Performance MoO3 Nanorods for 2.1 V Aqueous Asymmetric Supercapacitor. Nanomaterials, 14(24), 2029. https://doi.org/10.3390/nano14242029







