Magnetically Controlled Transport of Nanoparticles in Solid Tumor Tissues and Porous Media Using a Tumor-on-a-Chip Format
Abstract
1. Introduction
2. Materials and Methods
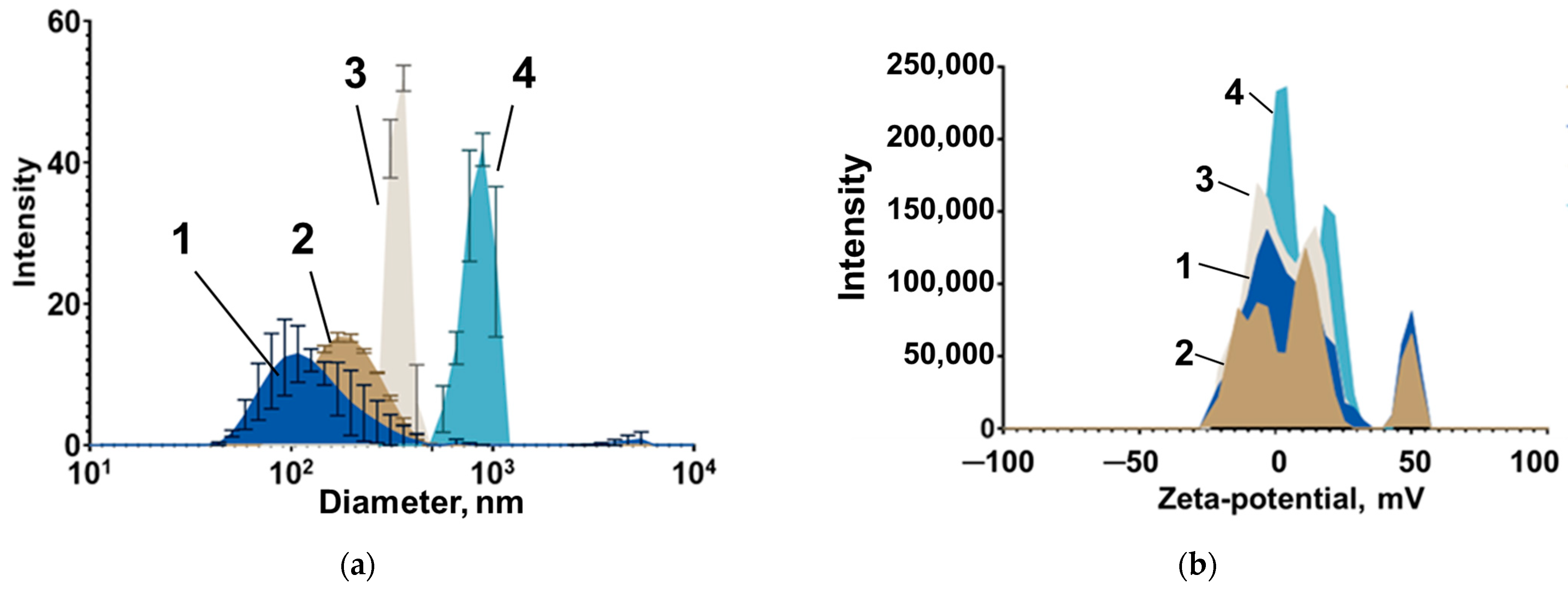
2.1. Magnetic Nanoparticles
2.1.1. Materials for Preparation of Magnetite Nanoparticles
2.1.2. A Procedure of Magnetite Nanoparticles Formation
2.1.3. Materials for Preparation of Carboxymethyl Dextran-Coated Superparamagnetic Iron Oxide Particles (CD-SPIONs)
2.1.4. Procedure of CD-SPIONs Preparation
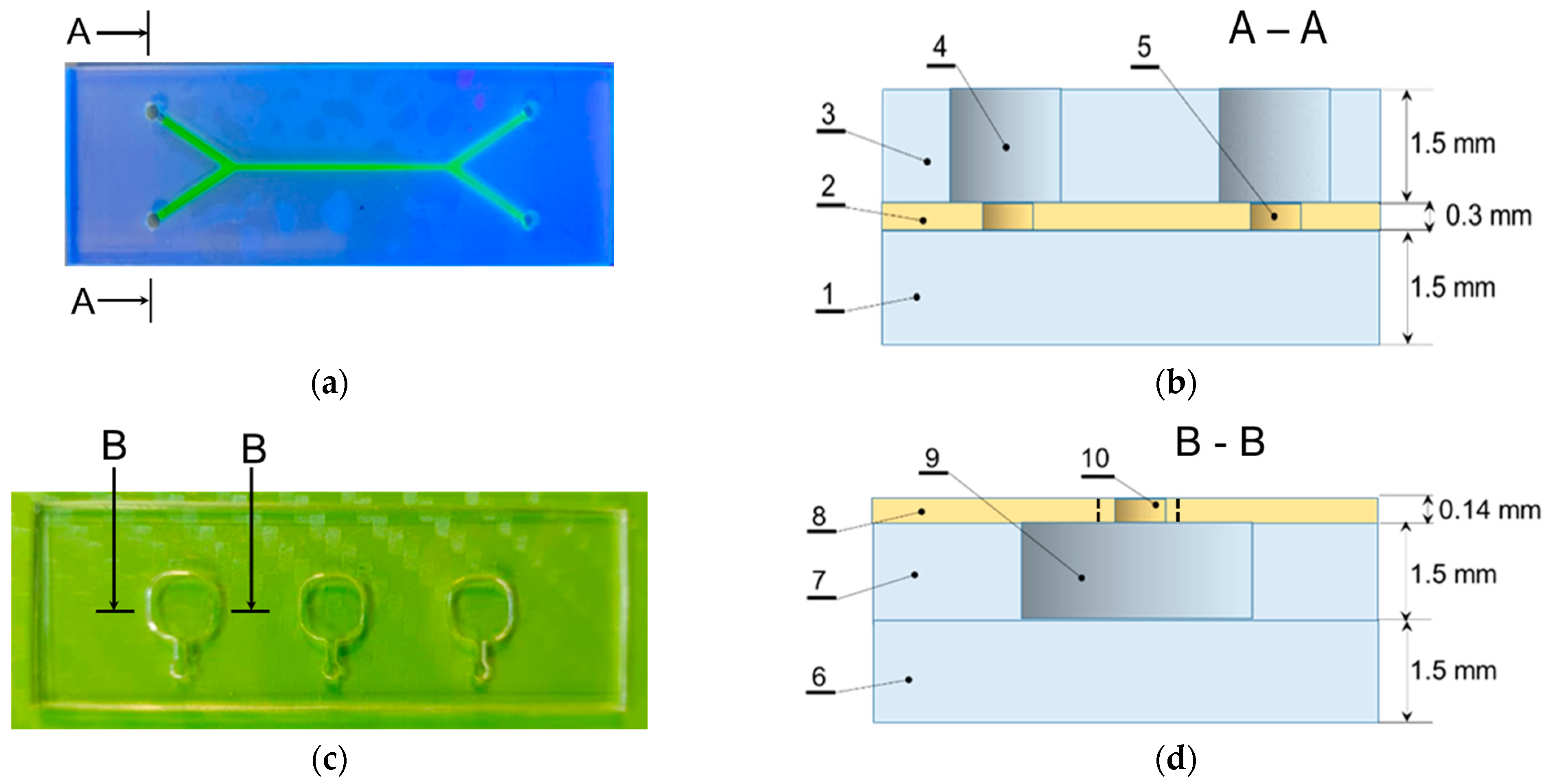
2.2. Microfluidic Chips
2.2.1. Materials for Microfluidic Chips Fabrication
2.2.2. Microfluidic Chips Fabrication Technology
2.3. Porous Media of Differing Mean Pore Diameter
2.3.1. Materials for Preparation of Porous Media
2.3.2. Procedure of Porous Media Preparation
2.4. Testing Materials, Buffer Solutions
2.5. Methods
2.5.1. Collection of Laboratory Mouse Brain Tissue Sample
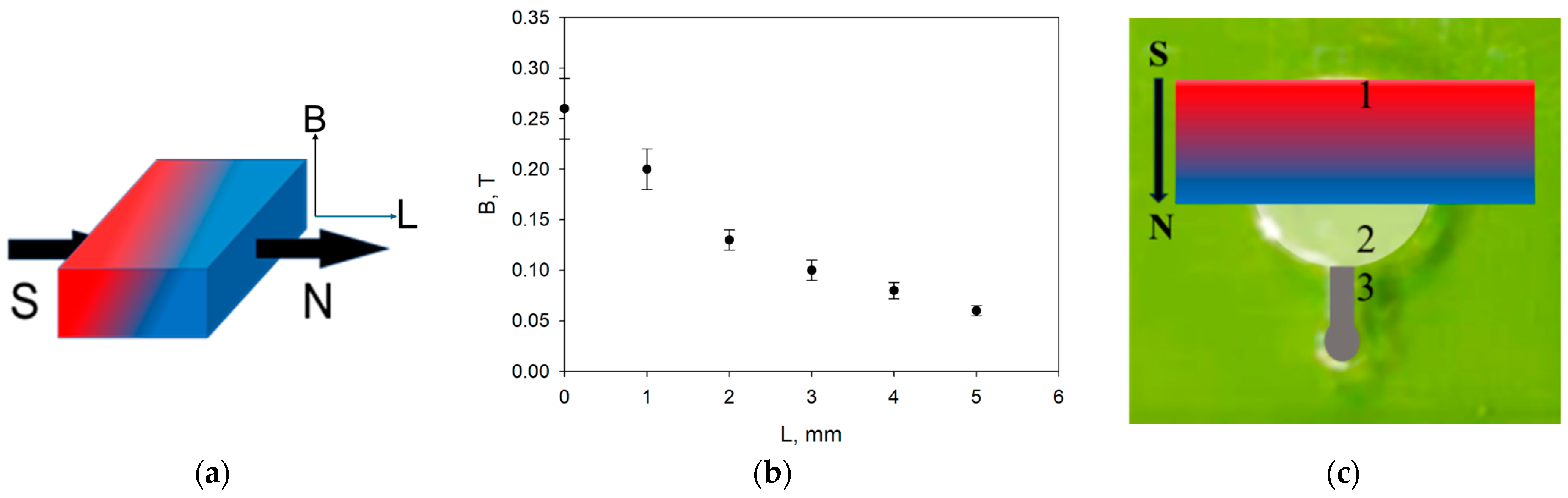
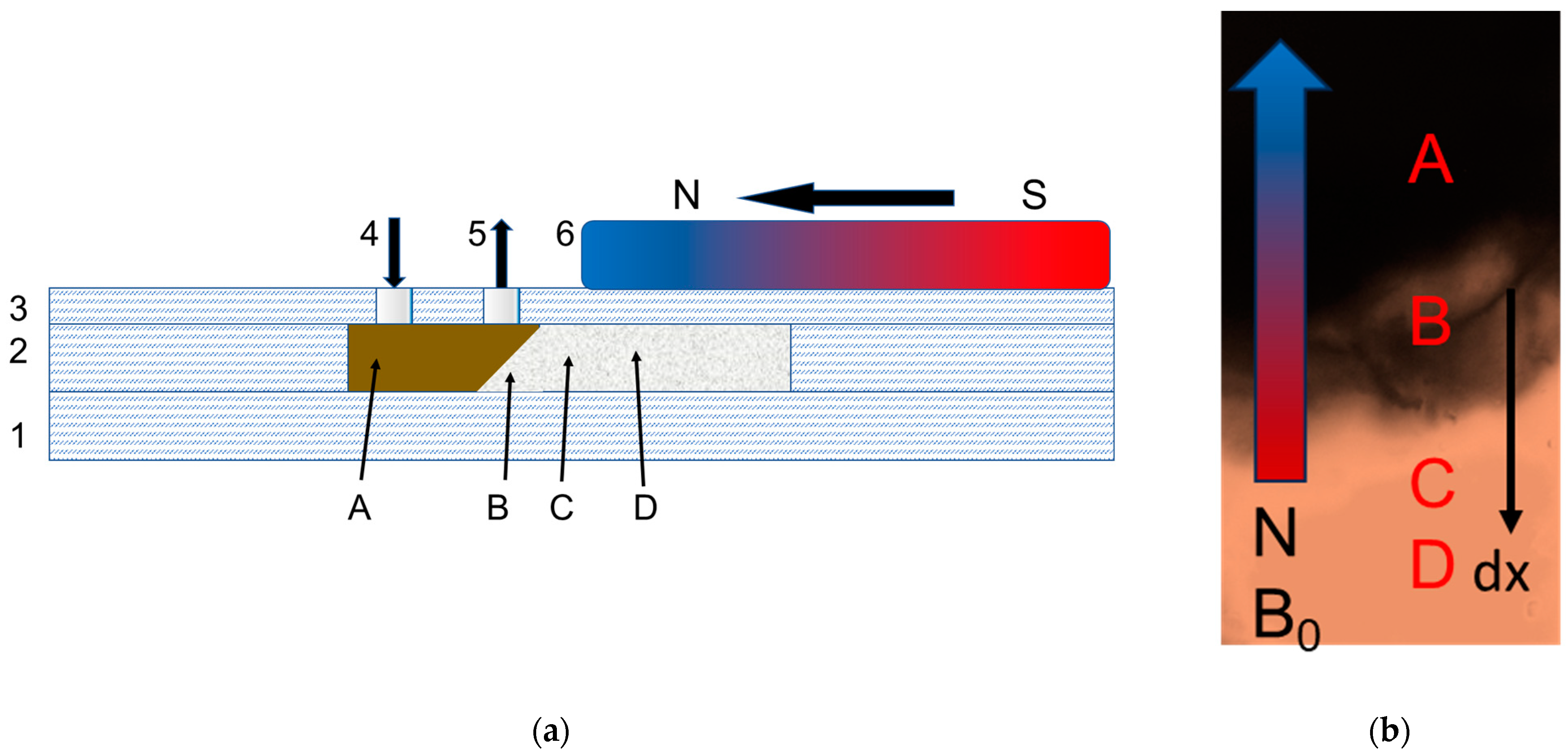
2.5.2. Application of Magnetic Field in the Microfluidic System
3. Results
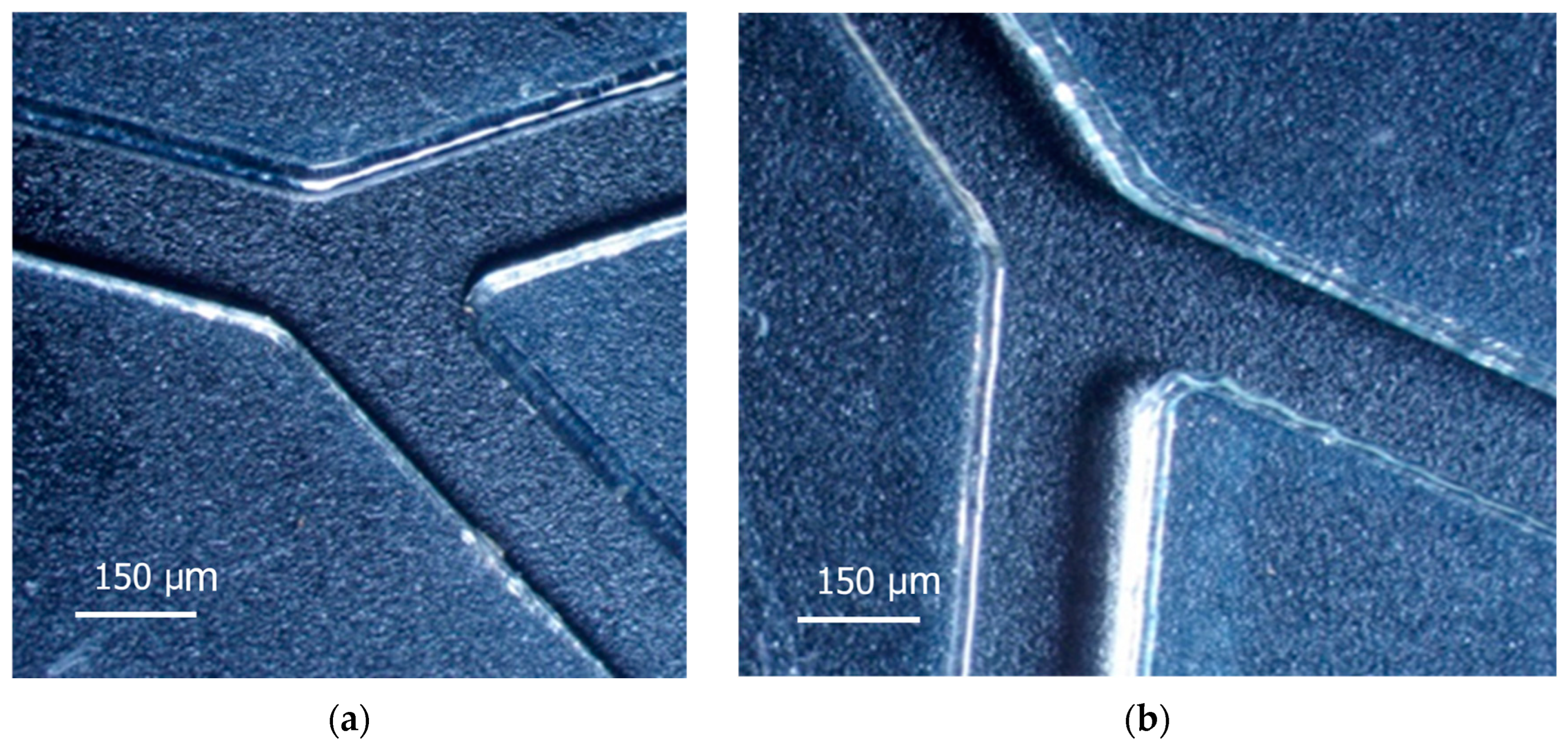
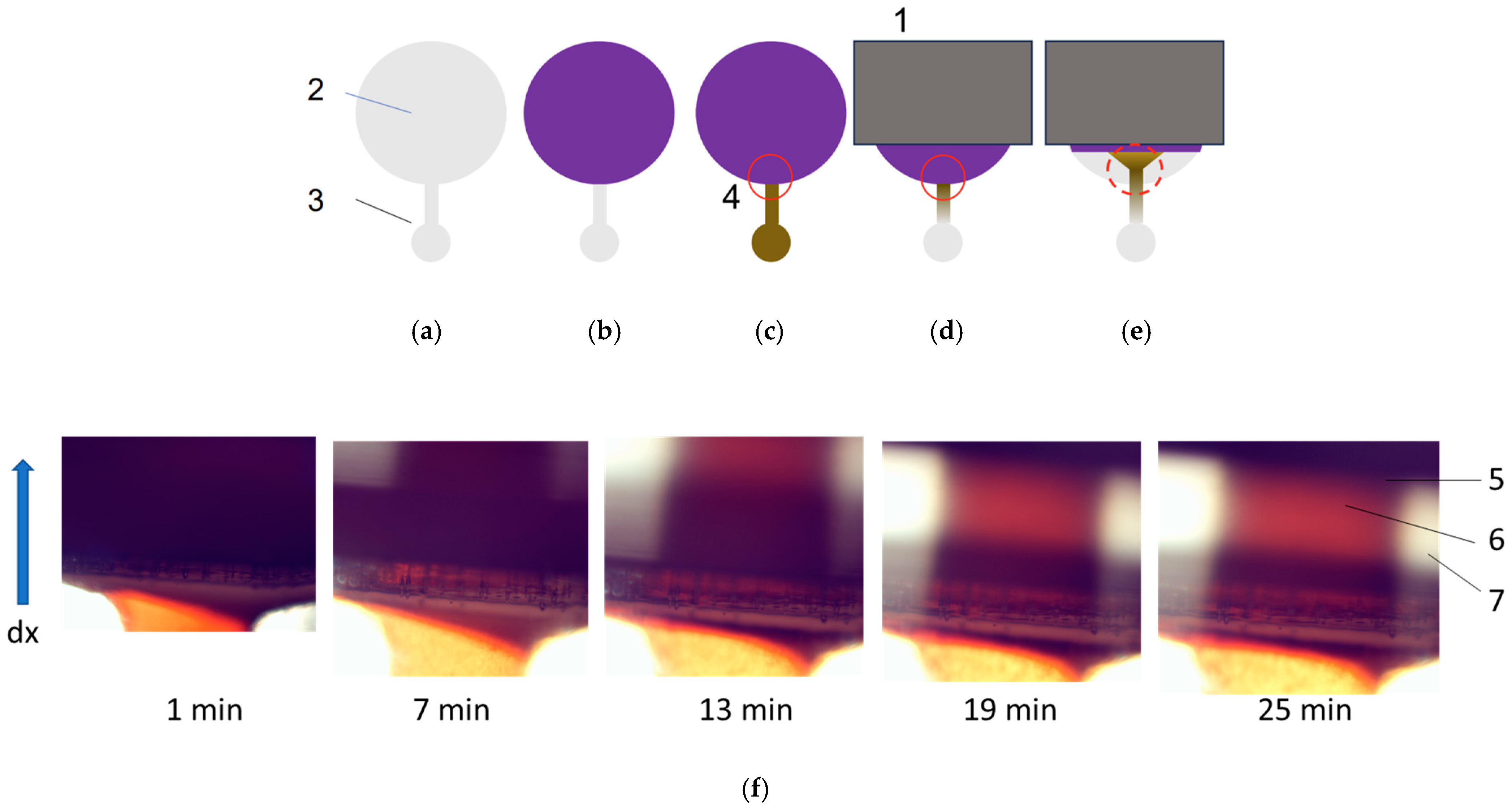
3.1. Features of Manufacturing Microfluidic Systems
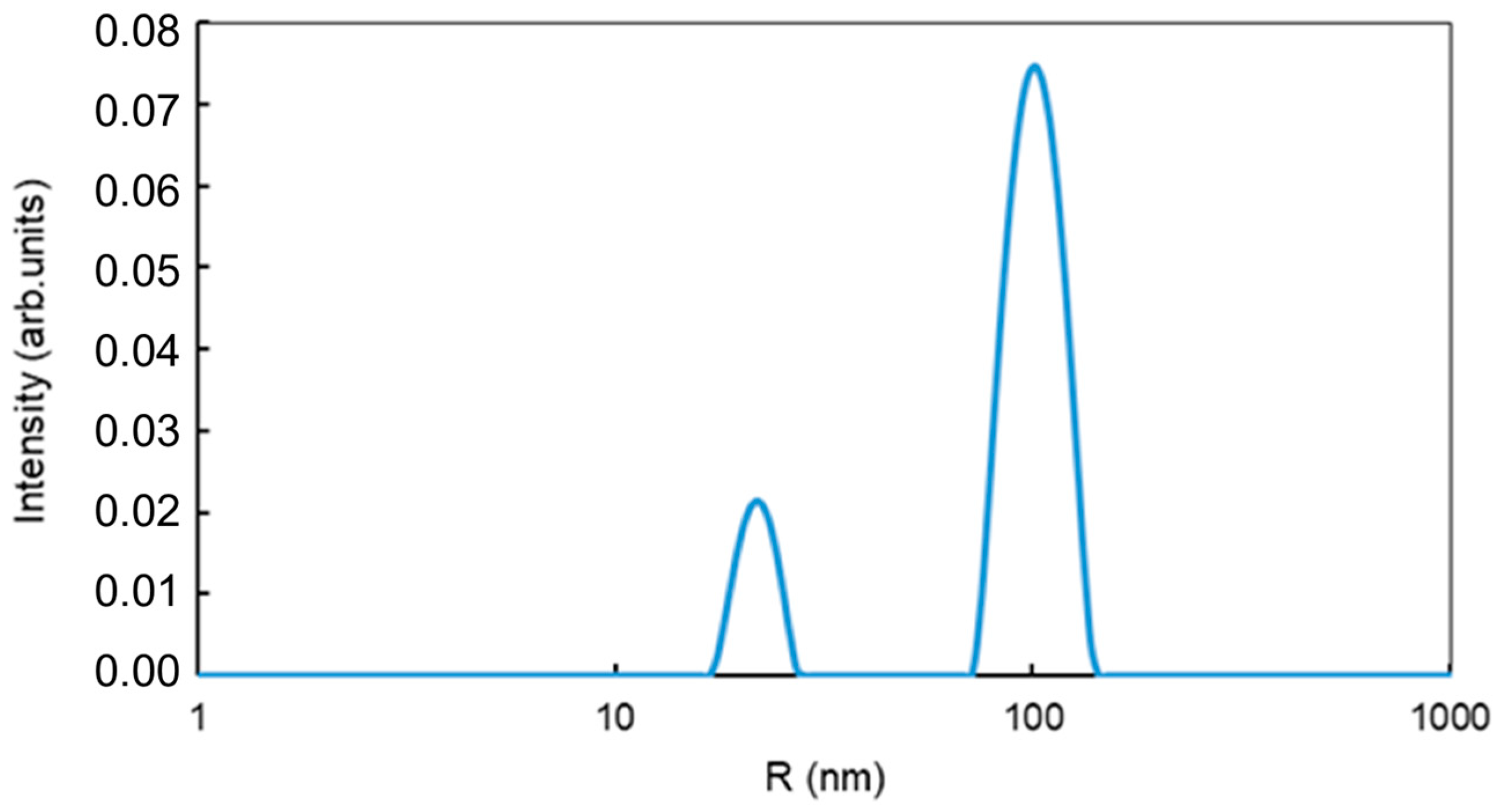
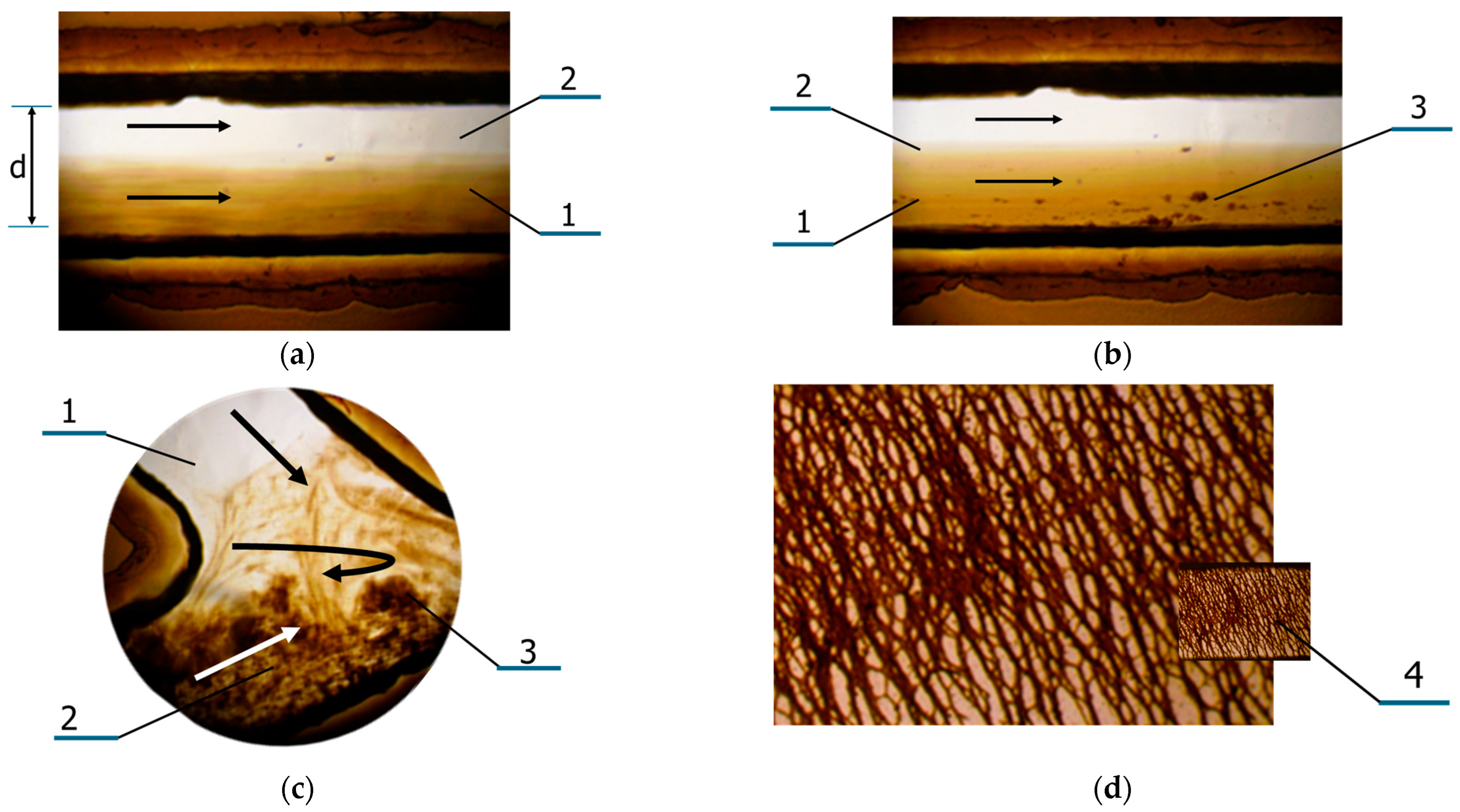
3.2. Features of Magnetic Nanoparticles Migrating in Aqueous Media Patterns
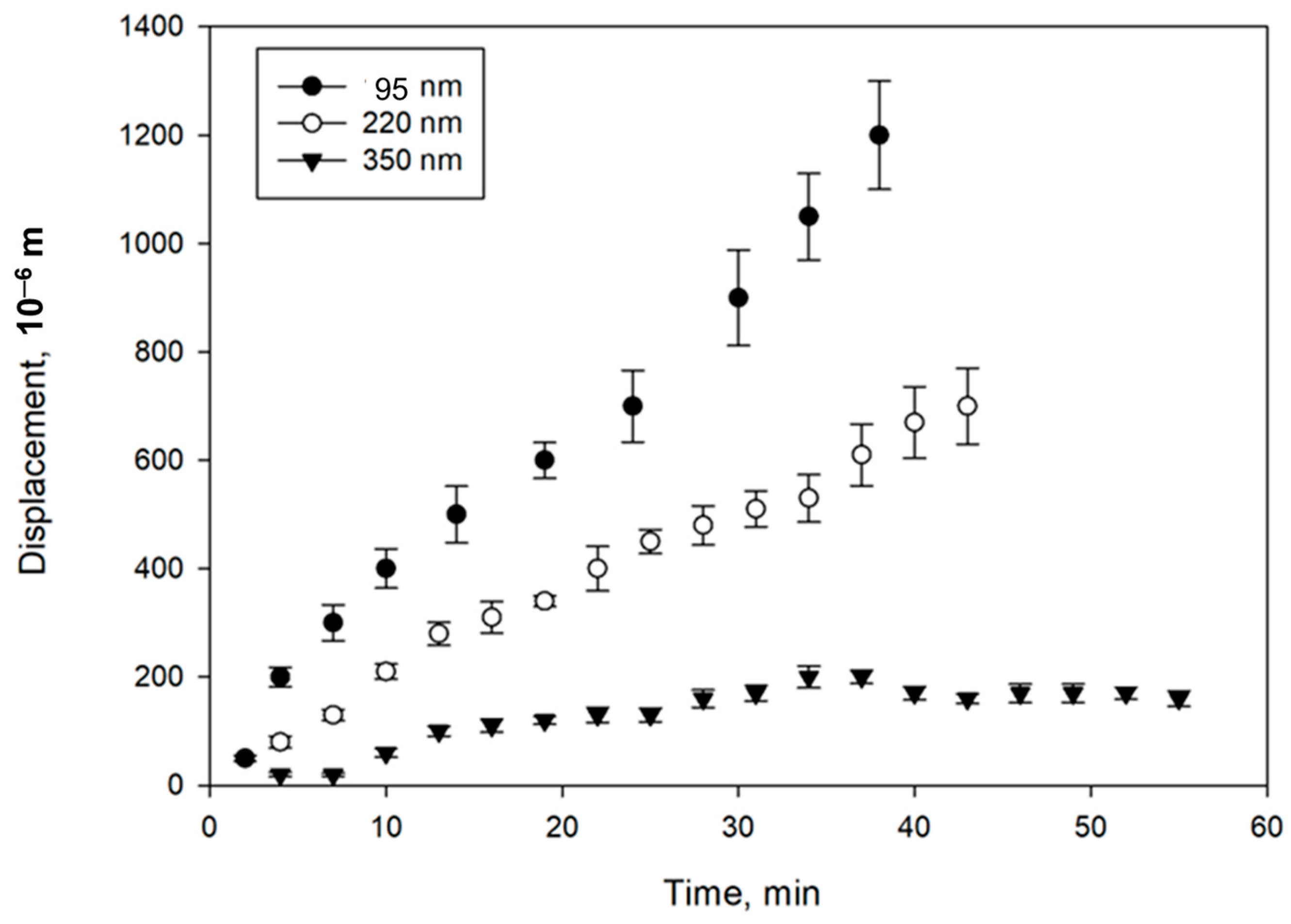
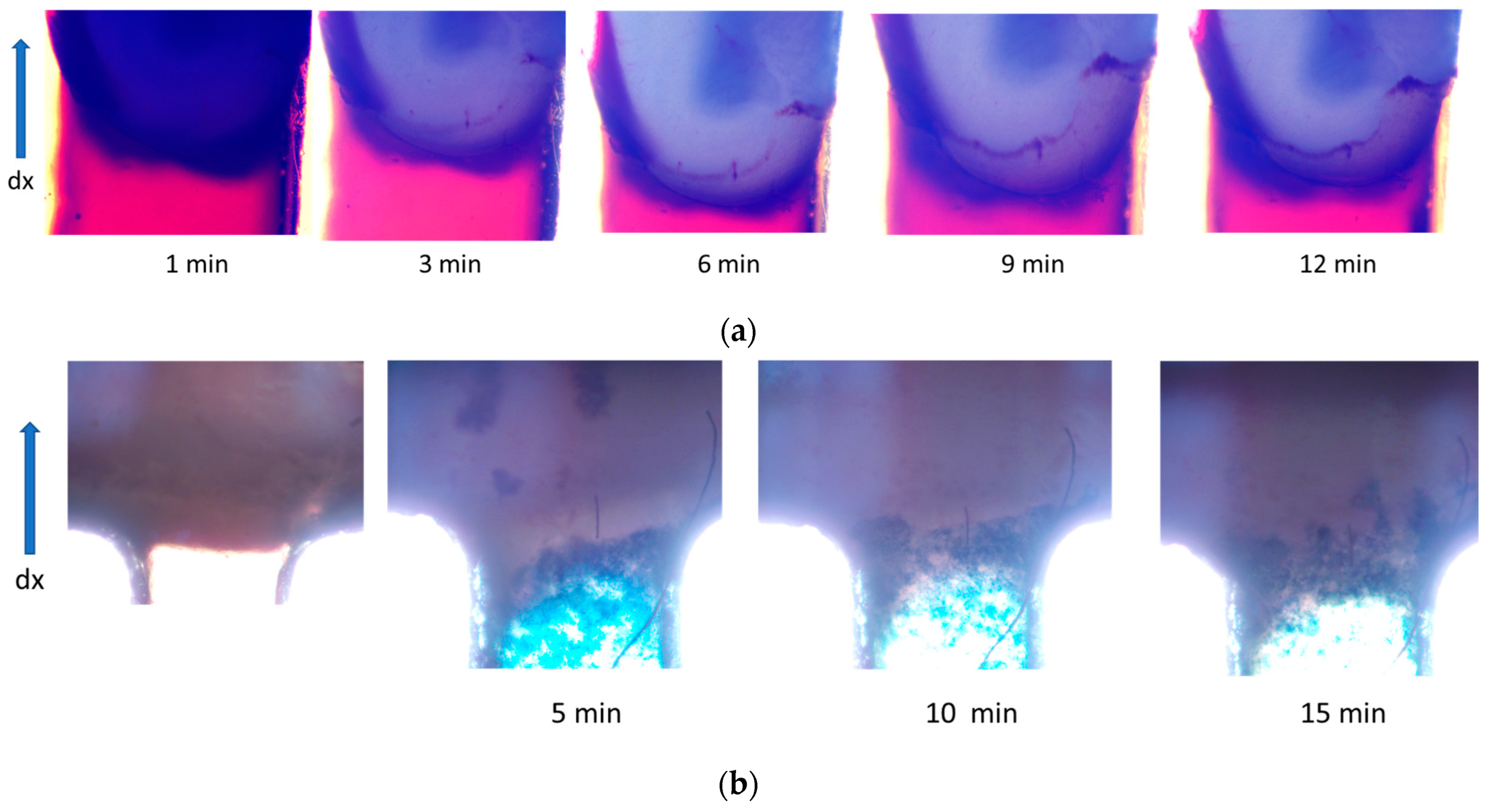
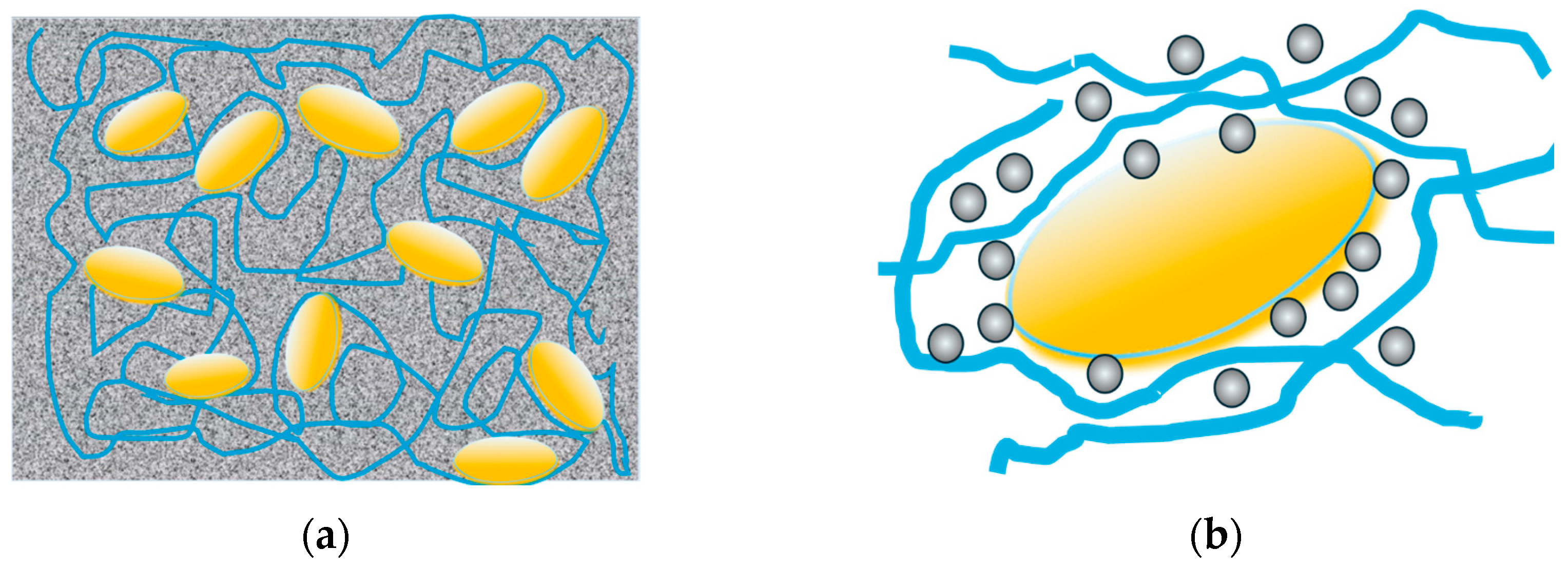
3.3. Study of Magnetically Controlled Migration of SPIONs in Porous Media Modeling Life Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 28 August 2024).
- Turner, J.H. An Introduction to the Clinical Practice of Theranostics in Oncology. Br. J. Radiol. 2018, 91, 20180440. [Google Scholar] [CrossRef]
- Arnold, C. Theranostics Could Be Big Business in Precision Oncology. Nat. Med. 2022, 28, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Tietze, R.; Zaloga, J.; Unterweger, H.; Lyer, S.; Friedrich, R.P.; Janko, C.; Pöttler, M.; Dürr, S.; Alexiou, C. Magnetic Nanoparticle-Based Drug Delivery for Cancer Therapy. Biochem. Biophys. Res. Commun. 2015, 468, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Zimina, T.M.; Sitkov, N.O.; Gareev, K.G.; Fedorov, V.; Grouzdev, D.S.; Koziaeva, V.V.; Gao, H.; Combs, S.E.; Shevtsov, M. Biosensors and Drug Delivery in Oncotheranostics Using Inorganic Synthetic and Biogenic Magnetic Nanoparticles. Biosensors 2022, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, B.; Yakovleva, L.; Fedorov, V.; Yudintceva, N.; Ryzhov, V.; Marchenko, Y.; Ischenko, A.; Zhakhov, A.; Dobrodumov, A.; Combs, S.E.; et al. Magnetic Relaxation Switching Assay Using IFNα-2b-Conjugated Superparamagnetic Nanoparticles for Anti-Interferon Antibody Detection. Biosensors 2023, 13, 624. [Google Scholar] [CrossRef] [PubMed]
- Yudintceva, N.; Lomert, E.; Mikhailova, N.; Tolkunova, E.; Agadzhanian, N.; Samochernych, K.; Multhoff, G.; Timin, G.; Ryzhov, V.; Deriglazov, V.; et al. Targeting Brain Tumors with Mesenchymal Stem Cells in the Experimental Model of the Orthotopic Glioblastoma in Rats. Biomedicines 2021, 9, 1592. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Xia, G.; Adilijiang, N.; Li, Y.; Hou, Z.; Fan, Z.; Li, J. Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy. Pharmaceutics 2023, 15, 2233. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.; Stangl, S.; Nikolaev, B.; Yakovleva, L.; Marchenko, Y.; Tagaeva, R.; Sievert, W.; Pitkin, E.; Mazur, A.; Tolstoy, P.; et al. Granzyme B Functionalized Nanoparticles Targeting Membrane Hsp70-Positive Tumors for Multimodal Cancer Theranostics. Small 2019, 15, e1900205. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.W.; Arrott, A.; Watson, J. Magnetism in Medicine. Available online: https://www.semanticscholar.org/paper/Magnetism-in-Medicine-Freeman-Arrott/488bd929580fc3650bc78f86bd434bcfca94ef7c (accessed on 2 December 2024).
- Khan, U.S.; Khattak, N.K.; Rahman, A.; Khan, F. Historical Development of Magnetite Nanoparticles Synthesis. J. Chem. Soc. Pak. 2011, 33, 793–804. [Google Scholar]
- Suwa, T.; Ozawa, S.; Ueda, M.; Ando, N.; Kitajima, M. Magnetic resonance imaging of esophageal squamous cell carcinoma using magnetite particles coated with anti-epidermal growth factor receptor antibody. Int. J. Cancer 1998, 75, 626–634. [Google Scholar] [CrossRef]
- De Paoli, V.M.; De Paoli Lacerda, S.H.; Spinu, L.; Ingber, B.; Rosenzweig, Z.; Rosenzweig, N. Effect of an Oscillating Magnetic Field on the Release Properties of Magnetic Collagen Gels. Langmuir 2006, 22, 5894–5899. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Park, S.; Yoon, H.; Huh, Y.; Haam, S. Preparation of Poly ɛ-Caprolactone Nanoparticles Containing Magnetite for Magnetic Drug Carrier. Int. J. Pharm. 2006, 324, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kohler, N.; Zhang, M. Surface Modification of Superparamagnetic Magnetite Nanoparticles and Their Intracellular Uptake. Biomaterials 2002, 23, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.; Cebers, A.; Bacri, J.-C.; Gazeau, F. Gazeau Deformation of Intracellular Endosomes under a Magnetic Field. Eur. Biophys. J. 2003, 32, 655–660. [Google Scholar] [CrossRef]
- Chen, Y.; Hou, S. Application of Magnetic Nanoparticles in Cell Therapy. Stem Cell Res. Ther. 2022, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Aslam, H.; Shukrullah, S.; Naz, M.Y.; Fatima, H.; Hussain, H.; Ullah, S.; Assiri, M.A. Current and Future Perspectives of Multifunctional Magnetic Nanoparticles Based Controlled Drug Delivery Systems. J. Drug Deliv. Sci. Technol. 2022, 67, 102946. [Google Scholar] [CrossRef]
- Chubarov, A.S. Serum Albumin for Magnetic Nanoparticles Coating. Magnetochemistry 2022, 8, 13. [Google Scholar] [CrossRef]
- Koksharov, Y.A.; Gubin, S.P.; Taranov, I.V.; Khomutov, G.B.; Gulyaev, Y.V. Magnetic Nanoparticles in Medicine: Progress, Problems, and Advances. J. Commun. Technol. Electron. 2022, 67, 101–116. [Google Scholar] [CrossRef]
- Hsieh, L.-C.; Le, T.-K.; Hu, F.-C.; Chen, Y.-T.; Hsieh, S.; Wu, C.-C.; Hsieh, S.-L. Targeted Colorectal Cancer Treatment: In Vitro Anti-Cancer Effects of Carnosine Nanoparticles Supported by Agar and Magnetic Iron Oxide. Eur. J. Pharm. Biopharm. 2024, 203, 114477. [Google Scholar] [CrossRef] [PubMed]
- Chircov, C.; Ștefan, R.-E.; Dolete, G.; Andrei, A.; Holban, A.M.; Oprea, O.-C.; Vasile, B.S.; Neacșu, I.A.; Tihăuan, B. Dextran-Coated Iron Oxide Nanoparticles Loaded with Curcumin for Antimicrobial Therapies. Pharmaceutics 2022, 14, 1057. [Google Scholar] [CrossRef]
- Alotaibi, I.; Alshammari, M.S.; Algessair, S.; Madkhali, N.; All, N.A.; Hjiri, M.; Alrub, S.A.; El Mir, L.; Lemine, O.M. Synthesis, Characterization and Heating Efficiency of Gd-Doped Maghemite (γ-Fe2O3) Nanoparticles for Hyperthermia Application. Phys. B Condens. Matter 2022, 625, 413510. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Lecomte, V.; Ternad, I.; Van Leuven, L.; Muller, R.N.; Stanicki, D.; Laurent, S. Superparamagnetic Iron Oxide Nanoparticles (SPION): From Fundamentals to State-of-The-Art Innovative Applications for Cancer Therapy. Pharmaceutics 2023, 15, 236. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Maurelli, A.M.; Giotta, L.; Catucci, L. Liposomes Containing Nanoparticles: Preparation and Applications. Colloids Surf. B Biointerfaces 2022, 218, 112737. [Google Scholar] [CrossRef]
- Kotakadi, S.M.; Borelli, D.P.R.; Nannepaga, J.S. Therapeutic Applications of Magnetotactic Bacteria and Magnetosomes: A Review Emphasizing on the Cancer Treatment. Front. Bioeng. Biotechnol. 2022, 10, 789016. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.; Arora, V.; Kabra, A.; Dureja, H.; Hamaal, S. Magnetosomes: A Tool for Targeted Drug Delivery in the Management of Cancer. J. Nanomater. 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- de Souza Cabral, A.; Verdan, M.; Presciliano, R.; Silveira, F.; Correa, T.; Abreu, F. Large-Scale Cultivation of Magnetotactic Bacteria and the Optimism for Sustainable and Cheap Approaches in Nanotechnology. Mar. Drugs 2023, 21, 60. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Neumann, C.A.; LeDuc, P.R. Tumor-on-a-Chip for Integrating a 3D Tumor Microenvironment: Chemical and Mechanical Factors. Lab Chip 2020, 20, 873–888. [Google Scholar] [CrossRef]
- Monteiro, M.V.; Zhang, Y.S.; Gaspar, V.M.; Mano, J.F. 3D-Bioprinted Cancer-On-a-Chip: Level-up Organotypic in Vitro Models. Trends Biotechnol. 2021, 40, 4. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Choudhary, N.K.; Parihar, D.S.; Khan, R. Tumor-On-a-Chip: Microfluidic Models of Hypoxic Tumor Microenvironment. In Hypoxia in Cancer: Significance and Impact on Cancer Therapy; Springer: Singapore, 2023; pp. 297–328. [Google Scholar] [CrossRef]
- Anderson, S.R.; Stagner, E.J.; Sivakumar, H.; Skardal, A. Three-Dimensional Bioprinting of in Vitro Tumor Organoid and Organ-on-a-Chip Models. MRS Bull. 2023, 48, 643–656. [Google Scholar] [CrossRef]
- Marino, A.; Battaglini, M.; Carmignani, A.; Pignatelli, F.; De Pasquale, D.; Tricinci, O.; Ciofani, G. Magnetic Self-Assembly of 3D Multicellular Microscaffolds: A Biomimetic Brain Tumor-On-a-Chip for Drug Delivery and Selectivity Testing. APL Bioeng. 2023, 7, 036103. [Google Scholar] [CrossRef] [PubMed]
- Ustun, M.; Rahmani Dabbagh, S.; Ilci, I.S.; Bagci-Onder, T.; Tasoglu, S. Glioma-on-a-Chip Models. Micromachines 2021, 12, 490. [Google Scholar] [CrossRef] [PubMed]
- Parlato, S.; Grisanti, G.; Sinibaldi, G.; Peruzzi, G.; Casciola, C.M.; Gabriele, L. Tumor-on-a-Chip Platforms to Study Cancer-Immune System Crosstalk in the Era of Immunotherapy. Lab Chip 2020, 21, 234–253. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the Enhanced Permeability and Retention Effect for Tumor Targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Gawali, P.; Saraswat, A.; Bhide, S.; Gupta, S.; Patel, K. Human Solid Tumors and Clinical Relevance of the Enhanced Permeation and Retention Effect: A “Golden Gate” for Nanomedicine in Preclinical Studies? Nanomedicine 2023, 18, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Volpe, A.; Gaudiuso, C.; Ancona, A. Bonding of PMMA to Silicon by Femtosecond Laser Pulses. Sci. Rep. 2023, 13, 5062. [Google Scholar] [CrossRef]
- Madadi, M.; Madadi, A.; Zareifar, R.; Nikfarjam, A. A Simple Solvent-Assisted Method for Thermal Bonding of Large-Surface, Multilayer PMMA Microfluidic Devices. Sens. Actuators A Phys. 2023, 349, 114077. [Google Scholar] [CrossRef]
- Nayak, N.C.; Yue, C.Y.; Lam, Y.C.; Tan, Y.L. Thermal Bonding of PMMA: Effect of Polymer Molecular Weight. Microsyst. Technol. 2009, 16, 487–491. [Google Scholar] [CrossRef]
- Zimina, T.M.; Gareev, K.G.; Sitkov, N.O.; Brusina, K.E.; Shubina, M.A.; Ganiev, Z.; Bobkov, D.E.; Likhomanova, R.B.; Yudintseva, N.M.; Potrakhov, N.N.; et al. A Device for Studying Heterogeneous Tumor Cell Cultures Based on a Hybrid Microfluidic System. Biomed. Eng. 2024, 58, 153–157. [Google Scholar] [CrossRef]
- Manshadi, M.K.D.; Saadat, M.; Mohammadi, M.; Shamsi, M.; Dejam, M.; Kamali, R.; Sanati-Nezhad, A. Delivery of Magnetic Micro/Nanoparticles and Magnetic-Based Drug/Cargo into Arterial Flow for Targeted Therapy. Drug Deliv. 2018, 25, 1963–1973. [Google Scholar] [CrossRef]
- Thomsen, L.B.; Thomsen, M.S.; Moos, T. Targeted Drug Delivery to the Brain Using Magnetic Nanoparticles. Ther. Deliv. 2015, 6, 1145–1155. [Google Scholar] [CrossRef]
- Hasan, M.; Choi, J.-G.; Lee, S.-S. External Magnet-Guided Targeted Delivery and Tissue Distribution Analysis of Anti-Cluster of Differentiation 3 Conjugated Magnetic Nanoparticles of Fe3O4 Using in Vivo Imaging System. AIP Adv. 2024, 14, 015128. [Google Scholar] [CrossRef]
- Al-Madhagi, H.; Yazbik, V.; Abdelwahed, W.; Alchab, L. Magnetite Nanoparticle Co-Precipitation Synthesis, Characterization, and Applications: Mini Review. BioNanoSci 2023, 13, 853–859. [Google Scholar] [CrossRef]
- Jiang, W.; Lai, K.-L.; Hu, H.; Zeng, X.-B.; Lan, F.; Liu, K.-X.; Wu, Y.; Gu, Z.-W. The Effect of [Fe3+]/[Fe2+] Molar Ratio and Iron Salts Concentration on the Properties of Superparamagnetic Iron Oxide Nanoparticles in the Water/Ethanol/Toluene System. J. Nanoparticle Res. 2011, 13, 5135–5145. [Google Scholar] [CrossRef]
- Wahajuddin, N.; Arora, S. Superparamagnetic Iron Oxide Nanoparticles: Magnetic Nanoplatforms as Drug Carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, B.; Yakovleva, L.; Fedorov, V.; Li, H.; Gao, H.; Shevtsov, M. Nano- and Microemulsions in Biomedicine: From Theory to Practice. Pharmaceutics 2023, 15, 1989. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, D.K. Carboxymethyl Group Activation of Dextran Cross-Linked Superparamagnetic Iron Oxide Nanoparticles. J. Korean Ceram. Soc. 2020, 58, 106–115. [Google Scholar] [CrossRef]
- Guo, H.; Chen, W.; Sun, X.; Liu, Y.-N.; Li, J.; Wang, J. Theranostic Magnetoliposomes Coated by Carboxymethyl Dextran with Controlled Release by Low-Frequency Alternating Magnetic Field. Carbohydr. Polym. 2015, 118, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Vasić, K.; Knez, Ž.; Konstantinova, E.A.; Kokorin, A.I.; Gyergyek, S.; Leitgeb, M. Structural and Magnetic Characteristics of Carboxymethyl Dextran Coated Magnetic Nanoparticles: From Characterization to Immobilization Application. React. Funct. Polym. 2020, 148, 104481. [Google Scholar] [CrossRef]
- Salvador, M.; Gutiérrez, G.; Noriega, S.; Moyano, A.; Blanco-López, M.C.; Matos, M. Microemulsion Synthesis of Superparamagnetic Nanoparticles for Bioapplications. Int. J. Mol. Sci. 2021, 22, 427. [Google Scholar] [CrossRef] [PubMed]
- Okoli, C.; Sanchez-Dominguez, M.; Boutonnet, M.; Järås, S.; Civera, C.; Solans, C.; Kuttuva, G.R. Comparison and Functionalization Study of Microemulsion-Prepared Magnetic Iron Oxide Nanoparticles. Langmuir 2012, 28, 8479–8485. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Liu, C.; Qiao, H.C.; Zhu, L.Y.; Chen, G.; Dai, X.D. Hot Embossing/Bonding of a Poly(Ethylene Terephthalate) (PET) Microfluidic Chip. J. Micromech. Microeng. 2007, 18, 015008. [Google Scholar] [CrossRef]
- Keng, P.Y.; Esterby, M.; van Dam, R.M. Emerging technologies for decentralized production of PET tracers. In Positron Emission Tomography-Current Clinical and Research Aspects; IntechOpen: London, UK, 2012; pp. 153–182. [Google Scholar]
- Yan, X.; Liu, W.; Yuan, Y.; Chen, C. Indium Tin Oxide Coated PET Film Contactless Conductivity Detector for Microchip Capillary Electrophoresis. Anal. Methods 2015, 7, 5295–5302. [Google Scholar] [CrossRef]
- Chou, J.; Du, N.; Ou, T.; Floriano, P.N.; Christodoulides, N.; McDevitt, J.T. Hot Embossed Polyethylene Through-Hole Chips for Bead-Based Microfluidicdevices. Biosens. Bioelectron. 2013, 42, 653–660. [Google Scholar] [CrossRef]
- Patil, S.M.; Rane, N.R.; Bankole, P.O.; Krishnaiah, P.; Ahn, Y.; Park, Y.-K.; Yadav, K.K.; Amin, M.A.; Jeon, B.-H. An Assessment of Micro- and Nanoplastics in the Biosphere: A Review of Detection, Monitoring, and Remediation Technology. Chem. Eng. J. 2022, 430, 132913. [Google Scholar] [CrossRef]
- Wang, K.; He, L.; Manz, A.; Wu, W. User-Friendly Microfabrication Method for Complex Topological Structure and Three-Dimensional Microchannel with the Application Prospect in Polymerase Chain Reaction (PCR). Anal. Chem. 2020, 93, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Pekgor, M.; Nikzad, M.; Arablouei, R.; Masood, S. Design of a 3D-Printable UHF RFID Hybrid Liquid Antenna for Biosensing Applications. Mater. Today Proc. 2020, 46, 4619–4624. [Google Scholar] [CrossRef]
- Matsuura, K.; Takata, K. Blood Cell Separation Using Polypropylene-Based Microfluidic Devices Based on Deterministic Lateral Displacement. Micromachines 2023, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Seok, S.; Park, H.; Kim, J. Scotch-Tape Surface Wrinkling Based Thin-Film Material Properties Extraction. J. Micromech. Microeng. 2022, 32, 045002. [Google Scholar] [CrossRef]
- Pradeep, A.; Raveendran, J.; Satheesh Babu, T.G. Design, Fabrication and Assembly of Lab-on-a-Chip and Its Uses. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2022; pp. 121–162. [Google Scholar] [CrossRef]
- Sun, H.; Chan, C.-W.; Wang, Y.; Yao, X.; Mu, X.; Lu, X.; Zhou, J.; Cai, Z.; Ren, K. Reliable and Reusable Whole Polypropylene Plastic Microfluidic Devices for a Rapid, Low-Cost Antimicrobial Susceptibility Test. Lab Chip 2019, 19, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Juang, Y.-J.; Chiu, Y.-J. Fabrication of Polymer Microfluidics: An Overview. Polymers 2022, 14, 2028. [Google Scholar] [CrossRef]
- Rohr, T.; Ogletree, D.F.; Svec, F.; Fréchet, J.M.J. Surface Functionalization of Thermoplastic Polymers for the Fabrication of Microfluidic Devices by Photoinitiated Grafting. Adv. Funct. Mater. 2003, 13, 264–270. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Zhao, Q.; Tan, B.; Chen, X.; Liao, J. Role of Hydrogels in Bone Tissue Engineering: How Properties Shape Regeneration. J. Biomed. Nanotechnol. 2020, 16, 1667–1686. [Google Scholar] [CrossRef]
- Rai, A.; Singh, A.K.; Bleimling, N.; Posern, G.; Vetter, I.R.; Goody, R.S. Rep15 Interacts with Several Rab GTPases and Has a Distinct Fold for a Rab Effector. Nat. Commun. 2022, 13, 4262. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhang, Z.; Guo, N.; Li, X.; Yang, M.; Peng, Y.; Ma, X.; Yu, K.; Wang, C. Effects of Sevoflurane Inhalation Anesthesia on the Intestinal Microbiome in Mice. Front. Cell. Infect. Microbiol. 2021, 11, 633527. [Google Scholar] [CrossRef] [PubMed]
- Weir, G.J.; Chisholm, G.; Leveneur, J. The Magnetic Field about a Three-Dimensional Block Neodymium Magnet. ANZIAM J. 2021, 62, 386–405. [Google Scholar] [CrossRef]
- Egea-Benavente, D.; Ovejero, J.G.; Morales, M.d.P.; Barber, D.F. Understanding MNPs Behaviour in Response to AMF in Biological Milieus and the Effects at the Cellular Level: Implications for a Rational Design That Drives Magnetic Hyperthermia Therapy toward Clinical Implementation. Cancers 2021, 13, 4583. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Ström, A.; Lopez-Sanchez, P.; Knutsen, S.H.; Ballance, S.; Zobel, H.K.; Sokolova, A.; Gilbert, E.P.; López-Rubio, A. Advanced Structural Characterisation of Agar-Based Hydrogels: Rheological and Small Angle Scattering Studies. Carbohydr. Polym. 2020, 236, 115655. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Choi, H.W.; Kim, H.E.; Hahn, J.; Choi, Y.J. Mimicking Animal Adipose Tissue Using a Hybrid Network-Based Solid-Emulsion Gel with Soy Protein Isolate, Agar, and Alginate. Food Hydrocoll. 2023, 145, 109043. [Google Scholar] [CrossRef]

| Material Name | Thickness, mm | Density, g/cm3 at 20 °C | Melting Point, °C | MW, kDa | Structural Formula |
|---|---|---|---|---|---|
| Poly(methylmeth-acrylate) (PMMA) [39,40] | 1.5 | 1.18 | >100 | 100–150 | 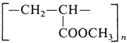 |
| Polyethylene terephthalate (PET) [54,55,56] | 0.5 | 1.38 | >245 | 150 | 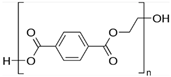 |
| Polyethylene (PE) [57,58,59] | 0.3 | 0.88–0.96 | 115–135 | <200 | 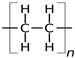 |
| Polypropylene (PP) [60,61] | 0.3 | – | 130–171 | – | 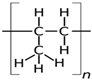 |
| 3M™ Scotch® Trans-parent Film Tape 600 (polyvinylchloride (PVC) film/acrylic adhesive) [62] | 0.52 0.38 (PVC base) | 1.35–1.43 | 150–220 | 100–170 | 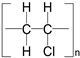 |
| Sample Name | Particle Size, nm | Particle Concentration, mg/mL | |
|---|---|---|---|
| 1 | mMNP | 46; 212 | 2 |
| 2 | CD-SPION-1 | 95 ± 3 | 2.2–4.0 |
| 3 | CD-SPION-2 | 220 ± 10 | 2.2–4.0 |
| 4 | CD-SPION-3 | 350 ± 15 | 2.2–4.0 |
| 5 | CD-SPION-4 | 820 ± 200 | 2.2–4.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimina, T.; Sitkov, N.; Brusina, K.; Fedorov, V.; Mikhailova, N.; Testov, D.; Gareev, K.; Samochernykh, K.; Combs, S.; Shevtsov, M. Magnetically Controlled Transport of Nanoparticles in Solid Tumor Tissues and Porous Media Using a Tumor-on-a-Chip Format. Nanomaterials 2024, 14, 2030. https://doi.org/10.3390/nano14242030
Zimina T, Sitkov N, Brusina K, Fedorov V, Mikhailova N, Testov D, Gareev K, Samochernykh K, Combs S, Shevtsov M. Magnetically Controlled Transport of Nanoparticles in Solid Tumor Tissues and Porous Media Using a Tumor-on-a-Chip Format. Nanomaterials. 2024; 14(24):2030. https://doi.org/10.3390/nano14242030
Chicago/Turabian StyleZimina, Tatiana, Nikita Sitkov, Ksenia Brusina, Viacheslav Fedorov, Natalia Mikhailova, Dmitriy Testov, Kamil Gareev, Konstantin Samochernykh, Stephanie Combs, and Maxim Shevtsov. 2024. "Magnetically Controlled Transport of Nanoparticles in Solid Tumor Tissues and Porous Media Using a Tumor-on-a-Chip Format" Nanomaterials 14, no. 24: 2030. https://doi.org/10.3390/nano14242030
APA StyleZimina, T., Sitkov, N., Brusina, K., Fedorov, V., Mikhailova, N., Testov, D., Gareev, K., Samochernykh, K., Combs, S., & Shevtsov, M. (2024). Magnetically Controlled Transport of Nanoparticles in Solid Tumor Tissues and Porous Media Using a Tumor-on-a-Chip Format. Nanomaterials, 14(24), 2030. https://doi.org/10.3390/nano14242030










