Ultrasmall Fe2O3 Tubular Nanomotors: The First Example of Swarming Photocatalytic Nanomotors Operating in High-Electrolyte Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Fe2O3 TNMs
2.2. Characterization
2.3. Light-Driven Propulsions and Swarming Behaviors of Fe2O3 TNMs
2.4. Photocatalytic Degradation of Rhodamine 6G
3. Results and Discussion
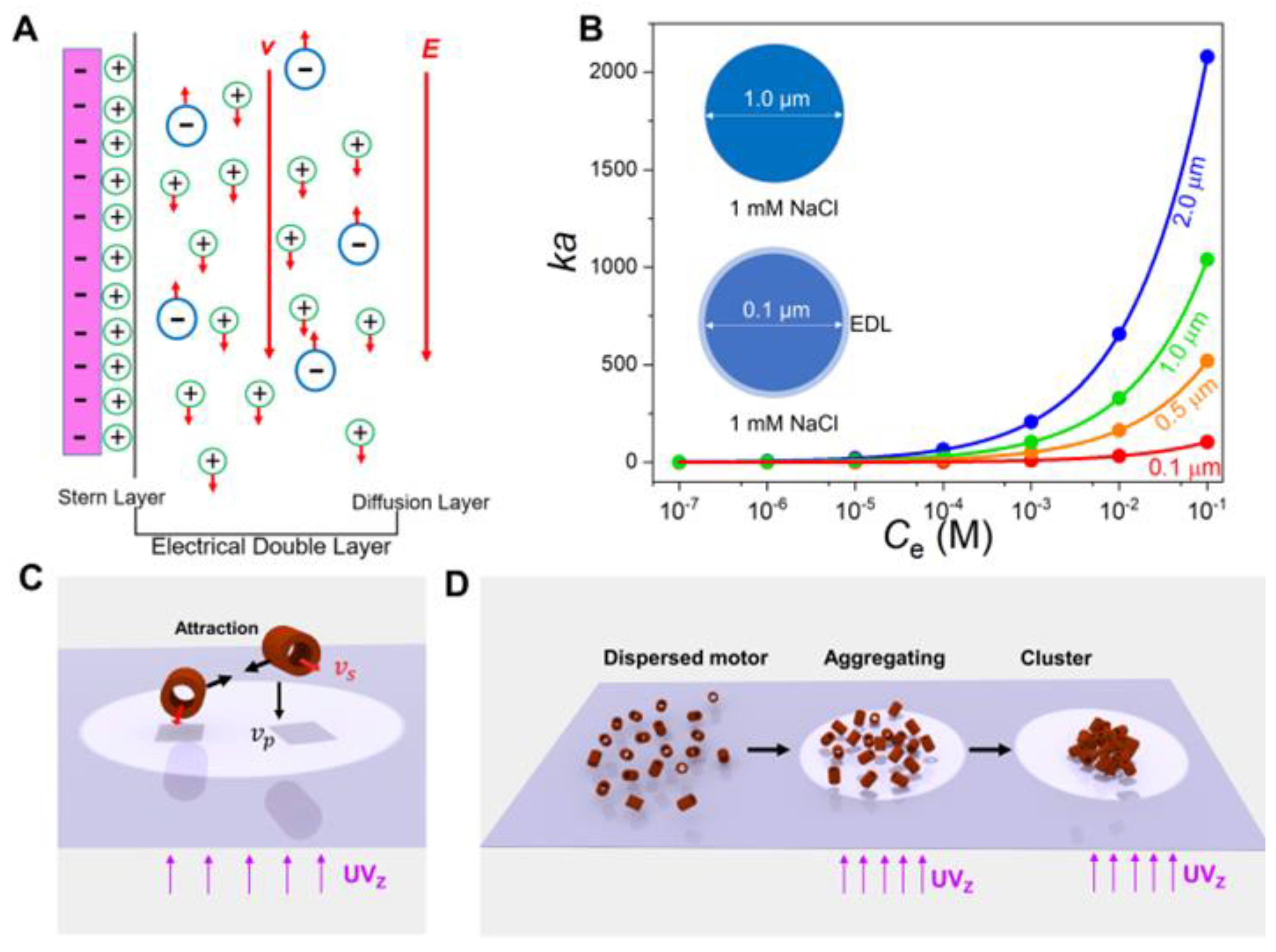
3.1. Conceptual Design of Ion-Tolerant Fe2O3 TNMs
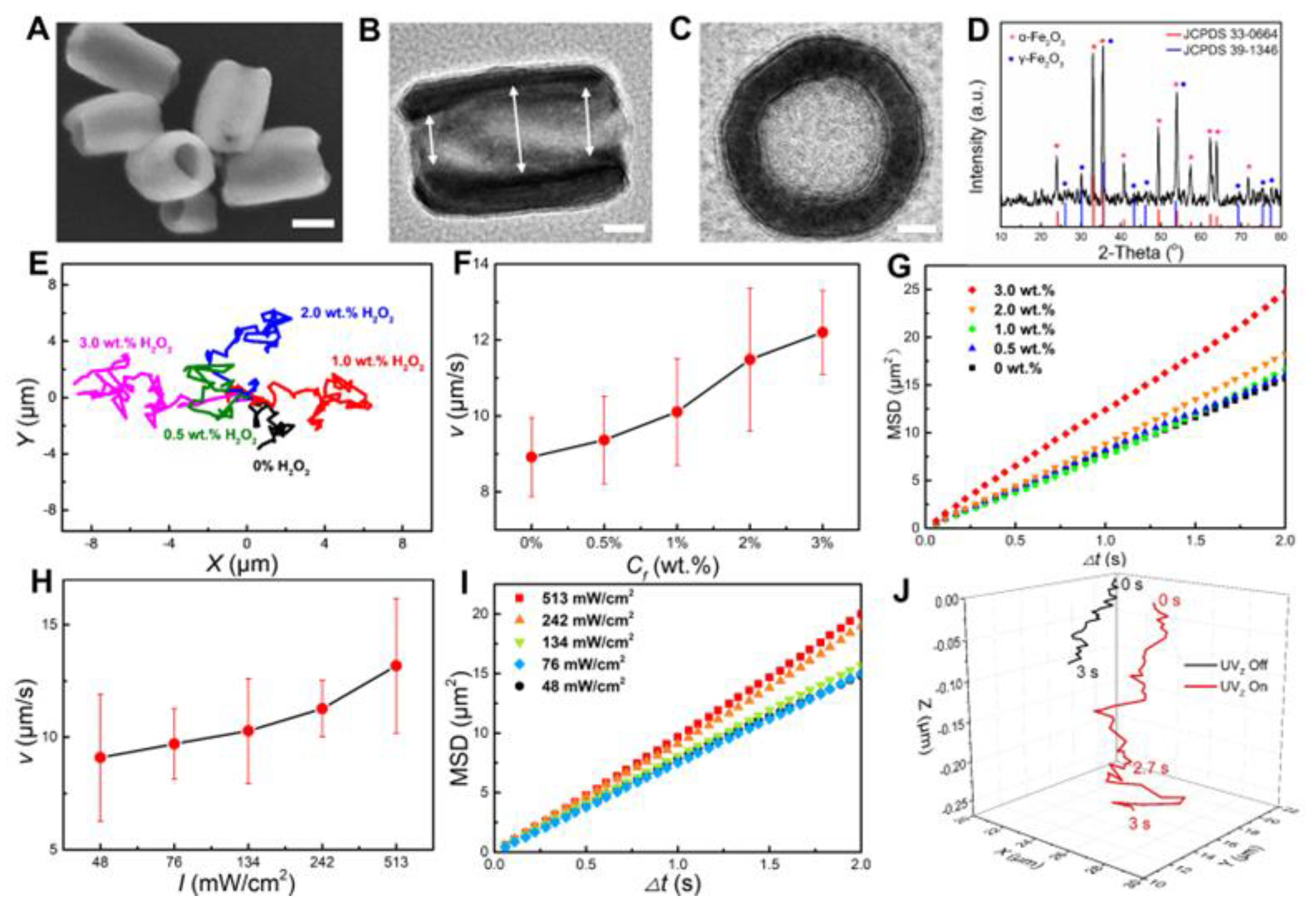
3.2. Characterization and Propulsions of Fe2O3 TNMs
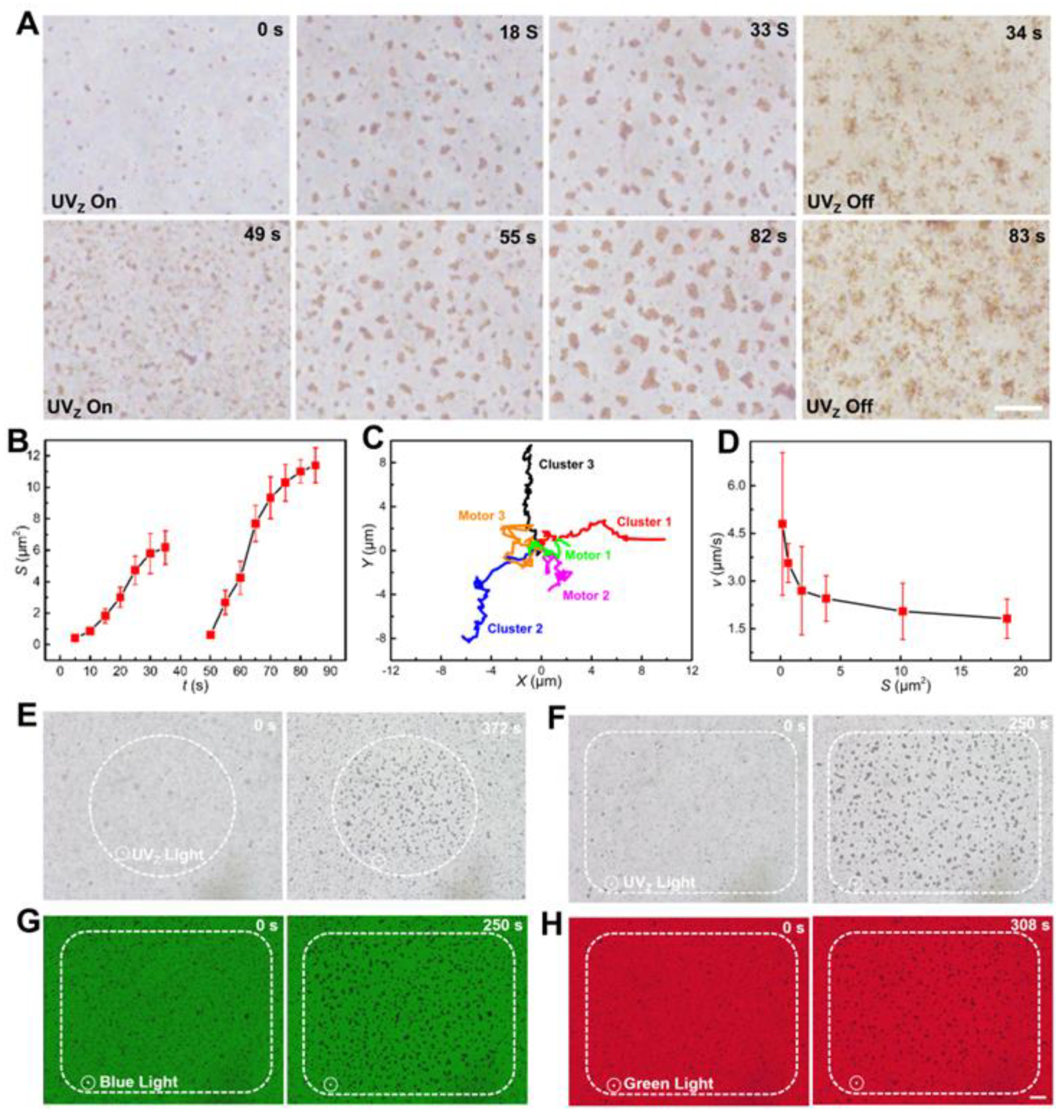
3.3. Swarming Behaviors of Fe2O3 TNMs
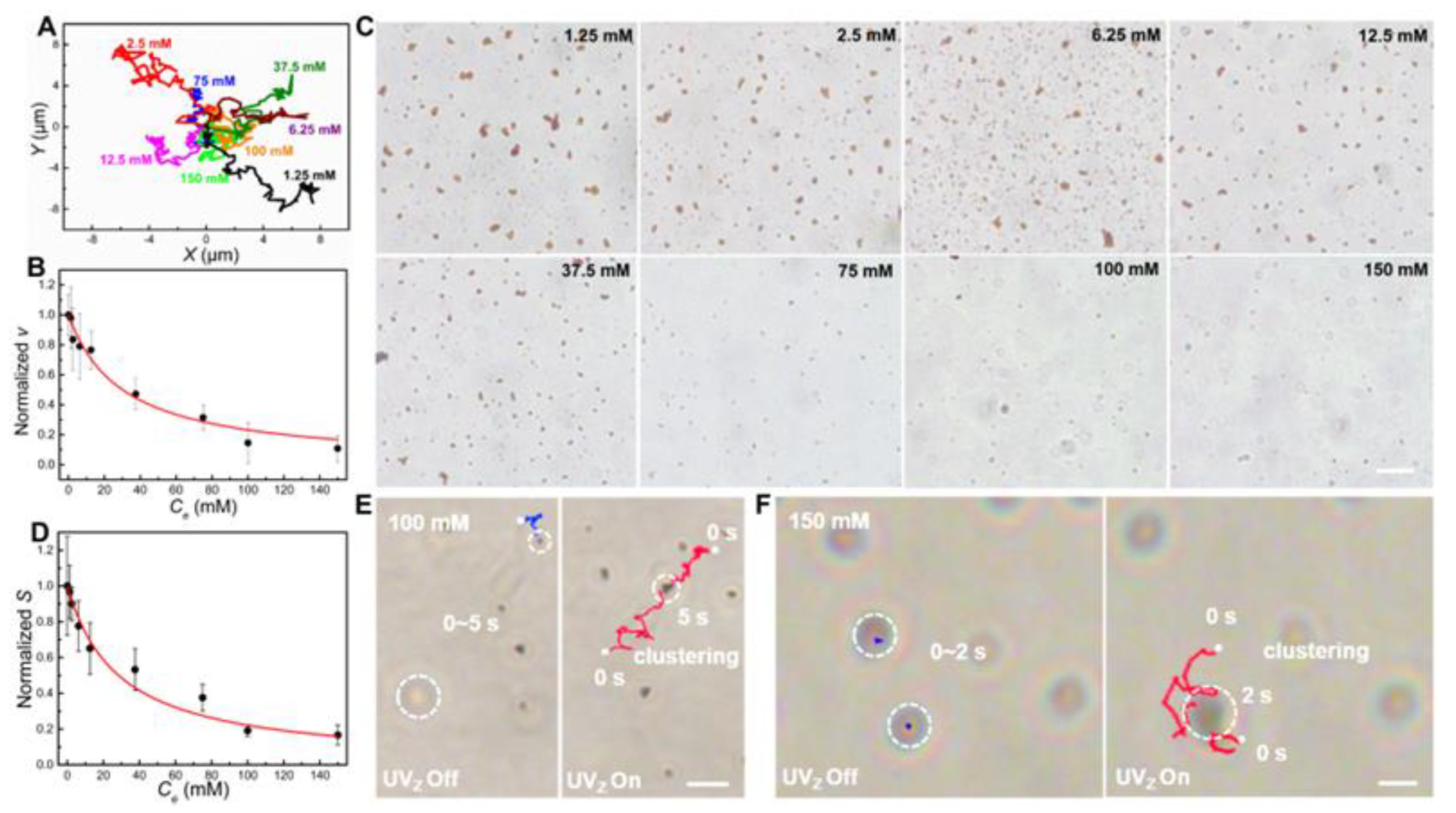
3.4. Ion Tolerance of Fe2O3 TNMs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, B.; Kostarelos, K.; Nelson, B.J.; Zhang, L. Trends in micro-/nanorobotics: Materials development, actuation, localization, and system integration for biomedical applications. Adv. Mater. 2021, 33, 2002047. [Google Scholar] [CrossRef] [PubMed]
- Safdar, M.; Khan, S.U.; Janis, J. Progress toward Catalytic Micro- and Nanomotors for Biomedical and Environmental Applications. Adv. Mater. 2018, 30, 1703660. [Google Scholar] [CrossRef] [PubMed]
- Parmar, J.; Vilela, D.; Villa, K.; Wang, J.; Sanchez, S. Micro- and Nanomotors as Active Environmental Microcleaners and Sensors. J. Am. Chem. Soc. 2018, 140, 9317–9331. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.Y.; Yu, W.Q.; Chen, X.C.; Gao, H.L. Self-Propelled Micro/Nanomotors for Tumor Targeting Delivery and Therapy. Adv. Healthc. Mater. 2020, 10, e2001212. [Google Scholar] [CrossRef] [PubMed]
- Soto, F.; Karshalev, E.; Zhang, F.; Esteban Fernandez de Avila, B.; Nourhani, A.; Wang, J. Smart Materials for Microrobots. Chem. Rev. 2022, 122, 5365–5403. [Google Scholar] [CrossRef]
- Liu, T.; Xie, L.; Price, C.-A.H.; Liu, J.; He, Q.; Kong, B. Controlled propulsion of micro/nanomotors: Operational mechanisms, motion manipulation and potential biomedical applications. Chem. Soc. Rev. 2022, 51, 10083–10119. [Google Scholar] [CrossRef]
- Yang, M.; Guo, X.; Mou, F.; Guan, J. Lighting up Micro-/Nanorobots with Fluorescence. Chem. Rev. 2022, 123, 3944–3975. [Google Scholar] [CrossRef]
- Yang, L.; Yu, J.; Yang, S.; Wang, B.; Nelson, B.J.; Zhang, L. A Survey on Swarm Microrobotics. IEEE Trans. Robot. 2022, 38, 1531–1551. [Google Scholar] [CrossRef]
- Wang, W.; Duan, W.; Ahmed, S.; Sen, A.; Mallouk, T.E. From One to Many: Dynamic Assembly and Collective Behavior of Self-Propelled Colloidal Motors. Accounts. Chem. Res. 2015, 48, 1938–1946. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L. External Power-Driven Microrobotic Swarm: From Fundamental Understanding to Imaging-Guided Delivery. ACS Nano 2021, 15, 149–174. [Google Scholar] [CrossRef]
- Wang, H.; Pumera, M. Coordinated behaviors of artificial micro/nanomachines: From mutual interactions to interactions with the environment. Chem. Soc. Rev. 2020, 49, 3211–3230. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Zhang, L. Collective Behaviors of Magnetic Active Matter: Recent progress toward reconfigurable, adaptive, and multifunctional swarming micro/Nanorobots. Accounts Chem. Res. 2021, 55, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, S.; Guan, J.; Mou, F. “Motile-targeting” drug delivery platforms based on micro/nanorobots for tumor therapy. Front. Bioeng. Biotech. 2022, 10, 1002171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mou, F.; Wu, Z.; Song, J.; Kauffman, J.E.; Sen, A.; Guan, J. Cooperative transport by flocking phototactic micromotors. Nanoscale Adv. 2021, 3, 6157–6163. [Google Scholar] [CrossRef]
- de Avila, B.E.F.; Martin, A.; Soto, F.; Lopez-Ramirez, M.A.; Campuzano, S.; Vasquez-Machado, G.M.; Gao, W.W.; Zhang, L.F.; Wang, J. Single Cell Real-Time miRNAs Sensing Based on Nanomotors. ACS Nano 2015, 9, 6756–6764. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, W.; Sun, Y. Self-propelled micro-/nanomotors as “on-the-move” platforms: Cleaners, sensors, and reactors. Adv. Funct. Mater. 2022, 32, 2109181. [Google Scholar] [CrossRef]
- Gultepe, E.; Randhawa, J.S.; Kadam, S.; Yamanaka, S.; Selaru, F.M.; Shin, E.J.; Kalloo, A.N.; Gracias, D.H. Biopsy with Thermally-Responsive Untethered Microtools. Adv. Mater. 2013, 25, 514–519. [Google Scholar] [CrossRef]
- Zhou, S.; Zhao, W.; Hu, J.; Mao, C.; Zhou, M. Application of Nanotechnology in Thrombus Therapy. Adv. Healthc. Mater. 2023, 12, 2202578. [Google Scholar] [CrossRef]
- He, W.; Frueh, J.; Hu, N.; Liu, L.; Gai, M.; He, Q. Guidable Thermophoretic Janus Micromotors Containing Gold Nanocolorifiers for Infrared Laser Assisted Tissue Welding. Adv. Sci. 2016, 3, 1600206. [Google Scholar] [CrossRef]
- Urso, M.; Ussia, M.; Pumera, M. Smart micro- and nanorobots for water purification. Nat. Rev. Bioeng. 2023, 1, 236–251. [Google Scholar] [CrossRef]
- Gao, C.; Feng, Y.; Wilson, D.A.; Tu, Y.; Peng, F. Micro-Nano Motors with Taxis Behavior: Principles, Designs, and Biomedical Applications. Small 2022, 18, 2106263. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Chen, C.; Xu, L.; Mou, F.; Guan, J. Intelligent Micro/nanomotors with Taxis. Accounts. Chem. Res. 2018, 51, 3006–3014. [Google Scholar] [CrossRef] [PubMed]
- Mou, F.; Xie, Q.; Liu, J.; Che, S.; Bahmane, L.; You, M.; Guan, J. ZnO-based micromotors fueled by CO2: The first example of self-reorientation-induced biomimetic chemotaxis. Natl. Sci. Rev. 2021, 8, nwab066. [Google Scholar] [CrossRef]
- Zhan, X.; Wang, J.; Xiong, Z.; Zhang, X.; Zhou, Y.; Zheng, J.; Chen, J.; Feng, S.-P.; Tang, J. Enhanced ion tolerance of electrokinetic locomotion in polyelectrolyte-coated microswimmer. Nat. Commun. 2019, 10, 3921. [Google Scholar] [CrossRef]
- Moran, J.L.; Posner, J.D. Phoretic Self-Propulsion. Annu. Rev. Fluid Mech. 2017, 49, 511–540. [Google Scholar] [CrossRef]
- Sridhar, V.; Podjaski, F.; Alapan, Y.; Kroger, J.; Grunenberg, L.; Kishore, V.; Lotsch, B.V.; Sitti, M. Light-driven carbon nitride microswimmers with propulsion in biological and ionic media and responsive on-demand drug delivery. Sci. Robot. 2022, 7, eabm1421. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.-J.; Sun, L.-D.; Luo, F.; Han, X.-D.; Heyderman, L.J.; Yan, Z.-G.; Yan, C.-H.; Zheng, K.; Zhang, Z.; Takano, M.; et al. Large-scale synthesis of single-crystalline iron oxide magnetic nanorings. J. Am. Chem. Soc. 2008, 130, 16968–16977. [Google Scholar] [CrossRef]
- Brown, M.A.; Goel, A.; Abbas, Z. Effect of Electrolyte Concentration on the Stern Layer Thickness at a Charged Interface. Angew Chem. Int. Ed. 2016, 55, 3790–3794. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.A.; Perez-Martinez, C.S.; Smith, A.M.; Perkin, S. Scaling Analysis of the Screening Length in Concentrated Electrolytes. Phys. Rev. Lett. 2017, 119, 026002. [Google Scholar] [CrossRef]
- Loukanov, A.R.; Basnakian, A.G.; Kawamura, R.; Udono, H.; Filipov, C.K.; Savenka, A.V.; Fite, T.; Nakabayashi, S. Light-Powered Nanoconverters Cytotoxic to Breast Cancer Cells. J. Phys. Chem. C 2018, 122, 7916–7924. [Google Scholar] [CrossRef]
- Safdar, M.; Simmchen, J.; Janis, J. Light-driven micro- and nanomotors for environmental remediation. Environ. Sci. Nano 2017, 4, 1602–1616. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, L.; Dong, X.; Zhang, W.; Gao, S.; Wang, X.; Li, L.; Yu, C.; Wang, Q.; Yuan, A.; et al. Visible-Light-Responsive Nanofibrous α-Fe2O3 Integrated FeOx Cluster-Templated Siliceous Microsheets for Rapid Catalytic Phenol Removal and Enhanced Antibacterial Activity. ACS Appl. Mater. Interfaces 2021, 13, 19803–19815. [Google Scholar] [CrossRef] [PubMed]
- Domacena, A.; Aquino, C.; Balela, M. Photo-Fenton Degradation of Methyl Orange Using Hematite (α-Fe2O3) of Various Morphologies. Mater. Today: Proc. 2020, 22, 248–254. [Google Scholar] [CrossRef]
- Tang, Z.; Ma, D.; Chen, Q.; Wang, Y.; Sun, M.; Lian, Q.; Shang, J.; Wong, P.K.; He, C.; Xia, D. Nanomaterial-enabled photothermal-based solar water disinfection processes: Fundamentals, recent advances, and mechanisms. J. Hazard. Mater. 2022, 437, 129373. [Google Scholar] [CrossRef]
- Che, S.; Zhang, J.; Mou, F.; Guo, X.; Kauffman, J.E.; Sen, A.; Guan, J. Light-Programmable Assemblies of Isotropic Micromotors. Research 2022, 2022, 9816562. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Y.; Mou, F.; Xie, Q.; Su, S.; Chen, C.; Guan, J. Phototactic micromotor assemblies in dynamic line formations for wide-range micromanipulations. J. Mater. Chem. C 2022, 10, 5079–5087. [Google Scholar] [CrossRef]
- Zhou, D.; Zhuang, R.; Chang, X.; Li, L.J.R. Enhanced Light-Harvesting Efficiency and Adaptation: A Review on Visible-Light-Driven Micro/Nanomotors. Research 2020, 2020, 1–25. [Google Scholar] [CrossRef]
- Xu, L.; Mou, F.; Gong, H.; Luo, M.; Guan, J. Light-driven micro/nanomotors: From fundamentals to applications. Chem. Soc. Rev. 2017, 46, 6905–6926. [Google Scholar] [CrossRef]
- Chen, C.; Duan, F.; Zhao, S.; Wang, W.; Yang, F.; Nuansing, W.; Zhang, B.; Qin, Y.; Knez, M. Porous Fe2O3 Nanotubes with α-γ Phase Junction for Enhanced Charge Separation and Photocatalytic Property Produced by Molecular Layer Deposition. Appl. Catal. B 2019, 248, 218–225. [Google Scholar] [CrossRef]
- Mestre, R.; Cadefau, N.; Hortelao, A.C.; Grzelak, J.; Gich, M.; Roig, A.; Sanchez, S. Nanorods Based on Mesoporous Silica Containing Iron Oxide Nanoparticles as Catalytic Nanomotors: Study of Motion Dynamics. ChemNanoMat 2021, 7, 134–140. [Google Scholar] [CrossRef]
- Xiong, K.; Xu, L.; Lin, J.; Mou, F.; Guan, J. Mg-Based Micromotors with Motion Responsive to Dual Stimuli. Research 2020, 2020, 6213981. [Google Scholar] [CrossRef]
- Howse, J.R.; Jones, R.A.; Ryan, A.J.; Gough, T.; Vafabakhsh, R.; Golestanian, R. Self-motile colloidal particles: From directed propulsion to random walk. Phys. Rev. Lett. 2007, 99, 048102. [Google Scholar] [CrossRef] [PubMed]
- Parrish, J.K.; Edelstein-Keshet, L. Complexity, Pattern, and Evolutionary Trade-Offs in Animal Aggregation. Science 1999, 284, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Vicsek, T.; Zafeiris, A. Collective motion. Phys. Rep. 2012, 517, 71–140. [Google Scholar] [CrossRef]
- Bunea, A.-I.; Martella, D.; Nocentini, S.; Parmeggiani, C.; Taboryski, R.; Wiersma, D.S. Light-Powered Microrobots: Challenges and Opportunities for Hard and Soft Responsive Microswimmers. Adv. Intell. Sys. 2021, 3, 2000256. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Yang, M.; Guan, J.; Mou, F. Ultrasmall Fe2O3 Tubular Nanomotors: The First Example of Swarming Photocatalytic Nanomotors Operating in High-Electrolyte Media. Nanomaterials 2023, 13, 1370. https://doi.org/10.3390/nano13081370
Yu L, Yang M, Guan J, Mou F. Ultrasmall Fe2O3 Tubular Nanomotors: The First Example of Swarming Photocatalytic Nanomotors Operating in High-Electrolyte Media. Nanomaterials. 2023; 13(8):1370. https://doi.org/10.3390/nano13081370
Chicago/Turabian StyleYu, Lingxia, Manyi Yang, Jianguo Guan, and Fangzhi Mou. 2023. "Ultrasmall Fe2O3 Tubular Nanomotors: The First Example of Swarming Photocatalytic Nanomotors Operating in High-Electrolyte Media" Nanomaterials 13, no. 8: 1370. https://doi.org/10.3390/nano13081370
APA StyleYu, L., Yang, M., Guan, J., & Mou, F. (2023). Ultrasmall Fe2O3 Tubular Nanomotors: The First Example of Swarming Photocatalytic Nanomotors Operating in High-Electrolyte Media. Nanomaterials, 13(8), 1370. https://doi.org/10.3390/nano13081370







