Investigation of Nanoscale Tungsten Carbide Enhanced Surface Carbon as a Platinum Support for the Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Experimental
2.1. Preparation of WC
2.2. Preparation of Pt/WC
2.3. Characterization of Electrocatalysts
2.4. Electrochemical Measurements
3. Results and Discussion
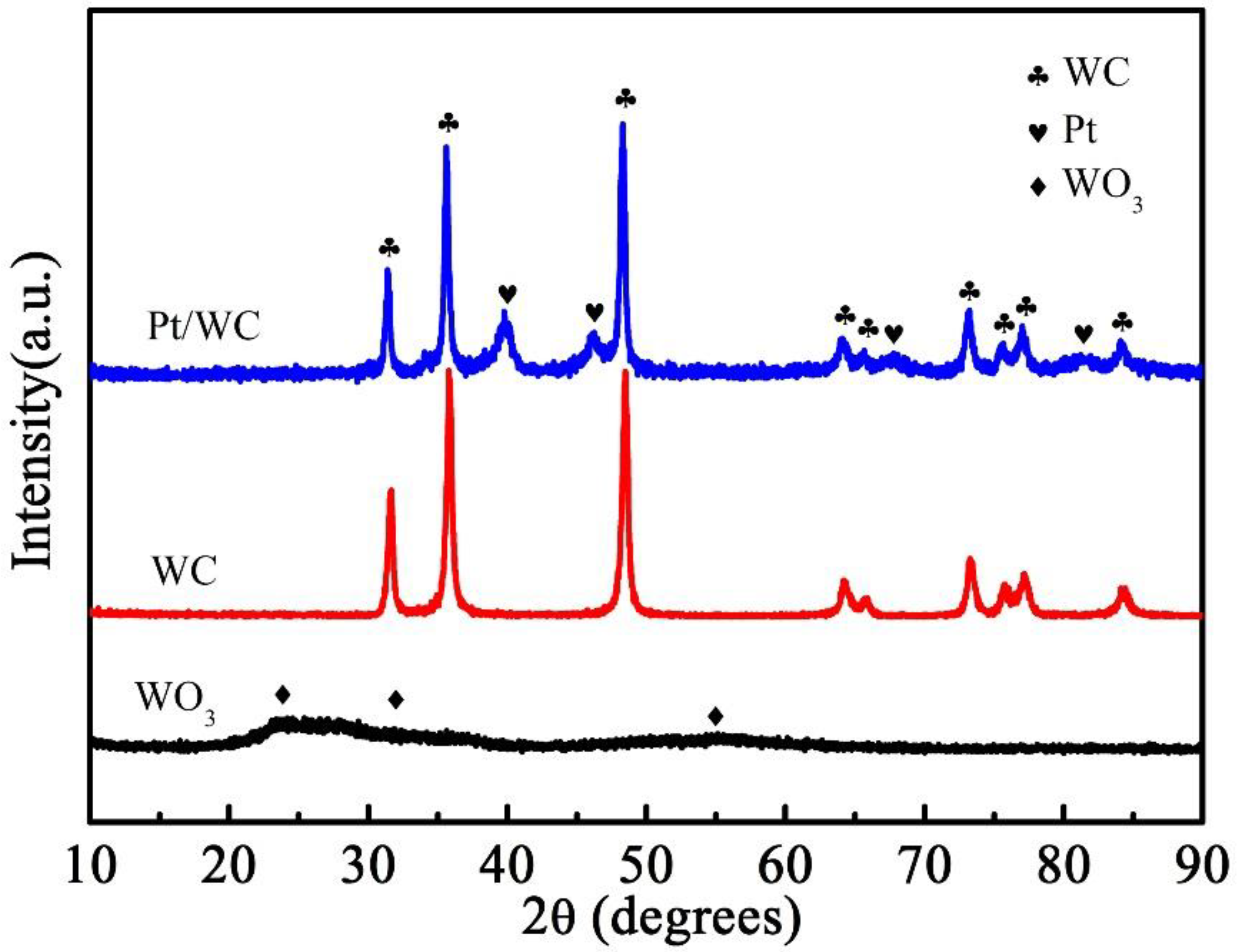
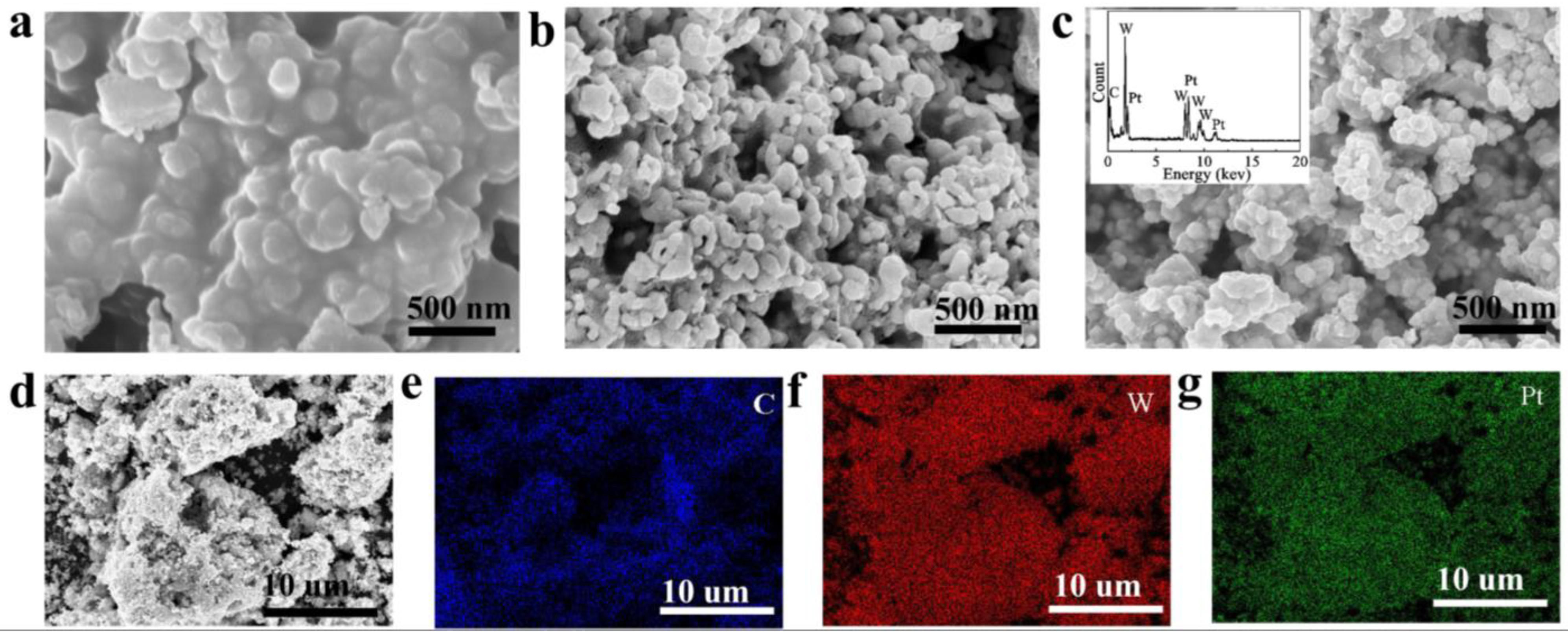
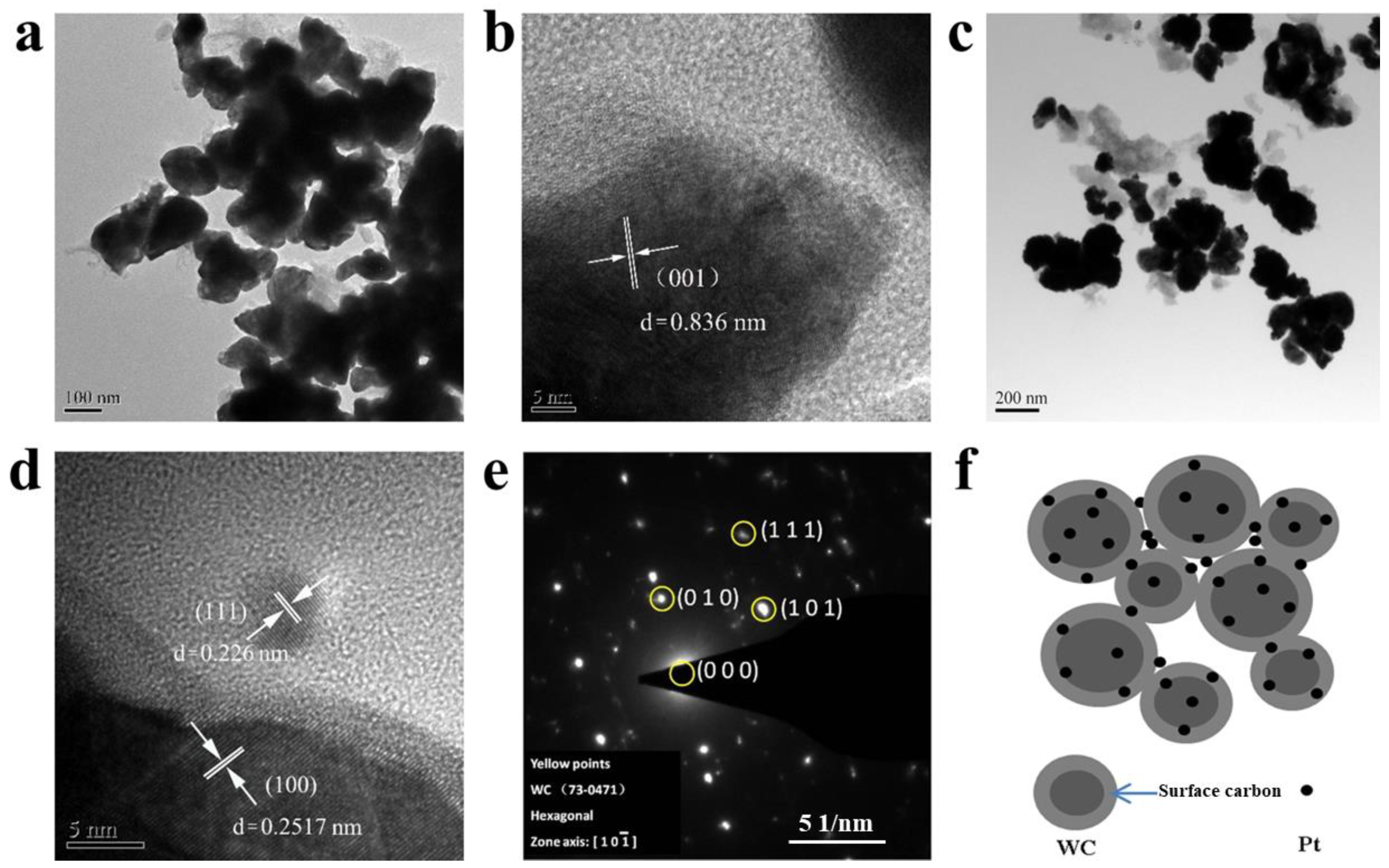
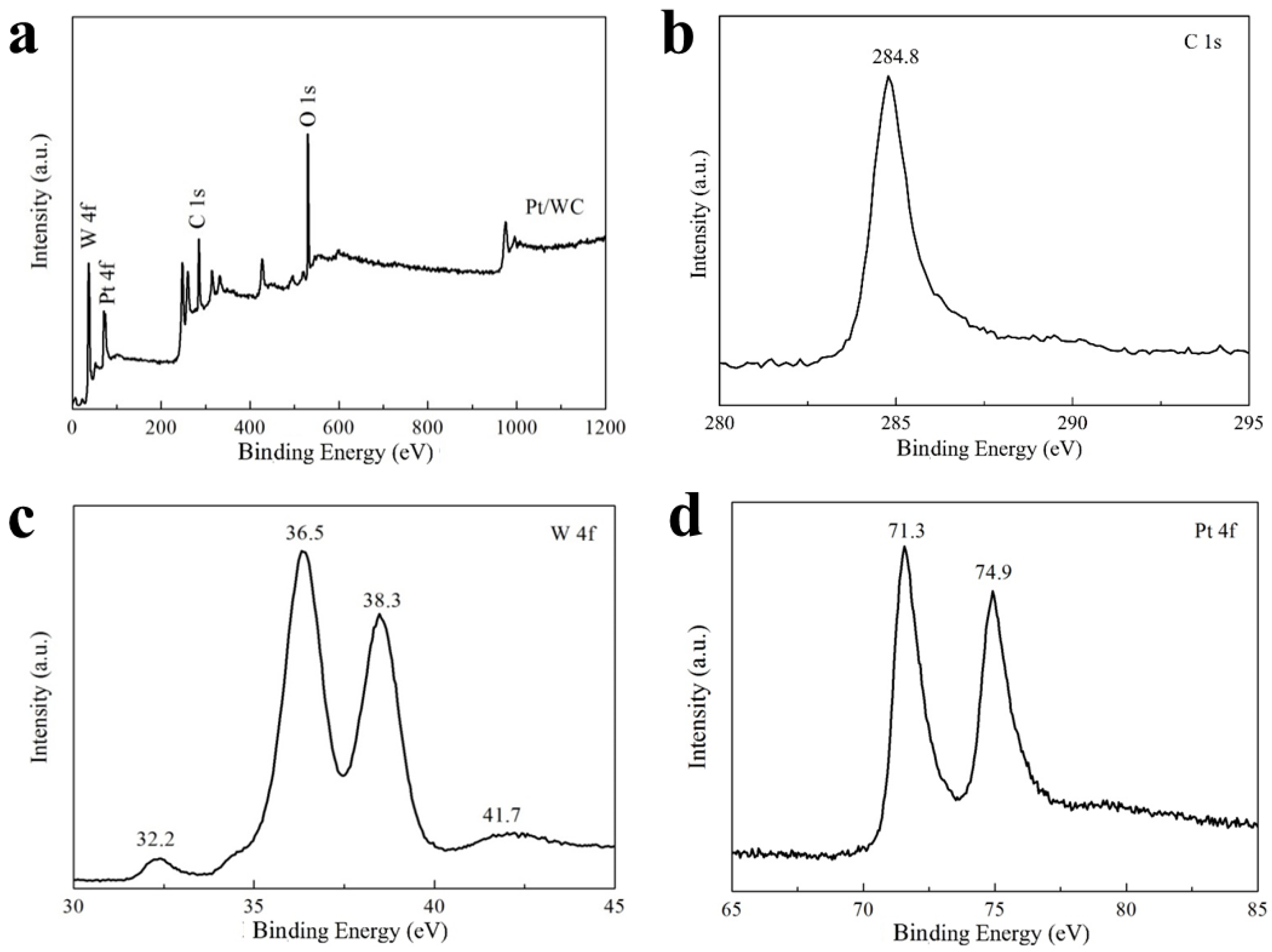
3.1. Phase and Structural Characterization of WC and Pt/WC Catalysts
3.2. Electrochemical Activity of Different Catalysts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cullen, D.A.; Neyerlin, K.; Ahluwalia, R.K.; Mukundan, R.; More, K.L.; Borup, R.L.; Weber, A.Z.; Myers, D.J.; Kusoglu, A. New roads and challenges for fuel cells in heavy-duty transportation. Nat. Energy 2021, 6, 462–474. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Priest, C.; Shi, Q.; Wu, G. Atomically dispersed metal-nitrogen-carbon catalysts for fuel cells: Advances in catalyst design, electrode performance, and durability improvement. Chem. Soc. Rev. 2020, 49, 3484–3524. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Liu, C.; Gao, Z.; Lu, B.; Tong, X.; Guo, X.; Yang, N. Atomic carbon layers supported Pt nanoparticles for minimized CO poisoning and maximized methanol oxidation. Small 2019, 15, 1902951. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.P.; Datta, M.K.; Jampani, P.H.; Hong, D.; Poston, J.A.; Manivannan, A.; Kumta, P.N. High performance and durable nanostructured TiN supported Pt50–Ru50 anode catalyst for direct methanol fuel cell (DMFC). J. Power Sources 2015, 293, 437–446. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Q.; Ling, Y.; Yang, Z.; Cheng, H. Promoted stability and electrocatalytic activity of PtRu electrocatalyst derived from coating by cerium oxide with high oxygen storage capacity. Appl. Surf. Sci. 2018, 455, 815–820. [Google Scholar] [CrossRef]
- Klein, J.; Argast, F.; Engstfeld, A.K.; Brimaud, S.; Behm, R.J. Electro-oxidation of methanol on Ru-core Pt-shell type model electrodes. Electrochim. Acta 2019, 311, 244–254. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, X.; Zhao, Z.; Zuo, W.; Chen, S.; Li, Z.; He, Y.; Liang, J.; Ma, F.; Wang, H.L. Improving the stability of non-noble-metal M–N–C catalysts for proton-exchange-membrane fuel cells through M–N bond length and coordination regulation. Adv. Mater. 2021, 33, 2006613. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, M.; Zhao, X.; Cai, J.; Yan, W.; Yen, J.C.; Chen, S.; Yu, Y.; Zhang, J. Advanced noncarbon materials as catalyst supports and non-noble electrocatalysts for fuel cells and metal–air batteries. Electrochem. Energy Rev. 2021, 4, 336–381. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.; Shao, Z.; Lim, J. Non-precious-metal catalysts for alkaline water electrolysis: Operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49, 9154–9196. [Google Scholar] [CrossRef]
- Shao-Horn, Y.; Sheng, W.; Chen, S.; Ferreira, P.J.; Holby, E.; Morgan, D. Instability of supported platinum nanoparticles in low-temperature fuel cells. Top. Catal. 2007, 46, 285–305. [Google Scholar] [CrossRef]
- Anićijević, D.D.V.; Nikolić, V.M.; Kaninski, M.P.M.; Pašti, I.A. Structure, chemisorption properties and electrocatalysis by Pd3Au overlayers on tungsten carbide–A DFT study. Int. J. Hydrog. Energy 2015, 40, 6085–6096. [Google Scholar] [CrossRef]
- Hassan, A.; Paganin, V.A.; Ticianelli, E.A. Pt modified tungsten carbide as anode electrocatalyst for hydrogen oxidation in proton exchange membrane fuel cell: CO tolerance and stability. Appl. Catal. B Environ. 2015, 165, 611–619. [Google Scholar] [CrossRef]
- Chu, Y.; Peng, R.; Chen, Z.; Li, L.; Zhao, F.; Zhu, Y.; Tong, S.; Zheng, H. Modulating Dominant Facets of Pt through Multistep Selective Anchored on WC for Enhanced Hydrogen Evolution Catalysis. ACS Appl. Mater. Interfaces 2023, 15, 9263–9272. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; You, H.; Kimmel, Y.C.; Esposito, D.V.; Chen, J.G.; Moffat, T.P. Self-terminating electrodeposition of Pt on WC electrocatalysts. Appl. Surf. Sci. 2020, 504, 144472. [Google Scholar] [CrossRef] [PubMed]
- Chhina, H.; Campbell, S.; Kesler, O. Thermal and electrochemical stability of tungsten carbide catalyst supports. J. Power Sources 2007, 164, 431–440. [Google Scholar] [CrossRef]
- Lu, J.L.; Li, Z.H.; Shen, P.K.; Li, L. Nanostructured tungsten carbide/carbon composites synthesized by a microwave heating method as supports of platinum catalysts for methanol oxidation. J. Power Sources 2012, 202, 56–62. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, M.; Xie, J.; Shen, P.K. Vanadium carbide and graphite promoted Pd electrocatalyst for ethanol oxidation in alkaline media. J. Power Sources 2013, 243, 336–342. [Google Scholar] [CrossRef]
- Ma, C.; Sheng, J.; Brandon, N.; Zhang, C.; Li, G. Preparation of tungsten carbide-supported nano Platinum catalyst and its electrocatalytic activity for hydrogen evolution. Int. J. Hydrog. Energy 2007, 32, 2824–2829. [Google Scholar] [CrossRef]
- Ham, D.J.; Ganesan, R.; Lee, J.S. Tungsten carbide microsphere as an electrode for cathodic hydrogen evolution from water. Int. J. Hydrog. Energy 2008, 33, 6865–6872. [Google Scholar] [CrossRef]
- Wu, M.; Shen, P.K.; Wei, Z.; Song, S.; Nie, M. High activity PtPd-WC/C electrocatalyst for hydrogen evolution reaction. J. Power Sources 2007, 166, 310–316. [Google Scholar] [CrossRef]
- Esposito, D.V.; Hunt, S.T.; Stottlemyer, A.L.; Dobson, K.D.; McCandless, B.E.; Birkmire, R.W.; Chen, J.G. Low-cost hydrogen-evolution catalysts based on monolayer platinum on tungsten monocarbide substrates. Angew. Chem. Int. Ed. 2010, 49, 9859–9862. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, H.; Hao, T.; Wang, P.; Zhang, X.; Zhang, H.; Xue, J.; Zhao, J.; Li, Y. In situ XRD and operando spectra-electrochemical investigation of tetragonal WO3-x nanowire networks for electrochromic supercapacitors. NPG Asia Mater. 2021, 13, 51. [Google Scholar] [CrossRef]
- Liu, Z.; Li, P.; Zhai, F.; Wan, Q.; Volinsky, A.A.; Qu, X. Amorphous carbon modified nano-sized tungsten carbide as a gas diffusion electrode catalyst for the oxygen reduction reaction. RSC Adv. 2015, 5, 70743–70748. [Google Scholar] [CrossRef]
- Rahsepar, M.; Pakshir, M.; Nikolaev, P.; Safavi, A.; Palanisamy, K.; Kim, H. Tungsten carbide on directly grown multiwalled carbon nanotube as a co-catalyst for methanol oxidation. Appl. Catal. B Environ. 2012, 127, 265–272. [Google Scholar] [CrossRef]
- Liu, Y.; Mustain, W.E. Evaluation of tungsten carbide as the electrocatalyst support for platinum hydrogen evolution/oxidation catalysts. Int. J. Hydrogen Energy 2012, 37, 8929–8938. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.; Zhang, L.; Tang, D.-M.; Ma, R.; Ren, C.-L.; Ding, F.; Liu, C.; Cheng, H.-M. Growth mechanism of carbon nanotubes from Co-WC alloy catalyst revealed by atmospheric environmental transmission electron microscopy. Sci. Adv. 2022, 8, eabo5686. [Google Scholar] [CrossRef]
- Liu, Z.; Huo, X.; Xi, K.; Li, P.; Yue, L.; Huang, M.; Suo, G.; Xu, L.; Wang, W.; Qu, X. Thickness controllable and mass produced WC@ C@ Pt hybrid for efficient hydrogen production. Energy Storage Mater. 2018, 10, 268–274. [Google Scholar] [CrossRef]
- Tang, C.; Wang, D.; Wu, Z.; Duan, B. Tungsten carbide hollow microspheres as electrocatalyst and platinum support for hydrogen evolution reaction. Int. J. Hydrogen Energy 2015, 40, 3229–3237. [Google Scholar] [CrossRef]
- Durst, J.; Simon, C.; Hasché, F.; Gasteiger, H.A. Hydrogen oxidation and evolution reaction kinetics on carbon supported Pt, Ir, Rh, and Pd electrocatalysts in acidic media. J. Electrochem. Soc. 2014, 162, F190. [Google Scholar] [CrossRef]
- Hsu, I.J.; Kimmel, Y.C.; Jiang, X.; Willis, B.G.; Chen, J.G. Atomic layer deposition synthesis of platinum–tungsten carbide core–shell catalysts for the hydrogen evolution reaction. Chem. Commun. 2012, 48, 1063–1065. [Google Scholar] [CrossRef]


| Element | Weight% | Atomic% |
|---|---|---|
| C | 15.04 | 63.07 |
| O | 4.88 | 15.36 |
| W | 57.09 | 15.64 |
| Pt | 22.99 | 5.93 |
| Totals | 100.00 | 100.00 |
| Catalysts | Onset η (mV versus RHE) | η at j = 10 mA·cm−2 (mV versus RHE) | Tafel Slope (mV per Decade) | Exchange Current Density (mA·cm−2) |
|---|---|---|---|---|
| WC | 136 | 238.3 | 88 | 0.202 |
| Pt/WC | 10 | 32.3 | 30 | 0.829 |
| Pt/C | 12 | 37.3 | 32 | 0.772 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, Y.; Fang, J.; Wan, Q. Investigation of Nanoscale Tungsten Carbide Enhanced Surface Carbon as a Platinum Support for the Hydrogen Evolution Reaction. Nanomaterials 2023, 13, 1369. https://doi.org/10.3390/nano13081369
Liu Z, Li Y, Fang J, Wan Q. Investigation of Nanoscale Tungsten Carbide Enhanced Surface Carbon as a Platinum Support for the Hydrogen Evolution Reaction. Nanomaterials. 2023; 13(8):1369. https://doi.org/10.3390/nano13081369
Chicago/Turabian StyleLiu, Zhiwei, Yang Li, Juan Fang, and Qi Wan. 2023. "Investigation of Nanoscale Tungsten Carbide Enhanced Surface Carbon as a Platinum Support for the Hydrogen Evolution Reaction" Nanomaterials 13, no. 8: 1369. https://doi.org/10.3390/nano13081369
APA StyleLiu, Z., Li, Y., Fang, J., & Wan, Q. (2023). Investigation of Nanoscale Tungsten Carbide Enhanced Surface Carbon as a Platinum Support for the Hydrogen Evolution Reaction. Nanomaterials, 13(8), 1369. https://doi.org/10.3390/nano13081369










