Exploring the Thermal-Oxidative Stability of Azithromycin Using a Thermoactivated Sensor Based on Cerium Molybdate and Multi-Walled Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cerium Molybdate Synthesis
2.3. Electrochemical Sensor Development
2.4. Physicochemical Characterization
2.5. Computer Simulations
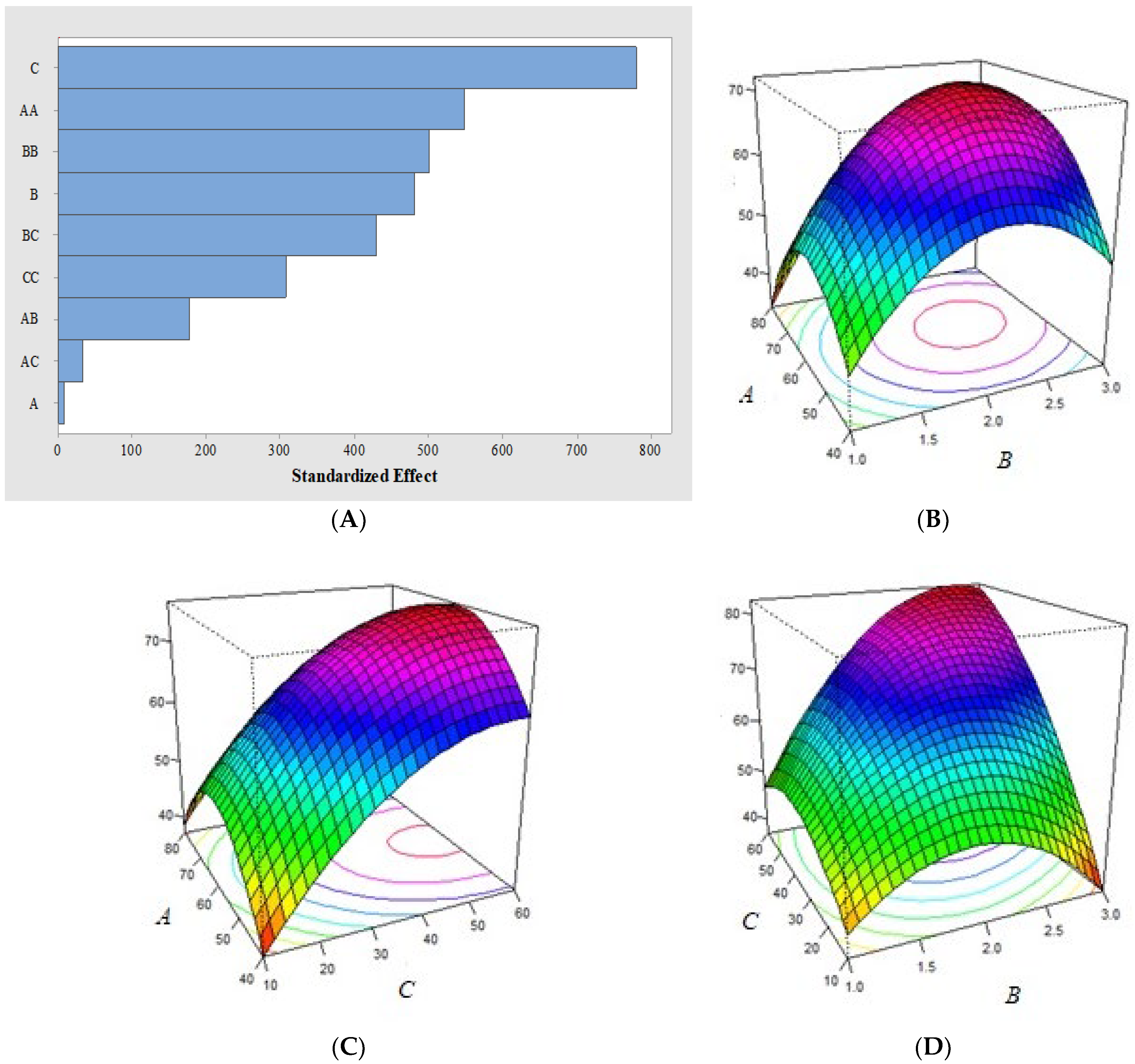
2.6. Electroanalytical Measurements and Performance
2.7. Studies on AZM’s Thermal-Oxidative Stability
3. Results and Discussion
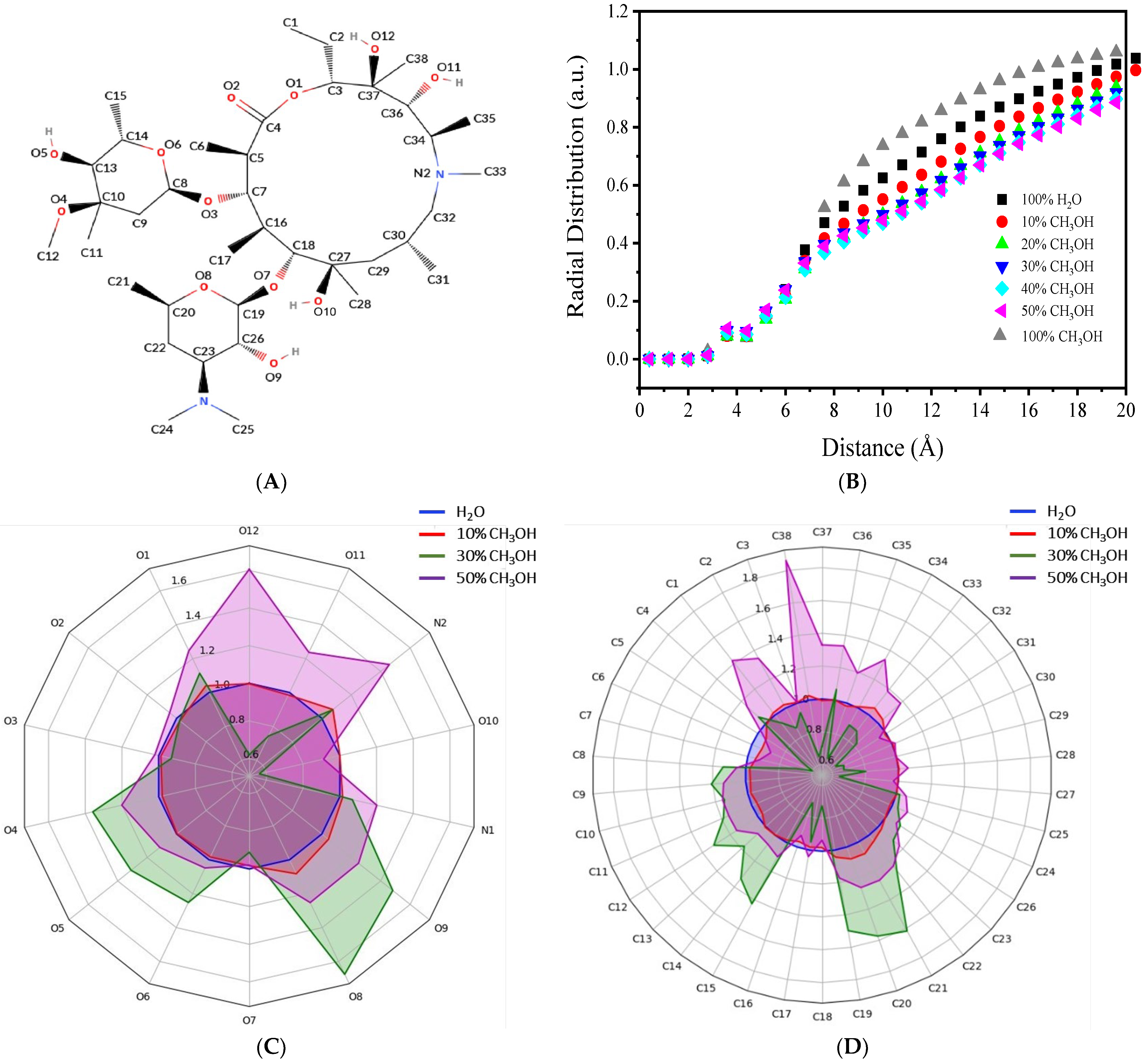
3.1. Relationship between AZM Solubility and Electroactivity
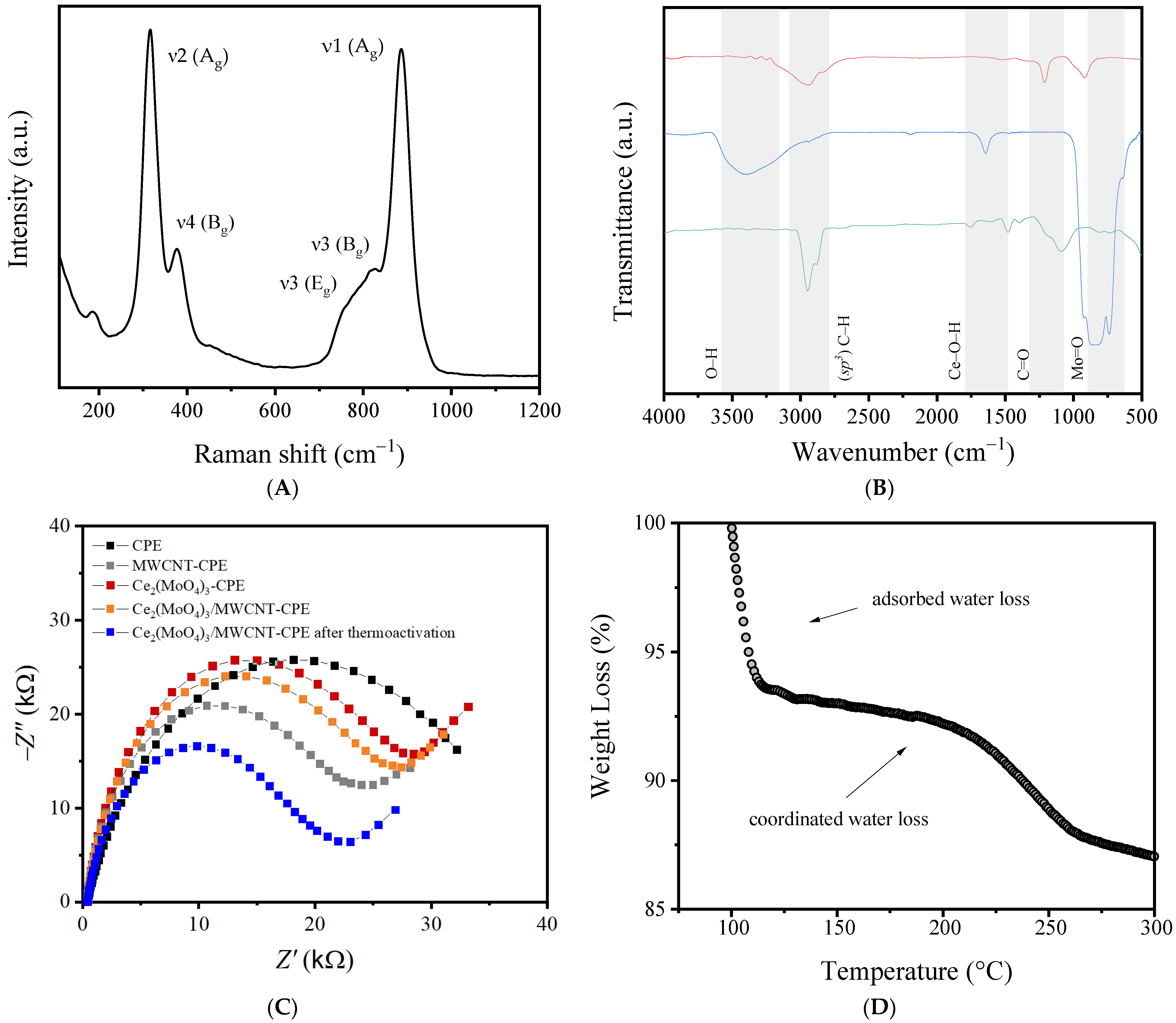
3.2. Characterization of Electrode Materials
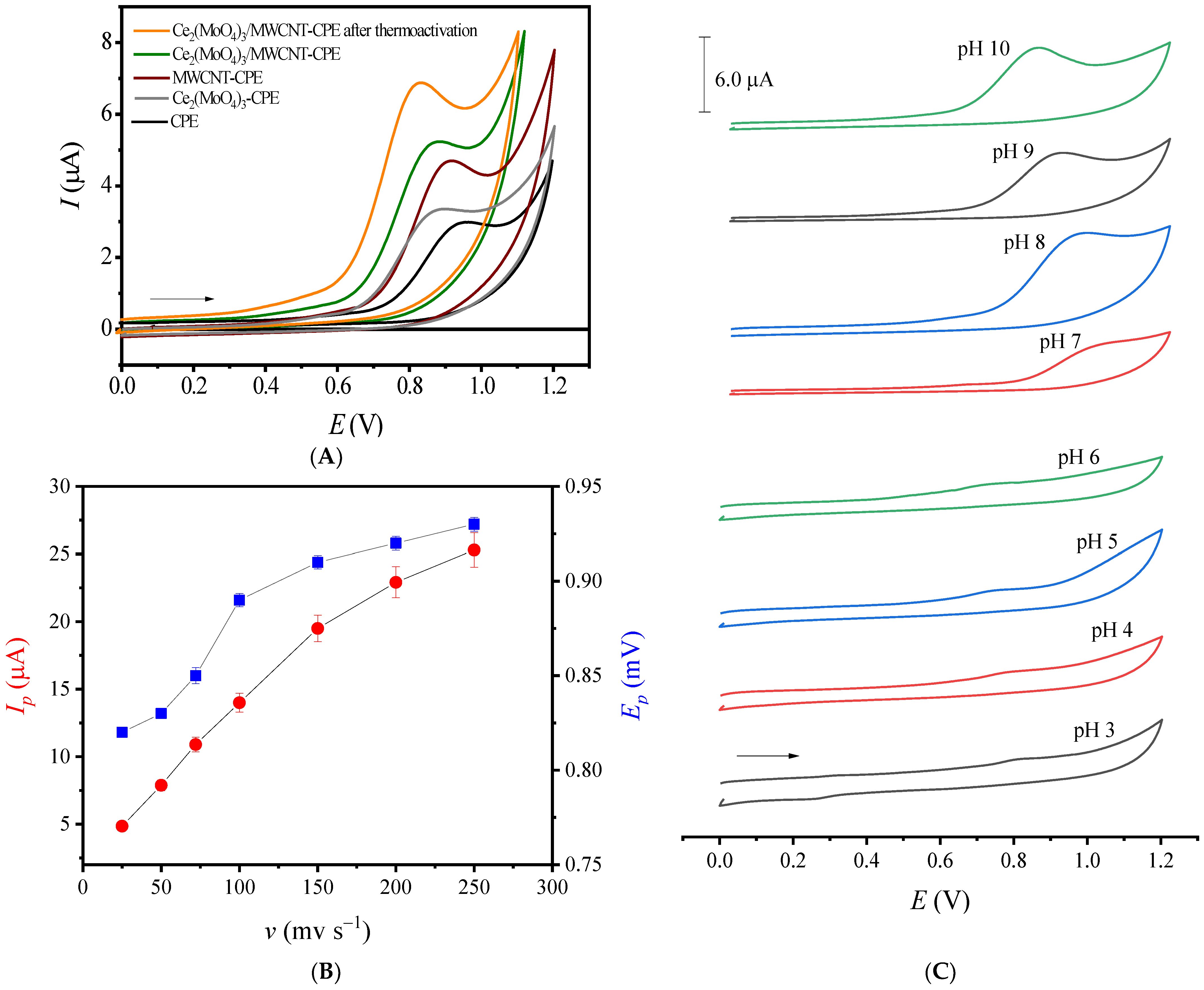
3.3. AZM Electroactivity
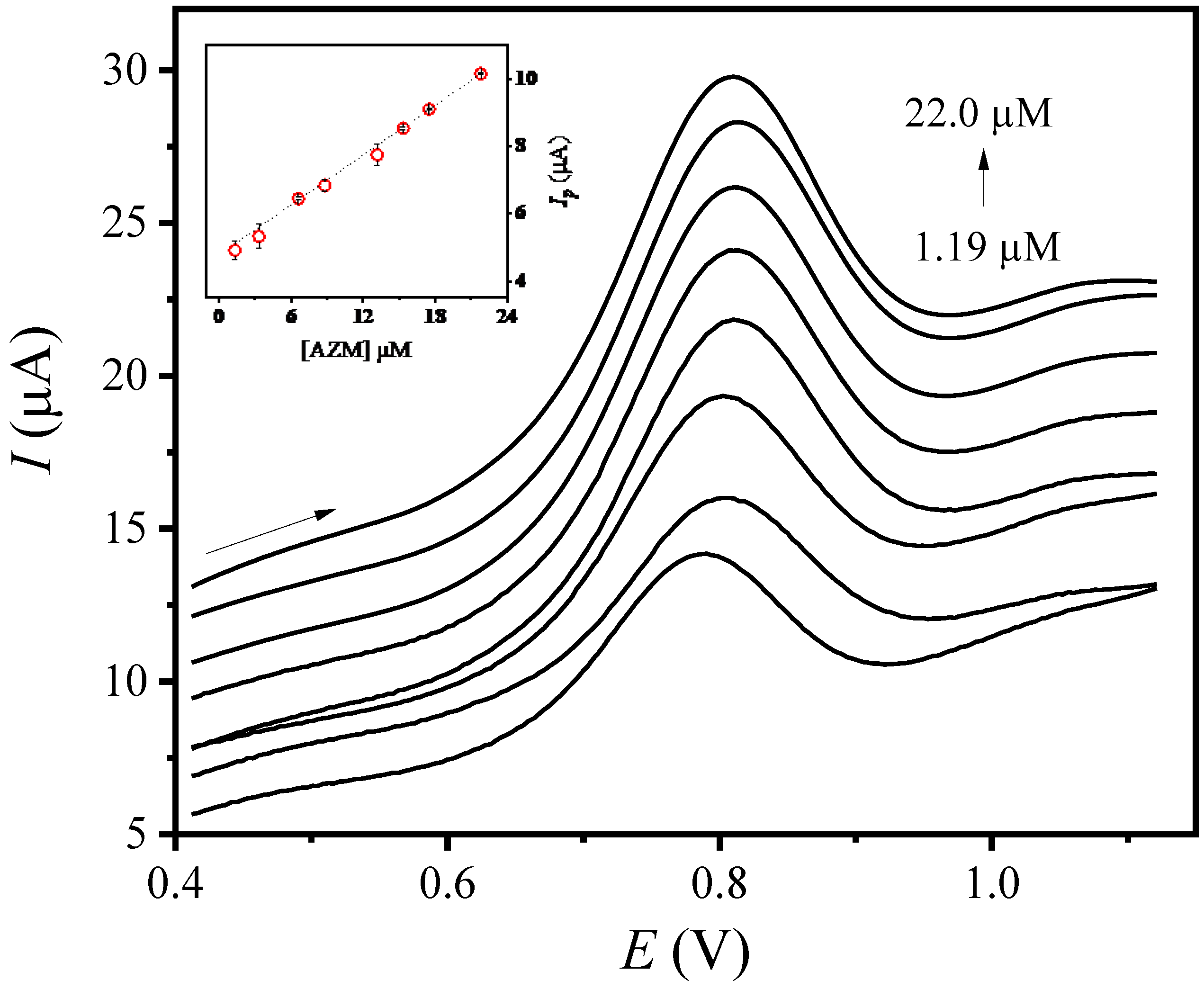
3.4. Electroanalytical Parameters
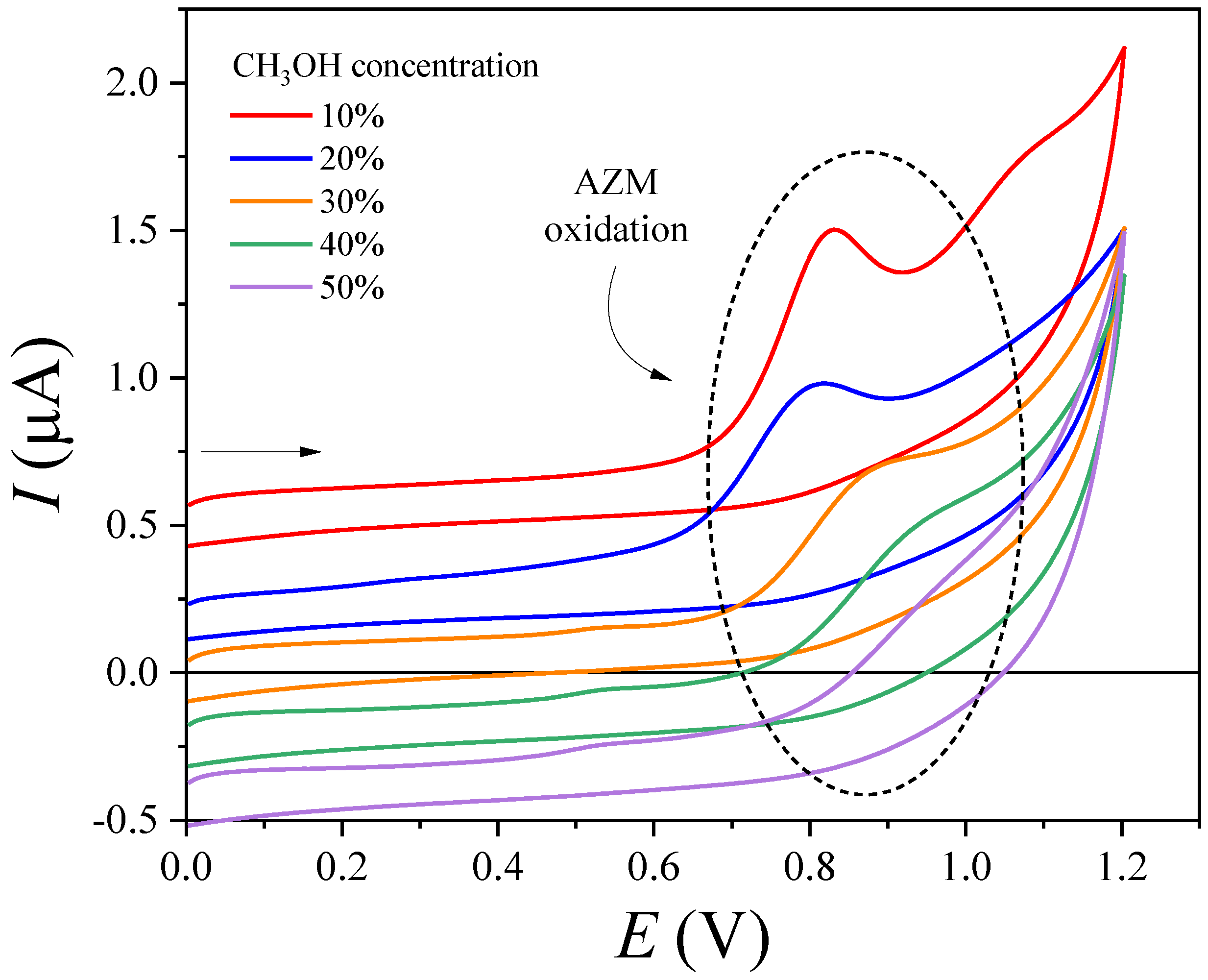
3.5. AZM Thermal-Oxidative Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bal, A.M. Macrolide Antibiotics. In Comprehensive Pharmacology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 170–184. [Google Scholar]
- Dhindwal, P.; Myziuk, I.; Ruzzini, A. Macrolide Esterases: Current Threats and Opportunities. Trends Microbiol. 2023, 31, 1199–1201. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.-Z.; Hiasa, H.; Lv, W.; Brody, S.; Yang, Z.-Y.; Aldrich, C.; Cushman, M.; Liang, J.-H. Design, Synthesis and Structure-Activity Relationships of Novel 15-Membered Macrolides: Quinolone/Quinoline-Containing Sidechains Tethered to the C-6 Position of Azithromycin Acylides. Eur. J. Med. Chem. 2020, 193, 112222. [Google Scholar] [CrossRef] [PubMed]
- Abou Assi, R.; Abdulbaqi, I.M.; Seok Ming, T.; Siok Yee, C.; Wahab, H.A.; Asif, S.M.; Darwis, Y. Liquid and Solid Self-Emulsifying Drug Delivery Systems (SEDDs) as Carriers for the Oral Delivery of Azithromycin: Optimization, In Vitro Characterization and Stability Assessment. Pharmaceutics 2020, 12, 1052. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, A.; Krzek, J.; Walczak, M. Stress Degradation Studies on Azithromycin and Development of a Validated Stability-Indicating TLC-Densitometric Method with HPLC/Electrospray Ionization-MS Analysis of Degradation Products. J. AOAC Int. 2012, 95, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Okaru, A.; Abuga, K.; Kamau, F.; Ndwigah, S.; Lachenmeier, D. A Robust Liquid Chromatographic Method for Confirmation of Drug Stability of Azithromycin in Bulk Samples, Tablets and Suspensions. Pharmaceutics 2017, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- El-Gindy, A.; Attia, K.A.; Nassar, M.W.; Al Abasawi, N.M.; Al-Shabrawi, M. Optimization and Validation of a Stability-Indicating RP-HPLC Method for Determination of Azithromycin and Its Related Compounds. J. AOAC Int. 2011, 94, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Breier, A.; Garcia, C.; Oppe, T.; Steppe, M.; Schapoval, E.E. Microbiological Assay for Azithromycin in Pharmaceutical Formulations. J. Pharm. Biomed. Anal. 2002, 29, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.S.; Bui, V.T.; Tong, T.T.V.; Le, D.C. Novel Approach for Infrared Spectroscopic Quantitation of Azithromycin in Commercial Tablets Employing Paracetamol as Matrix Modifier. Heliyon 2023, 9, e14647. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.H.; Pham, T.N.M.; Le, T.B.; Le, D.C.; Tran, T.T.P.; Nguyen, T.Q.H.; Nguyen, T.K.T.; Hauser, P.C.; Mai, T.D. Cost-Effective Capillary Electrophoresis with Contactless Conductivity Detection for Quality Control of Beta-Lactam Antibiotics. J. Chromatogr. A 2019, 1605, 360356. [Google Scholar] [CrossRef]
- Avramov Ivić, M.L.; Petrović, S.D.; Mijin, D.Ž.; Živković, P.M.; Kosović, I.M.; Drljević, K.M.; Jovanović, M.B. Studies on Electrochemical Oxidation of Azithromycin and Hemomycin® at Gold Electrode in Neutral Electrolyte. Electrochim. Acta 2006, 51, 2407–2416. [Google Scholar] [CrossRef]
- Oliveira, T.M.B.F.; Morais, S. New Generation of Electrochemical Sensors Based on Multi-Walled Carbon Nanotubes. Appl. Sci. 2018, 8, 1925. [Google Scholar] [CrossRef]
- Bae, S.; Jeon, I.; Mahmood, J.; Baek, J. Molybdenum-Based Carbon Hybrid Materials to Enhance the Hydrogen Evolution Reaction. Chem. A Eur. J. 2018, 24, 18158–18179. [Google Scholar] [CrossRef] [PubMed]
- Popovych, O.M.; Budzulyak, I.M.; Kotsyubynsky, V.O.; Yablon, L.S.; Popovych, O.V. Electrochemical and Electrical Properties of Nickel Molybdate/Carbon Material Composites. Phys. Chem. Solid State 2021, 22, 481–486. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Freire, C. Carbon Nanomaterial–Phosphomolybdate Composites for Oxidative Electrocatalysis. ChemElectroChem. 2015, 2, 269–279. [Google Scholar] [CrossRef]
- Krishnan, C.V.; Garnett, M.; Hsiao, B.; Chu, B. Electrochemical Measurements of Isopolyoxomolybdates: 1. PH Dependent Behavior of Sodium Molybdate. Int. J. Electrochem. Sci. 2007, 2, 29–51. [Google Scholar] [CrossRef]
- Nozaki, A.; Morishita, M.; Kinoshita, Y.; Yamamoto, H. Thermodynamic Properties of Cerium Molybdate. Int. J. Mater. Res. 2019, 110, 715–725. [Google Scholar] [CrossRef]
- Pryston, D.B.d.A.; Martins, T.V.d.S.; Vasconcelos Júnior, J.A.d.; Avelino, D.O.d.S.; Meneghetti, M.R.; Meneghetti, S.M.P. Investigation of CeO2, MoO3, and Ce2(MoO4)3, Synthesized by the Pechini Method, as Catalysts for Fructose Conversion. Catalysts 2022, 13, 4. [Google Scholar] [CrossRef]
- Shugurov, S.M.; Panin, A.I.; Lopatin, S.I. Thermodynamic Properties of Gaseous Cerium Molybdates and Tungstates Studied by Knudsen Effusion Mass Spectrometry. Rapid Commun. Mass Spectrom. 2018, 32, 1608–1616. [Google Scholar] [CrossRef]
- Oliveira, F.K.F.; Santiago, A.A.G.; Catto, A.C.; da Silva, L.F.; Tranquilin, R.L.; Longo, E.; Motta, F.V.; Bomio, M.R.D. Cerium Molybdate Nanocrystals: Microstructural, Optical and Gas-Sensing Properties. J. Alloys Compd. 2021, 857, 157562. [Google Scholar] [CrossRef]
- Gomes, N.M.C.; Guedes, V.H.M.C.; Costa, H.R.A.; Santos, A.O.; Moura, J.V.B.; da Luz-Lima, C.; Oliveira, T.M.B.F. Photoelectrochemical Sensor Based on Polymeric Composite Filled with Cerium(III) Molybdate to Monitor Pyriproxyfen Releases in Natural Waters. J. Photochem. Photobiol. A Chem. 2024, 446, 115113. [Google Scholar] [CrossRef]
- Karthik, R.; Vinoth Kumar, J.; Chen, S.-M.; Karuppiah, C.; Cheng, Y.-H.; Muthuraj, V. A Study of Electrocatalytic and Photocatalytic Activity of Cerium Molybdate Nanocubes Decorated Graphene Oxide for the Sensing and Degradation of Antibiotic Drug Chloramphenicol. ACS Appl. Mater. Interfaces 2017, 9, 6547–6559. [Google Scholar] [CrossRef]
- Dargahi, M.; Masteri-Farahani, M.; Shahsavarifar, S.; Feizi, M. Microemulsion-Mediated Preparation of Ce2(MoO4)3 Nanoparticles for Photocatalytic Degradation of Crystal Violet in Aqueous Solution. Environ. Sci. Pollut. Res. 2020, 27, 12047–12054. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.V.B.; Pinheiro, G.S.; Silveira, J.V.; Freire, P.T.C.; Viana, B.C.; Luz-Lima, C. NaCe(MoO4)2 Microcrystals: Hydrothermal Synthesis, Characterization and Photocatalytic Performance. J. Phys. Chem. Solids 2017, 111, 258–265. [Google Scholar] [CrossRef]
- Moura, J.V.B.; Souza, A.A.G.; Freire, P.T.C.; Luz-Lima, C.; Oliveira, T.M.B.F. Blue-light-excited NaCe(MoO4)2 Microcrystals for Photoelectrochemical Water Splitting. Int. J. Appl. Ceram. Technol. 2021, 18, 615–621. [Google Scholar] [CrossRef]
- Gajraj, V.; Devi, P.; Kumar, R.; Sundriyal, N.; Mariappan, C.R. Fabrication of Nanocluster-Aggregated Dense Ce2(MoO4)3 Microspherical Architectures for High-Voltage Energy Storage and High Catalytic Energy Conversion Applications. Energy Fuels 2022, 36, 7841–7853. [Google Scholar] [CrossRef]
- Morassaei, M.S.; Salehabadi, A.; Salavati-Niasari, M.; Akbari, A. Preparation, Structural Analysis, and Assessing the Impacts of Holmium and Ytterbium on Electrochemical Hydrogen Storage Property of Strontium Cerium Molybdate Nanostructures. Electrochim. Acta 2020, 356, 136851. [Google Scholar] [CrossRef]
- Santhoshkumar, P.; Vikraman, D.; Hussain, S.; Karuppasamy, K.; Kathalingam, A.; Kim, H.-S. 3D-Architectured Spherical Ce2Mo5O16 by a Time-Dependent Hydrothermal Process and Their Energy Storage Application. J. Alloys Compd. 2022, 928, 167215. [Google Scholar] [CrossRef]
- Yari, A.; Heidari Fathabad, S. A High-Performance Supercapacitor Based on Cerium Molybdate Nanoparticles Anchored on N, P Co-Doped Reduced Graphene Oxide Nanocomposite as the Electrode. J. Mater. Sci. Mater. Electron. 2020, 31, 13051–13062. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 7th ed.; Pearson: Harlow, UK, 2018; ISBN 1292186712. [Google Scholar]
- Jayanna, B.; Nagendrappa, G.; Arunkumar; Gowda, N. Spectrophotometric Estimation of Azithromycin in Tablets. Indian J. Pharm. Sci. 2012, 74, 365. [Google Scholar] [CrossRef]
- Vadde, K.K.; Syrotiuk, V.R.; Montgomery, D.C. Optimizing Protocol Interaction Using Response Surface Methodology. IEEE Trans. Mob. Comput. 2006, 5, 627–639. [Google Scholar] [CrossRef]
- Lenth, R.V. Response-Surface Methods in R, Using Rsm. J. Stat. Softw. 2009, 32, 1–17. [Google Scholar] [CrossRef]
- Horowitz, S.; Trievel, R.C. Carbon-Oxygen Hydrogen Bonding in Biological Structure and Function. J. Biol. Chem. 2012, 287, 41576–41582. [Google Scholar] [CrossRef] [PubMed]
- Mladenović, M.; Arsić, B.; Stanković, N.; Mihović, N.; Ragno, R.; Regan, A.; Milićević, J.; Trtić-Petrović, T.; Micić, R. The Targeted Pesticides as Acetylcholinesterase Inhibitors: Comprehensive Cross-Organism Molecular Modelling Studies Performed to Anticipate the Pharmacology of Harmfulness to Humans In Vitro. Molecules 2018, 23, 2192. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ji, S.; Kuang, W.; Liao, A.; Lan, P.; Zhang, J. Solubility Determination and Correlation for Azithromycin Monohydrate and Dihydrate in Solvent Mixtures. J. Mol. Liq. 2020, 301, 112398. [Google Scholar] [CrossRef]
- Cavalcante, L.S.; Sczancoski, J.C.; Lima, L.F.; Espinosa, J.W.M.; Pizani, P.S.; Varela, J.A.; Longo, E. Synthesis, Characterization, Anisotropic Growth and Photoluminescence of BaWO4. Cryst. Growth Des. 2009, 9, 1002–1012. [Google Scholar] [CrossRef]
- Yousefi, T.; Khanchi, A.R.; Ahmadi, S.J.; Rofouei, M.K.; Yavari, R.; Davarkhah, R.; Myanji, B. Cerium(III) Molybdate Nanoparticles: Synthesis, Characterization and Radionuclides Adsorption Studies. J. Hazard. Mater. 2012, 215–216, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Paiva, W.D.A.; Oliveira, T.M.B.F.; Sousa, C.P.; Neto, P.D.L.; Correia, A.N.; Morais, S.; Silva, D.R.; Castro, S.S.L. Electroanalysis of Imidacloprid Insecticide in Riverwaters Using Functionalized Multi-Walled Carbon Nanotubes Modified Glassy Carbon Electrode. J. Electrochem. Soc. 2018, 165, B431. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, Q.; Fan, Y. Research on Corrosion Resistance and Formation Mechanism of Molybdate Composite Film. Crystals 2022, 12, 1559. [Google Scholar] [CrossRef]
- Oliveira, T.M.B.F.; Ribeiro, F.W.P.; Do Nascimento, J.M.; Soares, J.E.S.; Freire, V.N.; Becker, H.; De Lima-Netoa, P.; Correia, A.N. Direct Electrochemical Analysis of Dexamethasone Endocrine Disruptor in Raw Natural Waters. J. Braz. Chem. Soc. 2012, 23, 110–119. [Google Scholar] [CrossRef]
- Pogăcean, F.; Varodi, C.; Măgeruşan, L.; Stefan-van Staden, R.-I.; Pruneanu, S. Highly Sensitive Electrochemical Detection of Azithromycin with Graphene-Modified Electrode. Sensors 2022, 22, 6181. [Google Scholar] [CrossRef]
- Al-Hakkani, M.F.; Gouda, G.A.; Hassan, S.H.A.; Mohamed, M.M.A.; Nagiub, A.M. Environmentally Azithromycin Pharmaceutical Wastewater Management and Synergetic Biocompatible Approaches of Loaded Azithromycin@hematite Nanoparticles. Sci. Rep. 2022, 12, 10970. [Google Scholar] [CrossRef] [PubMed]
- Foulds, G.; Shepard, R.M.; Johnson, R.B. The Pharmacokinetics of Azithromycin in Human Serum and Tissues. J. Antimicrob. Chemother. 1990, 25, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, Y.; Dong, W.; Hu, Q.; Li, Y.; Shuang, S.; Dong, C.; Cai, L.; Gong, X. Azithromycin Detection in Cells and Tablets by N,S Co-Doped Carbon Quantum Dots. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119506. [Google Scholar] [CrossRef] [PubMed]
- El-Yazbi, A.F.; Khamis, E.F.; Youssef, R.M.; El-Sayed, M.A.; Aboukhalil, F.M. Green Analytical Methods for Simultaneous Determination of Compounds Having Relatively Disparate Absorbance; Application to Antibiotic Formulation of Azithromycin and Levofloxacin. Heliyon 2020, 6, e04819. [Google Scholar] [CrossRef]
- Gaolatlhe, L.; Barik, R.; Ray, S.C.; Ozoemena, K.I. Voltammetric Responses of Porous Co3O4 Spinels Supported on MOF-Derived Carbons: Effects of Porous Volume on Dopamine Diffusion Processes. J. Electroanal. Chem. 2020, 872, 113863. [Google Scholar] [CrossRef]
- Mostafazadeh, R.; Karimi-Maleh, H.; Ghaffarinejad, A.; Tajabadi, F.; Hamidian, Y. Highly Sensitive Electrochemical Sensor Based on Carbon Paste Electrode Modified with Graphene Nanoribbon–CoFe2O4@NiO and Ionic Liquid for Azithromycin Antibiotic Monitoring in Biological and Pharmaceutical Samples. Appl. Nanosci. 2023, 13, 5829–5838. [Google Scholar] [CrossRef] [PubMed]
- De Moreno, A.H.; da Silva, M.F.C.; Salgado, H.R.N. Stability Study of Azithromycin in Ophthalmic Preparations. Braz. J. Pharm. Sci. 2009, 45, 219–226. [Google Scholar] [CrossRef]
- Bhimani, S.; Sanghvi, G.; Pethani, T.; Dave, G.; Airao, V.; Sharma, T.; Sheth, N.; Vaishnav, D. Development of the UV Spectrophotometric Method of Azithromycin in API and Stress Degradation Studies. Int. Lett. Chem. Phys. Astron. 2016, 68, 48–53. [Google Scholar] [CrossRef]
- Zannat, A.; Uddin, M.N.; Mahmud, S.T.; Mia, R.; Ahmed, T. Natural Dyes and Pigments in Functional Finishing. In Renewable Dyes and Pigments; Elsevier: Amsterdam, The Netherlands, 2024; pp. 271–287. [Google Scholar]

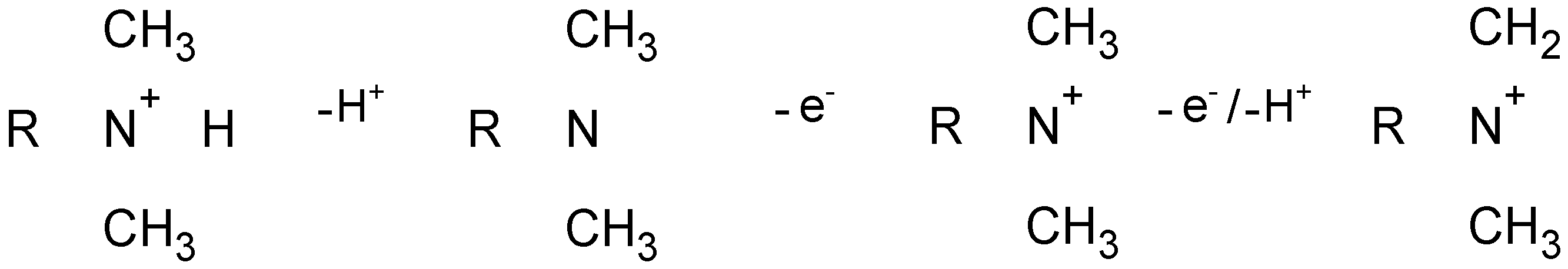
| Procedure | Intraday Repeatability (µM) | Mean (µM) | RSD (%) | Interday Repeatability (µM) | Mean (µM) | RSD (%) | Reproducibility (µM) | Mean (µM) | RSD (%) |
|---|---|---|---|---|---|---|---|---|---|
| Electroanalytical | 5.05 | 4.97 | 1.5 | 5.05 | 4.91 | 2.8 | 5.05 | 4.98 | 3.1 |
| 4.93 | 4.83 | 5.14 | |||||||
| 4.97 | 4.79 | 4.75 | |||||||
| 4.80 | 5.12 | ||||||||
| 4.79 | 4.78 | ||||||||
| 4.98 | |||||||||
| 5.08 | |||||||||
| 5.06 | |||||||||
| 5.05 | |||||||||
| 4.96 | |||||||||
| Spectroanalytical | 4.93 | 4.99 | 2.5 | 4.93 | 4.95 | 3.0 | 4.93 | 4.97 | 1.7 |
| 5.10 | 4.76 | 5.09 | |||||||
| 5.12 | 5.15 | 4.88 | |||||||
| 4.84 | 5.12 | ||||||||
| 4.83 | 4.78 | ||||||||
| 5.08 | |||||||||
| 5.05 | |||||||||
| 5.15 | |||||||||
| 4.87 | |||||||||
| 4.81 |
| Source | DF 1 | SS 2 | MS 3 | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 9 | 3.37 × 103 | 3.74 × 102 | 1.81 × 105 | 1.48 × 10−14 |
| Linear | 3 | 1.74 × 103 | 5.80 × 102 | 281 × 105 | 1.75 × 10−13 |
| A | 1 | 0.01 | 0.01 | 1.96 | 0.22 |
| B | 1 | 4.80 × 102 | 4.80 × 102 | 2.33 × 105 | 7.28 × 10−13 |
| C | 1 | 1.26 × 103 | 1.26 × 103 | 6.09 × 105 | 6.55 × 10−14 |
| Quadratic | 3 | 1.18 × 103 | 3.92 × 102 | 1.90 × 105 | 5.13 × 10−12 |
| A2 | 1 | 6.25 × 102 | 6.25 × 102 | 3.02 × 105 | 3.78 × 10−13 |
| B2 | 1 | 5.20 × 102 | 5.20 × 102 | 2.52 × 105 | 5.97 × 10−13 |
| C2 | 1 | 1.95 × 102 | 1.95 × 102 | 9.48 × 104 | 6.87 × 10−12 |
| Two-factor interaction | 3 | 4.50 × 102 | 1.50 × 102 | 7.27 × 104 | 4.65 × 10−13 |
| AB | 1 | 65.0 | 65.0 | 3.14 × 104 | 1.08 × 10−10 |
| AC | 1 | 2.37 | 2.37 | 1.15 × 103 | 4.22 × 10−7 |
| BC | 1 | 3.83 × 102 | 3.84 × 102 | 1.85 × 105 | 1.28 × 10−12 |
| Lack of fit | 3 | <0.01 | <0.01 | 0.15 | 0.923 |
| Pure error | 2 | 0.01 | – | – | – |
| Total (model + residual) | 14 | 3.37 × 103 | – | – | – |
| R2 | 0.999 | – | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, H.R.A.; Santos, A.O.; Teixeira, Y.N.; Silva, M.A.S.; Feitosa, V.A.; Morais, S.; Oliveira, T.M.B.F. Exploring the Thermal-Oxidative Stability of Azithromycin Using a Thermoactivated Sensor Based on Cerium Molybdate and Multi-Walled Carbon Nanotubes. Nanomaterials 2024, 14, 899. https://doi.org/10.3390/nano14110899
Costa HRA, Santos AO, Teixeira YN, Silva MAS, Feitosa VA, Morais S, Oliveira TMBF. Exploring the Thermal-Oxidative Stability of Azithromycin Using a Thermoactivated Sensor Based on Cerium Molybdate and Multi-Walled Carbon Nanotubes. Nanomaterials. 2024; 14(11):899. https://doi.org/10.3390/nano14110899
Chicago/Turabian StyleCosta, Heryka R. A., André O. Santos, Yago N. Teixeira, Maria A. S. Silva, Valker A. Feitosa, Simone Morais, and Thiago M. B. F. Oliveira. 2024. "Exploring the Thermal-Oxidative Stability of Azithromycin Using a Thermoactivated Sensor Based on Cerium Molybdate and Multi-Walled Carbon Nanotubes" Nanomaterials 14, no. 11: 899. https://doi.org/10.3390/nano14110899
APA StyleCosta, H. R. A., Santos, A. O., Teixeira, Y. N., Silva, M. A. S., Feitosa, V. A., Morais, S., & Oliveira, T. M. B. F. (2024). Exploring the Thermal-Oxidative Stability of Azithromycin Using a Thermoactivated Sensor Based on Cerium Molybdate and Multi-Walled Carbon Nanotubes. Nanomaterials, 14(11), 899. https://doi.org/10.3390/nano14110899








