Abstract
In recent years, the increasing energy requirement and consumption necessitates further improvement in energy storage technologies to obtain high cycling stability, power and energy density, and specific capacitance. Two-dimensional metal oxide nanosheets have gained much interest due to their attractive features, such as composition, tunable structure, and large surface area which make them potential materials for energy storage applications. This review focuses on the establishment of synthesis approaches of metal oxide nanosheets (MO nanosheets) and their advancements over time, as well as their applicability in several electrochemical energy storage systems, such as fuel cells, batteries, and supercapacitors. This review provides a comprehensive comparison of different synthesis approaches of MO nanosheets, as well their suitability in several energy storage applications. Among recent improvements in energy storage systems, micro-supercapacitors, and several hybrid storage systems are rapidly emerging. MO nanosheets can be employed as electrode and catalyst material to improve the performance parameters of energy storage devices. Finally, this review outlines and discusses the prospects, future challenges, and further direction for research and applications of metal oxide nanosheets.
1. Introduction
In recent times, the rapid development in technologies, commercialization, and industrialization have caused a substantial increment in the requirement of global energy consumption [1,2,3]. Fossil fuels are at the top of energy sources despite having a lot of negative issues. Even, according to statistics in 2019, 84% of the total global energy was generated from fossil fuels such as natural gas, coal, and oil. Due to the use of fossil fuels for energy production, several negative impacts are occurring, such as global warming, rise in sea level, soil pollution, water pollution, and air pollution which cause significant harm to flora and fauna. Researchers are working with great concern to minimize the use of such fossil fuels for which they have carried out approaches to utilize renewable sources for the fulfillment of energy requirements. However, the development of these approaches to utilize renewable sources are not sufficient. Additionally, to utilize green sources it’s required to develop efficient energy storage devices so that it can be possible to use the energy from such storage devices whenever power and energy are required. Nowadays, molten salt nanofluids are used as thermal energy storage (TES) materials for concentrated solar power (CSP) plants for large scale power generation. However, the appropriate synthesizing of molten salt for enhanced specific heat capacity is becoming an obstacle in its application [4]. Electrochemical energy storage is the most popular way to store energy for further use and there are several systems including fuel cells, supercapacitors, and batteries which are applied to store energy thermochemically. Researchers are working towards advancements in energy storage textiles to enhance the wearability and electrochemical performances and stability of batteries, and supercapacitors for improving their applicability for several purposes [5]. Wearable energy storage devices prepared using MO nanosheets can be integrated with bioenergy from biofluids, and bioenergy from human motions, and these integrated mechanisms possess attractive potential for building self-driven body-worn electronic devices [6]. Nano structure-enhanced electrochemical energy storage devices can be integrated with self-operated sensors toward self-sustainable gadgets for the management of health and wellbeing [7].
Due to possessing a high energy density and attractive kinetics, thermochemical energy storage is the preferred system. Fuel cells possess better energy conversion potential than supercapacitors and batteries, but the limitations of fuel cells include a shorter lifespan, less durability, and high cost due to applying several expensive catalysts [8,9]. Supercapacitors possess higher cyclic stability, better energy, and power density, and better kinetics for charging and discharging than batteries, but these are limited to lower energy density and lower specific capacitance [10,11]. To enhance the lifecycle, durability, and stability of the electrode material, the phase transition reaction should have potential reversibility and there should also be a proper space where atomic-level reactions can take place [12]. Therefore, to utilize the concept of electrochemical energy storage (EES), two-dimensional (2D) metal oxide electrodes are one of the best solutions that can increase the cyclic lifetime, power, and energy density of storage devices [13,14]. Two-dimensional MO nanosheets (MO-NSs) are rapidly emerging due to their applicability in EES devices and their ability to ameliorate performance parameters. Due to the variable valence states of these 2D materials, MO-NSs possess rich redox reactions. This feature assists to improve the storage capacity of suitable electrode materials used in batteries and supercapacitors. Two-dimensional MO nanosheets possess less volume change, more active sites, and less diffusion length compared to metal oxide nanostructures. These features assist to improve the electrochemical energy storage performance by enhancing cycling stability, specific capacity, surface area, and capacitance retention.
Two-dimensional metal oxide nanosheets possess a thickness of only a few atomic thin layers. Compared to 1D and 3D nanostructure materials, they possess several conveniences and limitations. Having a larger surface area is a major convenience of 2D nanosheets that exhibit much more reactivity than 1D and 3D nanostructures, and this feature makes them more promising for energy storage applications, such as fuel cells, supercapacitors, and batteries. These 2D nanosheets are also more suitable in sensors and flexible electronics compared to 1D and 3D nanostructures due to their flexibility feature [15,16]. However, the high cost of 2D nanosheets is the main barrier to the synthesis and fabrication of these nanosheets and these problems have limited the wide application of 2D metal oxide nanosheets in energy storage systems [17]. Overall, metal oxide (MO) nanosheets possess some uncommon attractive features that make them useful for electrochemical energy storage, but their preparation and manipulation processes may stand as a barrier during application in some cases.
Researchers have developed a lot of methodologies to synthesize 2D metal oxide nanosheets using top-down and bottom-up approaches [18,19,20,21]. Figure 1 illustrates the number of publications on metal oxide nanosheets from 2010 to 2022. The chemical vapor deposition, sol-gel method, and exfoliation with some merits and demerits are the most used approaches by researchers. In the sol-gel approach, a precursor is used to prepare a gel which is further heated to prepare nanosheets. In the case of chemical vapor deposition, the main advantages includes its capability to generate high-quality nanosheet materials with a large surface area. However, its low yield limits its wide application [22,23]. Exfoliation can also synthesize a massive amount of 2D nanosheets by applying mechanical or chemical intercalation to weaken the interlayer and molecular forces [24]. In recent years, hydrothermal and electrophoretic synthesis approaches have gained much research interest as these methodologies can provide a high yield, as well as good dimensional control [21,25,26].
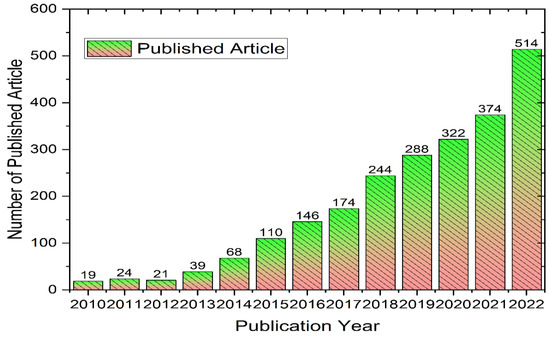
Figure 1.
Number of publications on “metal oxide nanosheets” from 2010–2022.
The previously published literature reveals massive conveniences which make MO nanosheets attractive for energy storage applications including energy density, chemical stability, semi-conductivity, redox properties, flexibility, power density, and larger surface area [14,27,28]. Moreover, researchers and scientists have published a lot of articles documenting the applications of MO nanosheets as electrode material in EESs such as supercapacitors, fuel cells, and batteries [29,30,31,32]. According to the author’s knowledge, there is no literature on the several types of MO nanosheets, their synthesis protocols, and applications. Therefore, this review paper approaches to provide sufficient information about MO nanosheet advancements over time, their applicability in several EES devices, synthesis, and manipulation approaches by reviewing more than 170 papers.
2. Synthesis Approaches of MO Nanosheets
2.1. Top-Down Synthesis
Top-down synthesis of MO nanosheets is a process of creating metal oxide nanosheets by breaking down the bulk metal oxide materials into smaller, nanoscale particles. This method contrasts with the bottom-up synthesis, that creates metal oxide nanosheets by assembling smaller molecules or atoms into larger structures. Mechanical exfoliation is one of the most used top-down techniques for producing MO-NSs. This method involves physically exfoliating the bulk metal oxide materials using techniques, such as sonication, grinding, or ball milling [33]. These techniques use mechanical forces to break down the bulk material into smaller, thinner sheets. The resulting metal oxide nanosheets are typically of high quality and have a high degree of crystallinity. Singh et al. [34] proposed a new top-down process to prepare 2D- MnO2 nanosheets in stirred media mills. They applied analytical grade manganese dioxide for nano milling experiments, and to increase the stability of the MnO2 nanosheet, they employed polyacrylic acid sodium salt. More details about the experiment can be found in Patel et al [35].
Wang et al. applied ball milling technology to fabricate Mn3O4 nanoparticles in large quantities for use as a cathode in Zn ion batteries [36]. To form a paste, ethanol was added with Mn3O4 and by using zirconia milling media the paste was ball-milled for 2 h at a rotational speed of 2000 rpm. Then, the composition was dried to separate C2H5OH via evaporation at 80 °C following several characterization approaches to obtain Mn3O4 powder. The pristine and ball-milled Mn3O4 are shown in Figure 2.
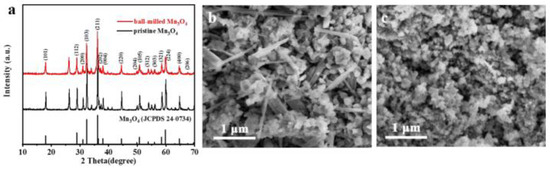
Figure 2.
Illustration of the Mn3O4 characterization process. (a) XRD patterns, (b) SEM image of the precursor Mn3O4, and (c) of the ball-milled Mn3O4, respectively. Reprinted with permission from [36]. Copyright 2018 American Chemical Society.
Template-assisted MO-NS preparation is another top-down approach to preparing nanosheets, nanowires, and nanocomposites. Template-assisted MO-NSs fabrication processes are receiving much attention recently and have distinguished themselves as superior to template-free technologies for the fabrication of nanosheets, as they possess improved cycling stability and control over the mechanism, composition, and shape of the finally produced nanosheet. Templates can be classified into two different types, such as soft templates and hard templates [37]. Soft templates, such as NH4+, amphophilic surfactants, ionic liquids, etc., do not have fixed stiff structures and this results in problems with the morphological product. Moreover, hard templates (colloidal silica spheres, mesoporous materials, microporous zeolites, anodic aluminum oxide membranes, etc.) exhibit good rigidity and stable structures that make these templates promising for utilization [38,39]. Barrer and Denny introduced the templates and templating influences through an experimental study in 196,1 where they prepared zeolites by using tetramethylammonium hydroxide (TMAOH) as the first organic cation [40]. Template-assisted synthesis procedures can be applied for preparing several metal oxide nanosheets for use as energy storage devices. This approach is also applicable to preparing 2D-TiO2 which is widely used in Li-ion batteries. Xue et al. experimented with a surfactant-supported exfoliation approach to synthesize 2D-TiO2 nanosheets [41]. TiO2 nanoparticles were mixed with tetrabutylammonium hydroxide (TBAOH) and reacted at 130 °C for 24 h to formulate a TiO2 nanosheet as shown in Figure 3A. The TBAOH not only acted as an alkaline solution but also as an agent to prevent the accumulation of nanosheets (Figure 3B) and the thickness of TiO2 was found as 0.40 nm.
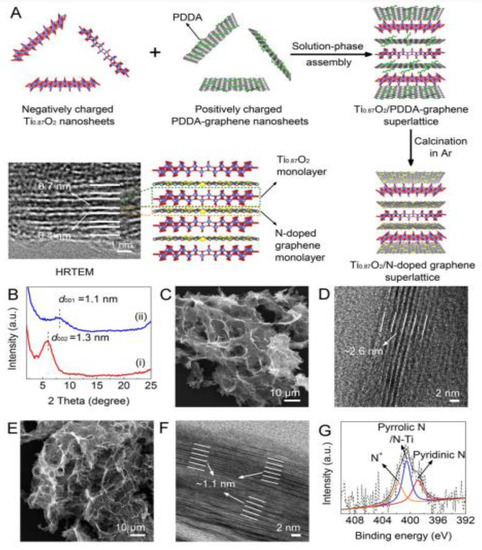
Figure 3.
(A) Illustration of the synthesis approaches of the Ti00.87O2/N-doped graphene superlattice. (B) XRD patterns of (i) Ti0.87O2/PDDA-graphene superlattices and (ii) Ti0.87O2/N-doped graphene superlattices. (C) SEM and (D) HRTEM images of Ti00.87O2/PDDA-graphene superlattices. (E) SEM and (F) HRTEM images of Ti0.87O2/N-doped graphene superlattices. (G) High-resolution spectrum of N 1s in Ti0.87O2/N-doped graphene superlattices. Reprinted with permission from [42]. Copyright 2018 American Chemical Society.
Xiong et al. synthesized Ti0.87O2 nanosheets via an exfoliation approach and they used layered titanate crystals as raw material [42]. Layered titanate crystals were also applied using this approach and for the same purpose in previous works [43,44]. A solid-state calcination approach was applied to obtain the layered titanate crystals of K0.8Ti1.73Li0.27O4. Following stirring K0.8Ti1.73Li0.27O4 in HCL solution for 2 days, the protonic form H1.07Ti1.73O4·H2O was achieved. Once the T0.87O2 nanosheet was obtained, it was mixed dropwise under continuous stirring on a hypothetical model from which a Ti0.87O2/PDDA-graphene superlattice was obtained and this superlattice was annealed for 2 hours at 600 °C to obtain a Ti0.87O4/N-doped graphene superlattice. The overall process is depicted in Figure 3 that includes several steps of the synthesis process.
Solid-state grinding is a mechanical process used to produce metal oxide nanosheets. It involves reducing the size of bulk metal oxide materials through the application of physical force. This force can be generated through various methods such as ball milling or mechanical grinding. Anbao et al. developed a novel approach to solid-state grinding reaction at room temperature where they prepared four MnO2 nanosheets using Mn(OAc)2·4H2O and (NH4)2C2O4·H2O as raw materials and they calcinated MnC2O4 at four different temperature ranging from 300–600 °C to obtain nano MnO2 [45]. Their experimental result revealed that calcination at 400 °C yielded the best result compared to other temperatures.
Another popular top-down method is chemical exfoliation. This method involves the use of a solvent or a chemical to dissolve the bulk metal oxide material and then separate the resulting solution into layers. This method is typically used for metal oxide materials that are not easily exfoliated mechanically. Soft chemical exfoliation is a well-developed process for synthesizing several metal oxide nanosheets such as KCa2Nb3O10, K0.45MnO2, Cs0.7Ti1.825O4, H1.07Ti1.73O4·H2O [46,47,48]. The conventional approach of this process includes four several steps such as synthesis of precursor layered crystals, protonation of the layered crystal through an acid exchange, osmotic swelling, and solution-phase exfoliation which are depicted in Figure 4.
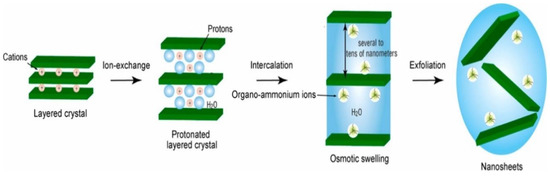
Figure 4.
Schematic illustration of the common soft chemical exfoliation approach for synthesizing metal oxide nanosheets. Reprinted with permission from [49]. Copyright 2018 Elsevier.
Top-down methods have some advantages over bottom-up approaches. For example, by applying top-down approaches, a huge amount of MO-NSs can be produced, and the resulting nanosheets are typically of high quality and have a high degree of crystallinity. Additionally, top-down methods are generally less time-consuming and less expensive than bottom-up methods. However, top-down methods also have some limitations. For example, the size and thickness of the MO-NSs produced by top-down methods are typically not as well-controlled as those produced by bottom-up methods. Additionally, top-down methods can be more difficult to scale up to industrial-level production. The TBAOH-based nanosheets and their applications in energy storage devices are listed in Table 1.

Table 1.
TBAOH-based nanosheets and their applications in energy storage devices.
2.2. Bottom-Up Synthesis
The bottom-up technologies for synthesizing MO nanosheets (MO-NSs) involve the use of small precursor molecules or atoms to build up the metal oxide structure from the bottom up. The major convenience of this method is that it permits precise control over the composition, morphology, and size of the nanosheets. One of the most common bottom-up methods for the preparation of MO-NSs is the sol-gel method. This method involves the use of metal alkoxides as precursors, which are dissolved in a solvent and then hydrolyzed to form metal oxide nanosheets. The sol-gel method permits scrutinizing control over the structure of the MO-NSs by adjusting the content ratio of the precursors. The sol-gel method is relatively facile and versatile, and it can be employed to produce a massive range of MO-NSs, including oxide nanosheets of transition metals, rare earth metals, and lanthanides. However, the sol-gel approach is usually limited to the production of small quantities of nanosheets. Paydar et al. synthesized LiAl0.5Co0.5O2 by using the sol-gel method where they applied aluminum nitrate (Al(NO3)·9H2O), Co(NO2)·6H2O, citric acid, and LiNO3 as raw materials with a high purity [64]. Then, by mixing the reacting agent, heating, and drying, the final product (LACO) was found which can be clearly observed in Figure 5.
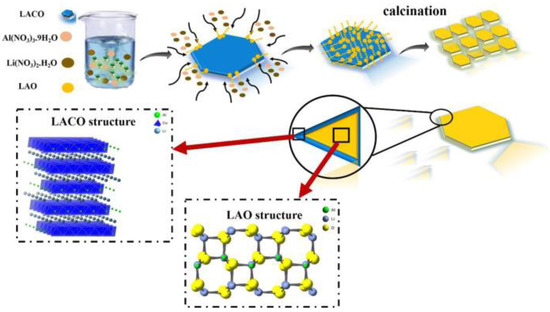
Figure 5.
Schematic showing the surface coating process of LAO on LACO. Reprinted with permission from [64]. Copyright 2021 Elsevier.
Another bottom-up method to synthesize the MO-NSs is the chemical vapor deposition (CVD) method. This approach involves the use of metal oxide precursors that are heated to high temperatures in the presence of a gas, causing the metal atoms to react with oxygen to form a thin film of metal oxide [65]. The CVD method also allows for scrutinizing control over the layer thickness, and the structure of the metal oxide nanosheets by adjusting the precursors, and the reaction conditions. However, CVD is a relatively complex and time-consuming method, and it is generally limited to the production of small quantities of nanosheets. Saenz et al. synthesized MoOx nanosheets using the CVD approach where MoO3 reacted with sulfur powder precursors, and nitrogen gas was applied as a transport gas [66]. Their prepared material showed high conductivity of nearly 6680.3 S cm−1 and an improved bolometric coefficient of 0.152 mS K−1. Li et al. synthesized ultrathin MoO2 nanosheets on a SiO2/Si substrate by using the CVD approach where they employed Mo2O3 and sublimated S as precursors [67]. Their prepared MnO2 nanosheet showed improved conductivity of 3600 S cm−1, and thermal stability retained above 200 °C.
Another bottom-up process for the fabrication of MO-NSs is the electrochemical deposition method. This method involves the use of an electrolyte solution, and a conductive substrate to deposit metal oxide onto the substrate [68]. The electrochemical method can be classified into two categories, namely anodic, and cathodic methods. In the anodic method, metal ions are oxidized to form a metal oxide on the substrate, whereas, in the cathodic method, metal ions are reduced to form metal on the substrate, which subsequently oxidizes to form metal oxide [69]. Kadam et al. reported an electrochemical synthesis approach to prepare a MnO2 nanosheet and in their experiment, they applied MnCl2·4H2O 0.2 m with 99.9% purity as a precursor and stainless steel of 304 grade as the conducting substrate. They annealed the electrodeposited manganese hydroxide at 350 °C for 3 h, which finally resulted in deposited reddish brown colored manganese oxide films [70].
Thermoregulated phase transition is an emerging bottom-up synthesis process in which a material changes its state (such as from solid to liquid or gas) as a result of temperature changes. The transition occurs when the temperature of the material reaches a specific threshold, known as the transition temperature, causing the material to rearrange its molecular structure, and exhibit different physical and chemical properties. This process is governed by the intermolecular forces, and thermodynamic properties of the material, and can be influenced by external factors, such as pressure and composition. Zhang et al. proposed a novel process to synthesize MO nanosheets based on the thermoregulated phase transition of the micelles [71]. For forming a solution with string, they mixed deionized water with metal salts, and to prevent hydrolysis of metal salts, they added several acids with it. Then, they dissolved 1.875 Pluronic P123 in the solution to be heated from 10 to 50 °C for 2 h, following the addition of ammonium hydroxide solution and shaken for 1 min. Finally, after several processes, such as centrifugation, washing, and annealing, the MO nanosheets were obtained. Their product showed high retention capacity and high reversible capacity. The electrochemical method allows for precise control over the composition and morphology of the metal oxide nanosheets by adjusting the precursors and the reaction conditions. However, the electrochemical method is generally limited to the production of small quantities of nanosheets.
Zhou et al. proposed a novel ultra-facile route to synthesize several metal oxide nanosheets, such as ZnO, Co3O4, Fe2O3, WO3, TiO2, and so on with a large surface area [72]. To synthesize the ZnO nanosheet in such a typical approach, firstly, the mixture of some precursors, such as Zn(NO3)2·6H2O, urea, and glucose were pre-calcined for 6 h at 140 °C. Then, the pre-calcined mixture was further calcined for 10 h at 500 °C, which finally resulted in ZnO nanosheets. They also noted that the metal oxide nanosheets synthesized in this way can be an attractive potential in energy storage applications.
Overall, the bottom-up approach to fabricating MO-NSs is a powerful method to prepare high-quality MO-NSs with precise control over their properties. The potential applications for metal oxide nanosheets are vast and varied, and the development of new bottom-up synthesis methods will continue to expand the possibilities for their use in a wide range of fields. Different methodologies for nanosheet synthesis with their advantages and limitations are listed in Table 2 for comparison.

Table 2.
Methodologies for nanosheet synthesis with their advantages and limitation.
3. Applications of MO Nanosheets in Batteries
3.1. MO Sheets Used in Li-S Batteries
Lithium sulfide batteries are increasing in popularity due to their massive convenient applications as electrochemical energy storage devices. During the transformation of sulfur (S) into sulfur ion (S−2), a high capacity (1675 mAh g−1) can be obtained, which is almost ten times that of commercial electrodes that are applied in lithium-ion batteries [73]. Sulfur possesses a much lower redox potential that allows these materials to enhance the operating voltage and energy density combined with alkali metals [74]. A high energy density, such as 2600 Wh kg−1, can easily be achieved by using a lithium-sulfur battery [75]. There are also some conveniences and limitations of sulfurs that can influence a Li-S battery. Among the conveniences, availability and biocompatibility are to be noted, while the inactivity of big particles caused by lower conductivity is considered the major barrier of sulfur-assisted batteries [76]. Another major limitation is the massive volume change during charging and discharging, which reduces the performance of a battery [77]. The cycle lifetime, which originated from the lithium-polysulfide (LIPS) shuttling process, is a major barrier of Li-S batteries. Researchers have taken a lot of approaches and have developed several systems to control this shuttling effect, but they have not found potential solutions for this. To approach an effective solution for this limitation, Patil et al. [78] synthesized a 2D lepidocrocite TiO2 nanosheet to apply in sulfur cathodes, which could minimize the polysulfide dissolution significantly. For this, the surface area was also improved, and the Li-S cell possessed 1023.5 mAh g−1 at 50 mA g−1 for just 80 wt% sulfur content. The capacity was also improved and after 300 cycles, an 82.3% capacity was retained.
3.2. MO Nanosheets for Zn-ion Batteries
Metal oxide nanosheets have emerged as an promising material to utilize in zinc-ion batteries, due to possessing some attractive features, such as a large surface area, high energy density and extradentary electrochemical properties, and low cost [79,80,81]. Zinc-ion batteries are being considered as a potential alternative to traditional lithium-ion batteries, due to the abundance and low cost of zinc, as well as the lack of safety concerns associated with lithium [82]. The large surface area of the nanosheets allows for the more efficient storage of zinc ions, leading to a higher capacity and better performance of the battery. Additionally, the high surface area also increases the rate of charge and discharge, making the battery more efficient. Another advantage of metal oxide nanosheets is their excellent electrochemical properties. Metal oxide nanosheets have been shown to have good conductivity, stability, and reversibility, which are all important factors for the performance of zinc-ion batteries. Additionally, the use of metal oxide nanosheets can improve the overall safety of the battery, as they have been shown to have a high thermal stability and good mechanical properties.
Different types of metal oxide nanosheets have been proposed for use in zinc-ion batteries. These include zinc oxide, titanium dioxide, and iron oxide nanosheets [83,84]. Each of these materials has its unique properties and advantages, and researchers are currently investigating which of these is the most promising for use in zinc-ion batteries. Zn/MnO2 batteries are also considered emerging in recent years due to their low cost, environmental benignity, scalable preparation, and safety. Wang et al. carried out a novel approach to prepare MnO2 from Mn3O4 based on the ball milling technology to utilize the product in Zn-ion batteries. The transformed akhtenskite MnO2 had a high retention capacity above 92% (after 500 cycles at 500 mA g−1) and a high reversible capacity of 221 mAh g−1 (at 100 mA g−1). In the meantime, the cathode showed a high specific energy of nearly 288 Wh kg−1, which is much higher compared to the available Pb-acid batteries [35]. They also found a small self-discharge limitation of their battery, but the source of this issue was unclear to them. However, they reported that concerning the long-time energy storage demand, their battery could be applied as a promising device. Figure 6 depicts the electrochemical performance of the Zn/ε-MnO2 cell, where Figure 6a shows cyclic-voltammetry (CV) curves between 0.6 to 1.9 V, Figure 6b shows a pair of redox peaks (at 1.7 and 1.32 V), and Figure 6c depicts the rate capacity of the transformed Zn/ε-MnO2 cathode, while the current density increased steadily from 100 to 2000 mA g−1.
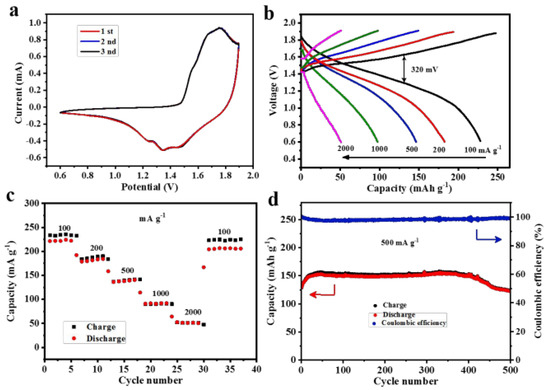
Figure 6.
Electrochemical performance of the Zn/ε-MnO2 coin cells. (a) Cyclic voltametric (CV) curves of the Zn/ε-MnO2 cell at a scan rate of 0.1 mV s−1. (b) The discharge/charge voltage profiles at various current densities between 0.6 and 1.9 V. (c) Rate capability. (d) Long-term cyclic performance and the corresponding Coulombic efficiency at 500 mA g−1. Reprinted with permission from [35]. Copyright 2018 American Chemical Society.
Vanadium-based oxides are gaining much attention for application in Zn-ion batteries, as they are environmentally friendly, easily available, and have a high specific capacity [85]. Liang et al. prepared a Ni-doped MxOy nanosheet for application in Zn ion batteries, and to form oxygen vacancies, they applied H2 annealing processes [86]. Their prepared device showed excellent performance with a capacity of 0.68 mAh cm−2, a current density of 2 mA cm−2, and capacity retention of over 6000 cycles. It also showed a high power and energy density of 3.34 mW cm−2 and 1.13 mW cm−2, respectively.
Co3O4 nanosheets are emerging materials for Zn-ion batteries. Wang et al. prepared Co3O4@Ni to use as a positive electrode in Zn//Co3O4 and they applied KOH as an electrolyte [87]. Their device provided a high energy density of 241 Wh kg−1 and revealed a high cycling stability. Zinc-manganese oxide nanosheets are very popular as electrodes for Zn-ion batteries. Zhang et al. reported ZnMn2O4 as an anode material to investigate its performance in Zn-ion batteries, where they applied Zn(CF3SO3)2 as an electrolyte [88]. Their system revealed excellent performance with a reversible capacity of 150 mAh g−1 and a retention capacity of 94% after 500 cycles.
3.3. MO Nanosheets for Zn-air Batteries
Zinc-air batteries can provide a potential solution where it is required for large-scale energy storage because Zn-air batteries have some attractive features, such as stability, durability, low expense, safety, high power, and energy density. Li et al. reported a nickel-doped CoO nanosheet as electrode material in Zn-air batteries for the first time and their battery showed great performance with a high power density of 377 mW cm−2, and it worked for more than 400 h with a high stability [89]. They also compared their battery with a Pt/C catalyst device and revealed that Ni-doped CoO nanosheets outperformed, based on the charge/discharge voltage. Tian et al. prepared oxygen defective amorphous crystalline CoO (ODAC-CoO) nanosheets from Co(OH)2 by using a vacuum-calcination approach and they found that their sample possessed an improved oxygen reduction reaction and oxygen evolution reaction, and it also showed a high stability [90].
To improve the retention capability and performance of Zn-air batteries, a bifunctional O2 catalyst plays a pivotal role. MnO2 is considered the most efficient material to apply as a catalyst in Zn-air batteries to improve the ORR. Zhong et al. employed a facile solution-based approach to modify MnO2-NS with nickel, cobalt, or iron [91]. They reported that by taking such an approach, they were able to enhance the ORR and OER for the Co-MnO2 sample, and it exhibited an improved power density of 167 mW cm−2.
3.4. MO Nanosheets for Li-Ion Batteries
Lithium-ion batteries (LIBs) are competitive options for EESs in the clean energy market and a lot of research has already been carried out due to their applicability as energy storage devices [92,93,94]. To ameliorate the Li-ion batteries, Zhang et al. prepared a V2O3@NC nanosheet to utilize as a free-standing cathode in a lithium-ion battery, and through TEM images, it was investigated that the V2O5 nanoparticles were layered homogeneously in the carbon region [95]. The charge and discharge specific capacities were found as 508 and 984 mAh g−1, respectively, and the reversible capacity after 400 cycles was found as 892 mAh g−1 at 0.1 A g−1. Li et al. prepared a GN/SnO2 nanosheet via a facile cold-quenching approach and reported that, by having the distinctive wrinkled surface property, the maintenance of the monodispersed state was facilitated. Their hybrid nanosheet material exhibited a high reversible capacity of 1147 mAh g−1 at 100 mA g−1 [96]. The synthesis process of SnO2/MG nanosheets is illustrated in Figure 7.
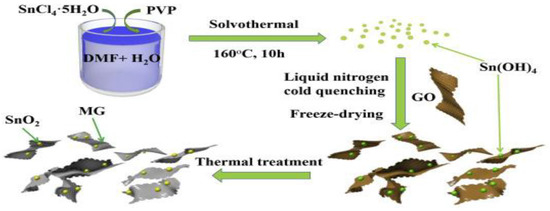
Figure 7.
The synthesis process of SnO2/MG nanosheets. Reprinted with permission from [96]. Copyright 2019 Elsevier.
Li et al. prepared a new 2D LiNi1/3Co1/3Mn1/3O2 nanosheet by using sol-gel technology [97]. They found that their prepared material showed high performance as a cathode and it showed a high discharge capacity of 137.7 mAh g−1 at 20C. Mei et al. [98] synthesized 2D Bi2O3 nanosheets for the first time by applying a solution-based self-assembly process and their nanosheet material supplied a high capacitance above 200 mAh g−1 and discharge capacity of 647.6 mAh g−1, when utilized as an anode in a Li-ion battery. Li et al. used an electrodeposition technique to synthesize a two-dimensional CoO nanosheet and found that their nanosheet possessed extraordinary performance with a high retention capacity of 1000 mAh g−1 at 1 A g−1 over 100 cycles [99].
3.5. NO Nanosheets for Na-ion Batteries
Xiong et al. reported a Ti-deficient 2D Ti0.87O2 nanosheet superlattice for application in Na-ion batteries [41]. They also reported that Ti0.87O2 with a N-doped graphene monolayer possessed attractive performance, that could be applied in Na-ion battery with a high capacity of nearly 490 mAh g−1 at 0.1 A g−1. The simulation results, which are shown in Figure 8, reveal conspicuously a lower diffusion barrier of energy for diffusing Na+ ions in Ti0.87O2/graphene, demonstrating its superiority for Na ion batteries.
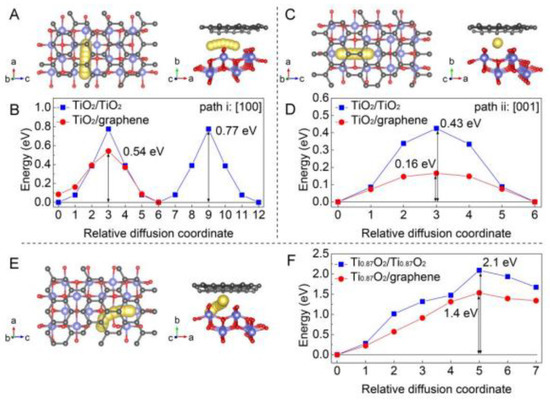
Figure 8.
(A) Diagram of the sodium diffusion path towards the [100] directions between the TiO2/graphene bilayers. (B) The energy of sodium diffusion along the [100] direction in TiO2 bilayers and TiO2/graphene bilayers. (C) Illustration of the Na diffusion path along the [001] directions between the TiO2/graphene bilayers. (D) The energy of Na diffusion along the [001] direction in TiO2 bilayers and TiO2/graphene bilayers. (E) Illustration of a possible Na diffusion path in Ti0.87O2/graphene bilayers. (F) The energy of Na diffusion in Ti0.87O2 bilayers and Ti0.87O2/graphene bilayers. Reprinted with permission from [41]. Copyright 2018 American Chemical Society.
Rubio et al. synthesized a hybrid MO nanosheet utilizing iron oxide and iron sulfide, to apply as an anode in Na-ion batteries [100]. There are also some previous studies where researchers worked with these two materials as an anode, but the materials had some disappointing properties when they were applied for battery applications [101,102]. At the time of charging and discharging, these materials showed an excessive fluctuation in volume, and another limitation of these materials was electron fragility, which limited their applications in electrochemical storage devices [103,104,105].
Chen et al. prepared a metal oxide anode for application in a sodium ion battery and MO K0.8Ti1.73Li0.27O4 (KTLO) was fabricated via a simple flux process that is based on ball-milling [57]. The KTLO, as a Na-ion battery, showed a high specific capacity of 119.6 mAh g−1 at 20 mA g−1 with a high retention capacity for more than 250 cycles. The carbon-coated KTLO enhances the structure stability and electronic conductivity which makes this material an attractive anode material for sodium-ion batteries.
Li et al. prepared a GN/SnO2 nanosheet via a facile cold-quenching approach and reported that by having the distinctive wrinkled surface property, the maintenance of the monodispersed state was facilitated [96]. Their hybrid nanosheet material exhibited a high reversible capacity of 314 mAh g−1 at 100 mA g−1.
Sun et al. prepared a two-dimensional FeOx nanosheet and applied it as an anode in Na-ion batteries [106]. They reported that their synthesized MO nanosheet showed an attractive output with a mean specific capacity of 408 mAh g−1 at a current density of 0.21 A g−1. Further application of their electrode revealed that after completing 100 cycles, the specific capacity was reduced to 263.4 mAh g−1. They also showed that amorphous FeOx nanosheets possessed a faster electron movement compared to crystalized Fe3O4 nanosheets, that showed a better capacity for FeOx than for Fe3O4.
3.6. Battery-Supercapacitor Hybrid (BSH) Device
For achieving a higher performance and cost-effective energy storage systems, researchers have developed several hybrid electrochemical energy storage technologies, such as battery-supercapacitor hybrid systems (BSH) [107]. A BSH system combines the advantages of batteries and supercapacitors by building battery-based and supercapacitor-based electrodes. Figure 9 demonstrates that Ni-MH and Ni-Cd batteries, which have been utilized in secondary batteries for over a century due to their greater specific capacities, are still commonly used today, and have an energy density of 30–70 Wh kg−1 [108]. By using inorganic carbon hybrid electrodes, recently developed Ni-Fe alkaline batteries can also charge extremely quickly and can deliver specific energy densities greater than 100 Wh kg−1 [109].
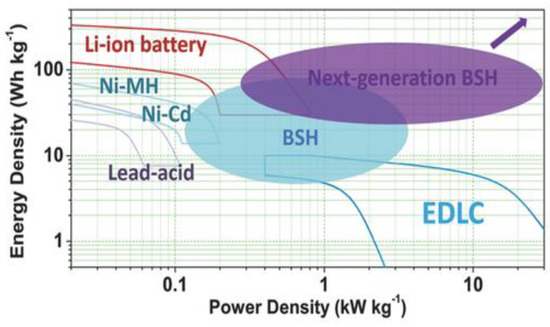
Figure 9.
Ragone plots of various rechargeable batteries and EDLC, and the comparison with BSHs. Reprinted with permission from [110]. Copyright 2017 Willey online library.
A typical BSH energy storage device is depicted in Figure 10a, where anions and cations migrate to the two electrodes during charging and discharging, where the battery electrode undergoes the redox reaction, and the Sc electrode accumulates ions. There are lots of conveniences to modeling different types of BSH devices, such as diverse types of electrodes, device configurations, and electrolytes. The potential candidate for electrodes and electrolytes in BSH devices are captured in Figure 10b.
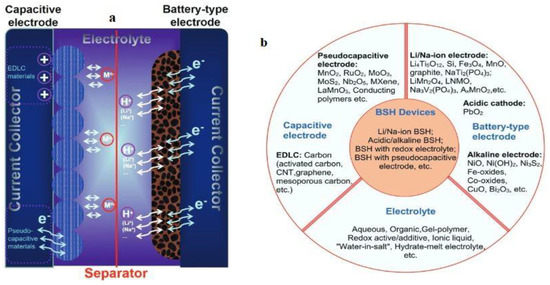
Figure 10.
(a) A typical hybrid energy storage device with a working process. (b) Different types of hybrid devices and their electrolyte and electrode materials. Reprinted with permission from [110]. Copyright 2017 Willey online library.
Huang et al. [111] developed such BSH devices that included activated carbon as the anode and the NixCoyMozO as a cathode. They measured the optimized potential window for the anode and cathode. Figure 11a shows the current-potential curves measured for both electrodes, and Figure 11b illustrates the current-potential curves of the cathode and anode using several potential windows, and it revealed that a window with 1.8 V showed the best scan rates. Figure 11d,e illustrate the GC/D plot which also suggests a window of 1.8 V for application in hybrid devices. They found power and energy densities of 3.5 W kg−1 and 22.02 Wh kg−1, respectively, and at a current density of 10 mA cm−2, they found a CF value of 126 mF cm−2. Figure 11f illustrates the Ragone plot of the BSH.
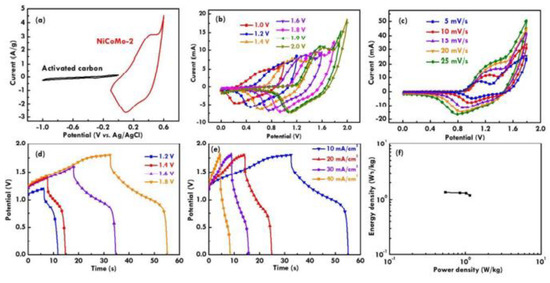
Figure 11.
The current-potential curves for (a) the anodes and cathodes, (b) the current-potential curves measured using several potential windows, (c) different scan rates, (d) the GC/D plots measured using several potential windows, (e) different current densities, and (f) the Ragone plot of the BSH. Reprinted with permission from [111]. Copyright 2018 American Chemical Society.
Researchers have developed several Li-ion-based, Na-ion-based, alkaline, and acidic BSH systems which have been increasing in popularity in recent years. A brief comparison is carried out in Table 3 for a proper understanding of different BSH devices.

Table 3.
Battery-supercapacitor hybrid (BSH) systems with their performance parameters.
4. Applications of Metal Oxide Nanosheets for Fuel Cells
4.1. Solid Oxide Fuel Cell
Solid oxide fuel cells (SOFCs) are the type of cells that can generate energy by utilizing hydrogen fuel, and in recent years with the increasing amount of hydrogen fuel generation, the application of SOFCs is increasing steadily [125,126]. The major limitation of these fuel cells for application is their poor reliability at high temperatures due to the thermal corrosion in their components [127,128]. In this regard, lowering the cell operating temperature cannot be a potential solution as at a lower temperature the charge conductivity of the cathode decreases. To find a potential solution, researchers have developed cobalt (Co) containing perovskite, which possesses high charge conductivity [129,130,131,132]. Kim et al. prepared a SOFC device using a La0.6Sr0.4CoO3 − δ nanosheet which facilitated the conduction of charge in the cathode material [133]. They prepared the MO nanosheet in several steps (Figure 12) and found that the cell power density enhanced to 1.2 W cm−2 at 600 °C.
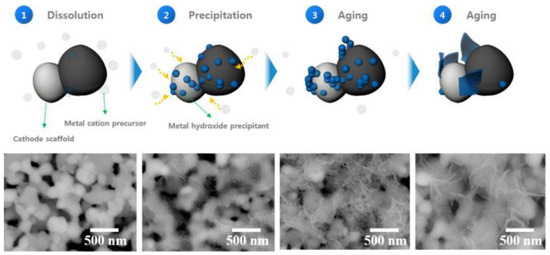
Figure 12.
Schematic of the step-wise synthesis process of the catalyst and corresponding SEM images. Reprinted with permission from Ref. [134]. Copyright 2021 Elsevier.
4.2. Direct Methanol Fuel Cell
Jia et al. synthesized an ultra-thin 2D NiO nanosheet to use as an electrocatalyst in direct methanol fuel cell devices [134]. They prepared this NiO nanosheet via a hydrothermal approach with four several steps as shown in Figure 13. They tested the NiO precursor at four different annealing temperatures ranging from 350 to 650 °C and gave those names as 350-NiO, 450-NiO, 550-NiO, and 650-NiO respectively. They found that with increasing annealing temperature, the surface area of this nanosheet decreases, which causes a deterioration in the performance of the catalyst. In this regard, NiO at 350 °C annealing temperature showed the best catalytic performance compared to NiO at other temperatures.
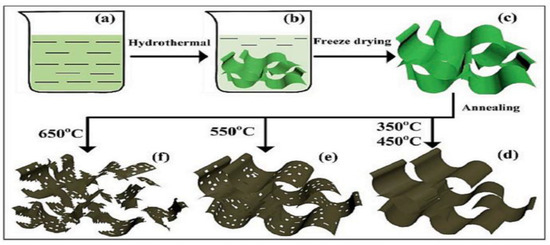
Figure 13.
Synthesis flow diagram of NiO. Reprinted with permission from [134]. Copyright 2021 Elsevier.
TiO2 nanosheets with Pt-derived catalysts are increasing in popularity, for utilization in direct methanol fuel cells. Saida et al. experimented with TiO2 applicability in a methanol fuel cell where they synthesized TiO2 nanosheet from layered H2Ti4O9 and then mixed the product with PtRu/C catalyst, which was prepared by the impregnation method in disparate compositions. The modified composite catalyst enhanced the electrooxidation of CO2 and methanol, which caused a rise in the electrolyte and PtRu catalyst. The TiO2 nanosheet-modified PtRu/C showed higher ECSA compared to unmodified PtRu/C [54].
4.3. PEM Fuel Cells
The proton exchange membrane (PEM) fuel cell is the most widely applied fuel cell and in recent years, researchers have focused on it due to its higher capacity compared to other electrochemical energy storage devices. The working mechanism of the PEM fuel cells includes the transformation of the proton from the anode side to the cathode side and the electricity is produced during the half-reaction. There are some attractive conveniences, such as no vibration, no mechanical parts, and no CO2 emissions during operation with H2 as fuel, and this system is also suitable for various temperature ranges. In recent years, researchers have felt the requirement for proper electrocatalyst materials that can replace Pt and Pb-based catalysts to obtain a clean energy storage system [135,136,137]. In this regard, they found the MO nanosheet to be a suitable material that can be applied as an electrocatalyst due to its large active sites, and attractive mechanical properties that can enhance the capability of a speedy charge transfer between two electrodes [138]. Tiido et al. reported Pt/TiO2 -graphene as an attractive and potential cathode catalyst for application in PEM fuel cells [139]. In their experiment, they prepared TiO2 functionalized graphene nanosheets to employ as support for Pt nanoparticles. Their microscopic and electrochemical investigation revealed that the electrode exhibited a high electrocatalytic performance.
Naik et al. developed an oxygen-deficient TiO2 nanosheet to apply as a supporting material catalyst in PEM fuel cells, as shown in Figure 14. In addition, they found that Pt-TiO2 − xNS, as illustrated in Figure 15, showed extraordinary performance to improve the stability and activity of oxygen reduction reaction [140]. They reported that TiO2 − xNS to be a promising support that could be used in future PEM fuel cells, as its performance was experimentally proved. They carried out a square-wave potential cycle test at 80 °C with their catalyst support and found that it possessed durability over 10,000 cycles and excellent stability for more than 100 h. Pt-NiO2 − xNSs assisted fuel cells showed a higher power density of 958 mW cm−2, as compared with Pt-C catalysts.
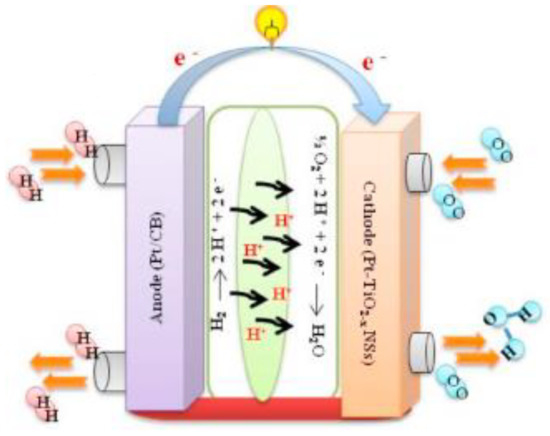
Figure 14.
Schematic representation of a PEM fuel cell [140]. Copyright permission 2020, Elsevier.
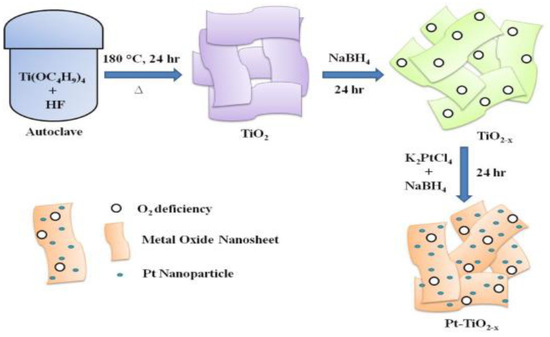
Figure 15.
Schematic illustration of the preparation of Pt–TiO2 − x NS. Reprinted with permission from [140]. Copyright 2020 Elsevier.
In addition to the above-mentioned metal oxide nanosheets, other metal oxides, such as cobalt oxide (Co3O4), manganese oxide (MnO2), and vanadium oxide (V2O5), have also been studied as catalysts for PEM fuel cells. These metal oxide nanosheets have been found to have a high surface area and good electronic conductivity, which can improve the performance and durability of PEM fuel cells. Lejing et al. reported that Co3O4 applied in a PEM fuel cell can enhance the oxygen evolution reaction (OER) [141]. They investigated Co3-xCexO4 and Co3O4 nanosheets to compare their potential in electrodes and found that the first material had less potential to reach a current density of 100 mA cm−2 and the addition of Ce resulted in the exposure of more active sites.
4.4. Microbial Fuel Cells
Over the last decade, the application of microbial fuel cells (MFCs) has risen with proper understanding related to their structure, working procedure, and advancement strategies [142]. In regards to MFC, some researchers have been more conscious of the power that this system consumes, others have been worried about its applications, other researchers identify it as a toxicity conveyer, and it has also been considered as a system through which wastes can be utilized while ignoring environmental pollution [143,144]. Moreover, H2O2 is considered a pivotal element for various applications that can be generated through MFC when ORR is facilitated by a two-electron mechanism instead of the four-electron [145,146]. Chakraborty et al. synthesized a multilayer CeO2 nanosheet to investigate its performance as a cathode material in a dual chamber MFC (Figure 16) [147]. They aimed to minimize the reduction of O2 to H2O2 in the cathode and their result revealed that MFC with CeO2 as catalyst showed the highest yield of H2O2 of nearly 221.4 mg L−1 in one day compared to the bare C electrode that yielded nearly 133.4 mg L−1 H2O2 in similar operating conditions.
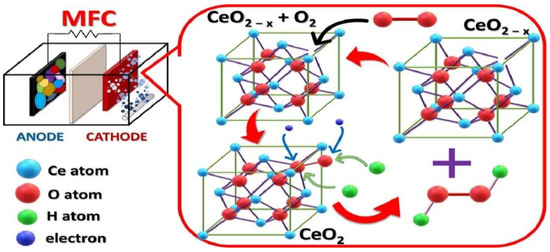
Figure 16.
Schematic diagram of a microbial fuel cell. Reprinted with permission from [147]. Copyright 2021 Elsevier.
One of the metal oxide nanosheet that have been studied for use in MFCs is titanium dioxide (TiO2) nanosheets. TiO2 nanosheets have been found to have a high surface area, which can increase the amount of active material that can be loaded onto the anode. Additionally, TiO2 nanosheets have been found to have good electronic conductivity, which can improve the rate performance of MFCs. Research has shown that the use of TiO2 nanosheets can significantly improve the power density and durability of MFCs by increasing the microbial adhesion and reducing electron transfer resistance. Yin et al. suggested the application of composite materials in electrodes that can enhance the performance of electrodes via complementary elements [148]. For this purpose, they studied modified conductive polyaniline (PANI) TiO2 nanosheets (TiO2-NSs), where they deposited disparate amounts of PANI on TiO2-NSs during different cycles, such as 5, 10, 15, 20, and 25. Their result revealed that 63.6% of power density can be increased by using TiO2-20PANI/CP (813 mW m−2) compared to a TiO2-NSs/CP anode.
Another type of metal oxide nanosheet that has been investigated for use in MFCs is nickel oxide (NiO) nanosheets. NiO nanosheets have been found to have a high surface area and good electronic conductivity, which can improve the performance of MFCs. Research has shown that the use of NiO nanosheets can significantly improve the power density and durability of MFCs by increasing the microbial adhesion and reducing electron transfer resistance. Furthermore, NiO nanosheets have been found to have a high electrocatalytic activity for the oxygen reduction reaction (ORR), which is an important reaction in MFCs. This increased ORR activity can lead to improved MFC performance. Huang et al. [149] took the first attempt to apply NiO/CNT as a cathode catalyst and reported that 77% NiO/CNT showed maximized power density of 670 mW m−2. They also stated that increasing the growth of the content of NiO in CNT, steadily enhanced the performance of MFCs.
Another MO-NS that has been studied for use in MFCs is manganese oxide (MnO2) nanosheets. MnO2 nanosheets have been found to have a large surface area, good electronic conductivity, and a high electrocatalytic activity for the ORR, which makes them a promising material for use in MFCs. Research has shown that the use of MnO2 nanosheets can significantly improve the power density and durability of MFCs by increasing microbial adhesion, reducing electron transfer resistance, and increasing the ORR activity. Zhang et al. synthesized a mesoporous layered α-MnO2 nanosheet of 2 nm to maximize the performance by utilizing the conveniences of porous materials and 2D nanosheets [150]. During its application as an ORR catalyst in MFC, it showed excellent electrocatalytic performance compared to conventional Pt/C as α-MnO2 exposed an ultra-large surface area of 339 m2 g−1. CoO was reported as a potential catalyst for microbial fuel cells by Huang et al [151]. They prepared a catalyst of CoO nanosheets which was supported by N-doped activated carbon and their prepared nanocomposite exhibited low total resistance of 9.26 Ω, 4-electron ORR pathway, and a large surface area which made them preferable for application in MFCs as a cathodic catalyst.
5. Application of MO Nanosheets in Supercapacitors
Primary metal oxide nanosheets are ultra-thin two-dimensional sheets made up of metal oxide materials. They have thicknesses in the order of a few nanometers and are considered to be a type of nanomaterial due to their small size and unique properties. They have potential applications in several fields, such as energy, electronics, and catalysis. The properties of metal oxide nanosheets can be tailored by controlling their thickness, surface area, and defects, leading to potential opportunities for new and improved technologies. Primary metal oxide nanosheets are suitable for supercapacitor applications as they possess a large surface area and electrochemical stability. The large surface area of the nanosheets supplies ample space for the storage and transfer of electrical charge, making them highly effective in energy storage. Additionally, the metal oxide materials used in the nanosheets have a high stability, preventing degradation and ensuring long-term performance in energy storage applications. These properties make primary metal oxide nanosheets an attractive option for use as electrodes in supercapacitors, where they can deliver high energy density and a long cycle life.
Rubidium oxide (RuO2) nanosheet is considered a potential and attractive element to apply in supercapacitors due to its high electrical conductivity and capacitance. RuO2 derived from layered ruthenic acid hydrate (H0.2RuO2.1.nH2O through exfoliation approach and experimental testing revealed that this RuO2 nanosheet had a high specific capacitance of 658 F g−1 that is ten times higher compared to rutile type RuO2 [152,153]. 2D MnO2 nanosheets are also attractive materials that can be applied as an electrode in supercapacitors. Research has been carried out on approaches to increase the performance of this type of electrode and has tested the intercalation attitudes of different metal ions, such as Na+, Mg2+, Li+, and K+ in layered MnO2 [154]. Two-dimensional MnO2 nanosheets are also attractive materials that can be applied as an electrode in supercapacitors. Research has been carried approaches to rise the performance of this type of electrodes and tested intercalation attitudes of different metal ions such as Na+, Mg2+, Li+, and K+ in layered MnO2 [154]. 2D MnO2 nanosheet as an electrode of supercapacitor showed an attractive specific capacitance of 171 F g−1 at a current density of 3 A g−1 [155]. Jin et al. prepared a MnO2-Ti3C2 nanosheet to investigate its performance and compared it with the capacitance and performance of MnO2-rGO nanohybrids [156]. The porosity and ion diffusivity of Ti3C2 was found to be higher than that of the rGO-assisted nanosheet and the specific capacitance and surface area of MnO2-Ti3C2 were also greater compared to the other material.
To enhance the performance and capacitance of supercapacitors, Lin et al. prepared a MoO3/NiCo2O4 nanosheet electrode to apply as cathode material, and α-FeOOH/rGO as anode material and they applied a hydrothermal approach (Figure 17) to synthesize these materials [157]. The cathode showed an attractive specific capacitance of 1042 F g−1 at a current density of 1 A g−1 and the retention capacity was 110.8% after 2000 cycles.
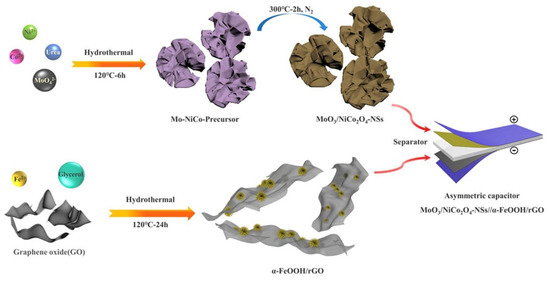
Figure 17.
Schematic of MoO3/NiCo2O4-NSs, α-FeOOH/rGO synthesis (hydrothermal) process, and asymmetric supercapacitor. Reprinted with permission from [157]. Copyright 2019 Elsevier.
Peng et al. proposed that NiMoO4 nanosheets exhibited a higher capacitance compared to NiMoO4 nanorods based on their application as electrodes for supercapacitors [123]. They prepared an asymmetric supercapacitor with activated carbon as anode and NiMoO4 as cathode and found that their device showed an excellent electrochemical property with a high-power density of 850 W kg−1 and energy density of 60.9 Wh kg−1. Moreover, the device retained 85.7% after 10,000 cycles which showed its stability and durability.
Researchers have developed another type of supercapacitor that is a flexible fiber-shaped supercapacitor that has become popular due to its portability and wearable electronics. However, these devices cannot provide much operating voltage, and the specific capacitance is not high, which limits their wide application and use. Neng et al. prepared an asymmetric supercapacitor (ASC) where they applied a MnO2 nanosheet as a cathode and carbon fiber as an anode [158]. Their ASC showed an extraordinary performance with a high energy density of 27.2 Wh kg−1, a specific capacitance of 87.1 F g−1, and 95.2% capacitance retention after 3000 cycles.
Micro supercapacitors (MSCs) are also emerging EES devices with some attractive conveniences, such as high specific capacitance, less expensive, thin atomic layers, etc., and MO nanosheets are an appropriate material as an electrode for MSCs. Wang et al. prepared one such MSC device based on δ-MnO2 nanosheet ink [159]. On glass and polyimide film substrates treated with O2 plasma, a highly concentrated ink comprising 2D δ-MnO2 nanosheets was inkjet printed to create δ-MnO2 shapes without the negative “coffee ring” effect. Their device showed extraordinary performance with a power density of 0.018 W cm−3 and energy density of 1.8 10−4 Wh cm−3, capacitance retention of 88% over 3600 cycles. Applications of several MO nanosheets in different EES systems are given in Table 4.

Table 4.
Applications of several MO nanosheets in different EES systems.
6. Challenges and Prospects
Metal oxide nanosheets have emerged as potential elements for enhancing electrochemical energy storage systems because of their extraordinary physical and chemical features. Researchers have proved their potential as a catalyst in fuel cells and electrodes in batteries and supercapacitors. However, the major concern is the limitation of large-scale production of MO nanosheets that restricted their production and applications. Following a review of the previously published works, we came to realize that the other limitation is the limited power and energy density of MO-NS-based energy storage technologies. Therefore, further study and research is required to utilize MO nanosheets in energy storage technology. Moreover, more research efforts are required to obtain materials with more reliability, stability, and composition tuneability via eco-environmentally friendly and facile approaches. Several additives and nanocomposites should be derived to enhance the performance of various MO-NSs.
There are also some prospects in the utilization of metal oxide nanosheets for EESs that should be noted.
- Researchers are performing a lot of studies and experiments for synthesizing novel MO nanosheet materials with improved physical and electrochemical properties, such as enhanced cyclic stability, high retention capacity, high power and energy density, and speedy charging-discharging rates. Additionally, researchers have also carried out improvements in hybrid MO nanosheets which may provide better performance in the near future compared to the nanosheets prepared up to now;
- MO nanosheets can be utilized in several electrochemical energy storage devices, conveying the aim that these devices can capture and store renewable energy in a portable device for further use. This concept inspires us to utilize renewable energy sources and particularly, this can negate the application of carbon in supercapacitors and can make the energy system more reliable and environmentally friendly in the near future;
- MO nanosheets can be integrated with different device structures as they possess some potential physical and chemical properties and this attracted great interest among researchers to apply them for preparing wearable and portable energy storage devices.
Overall, further improvement in metal oxide nanosheets will have a potential role in the significant development of EESs in the upcoming years.
7. Conclusions
This article reviews and discusses metal oxide nanosheets and their suitable applications in several electrochemical energy storage devices. With the rising energy demand, the requirement for advancements in energy generation and energy storage materials is also increasing and these necessities inspire researchers to carry out effective and potential research. This work reviews several MO synthesis processes that are based on top-down and bottom-up processes, as well as their advancements over time. Researchers are primarily focused on preparing MO nanosheets in a pathway that can improve power and energy density in supercapacitors, retention ability in batteries, and surface area and electrocatalytic performance in fuel cells. This paper reviews extensively the applicability, and variation of the performance of several MO nanosheets in different operating conditions for various applications, such as the battery, supercapacitors, and fuel cells. For further study and improvement of MO nanosheets, this article gives detailed information and guidelines from which researchers can obtain a lot of information on MO nanosheets and their applicability and performance parameters in different electrochemical energy storage devices.
Author Contributions
Conceptualization, A.D., S.D.P. and M.S.H.; Resources, A.D., S.D.P., M.S.H. and M.M.S.; Formal Analysis, A.D., S.D.P., M.A.M.A. and M.R.; Writing—Original Draft, A.D., S.D.P., M.S.H., M.A.M.A., M.M.H.A. and M.R.; Writing—review & editing, A.D., M.M.S., M.M.H.A. and B.K.D.; Supervision, M.M.S. and B.K.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not apply to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Das, A.; Peu, S.D. A Comprehensive Review on Recent Advancements in Thermochemical Processes for Clean Hydrogen Production to Decarbonize the Energy Sector. Sustainability 2022, 14, 11206. [Google Scholar] [CrossRef]
- Das, A.; Peu, S.D.; Akanda, A.M.; Islam, A.R.M.T. Peer-to-Peer Energy Trading Pricing Mechanisms: Towards a Comprehensive Analysis of Energy and Network Service Pricing (NSP) Mechanisms to Get Sustainable Enviro-Economical Energy Sector. Energies 2023, 16, 2198. [Google Scholar] [CrossRef]
- Das, A.; Peu, S.D.; Akanda, M.A.M.; Salah, M.M.; Hossain, M.S.; Das, B.K. Numerical Simulation and Optimization of Inorganic Lead-Free Cs3Bi2I9-Based Perovskite Photovoltaic Cell: Impact of Various Design Parameters. Energies 2023, 16, 2328. [Google Scholar] [CrossRef]
- Akanda, A.M.; Shin, D. A synthesis parameter of molten salt nanofluids for solar thermal energy storage applications. J. Energy Storage 2023, 60, 106608. [Google Scholar] [CrossRef]
- Liu, Z.; Mo, F.; Li, H.; Zhu, M.; Wang, Z.; Liang, G.; Zhi, C. Advances in Flexible and Wearable Energy-Storage Textiles. Small Methods 2018, 2. [Google Scholar] [CrossRef]
- Lv, J.; Chen, J.; Lee, P.S. Sustainable wearable energy storage devices self-charged by human-body bioenergy. Susmat 2021, 1, 285–302. [Google Scholar] [CrossRef]
- Parrilla, M.; De Wael, K. Wearable Self-Powered Electrochemical Devices for Continuous Health Management. Adv. Funct. Mater. 2021, 31. [Google Scholar] [CrossRef]
- Hassan, N. Catalytic performance of nanostructured materials recently used for developing fuel cells’ electrodes. Int. J. Hydrogen Energy 2021, 46, 39315–39368. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Alarab, S.; Al-Othman, A.; Javed, R.M.N. The Operating Parameters, Structural Composition, and Fuel Sustainability Aspects of PEM Fuel Cells: A Mini Review. Fuels 2022, 3, 449–474. [Google Scholar] [CrossRef]
- A molecular cross-linking approach for hybrid metal oxides|Nature Materials. Available online: https://www.nature.com/articles/s41563-018-0021-9 (accessed on 3 February 2023).
- Tawalbeh, M.; Murtaza, S.Z.; Al-Othman, A.; Alami, A.H.; Singh, K.; Olabi, A.G. Ammonia: A versatile candidate for the use in energy storage systems. Renew. Energy 2022, 194, 955–977. [Google Scholar] [CrossRef]
- Mahmood, N.; Zhang, C.; Hou, Y. Nickel Sulfide/Nitrogen-Doped Graphene Composites: Phase-Controlled Synthesis and High Performance Anode Materials for Lithium Ion Batteries. Small 2013, 9, 1321–1328. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, L.; Xu, X.; Sun, Z.; Liao, T.; Dou, S.X. Atomically thin non-layered nanomaterials for energy storage and conversion. Chem. Soc. Rev. 2017, 46, 7338–7373. [Google Scholar] [CrossRef] [PubMed]
- Scopus—Document Details—Holey Two-Dimensional Transition Metal Oxide Nanosheets for Efficient Energy Storage|Signed in. Available online: https://www.scopus.com/record/display.uri?eid=2-s2.0-85029922186&origin=inward (accessed on 3 February 2023).
- Two-Dimensional Flexible Nanoelectronics|Nature Communications. Available online: https://www.nature.com/articles/ncomms6678 (accessed on 3 February 2023).
- Rabani, I.; Yoo, J.; Kim, H.-S.; Van Lam, D.; Hussain, S.; Karuppasamy, K.; Seo, Y.-S. Highly dispersive Co3O4 nanoparticles incorporated into a cellulose nanofiber for a high-performance flexible supercapacitor. Nanoscale 2020, 13, 355–370. [Google Scholar] [CrossRef]
- Shanmugam, V.; Mensah, R.A.; Babu, K.; Gawusu, S.; Chanda, A.; Tu, Y.; Neisiany, R.E.; Försth, M.; Sas, G.; Das, O. A Review of the Synthesis, Properties, and Applications of 2D Materials. Part. Part. Syst. Charact. 2022, 39. [Google Scholar] [CrossRef]
- Ultrathin Two-Dimensional Nanomaterials|ACS Nano. Available online: https://pubs.acs.org/doi/10.1021/acsnano.5b05040 (accessed on 5 February 2023).
- Balan, A.P.; Radhakrishnan, S.; Woellner, C.F.; Sinha, S.K.; Deng, L.; Reyes, C.D.L.; Rao, B.M.; Paulose, M.; Neupane, R.; Apte, A.; et al. Exfoliation of a non-van der Waals material from iron ore hematite. Nat. Nanotechnol. 2018, 13, 602–609. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Rice, S.B.; Jacobson, A.J.; Lewandowski, J.T. Electron microscopy study of delamination in dispersions of the perovskite-related layered phases K[Ca2Nan-3NbnO3n-1]: Evidence for single-layer formation. Chem. Mater. 1990, 2, 279–286. [Google Scholar] [CrossRef]
- Scopus—Document Details—Wet-Chemical Synthesis and Applications of Non-Layer Structured Two-Dimensional Nanomaterials|Signed In. Available online: https://www.scopus.com/record/display.uri?eid=2-s2.0-84940055189&origin=inward (accessed on 5 February 2023).
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-J.; Sung, Y.-M. Synthesis of Anatase Nanosheets with Exposed (001) Facets via Chemical Vapor Deposition. Cryst. Growth Des. 2012, 12, 5792–5795. [Google Scholar] [CrossRef]
- Mounet, N.; Gibertini, M.; Schwaller, P.; Campi, D.; Merkys, A.; Marrazzo, A.; Sohier, T.; Castelli, I.E.; Cepellotti, A.; Pizzi, G.; et al. Two-dimensional materials from high-throughput computational exfoliation of experimentally known compounds. Nat. Nanotechnol. 2018, 13, 246–252. [Google Scholar] [CrossRef]
- Xiao, X.; Song, H.; Lin, S.; Zhou, Y.; Zhan, X.; Hu, Z.; Zhang, Q.; Sun, J.; Yang, B.; Li, T.; et al. Scalable salt-templated synthesis of two-dimensional transition metal oxides. Nat. Commun. 2016, 7, 11296. [Google Scholar] [CrossRef]
- Hou, C.; Zhang, M.; Zhang, L.; Tang, Y.; Wang, H.; Chi, Q. Reagent-Free Electrophoretic Synthesis of Few-Atom-Thick Metal Oxide Nanosheets. Chem. Mater. 2017, 29, 1439–1446. [Google Scholar] [CrossRef]
- JElshof, J.E.T.; Yuan, H.; Rodriguez, P.G. Two-Dimensional Metal Oxide and Metal Hydroxide Nanosheets: Synthesis, Controlled Assembly and Applications in Energy Conversion and Storage. Adv. Energy Mater. 2016, 6, 1600355. [Google Scholar] [CrossRef]
- Generalized Self-Assembly of Scalable Two-Dimensional Transition Metal Oxide Nanosheets|Nature Communications. Available online: https://www.nature.com/articles/ncomms4813 (accessed on 3 February 2023).
- Li, Y.; Dai, H. Recent advances in zinc–air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef]
- Gu, P.; Zheng, M.; Zhao, Q.; Xiao, X.; Xue, H.; Pang, H. Rechargeable zinc–air batteries: A promising way to green energy. J. Mater. Chem. A 2017, 5, 7651–7666. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Geng, D.; Liu, H.; Meng, X. Cobalt oxide nanosheets anchored onto nitrogen-doped carbon nanotubes as dual purpose electrodes for lithium-ion batteries and oxygen evolution reaction. Int. J. Energy Res. 2018, 42, 853–862. [Google Scholar] [CrossRef]
- Transition Metal Oxide Nanocatalysts for Oxygen Reduction Reaction—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S2589299118300430?via%3Dihub (accessed on 3 February 2023).
- Chu, T.; Liu, D.; Tian, Y.; Li, Y.; Liu, W.; Li, G.; Song, Z.; Jian, Z.; Cai, X. Cationic Hexagonal Boron Nitride, Graphene, and MoS2 Nanosheets Heteroassembled with Their Anionic Counterparts for Photocatalysis and Sodium-Ion Battery Applications. ACS Appl. Nano Mater. 2020, 3, 5327–5334. [Google Scholar] [CrossRef]
- Singh, V.K.; Patel, C.M. Preparation of two-dimensional manganese dioxide nanosheets by stirred media milling and its application as supercapacitor electrode materials. Inorg. Chem. Commun. 2023, 149. [Google Scholar] [CrossRef]
- Enhancement of Stirred Media Mill Performance by a New Mixed Media Grinding Strategy—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S1226086X13004607 (accessed on 1 February 2023).
- Wang, L.; Cao, X.; Xu, L.; Chen, J.; Zheng, J. Transformed Akhtenskite MnO2 from Mn3O4 as Cathode for a Rechargeable Aqueous Zinc Ion Battery. ACS Sustain. Chem. Eng. 2018, 6, 16055–16063. [Google Scholar] [CrossRef]
- Takahashi, K.; Limmer, S.J.; Wang, Y.; Cao, G. Synthesis and Electrochemical Properties of Single-Crystal V2O5 Nanorod Arrays by Template-Based Electrodeposition. J. Phys. Chem. B 2004, 108, 9795–9800. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, Q. Agar Hydrogel Template Synthesis of Mn3O4 Nanoparticles through an Ion Diffusion Method Controlled by Ion Exchange Membrane and Electrochemical Performance. Nanomaterials 2019, 9, 503. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Wang, K.-X.; Chen, J.-S. Template-directed metal oxides for electrochemical energy storage. Energy Storage Mater. 2016, 3, 1–17. [Google Scholar] [CrossRef]
- Barrer, R.M.; Denny, P.J. 202. Hydrothermal chemistry of the silicates. Part X. A partial study of the field CaO–Al2O3–SiO2–H2O. J. Chem. Soc. 1961, 983–1000. [Google Scholar] [CrossRef]
- Leng, M.; Chen, Y.; Xue, J. Synthesis of TiO2 nanosheets via an exfoliation route assisted by a surfactant. Nanoscale 2014, 6, 8531–8534. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, X.; Zhang, F.; Yi, D.; Zhang, J.; Sun, B.; Tian, H.; Shanmukaraj, D.; Rojo, T.; Armand, M.; et al. Two-Dimensional Unilamellar Cation-Deficient Metal Oxide Nanosheet Superlattices for High-Rate Sodium Ion Energy Storage. ACS Nano 2018, 12, 12337–12346. [Google Scholar] [CrossRef]
- Sasaki, T.; Watanabe, M.; Hashizume, H.; Yamada, H.; Nakazawa, H. Macromolecule-like Aspects for a Colloidal Suspension of an Exfoliated Titanate. Pairwise Association of Nanosheets and Dynamic Reassembling Process Initiated from It. J. Am. Chem. Soc. 1996, 118, 8329–8335. Available online: https://pubs.acs.org/doi/abs/10.1021/ja960073b (accessed on 2 February 2023). [CrossRef]
- Tanaka, T.; Ebina, Y.; Takada, K.; Kurashima, K.; Sasaki, T. Oversized Titania Nanosheet Crystallites Derived from Flux-Grown Layered Titanate Single Crystals. Chem. Mater. 2003, 15, 3564–3568. Available online: https://pubs.acs.org/doi/10.1021/cm034307j (accessed on 2 February 2023). [CrossRef]
- Yuan, A.; Wang, X.; Wang, Y.; Hu, J. Comparison of nano-MnO2 derived from different manganese sources and influence of active material weight ratio on performance of nano-MnO2/activated carbon supercapacitor. Energy Convers. Manag. 2010, 51, 2588–2594. [Google Scholar] [CrossRef]
- Geng, F.; Ma, R.; Ebina, Y.; Yamauchi, Y.; Miyamoto, N.; Sasaki, T. Gigantic Swelling of Inorganic Layered Materials: A Bridge to Molecularly Thin Two-Dimensional Nanosheets. J. Am. Chem. Soc. 2014, 136, 5491–5500. [Google Scholar] [CrossRef] [PubMed]
- Maluangnont, T.; Matsuba, K.; Geng, F.; Ma, R.; Yamauchi, Y.; Sasaki, T. Osmotic Swelling of Layered Compounds as a Route to Producing High-Quality Two-Dimensional Materials. A Comparative Study of Tetramethylammonium versus Tetrabutylammonium Cation in a Lepidocrocite-type Titanate. Chem. Mater. 2013, 25, 3137–3146. [Google Scholar] [CrossRef]
- Song, Y.; Iyi, N.; Hoshide, T.; Ozawa, T.C.; Ebina, Y.; Ma, R.; Miyamoto, N.; Sasaki, T. Accordion-like swelling of layered perovskite crystals via massive permeation of aqueous solutions into 2D oxide galleries. Chem. Commun. 2015, 51, 17068–17071. [Google Scholar] [CrossRef]
- Xiong, P.; Ma, R.; Wang, G.; Sasaki, T. Progress and perspective on two-dimensional unilamellar metal oxide nanosheets and tailored nanostructures from them for electrochemical energy storage. Energy Storage Mater. 2018, 19, 281–298. [Google Scholar] [CrossRef]
- Wang, C.; Osada, M.; Ebina, Y.; Li, B.-W.; Akatsuka, K.; Fukuda, K.; Sugimoto, W.; Ma, R.; Sasaki, T. All-Nanosheet Ultrathin Capacitors Assembled Layer-by-Layer via Solution-Based Processes. ACS Nano 2014, 8, 2658–2666. [Google Scholar] [CrossRef] [PubMed]
- Saida, T.; Sugimoto, W.; Takasu, Y. Enhanced activity and stability of Pt/C fuel cell anodes by the modification with ruthenium-oxide nanosheets. Electrochim. Acta 2010, 55, 857–864. [Google Scholar] [CrossRef]
- Suzuki, S.; Miyayama, M. Lithium Intercalation Properties of Octatitanate Synthesized through Exfoliation/Reassembly. J. Phys. Chem. B 2006, 110, 4731–4734. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Miyayama, M. Microstructural controls of titanate nanosheet composites using carbon fibers and high-rate electrode properties for lithium ion secondary batteries. J. Power Sources 2011, 196, 2269–2273. [Google Scholar] [CrossRef]
- Saida, T.; Ogiwara, N.; Takasu, Y.; Sugimoto, W. Titanium Oxide Nanosheet Modified PtRu/C Electrocatalyst for Direct Methanol Fuel Cell Anodes. J. Phys. Chem. C 2010, 114, 13390–13396. [Google Scholar] [CrossRef]
- Harada, M.; Sasaki, T.; Ebina, Y.; Watanabe, M. Preparation and characterizations of Fe- or Ni-substituted titania nanosheets as photocatalysts. J. Photochem. Photobiol. A Chem. 2002, 148, 273–276. [Google Scholar] [CrossRef]
- Lee, S.; Park, H.; Paik, U.; Han, T.H. Exfoliation of titanium oxide powder into nanosheets using hydrothermal reaction and its reassembly into flexible papers for thin-film capacitors. J. Solid State Chem. 2015, 224, 76–81. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Zhang, W.-X.; Liu, Y.; Zhu, H.-P.; Duan, J.; Xiang, X.-H.; Xue, L.-H.; Huang, Y.-H. Carbon coated K0.8Ti1.73Li0.27O4: A novel anode material for sodium-ion batteries with a long cycle life. Chem. Commun. 2014, 51, 1608–1611. [Google Scholar] [CrossRef]
- Tanaka, T.; Fukuda, K.; Ebina, Y.; Takada, K.; Sasaki, T. Highly Organized Self-Assembled Monolayer and Multilayer Films of Titania Nanosheets. Adv. Mater. 2004, 16, 872–875. [Google Scholar] [CrossRef]
- Xu, X.; Takada, K.; Fukuda, K.; Ohnishi, T.; Akatsuka, K.; Osada, M.; Hang, B.T.; Kumagai, K.; Sekiguchi, T.; Sasaki, T. Tantalum oxide nanomesh as self-standing one nanometre thick electrolyte. Energy Environ. Sci. 2011, 4, 3509–3512. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kim, I.Y.; Patil, S.B.; Oh, S.M.; Lee, N.-S.; Hwang, S.-J. Composition-Tailored 2 D Mn1−xRuxO2Nanosheets and Their Reassembled Nanocomposites: Improvement of Electrode Performance upon Ru Substitution. Chem. A Eur. J. 2014, 20, 5132–5140. [Google Scholar] [CrossRef] [PubMed]
- Omomo, Y.; Sasaki, T.; Wang, L.; Watanabe, M. Redoxable Nanosheet Crystallites of MnO2 Derived via Delamination of a Layered Manganese Oxide. J. Am. Chem. Soc. 2003, 125, 3568–3575. [Google Scholar] [CrossRef]
- Song, M.-S.; Lee, K.M.; Lee, Y.R.; Kim, I.Y.; Kim, T.W.; Gunjakar, J.L.; Hwang, S.-J. Porously Assembled 2D Nanosheets of Alkali Metal Manganese Oxides with Highly Reversible Pseudocapacitance Behaviors. J. Phys. Chem. C 2010, 114, 22134–22140. Available online: https://pubs.acs.org/doi/abs/10.1021/jp108969s (accessed on 3 February 2023). [CrossRef]
- Ida, S.; Thapa, A.K.; Hidaka, Y.; Okamoto, Y.; Matsuka, M.; Hagiwara, H.; Ishihara, T. Manganese oxide with a card-house-like structure reassembled from nanosheets for rechargeable Li-air battery. J. Power Sources 2012, 203, 159–164. [Google Scholar] [CrossRef]
- Paydar, S.; Peng, J.; Huang, L.; Shi, Q.; Akbar, N.; Islam, Q.A.; Muhammad, A.; Xing, Y.; Kim, J.-S.; Wu, Y. Performance analysis of LiAl0.5Co0.5O2 nanosheets for intermediate-temperature fuel cells. Int. J. Hydrogen Energy 2021, 46, 26478–26488. [Google Scholar] [CrossRef]
- Wu, H.; Tong, P.; Li, N.; Zhou, X.; Wei, N.; Zhao, J. Chemical Vapor Deposition of Oriented Vertical MoO2 Nanofins in a Confined Space for Conductive Electrodes. ACS Appl. Nano Mater. 2022, 5, 16633–16641. [Google Scholar] [CrossRef]
- Saenz, G.A.; Kaul, A.B. Nanosheets of MoOx crystallites synthesized via chemical vapor deposition and its potential in bolometric applications. Surf. Coat. Technol. 2020, 382, 125031. [Google Scholar] [CrossRef]
- Li, J.; Yin, J.; Li, X.; Zhou, J.; Guo, W. Chemical vapor deposition of ultra-thin molybdenum dioxide nanosheets. Mater. Lett. 2016, 174, 188–191. [Google Scholar] [CrossRef]
- Grote, F.; Yu, Z.-Y.; Wang, J.-L.; Yu, S.-H.; Lei, Y. Self-Stacked Reduced Graphene Oxide Nanosheets Coated with Cobalt-Nickel Hydroxide by One-Step Electrochemical Deposition toward Flexible Electrochromic Supercapacitors. Small 2015, 11, 4666–4672. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/smll.201501037 (accessed on 5 February 2023). [CrossRef]
- Younis, A.; Chu, D.; Lin, X.; Lee, J.; Li, S. Bipolar resistive switching in p-type Co3O4 nanosheets prepared by electrochemical deposition. Nanoscale Res. Lett. 2013, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.L.; Padwal, P.M.; Mane, S.M.; Kulkarni, S.B. Electrochemical synthesis and investigation of nano MnO2 electrode material for supercapacitor application. Adv. Mater. Proc. 2016, 1, 205–209. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, X.; Xue, D.; Xu, B.; Long, D.; Xue, F.; Duan, X.; Ye, W.; Wang, M.; Li, Q. A generalized strategy for the synthesis of two-dimensional metal oxide nanosheets based on a thermoregulated phase transition. Nanoscale 2019, 11, 3200–3207. [Google Scholar] [CrossRef]
- Synthesis of Metal Oxide Nanosheets through a Novel Approach for Energy Applications—Journal of Materials Chemistry A (RSC Publishing). Available online: https://pubs.rsc.org/en/content/articlelanding/2016/ta/c5ta08688d (accessed on 1 February 2023).
- Scopus—Document Details—Cell Concepts of Metal–Sulfur Batteries (Metal = Li, Na, K, Mg): Strategies for Using Sulfur in Energy Storage Applications|Signed In. Available online: https://www.scopus.com/record/display.uri?eid=2-s2.0-85030180613&origin=inward (accessed on 4 February 2023).
- Gu, X.Y.; Dong, C.Y.; Li, J.L.; Liu, Z.Y.; Xu, J.Y. MPM simulations of high-speed and ultra high-speed machining of titanium alloy (Ti–6Al–4V) based on fracture energy approach. Eng. Anal. Bound. Elem. 2015, 59, 129–143. [Google Scholar] [CrossRef]
- Rehman, S.; Gu, X.; Khan, K.; Mahmood, N.; Yang, W.; Huang, X.; Guo, S.; Hou, Y. 3D Vertically Aligned and Interconnected Porous Carbon Nanosheets as Sulfur Immobilizers for High Performance Lithium-Sulfur Batteries. Adv. Energy Mater. 2016, 6, 1502518. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.Q.; Zhang, Q.; Mai, L. Nanostructured Metal Oxides and Sulfides for Lithium-Sulfur Batteries. Adv. Mater. 2017, 29, 1601759. Available online: https://onlinelibrary.wiley.com/doi/10.1002/adma.201601759 (accessed on 4 February 2023). [CrossRef]
- Mahmood, N.; Zhang, C.; Yin, H.; Hou, Y. Graphene-based nanocomposites for energy storage and conversion in lithium batteries, supercapacitors and fuel cells. J. Mater. Chem. A 2014, 2, 15–32. [Google Scholar] [CrossRef]
- Patil, S.B.; Kim, H.J.; Lim, H.-K.; Oh, S.M.; Kim, J.; Shin, J.; Kim, H.; Choi, J.W.; Hwang, S.-J. Exfoliated 2D Lepidocrocite Titanium Oxide Nanosheets for High Sulfur Content Cathodes with Highly Stable Li–S Battery Performance. ACS Energy Lett. 2018, 3, 412–419. [Google Scholar] [CrossRef]
- Guan, C.; Zhao, W.; Hu, Y.; Ke, Q.; Li, X.; Zhang, H.; Wang, J. High-Performance Flexible Solid-State Ni/Fe Battery Consisting of Metal Oxides Coated Carbon Cloth/Carbon Nanofiber Electrodes. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Lu, X.; Zeng, Y.; Yu, M.; Zhai, T.; Liang, C.; Xie, S.; Balogun, M.-S.; Tong, Y. Oxygen-Deficient Hematite Nanorods as High-Performance and Novel Negative Electrodes for Flexible Asymmetric Supercapacitors. Adv. Mater. 2014, 26, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Han, Y.; Zhao, Y.; Zeng, Y.; Yu, M.; Liu, Y.; Tang, H.; Tong, Y.; Lu, X. Advanced Ti-Doped Fe2O3@PEDOT Core/Shell Anode for High-Energy Asymmetric Supercapacitors. Adv. Energy Mater. 2015, 5, 1402176. [Google Scholar] [CrossRef]
- Liu, J.; Guan, C.; Zhou, C.; Fan, Z.; Ke, Q.; Zhang, G.; Liu, C.; Wang, J. A Flexible Quasi-Solid-State Nickel-Zinc Battery with High Energy and Power Densities Based on 3D Electrode Design. Adv. Mater. 2016, 28, 8732–8739. Available online: https://onlinelibrary.wiley.com/doi/10.1002/adma.201603038 (accessed on 5 February 2023). [CrossRef]
- Zeng, Y.; Zhang, X.; Qin, R.; Liu, X.; Fang, P.; Zheng, D.; Tong, Y.; Lu, X. Dendrite-Free Zinc Deposition Induced by Multifunctional CNT Frameworks for Stable Flexible Zn-Ion Batteries. Adv. Mater. 2019, 31, e1903675. Available online: https://onlinelibrary.wiley.com/doi/10.1002/adma.201903675 (accessed on 5 February 2023). [CrossRef] [PubMed]
- Achieving Ultrahigh Energy Density and Long Durability in a Flexible Rechargeable Quasi-Solid-State Zn–MnO2 Battery—Zeng—2017—Advanced Materials—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/adma.201700274 (accessed on 5 February 2023).
- Jia, B.-R.; Qin, M.-L.; Zhang, Z.-L.; Li, S.-M.; Zhang, D.-Y.; Wu, H.-Y.; Zhang, L.; Lu, X.; Qu, X.-H. Hollow Porous VOx/C Nanoscrolls as High-Performance Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 25954–25961. Available online: https://pubs.acs.org/doi/10.1021/acsami.6b07439 (accessed on 5 February 2023). [CrossRef]
- Liang, X.; Zou, X.; Ren, X.; Xiang, B.; Li, W.; Chen, F.; Hao, J.; Hu, Q.; Feng, L. Ultrathin nickel manganese nanosheets with rich oxygen-vacancy as a durability electrode for aqueous Ni//Zn batteries. J. Colloid Interface Sci. 2020, 578, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, F.; Wang, L.; Li, M.; Wang, Y.; Chen, B.; Zhu, Y.; Fu, L.; Zha, L.; Zhang, L.; et al. An Aqueous Rechargeable Zn//Co3O4Battery with High Energy Density and Good Cycling Behavior. Adv. Mater. 2016, 28, 4904–4911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Cheng, F.; Liu, Y.; Zhao, Q.; Lei, K.; Chen, C.; Liu, X.; Chen, J. Cation-Deficient Spinel ZnMn2O4 Cathode in Zn(CF3SO3)2 Electrolyte for Rechargeable Aqueous Zn-Ion Battery. J. Am. Chem. Soc. 2016, 138, 12894–12901. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cui, L.; Da, P.; Qiu, K.; Qin, W.; Hu, W.; Du, X.; Davey, K.; Ling, T.; Qiao, S. Multiscale Structural Engineering of Ni-Doped CoO Nanosheets for Zinc–Air Batteries with High Power Density. Adv. Mater. 2018, 30, e1804653. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liu, X.; Xu, L.; Yuan, D.; Dou, Y.; Qiu, J.; Li, H.; Ma, J.; Wang, Y.; Su, D.; et al. Engineering Crystallinity and Oxygen Vacancies of Co(II) Oxide Nanosheets for High Performance and Robust Rechargeable Zn–Air Batteries. Adv. Funct. Mater. 2021, 31. [Google Scholar] [CrossRef]
- Zhong, Y.; Dai, J.; Xu, X.; Su, C.; Shao, Z. Facilitating Oxygen Redox on Manganese Oxide Nanosheets by Tuning Active Species and Oxygen Defects for Zinc-Air Batteries. ChemElectroChem 2020, 7, 4949–4955. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. Available online: https://onlinelibrary.wiley.com/doi/10.1002/adma.201800561 (accessed on 5 February 2023). [CrossRef]
- Building Better Batteries|Nature. Available online: https://www.nature.com/articles/451652a (accessed on 5 February 2023).
- Jin, J.; Wu, L.; Huang, S.; Yan, M.; Wang, H.; Chen, L.; Hasan, T.; Li, Y.; Su, B.-L. Hierarchy Design in Metal Oxides as Anodes for Advanced Lithium-Ion Batteries. Small Methods 2018, 2. Available online: https://onlinelibrary.wiley.com/doi/10.1002/smtd.201800171 (accessed on 5 February 2023). [CrossRef]
- Zhang, X.; Xun, L.; Gao, S.; Xu, Y.; Cheng, X.; Zhao, H.; Huo, L. Facile synthesis of V2O3@N-doped carbon nanosheet arrays on nickel foam as free-standing electrode for high performance lithium ion batteries. Catal. Today 2020, 374, 117–123. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Ma, C.; Shi, J.; Zhao, Y. Highly monodispersed graphene/SnO2 hybrid nanosheets as bifunctional anode materials of Li-ion and Na-ion batteries. J. Alloys Compd. 2020, 821, 153506. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhao, J.; Chen, J.; Jia, T.; Cao, C. Porous lithium nickel cobalt manganese oxide hierarchical nanosheets as high rate capability cathodes for lithium ion batteries. J. Power Sources 2016, 307, 731–737. [Google Scholar] [CrossRef]
- Mei, J.; Liao, T.; Ayoko, G.A.; Sun, Z. Two-Dimensional Bismuth Oxide Heterostructured Nanosheets for Lithium- and Sodium-Ion Storages. ACS Appl. Mater. Interfaces 2019, 11, 28205–28212. [Google Scholar] [CrossRef]
- Li, D.; Ding, X.-L.; Wang, S.; Cai, D.; Wang, H. Ultrathin and highly-ordered CoO nanosheet arrays for lithium-ion batteries with high cycle stability and rate capability. J. Mater. Chem. A 2014, 2, 5625–5630. [Google Scholar] [CrossRef]
- Rubio, S.; Maça, R.R.; Ortiz, G.F.; Vicente, C.P.; Lavela, P.; Etacheri, V.; Tirado, J.L. Iron Oxide–Iron Sulfide Hybrid Nanosheets as High-Performance Conversion-Type Anodes for Sodium-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 10765–10775. [Google Scholar] [CrossRef]
- Array of Nanosheets Render Ultrafast and High-Capacity Na-Ion Storage by Tunable Pseudocapacitance|Nature Communications. Available online: https://www.nature.com/articles/ncomms12122 (accessed on 3 February 2023).
- Etacheri, V.; Haik, O.; Goffer, Y.; Roberts, G.A.; Stefan, I.C.; Fasching, R.; Aurbach, D. Effect of Fluoroethylene Carbonate (FEC) on the Performance and Surface Chemistry of Si-Nanowire Li-Ion Battery Anodes. Langmuir 2011, 28, 965–976. Available online: https://pubs.acs.org/doi/10.1021/la203712s (accessed on 3 February 2023). [CrossRef]
- Atomic Layer Deposition an Innovative Technology to Improve Corrosion and Surface Functionalities of Alloys—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/B9780124095472134407?via%3Dihub (accessed on 3 February 2023).
- Bishop, C.A. 19—Atomic Layer Deposition. In Vacuum Deposition onto Webs, Films and Foils (Second Edition); Bishop, C.A., Ed.; William Andrew Publishing: Oxford, UK, 2011; pp. 331–336. [Google Scholar] [CrossRef]
- Etacheri, V.; Yourey, J.E.; Bartlett, B.M. Chemically Bonded TiO2–Bronze Nanosheet/Reduced Graphene Oxide Hybrid for High-Power Lithium Ion Batteries. ACS Nano 2014, 8, 1491–1499. Available online: https://pubs.acs.org/doi/10.1021/nn405534r (accessed on 3 February 2023). [CrossRef]
- Sun, R.; Gao, J.; Wu, G.; Liu, P.; Guo, W.; Zhou, H.; Ge, J.; Hu, Y.; Xue, Z.; Li, H.; et al. Amorphous Metal Oxide Nanosheets Featuring Reversible Structure Transformations as Sodium-Ion Battery Anodes. Cell Rep. Phys. Sci. 2020, 1, 100118. [Google Scholar] [CrossRef]
- Dubal, D.P.; Ayyad, O.; Ruiz, V.; Gómez-Romero, P. Hybrid energy storage: The merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 2015, 44, 1777–1790. [Google Scholar] [CrossRef]
- Challenge of Portable Power|Nature. Available online: https://www.nature.com/articles/373557a0 (accessed on 2 February 2023).
- Wang, H.; Liang, Y.; Gong, M.; Li, Y.; Chang, W.; Mefford, T.; Zhou, J.; Wang, J.; Regier, T.; Wei, F.; et al. An ultrafast nickel–iron battery from strongly coupled inorganic nanoparticle/nanocarbon hybrid materials. Nat. Commun. 2012, 3, 917. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-Supercapacitor Hybrid Devices: Recent Progress and Future Prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Lin, L.-Y. Synthesis of Ternary Metal Oxides for Battery-Supercapacitor Hybrid Devices: Influences of Metal Species on Redox Reaction and Electrical Conductivity. ACS Appl. Energy Mater. 2018, 1, 2979–2990. [Google Scholar] [CrossRef]
- Qu, Q.T.; Shi, Y.; Tian, S.; Chen, Y.H.; Wu, Y.P.; Holze, R. A new cheap asymmetric aqueous supercapacitor: Activated carbon//NaMnO2. J. Power Sources 2009, 194, 1222–1225. [Google Scholar] [CrossRef]
- Chen, Z.; Augustyn, V.; Jia, X.; Xiao, Q.; Dunn, B.; Lu, Y. High-Performance Sodium-Ion Pseudocapacitors Based on Hierarchically Porous Nanowire Composites. ACS Nano 2012, 6, 4319–4327. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, N.; Ni, J.; Gao, L. Improved electrochemical performance of sol-gel method prepared Na4Mn9O18 in aqueous hybrid Na-ion supercapacitor. J. Solid State Electrochem. 2013, 17, 1939–1944. [Google Scholar] [CrossRef]
- Lu, K.; Song, B.; Gao, X.; Dai, H.; Zhang, J.; Ma, H. High-energy cobalt hexacyanoferrate and carbon micro-spheres aqueous sodium-ion capacitors. J. Power Sources 2016, 303, 347–353. [Google Scholar] [CrossRef]
- Brandt, A.; Balducci, A.; Rodehorst, U.; Menne, S.; Winter, M.; Bhaskar, A. Investigations about the Use and the Degradation Mechanism of LiNi0.5Mn1.5O4in a High Power LIC. J. Electrochem. Soc. 2014, 161, A1139–A1143. [Google Scholar] [CrossRef]
- Nanostructured Spinel LiNi0.5Mn1.5O4 as New Insertion Anode for Advanced Li-Ion Capacitors with High Power Capability—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S221128551400278X (accessed on 2 February 2023).
- Zhang, F.; Zhang, T.; Yang, X.; Zhang, L.; Leng, K.; Huang, Y.; Chen, Y. A high-performance supercapacitor-battery hybrid energy storage device based on graphene-enhanced electrode materials with ultrahigh energy density. Energy Environ. Sci. 2013, 6, 1623–1632. [Google Scholar] [CrossRef]
- Fan, Q.; Yang, M.; Meng, Q.; Cao, B.; Yu, Y. Activated-Nitrogen-Doped Graphene-Based Aerogel Composites as Cathode Materials for High Energy Density Lithium-Ion Supercapacitor. J. Electrochem. Soc. 2016, 163, A1736–A1742. [Google Scholar] [CrossRef]
- Ni, J.; Wang, H.; Qu, Y.; Gao, L. PbO2 electrodeposited on graphite for hybrid supercapacitor applications. Phys. Scr. 2013, 87, 045802. [Google Scholar] [CrossRef]
- Electrodeposited PbO2 Thin Film as Positive Electrode in PbO2/AC Hybrid Capacitor—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0013468609002217 (accessed on 2 February 2023).
- Perret, P.; Khani, Z.; Brousse, T.; Bélanger, D.; Guay, D. Carbon/PbO2 asymmetric electrochemical capacitor based on methanesulfonic acid electrolyte. Electrochim. Acta 2011, 56, 8122–8128. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Wu, H.B.; Madhavi, S.; (David) Lou, X.W. Controlled Growth of NiMoO4 Nanosheet and Nanorod Arrays on Various Conductive Substrates as Advanced Electrodes for Asymmetric Supercapacitors. Adv. Energy Mater. 2015, 5, 1401172. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Zhou, C.; Wang, C.; Ba, X.; Li, Y.; Huang, X.; Liu, J. Carbon-Stabilized High-Capacity Ferroferric Oxide Nanorod Array for Flexible Solid-State Alkaline Battery-Supercapacitor Hybrid Device with High Environmental Suitability. Adv. Funct. Mater. 2015, 25, 5384–5394. [Google Scholar] [CrossRef]
- Choudhury, A.; Chandra, H.; Arora, A. Application of solid oxide fuel cell technology for power generation—A review. Renew. Sustain. Energy Rev. 2013, 20, 430–442. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, M.; Wang, N.; Ma, C.; Wang, J.; Han, M. A short review of cathode poisoning and corrosion in solid oxide fuel cell. Int. J. Hydrog. Energy 2017, 42, 24948–24959. [Google Scholar] [CrossRef]
- Kofstad, P.; Bredesen, R. High temperature corrosion in SOFC environments. Solid State Ionics 1992, 52, 69–75. [Google Scholar] [CrossRef]
- Electrochemical Performance and Degradation of (La0.6Sr0.4)0.99CoO3 − δ as Porous SOFC-Cathode—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0167273807003517 (accessed on 5 February 2023).
- A High-Performance Cathode for the Next Generation of Solid-Oxide Fuel Cells|Nature. Available online: https://www.nature.com/articles/nature02863 (accessed on 5 February 2023).
- Baumann, F.S.; Fleig, J.; Habermeier, H.-U.; Maier, J. Impedance spectroscopic study on well-defined (La,Sr)(Co,Fe)O3-δ model electrodes. Solid State Ionics 2006, 177, 1071–1081. [Google Scholar] [CrossRef]
- Rossignol, C.; Ralph, J.; Bae, J.-M.; Vaughey, J. Ln1 − xSrxCoO3 (Ln = Gd, Pr) as a cathode for intermediate-temperature solid oxide fuel cells? Solid State Ionics 2004, 175, 59–61. [Google Scholar] [CrossRef]
- Kim, C.; Lee, H.; Jang, I.; Kim, S.; Yoon, H.; Paik, U.; Song, T. Subcontinuous 2D La0.6Sr0.4CoO3-δ nanosheet as an efficient charge conductor for boosting the cathodic activity of solid oxide fuel cells. Electrochim. Acta 2020, 366, 137371. [Google Scholar] [CrossRef]
- Jia, Z.; Rondiya, S.R.; Cross, R.W.; Wang, C.; Dzade, N.Y.; Zhang, C. Highly active methanol oxidation electrocatalyst based on 2D NiO porous nanosheets: A combined computational and experimental study. Electrochim. Acta 2021, 394, 139143. [Google Scholar] [CrossRef]
- Javed, R.M.N.; Al-Othman, A.; Tawalbeh, M.; Olabi, A.G. Recent developments in graphene and graphene oxide materials for polymer electrolyte membrane fuel cells applications. Renew. Sustain. Energy Rev. 2022, 168. [Google Scholar] [CrossRef]
- Alashkar, A.; Al-Othman, A.; Tawalbeh, M.; Qasim, M. A Critical Review on the Use of Ionic Liquids in Proton Exchange Membrane Fuel Cells. Membranes 2022, 12, 178. [Google Scholar] [CrossRef]
- The Novel Advancements of Nanomaterials in Biofuel Cells with a Focus on Electrodes’ Applications—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0016236122010924?via%3Dihub (accessed on 3 February 2023).
- Revealing the Nature of Active Sites in Electrocatalysis—Chemical Science (RSC Publishing). Available online: https://pubs.rsc.org/en/content/articlelanding/2019/SC/C9SC02654A (accessed on 3 February 2023).
- Tiido, K.; Alexeyeva, N.; Couillard, M.; Bock, C.; MacDougall, B.R.; Tammeveski, K. Graphene–TiO2 composite supported Pt electrocatalyst for oxygen reduction reaction. Electrochim. Acta 2013, 107, 509–517. [Google Scholar] [CrossRef]
- Naik, K.M.; Higuchi, E.; Inoue, H. Two-dimensional oxygen-deficient TiO2 nanosheets-supported Pt nanoparticles as durable catalyst for oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2020, 455, 227972. [Google Scholar] [CrossRef]
- Li, L.; Hu, Z.; Tao, L.; Xu, J.; Yu, J.C. Efficient Electronic Transport in Partially Disordered Co3O4 Nanosheets for Electrocatalytic Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2020, 3, 3071–3081. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Trapero, J.R.; Horcajada, L.; Linares, J.J.; Lobato, J. Is microbial fuel cell technology ready? An economic answer towards industrial commercialization. Appl. Energy 2017, 185, 698–707. [Google Scholar] [CrossRef]
- Microbial Fuel Cell-Based Biosensor for Online Monitoring Wastewater Quality: A Critical Review—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0048969719356074?via%3Dihub (accessed on 3 February 2023).
- Highly Efficient Electro-Generation of H2O2 by Adjusting Liquid-Gas-Solid Three Phase Interfaces of Porous Carbonaceous Cathode during Oxygen Reduction Reaction—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0043135419307079?via%3Dihub (accessed on 3 February 2023).
- Liu, Y.; Quan, X.; Fan, X.; Wang, H.; Chen, S. High-Yield Electrosynthesis of Hydrogen Peroxide from Oxygen Reduction by Hierarchically Porous Carbon. Angew. Chem. 2015, 127, 6941–6945. Available online: https://onlinelibrary.wiley.com/doi/10.1002/ange.201502396 (accessed on 3 February 2023). [CrossRef]
- Chakraborty, I.; Ghosh, D.; Sathe, S.M.; Dubey, B.K.; Pradhan, D.; Ghangrekar, M.M. Investigating the efficacy of CeO2 multi-layered triangular nanosheets for augmenting cathodic hydrogen peroxide production in microbial fuel cell. Electrochim. Acta 2021, 398, 139341. [Google Scholar] [CrossRef]
- Yin, T.; Zhang, H.; Yang, G.; Wang, L. Polyaniline composite TiO2 nanosheets modified carbon paper electrode as a high performance bioanode for microbial fuel cells. Synth. Met. 2019, 252, 8–14. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, N.; Yang, T.; Zhang, T.; Wu, P.; Dang, Z. Nickel oxide and carbon nanotube composite (NiO/CNT) as a novel cathode non-precious metal catalyst in microbial fuel cells. Biosens. Bioelectron. 2015, 72, 332–339. [Google Scholar] [CrossRef]
- Zhang, S.; Su, W.; Wei, Y.; Liu, J.; Li, K. Mesoporous MnO2 structured by ultrathin nanosheet as electrocatalyst for oxygen reduction reaction in air-cathode microbial fuel cell. J. Power Sources 2018, 401, 158–164. [Google Scholar] [CrossRef]
- Huang, Q.; Zhou, P.; Yang, H.; Zhu, L.; Wu, H. CoO nanosheets in situ grown on nitrogen-doped activated carbon as an effective cathodic electrocatalyst for oxygen reduction reaction in microbial fuel cells. Electrochim. Acta 2017, 232, 339–347. [Google Scholar] [CrossRef]
- Sugimoto, W.; Iwata, H.; Yasunaga, Y.; Murakami, Y.; Takasu, Y. Preparation of ruthenic acid nanosheets and utilization of its interlayer surface for electrochemical energy storage. Angew. Chem. Int. Ed. 2003, 42, 4092–4096. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Saida, T.; Sato, J.; Yonezawa, M.; Takasu, Y.; Sugimoto, W. Synthesis of Nanosheet Crystallites of Ruthenate with an α-NaFeO2-Related Structure and Its Electrochemical Supercapacitor Property. Inorg. Chem. 2010, 49, 4391–4393. [Google Scholar] [CrossRef]
- Xiong, P.; Ma, R.; Sakai, N.; Bai, X.; Li, S.; Sasaki, T. Redox Active Cation Intercalation/Deintercalation in Two-Dimensional Layered MnO2 Nanostructures for High-Rate Electrochemical Energy Storage. ACS Appl. Mater. Interfaces 2017, 9, 6282–6291. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, K.; Sun, H.; Yin, S. One-Step Synthesis of Single-Layer MnO2 Nanosheets with Multi-Role Sodium Dodecyl Sulfate for High-Performance Pseudocapacitors. Small 2015, 11, 2182–2191. [Google Scholar] [CrossRef] [PubMed]
- Superior Role of MXene Nanosheet as Hybridization Matrix over Graphene in Enhancing Interfacial Electronic Coupling and Functionalities of Metal Oxide—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S2211285518306992 (accessed on 1 February 2023).
- Lin, F.; Yuan, M.; Chen, Y.; Huang, Y.; Lian, J.; Qiu, J.; Xu, H.; Li, H.; Yuan, S.; Zhao, Y.; et al. Advanced asymmetric supercapacitor based on molybdenum trioxide decorated nickel cobalt oxide nanosheets and three-dimensional α-FeOOH/rGO. Electrochim. Acta 2019, 320, 134580. [Google Scholar] [CrossRef]
- Yu, N.; Yin, H.; Zhang, W.; Liu, Y.; Tang, Z.; Zhu, M.-Q. High-Performance Fiber-Shaped All-Solid-State Asymmetric Supercapacitors Based on Ultrathin MnO2Nanosheet/Carbon Fiber Cathodes for Wearable Electronics. Adv. Energy Mater. 2015, 6. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.-Z.; Dubbink, D.; Elshof, J.E.T. Inkjet printing of δ-MnO2 nanosheets for flexible solid-state micro-supercapacitor. Nano Energy 2018, 49, 481–488. [Google Scholar] [CrossRef]
- Ma, W.; Ma, R.; Wu, J.; Sun, P.; Liu, X.; Zhou, K.; Sasaki, T. Development of efficient electrocatalysts via molecular hybridization of NiMn layered double hydroxide nanosheets and graphene. Nanoscale 2016, 8, 10425–10432. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Hu, Z.; Xiang, Z.; Ma, J.; Song, B.; Zhang, J.; Ma, H. Cation Intercalation in Manganese Oxide Nanosheets: Effects on Lithium and Sodium Storage. Angew. Chem. Int. Ed. 2016, 55, 10448–10452. Available online: https://onlinelibrary.wiley.com/doi/10.1002/anie.201605102 (accessed on 31 January 2023). [CrossRef]
- Jin, X.; Shin, S.-J.; Kim, J.; Lee, N.-S.; Kim, H.; Hwang, S.-J. Heterolayered 2D nanohybrids of uniformly stacked transition metal dichalcogenide–transition metal oxide monolayers with improved energy-related functionalities. J. Mater. Chem. A 2018, 6, 15237–15244. [Google Scholar] [CrossRef]
- Wang, L.; Takada, K.; Kajiyama, A.; Onoda, M.; Michiue, Y.; Zhang, L.; Watanabe, M.; Sasaki, T. Synthesis of a Li−Mn-oxide with Disordered Layer Stacking through Flocculation of Exfoliated MnO2 Nanosheets, and Its Electrochemical Properties. Chem. Mater. 2003, 15, 4508–4514. [Google Scholar] [CrossRef]
- Su, Y.; Pan, A.; Wang, Y.; Huang, J.; Nie, Z.; An, X.; Liang, S. Template-assisted formation of porous vanadium oxide as high performance cathode materials for lithium ion batteries. J. Power Sources 2015, 295, 254–258. [Google Scholar] [CrossRef]
- Divya, P.; Vadivel, S.; Rosaiah, P. Synthesis and characterization of graphene oxide based metal oxides for high performance Li-ion batteries. Mater. Today Proc. 2022, 66, 1819–1822. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Jiang, N.; An, L.; Song, J.; Zuo, Y.; Ning, F.; Shang, H.; Xia, D. Electrolytic-anion-redox adsorption pseudocapacitance in nanosized lithium-free transition metal oxides as cathode materials for Li-ion batteries. Nano Energy 2020, 72, 104727. [Google Scholar] [CrossRef]
- Lai, Y.; Wang, P.; Qin, F.; Xu, M.; Li, J.; Zhang, K.; Zhang, Z. A carbon nanofiber@mesoporous δ-MnO2 nanosheet-coated separator for high-performance lithium-sulfur batteries. Energy Storage Mater. 2017, 9, 179–187. [Google Scholar] [CrossRef]
- Liang, X.; Hart, C.; Pang, Q.; Garsuch, A.; Weiss, T.; Nazar, L.F. A highly efficient polysulfide mediator for lithium–sulfur batteries. Nat. Commun. 2015, 6, 5682. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Ma, R.; Sakai, N.; Sasaki, T. Genuine Unilamellar Metal Oxide Nanosheets Confined in a Superlattice-like Structure for Superior Energy Storage. ACS Nano 2018, 12, 1768–1777. Available online: https://pubs.acs.org/doi/10.1021/acsnano.7b08522 (accessed on 1 February 2023). [CrossRef]
- Xie, Q.; Cheng, G.; Xue, T.; Huang, L.; Chen, S.; Sun, Y.; Sun, M.; Wang, H.; Yu, L. Alkali ions pre-intercalation of δ-MnO2 nanosheets for high-capacity and stable Zn-ion battery. Mater. Today Energy 2022, 24, 100934. [Google Scholar] [CrossRef]
- Li, X.; Qu, J.; Xu, J.; Zhang, S.; Wang, X.; Wang, X.; Dai, S. K-preintercalated MnO2 nanosheets as cathode for high-performance Zn-ion batteries. J. Electroanal. Chem. 2021, 895, 115529. [Google Scholar] [CrossRef]
- Qi, J.; Wang, P.; Yan, Y.; Zheng, X.; Cao, J.; Feng, J. MCo2O4 (M=Co, Mn, Ni, Zn) nanosheet arrays constructed by two-dimension metal-organic frameworks as binder-free electrodes for lithium-ion batteries. Vacuum 2019, 169, 108959. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, P.; Liang, J.; Xia, X.; Ren, L.; Song, L.; Liu, W.; Sun, X. Uncovering sulfur doping effect in MnO2 nanosheets as an efficient cathode for aqueous zinc ion battery. Energy Storage Mater. 2022, 47, 424–433. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, K.; Cen, Z.; Xu, K. Rational construction of multidimensional oxygen-deficient Co3O4 nanosheet/nanowire arrays as high-performance electrodes for aqueous Zn-ion batteries and asymmetric supercapacitors. J. Alloys Compd. 2021, 879, 160439. [Google Scholar] [CrossRef]
- Li, Y.; Talib, S.H.; Liu, D.; Zong, K.; Saad, A.; Song, Z.; Zhao, J.; Liu, W.; Liu, F.; Ji, Q.; et al. Improved oxygen evolution reaction performance in Co0.4Mn0.6O2 nanosheets through Triple-doping (Cu, P, N) strategy and its application to Zn-air battery. Appl. Catal. B Environ. 2023, 320. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.; Liu, K.; Yin, J.; Li, Y.; Fu, G.; Zhang, Y.; Tang, Y. Hollow yolk-shell nanoboxes assembled by Fe-doped Mn3O4 nanosheets for high-efficiency electrocatalytic oxygen reduction in Zn-Air battery. Chem. Eng. J. 2021, 427, 131992. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Su, Y.; Li, Q.; Zhao, Z.; Geng, F. Molecularly Stacking Manganese Dioxide/Titanium Carbide Sheets to Produce Highly Flexible and Conductive Film Electrodes with Improved Pseudocapacitive Performances. Adv. Energy Mater. 2017, 7. Available online: https://onlinelibrary.wiley.com/doi/10.1002/aenm.201602834 (accessed on 1 February 2023). [CrossRef]
- Li, C.; Jiang, G.; Liu, T.; Zeng, Z.; Li, P.; Wang, R.; Zhang, X. NiCoO2 nanosheets interlayer network connected in reduced graphene oxide and MXene for high-performance asymmetric supercapacitors. J. Energy Storage 2022, 49, 104176. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, J.; Yuan, Z.; Zhao, L.; Wang, L.; Han, W. Assembling Co3O4 Nanoparticles into MXene with Enhanced electrochemical performance for advanced asymmetric supercapacitors. J. Colloid Interface Sci. 2021, 599, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gong, J.; Zhang, H.; Lv, L.; Liu, Y.; Dai, Y. Construction of hexagonal nickel-cobalt oxide nanosheets on metal-organic frameworks based on MXene interlayer ion effect for hybrid supercapacitors. J. Alloys Compd. 2021, 870, 159466. [Google Scholar] [CrossRef]
- Nakate, U.T.; Patil, P.; Nakate, Y.T.; Na, S.-I.; Yu, Y.; Hahn, Y.-B. Ultrathin ternary metal oxide Bi2MoO6 nanosheets for high performance asymmetric supercapacitor and gas sensor applications. Appl. Surf. Sci. 2021, 551, 149422. [Google Scholar] [CrossRef]
- Pendashteh, A.; Moosavifard, S.E.; Rahmanifar, M.S.; Wang, Y.; El-Kady, M.F.; Kaner, R.B.; Mousavi, M.F. Highly Ordered Mesoporous CuCo2O4 Nanowires, a Promising Solution for High-Performance Supercapacitors. Chem. Mater. 2015, 27, 3919–3926. Available online: https://pubs.acs.org/doi/10.1021/acs.chemmater.5b00706 (accessed on 1 February 2023). [CrossRef]
- Xu, K.; Ma, S.; Shen, Y.; Ren, Q.; Yang, J.; Chen, X.; Hu, J. CuCo2O4 nanowire arrays wrapped in metal oxide nanosheets as hierarchical multicomponent electrodes for supercapacitors. Chem. Eng. J. 2019, 369, 363–369. [Google Scholar] [CrossRef]
- Qiu, K.; Lu, M.; Luo, Y.; Du, X. Engineering hierarchical nanotrees with CuCo2O4 trunks and NiO branches for high-performance supercapacitors. J. Mater. Chem. A 2017, 5, 5820–5828. [Google Scholar] [CrossRef]
- Kuang, M.; Liu, X.Y.; Dong, F.; Zhang, Y.X. Tunable design of layered CuCo2O4 nanosheets@MnO2 nanoflakes core–shell arrays on Ni foam for high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 21528–21536. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Zhang, Q.; Cheng, Y.; Zhao, B.; Dai, S.; Wu, H.-H.; Zhang, K.; Ding, D.; Wu, Y.; et al. Anion and cation substitution in transition-metal oxides nanosheets for high-performance hybrid supercapacitors. Nano Energy 2018, 57, 22–33. [Google Scholar] [CrossRef]
- Lin, J.-S.; Kumar, S.R.; Ma, W.-T.; Shih, C.-M.; Teng, L.-W.; Yang, C.-C.; Lue, S.J. Gradiently distributed iron oxide@graphene oxide nanofillers in quaternized polyvinyl alcohol composite to enhance alkaline fuel cell power density. J. Membr. Sci. 2017, 543, 28–39. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).