Rational Construction of Pt Incorporated Co3O4 as High-Performance Electrocatalyst for Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Experimental Section
3. Material Characterization
4. Electrochemical Test
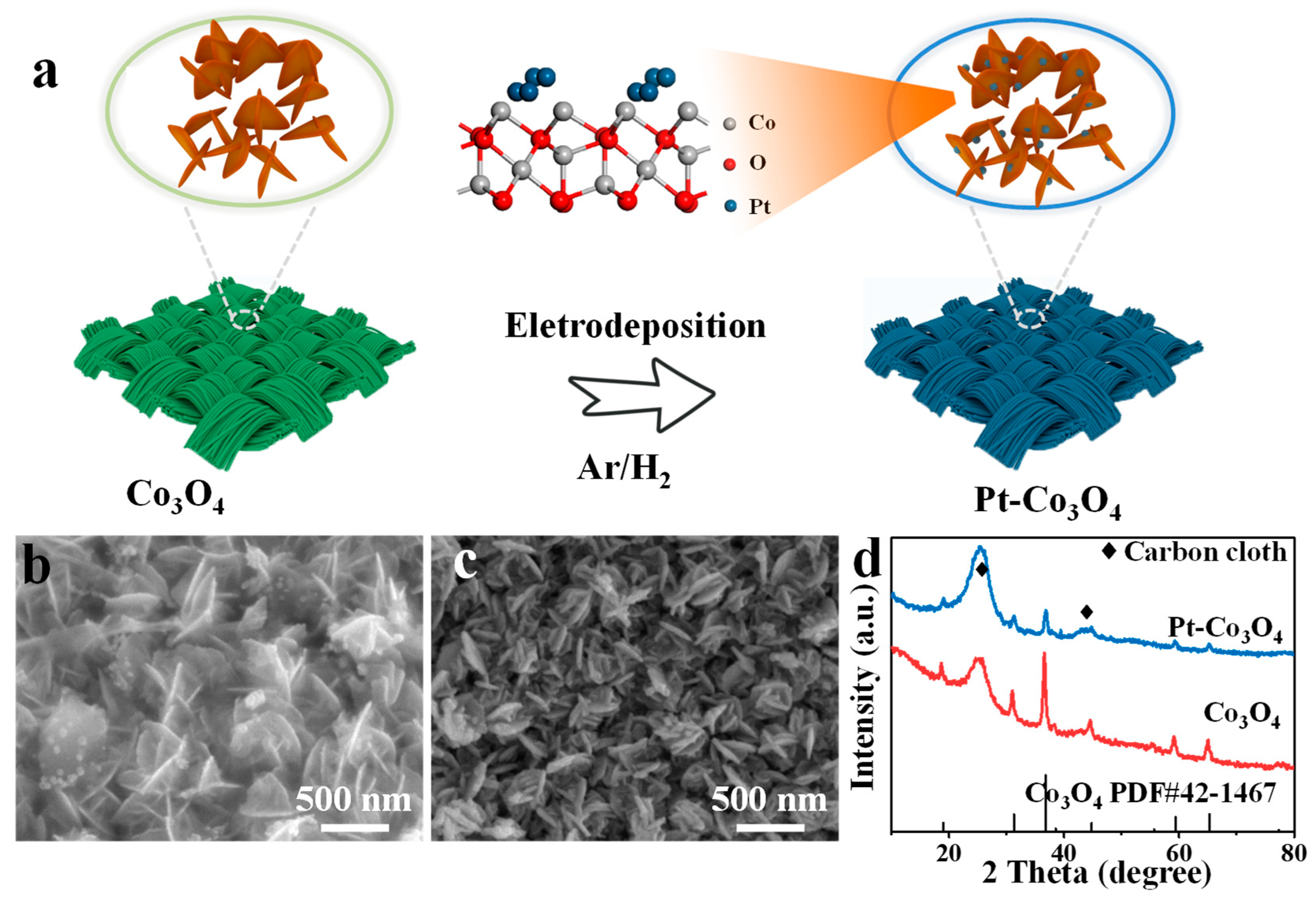
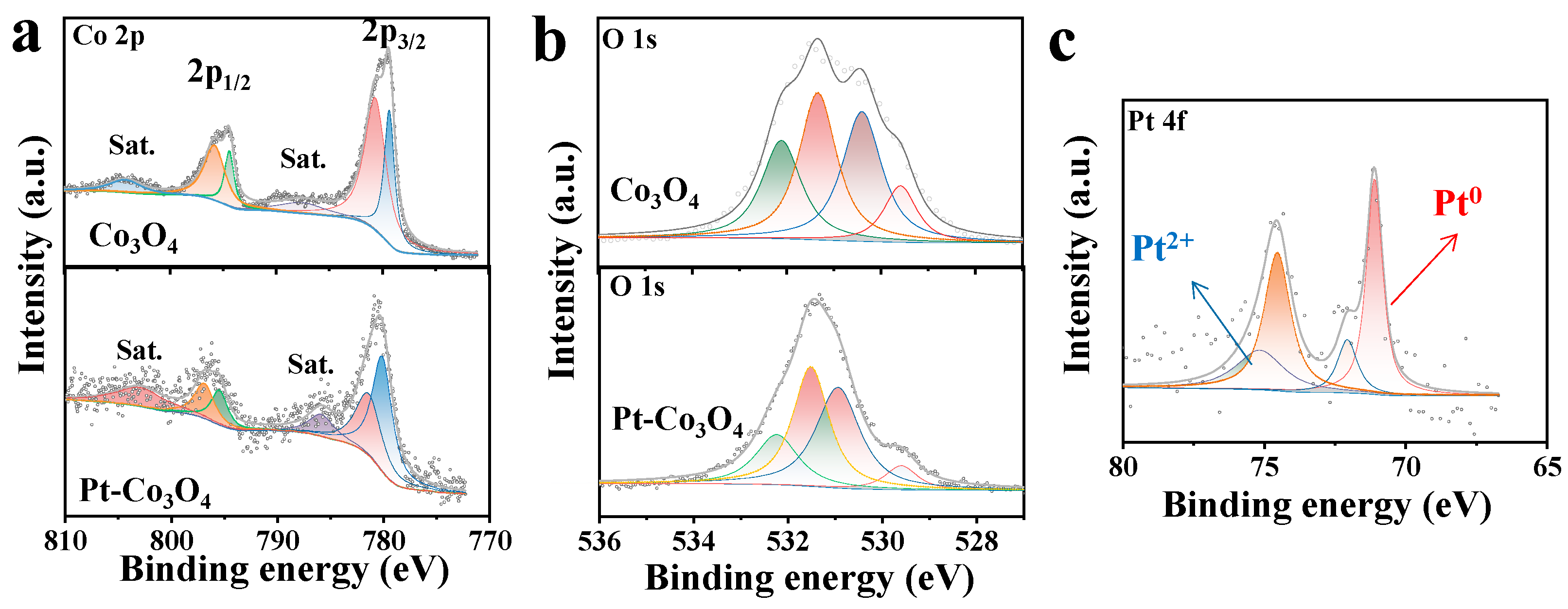
5. Results and Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chung, Y.J.; Yang, C.S.; Lee, J.T.; Wu, G.H.; Wu, J.M. Coupling Effect of Piezo-Flexocatalytic Hydrogen Evolution with Hybrid 1T- and 2H-Phase Few-Layered MoSe2 Nanosheets. Adv. Energy Mater. 2020, 10, 2002082. [Google Scholar] [CrossRef]
- Nerini, F.F.; Tomei, J.; To, L.S.; Bisaga, I.; Parikh, P.; Black, M.; Borrion, A.; Spataru, C.; Broto, V.C.; Anandarajah, G.; et al. Mapping synergies and trade-offs between energy and the Sustainable Development Goals. Nat. Energy 2018, 3, 10–15. [Google Scholar] [CrossRef]
- Fu, H.Q.; Zhou, M.; Liu, P.F.; Liu, P.; Yin, H.; Sun, K.Z.; Yang, H.G.; Al-Mamun, M.; Hu, P.; Wang, H.; et al. Hydrogen Spillover-Bridged Volmer/Tafel Processes Enabling Ampere-Level Current Density Alkaline Hydrogen Evolution Reaction under Low Overpotential. J. Am. Chem. Soc. 2022, 144, 6028–6039. [Google Scholar] [CrossRef]
- Lin, Z.; Zhou, Y.; Fu, J.; Yan, X.; Fan, R.; Li, M.; Liu, H.; Yu, J.; Chai, Y.; Dong, B. Ultrahigh activity of molybdenum/vanadium-doped Ni-Co phosphides nanoneedles based on ion-exchange for hydrogen evolution at large current density. J. Colloid Interface Sci. 2021, 604, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hu, W.; Zhen, Y.; Dong, B.; Dong, Y.; Fan, R.; Liu, B.; Liu, D.; Chai, Y. Metallic MoO layer promoting high-valence Mo doping into CoP nanowires with ultrahigh activity for hydrogen evolution at 2000 mA cm−2. Appl. Catal. B Environ. 2022, 309, 121230. [Google Scholar] [CrossRef]
- Jiang, W.; Tang, T.; Zhang, Y.; Hu, J. Synergistic Modulation of Non-Precious-Metal Electrocatalysts for Advanced Water Splitting. Acc. Chem. Res. 2020, 53, 1111–1123. [Google Scholar] [CrossRef]
- Choudhary, N.; Islam, M.A.; Kim, J.H.; Ko, T.; Schropp, A.; Hurtado, L.; Weitzman, D.; Zhai, L.; Jung, Y. Two-dimensional transition metal dichalcogenide hybrid materials for energy applications. Nano Today 2018, 19, 16–40. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, W.; Ren, J.; Tang, Y. Cu-Co bimetal oxide hierarchical nanostructures as high-performance electrocatalyst for oxygen evolution reaction. Mater. Today Energy 2021, 21, 100703. [Google Scholar] [CrossRef]
- Raja, D.S.; Cheng, P.; Cheng, C.; Chang, S.; Huang, C.; Lu, S. In-situ grown metal-organic framework-derived carbon-coated Fe-doped cobalt oxide nanocomposite on fluorine-doped tin oxide glass for acidic oxygen evolution reaction. Appl. Catal. B Environ. 2022, 303, 120899. [Google Scholar] [CrossRef]
- Wang, T.; Wang, P.; Zang, W.; Li, X.; Chen, D.; Kou, Z.; Mu, S.; Wang, J. Nanoframes of Co3O4-Mo2N Heterointerfaces Enable High-Performance Bifunctionality toward Both Electrocatalytic HER and OER. Adv. Funct. Mater. 2022, 32, 2107382. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, T.; Dong, C.L.; Yang, C.; Zhou, L.; Huang, Y.C.; Li, Y.; Zhou, B.; Zou, Y.; Wang, S. Tailoring Competitive Adsorption Sites by Oxygen-Vacancy on Cobalt Oxides to Enhance the Electrooxidation of Biomass. Adv. Mater. 2022, 34, 2107185. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zheng, D.; Li, Q.; Xiao, W.; Ma, T.; Fu, Y.; Wu, Z.; Wang, L. 3D Co3O4-RuO2 Hollow Spheres with Abundant Interfaces as Advanced Trifunctional Electrocatalyst for Water-Splitting and Flexible Zn–Air Battery. Adv. Funct. Mater. 2022, 32, 2203206. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Duan, Y.; Feng, X.Y.; Yu, X.; Gao, M.R.; Yu, S.H. Clean and Affordable Hydrogen Fuel from Alkaline Water Splitting: Past, Recent Progress, and Future Prospects. Adv. Mater. 2021, 33, 2007100. [Google Scholar] [CrossRef]
- Wang, G.; Tao, S.; Bu, X. A systematic theoretical study of water dissociation on clean and oxygen-preadsorbed transition metals. J. Catal. 2006, 244, 10–16. [Google Scholar] [CrossRef]
- Sun, J.; Xu, W.; Lv, C.; Zhang, L.; Shakouri, M.; Peng, Y.; Wang, Q.; Yang, X.; Yuan, D.; Huang, M.; et al. Co/MoN hetero-interface nanoflake array with enhanced water dissociation capability achieves the Pt-like hydrogen evolution catalytic performance. Appl. Catal. B Environ. 2021, 286, 119882. [Google Scholar] [CrossRef]
- McCrum, I.T.; Koper, M.T.M. The role of adsorbed hydroxide in hydrogen evolution reaction kinetics on modified platinum. Nat. Energy 2020, 5, 891–899. [Google Scholar] [CrossRef]
- Fajín, J.L.C.; Cordeiro, M.N.D.S.; Gomes, J.R.B. Density Functional Theory Study of the Water Dissociation on Platinum Surfaces: General Trends. J. Phys. Chem. A 2014, 118, 5832–5840. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Hu, Z.; Chen, Z.; Liu, J.; Sun, J.; Song, Y.; Dong, S.; Zhang, L. Atomic-Level Tailoring of the Electronic Metal-Support Interaction Between Pt-Co3O4 Interfaces for High Hydrogen Evolution Performance. J. Phys. Chem. Lett. 2024, 15, 3486–3492. [Google Scholar] [CrossRef]
- Li, J.; Guan, Q.; Wu, H.; Liu, W.; Lin, Y.; Sun, Z.; Ye, X.; Zheng, X.; Pan, H.; Zhu, J.; et al. Highly Active and Stable Metal Single-Atom Catalysts Achieved by Strong Electronic Metal–Support Interactions. J. Am. Chem. Soc. 2019, 141, 14515–14519. [Google Scholar] [CrossRef]
- Jana, R.; Chowdhury, C.; Malik, S.; Datta, A. Pt/Co3O4 Surpasses Benchmark Pt/C: An Approach Toward Next Generation Hydrogen Evolution Electrocatalyst. ACS Appl. Energy Mater. 2019, 2, 5613–5621. [Google Scholar] [CrossRef]
- Gu, M.; Jia, Q.; Zhu, Y.; Xu, L.; Tang, Y. In Situ Growth of Ultrafine Pt Nanoparticles onto Hierarchical Co3O4 Nanosheet-Assembled Microflowers for Efficient Electrocatalytic Hydrogen Evolution. Chem. Eur. J. 2020, 26, 15103–15108. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Liu, C.; Feng, X.; Wu, H.; Liu, X. Aromatic polymer dual-confined magnetic metal-organic framework microspheres enable highly efficient removal of dyes, heavy metals, and antibiotics. Chem. Eng. J. 2023, 472, 145159. [Google Scholar] [CrossRef]
- Kwon, I.S.; Kwak, I.H.; Kim, J.Y.; Debela, T.T.; Park, Y.C.; Park, J.; Kang, H.S. Concurrent Vacancy and Adatom Defects of Mo1-xNbxSe2 Alloy Nanosheets Enhance Electrochemical Performance of Hydrogen Evolution Reaction. ACS Nano 2021, 15, 5467–5477. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, H.; Jiang, X.; Jiang, M.; Zhan, X.; Fu, G.; Lee, J.; Tang, Y. Gd-induced electronic structure engineering of a NiFe-layered double hydroxide for efficient oxygen evolution. J. Mater. Chem. A 2021, 9, 2936–2999. [Google Scholar] [CrossRef]
- Biswal, A.; Panda, P.K.; Acharya, A.N.; Mohapatra, S.; Swain, N.; Tripathy, B.C.; Jiang, Z.; Sundaram, M.M. Role of Additives in Electrochemical Deposition of Ternary Metal Oxide Microspheres for Supercapacitor Applications. ACS Omega 2020, 5, 3405–3417. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, Z.; Zhang, D.; Qin, Y.; Wang, M.; Han, Y.; Zhan, T.; Yang, B.; Li, S.; Lai, J.; et al. Solvent-free microwave synthesis of ultra-small Ru-Mo2C@CNT with strong metal-support interaction for industrial hydrogen evolution. Nat. Commun. 2021, 12, 4018. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Tharani, D.S.; Manickam, M.; Sivasubramanian, R. A sol-gel derived LaCoO3 perovskite as an electrocatalyst for Al-air batteries. Dalton Trans. 2024, 53, 3713–3721. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal–Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, J.; Wan, J.; Yu, C. MOF-on-MOF hybrids: Synthesis; applications. Coordin. Chem. Rev. 2021, 432, 213743. [Google Scholar] [CrossRef]
- Qiu, L.; Li, H.; He, L. Incorporating Catalytic Units into Nanomaterials: Rational Design of Multipurpose Catalysts for CO2 Valorization. Acc. Chem. Res. 2023, 56, 2225–2240. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tang, C.; Wang, H.; Li, B.; Zhang, Q.; Li, C.; Yang, C.; Wei, F. Monolithic-structured ternary hydroxides as freestanding bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2016, 4, 7245–7250. [Google Scholar] [CrossRef]
- Zhuang, L.; Ge, L.; Yang, Y.; Li, M.; Jia, Y.; Yao, X.; Zhu, Z. Ultrathin Iron-Cobalt Oxide Nanosheets with Abundant Oxygen Vacancies for the Oxygen Evolution Reaction. Adv. Mater. 2017, 29, 1606793. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, X.; Wang, X.; Hu, J.; Liu, Y.; Fu, G.; Tang, Y. Concave PtCo nanocrosses for methanol oxidation reaction. Appl. Catal. B Environ. 2020, 277, 119135. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, X.; Fu, G.; Li, Y.; Tang, Y.; Lee, J.; Tang, Y. Cu5Pt Dodecahedra with Low-Pt Content: Facile Synthesis and Outstanding Formic Acid Electrooxidation. ACS Appl. Mater. Interfaces 2019, 11, 34869–34877. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Liang, M.; Kumar, A.; Liu, X.; Jin, H.; Ajmal, S.; Bui, V.Q.; Bui, H.T.D.; Lee, J.; Tran, N.Q.; et al. Amorphization of Metal Nanoparticles by 2D Twisted Polymer for Super Hydrogen Evolution Reaction. Adv. Energy Mater. 2022, 12, 2102257. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Jaramillo, T.F.; Phosphosulfide, M. Acid-Stable, Earth-Abundant Catalyst for the Hydrogen Evolution Reaction. Angew. Chem. Int. Edit. 2014, 53, 14433–14437. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Zhang, D.; Qin, Y.; Xiong, J.; Lai, J.; Wang, L. Systematic Engineering on Ni-Based Nanocatalysts Effectively Promote Hydrogen Evolution Reaction. Small 2022, 18, 2108072. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, J.; Zhang, M.; Gou, W.; Zhang, S.; Chen, Z.; Qu, Y.; Ma, Y. A fundamental viewpoint on the hydrogen spillover phenomenon of electrocatalytic hydrogen evolution. Nat. Commun. 2021, 12, 3502. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Norskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Norskov, J.K. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, J23. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Yan, Y.; Qin, B.; Zheng, X.; Cai, W.; Qi, J. Rational Construction of Pt Incorporated Co3O4 as High-Performance Electrocatalyst for Hydrogen Evolution Reaction. Nanomaterials 2024, 14, 898. https://doi.org/10.3390/nano14110898
Wang P, Yan Y, Qin B, Zheng X, Cai W, Qi J. Rational Construction of Pt Incorporated Co3O4 as High-Performance Electrocatalyst for Hydrogen Evolution Reaction. Nanomaterials. 2024; 14(11):898. https://doi.org/10.3390/nano14110898
Chicago/Turabian StyleWang, Peijia, Yaotian Yan, Bin Qin, Xiaohang Zheng, Wei Cai, and Junlei Qi. 2024. "Rational Construction of Pt Incorporated Co3O4 as High-Performance Electrocatalyst for Hydrogen Evolution Reaction" Nanomaterials 14, no. 11: 898. https://doi.org/10.3390/nano14110898
APA StyleWang, P., Yan, Y., Qin, B., Zheng, X., Cai, W., & Qi, J. (2024). Rational Construction of Pt Incorporated Co3O4 as High-Performance Electrocatalyst for Hydrogen Evolution Reaction. Nanomaterials, 14(11), 898. https://doi.org/10.3390/nano14110898





