Immobilization of Dextranase Obtained from the Marine Cellulosimicrobium sp. Y1 on Nanoparticles: Nano-TiO2 Improving Hydrolysate Properties and Enhancing Reuse
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Selection of Nanomaterials
2.2.2. Optimization of the Immobilization Process
Effect of TiO2 Content
Effect of Adsorption Time
Effect of Temperature and pH
2.2.3. Properties of Immobilized Dextranase
Effect of Temperature on Enzyme Activity and Stability
Effect of pH on Enzyme Activity and Stability
2.2.4. Characterization of Immobilized Dextranase
2.2.5. Analysis of the Hydrolysates of Immobilized Dextranase
2.2.6. Reproducibility and Storage of Immobilized Dextranase
2.2.7. Adsorption Kinetics
2.2.8. Data Analysis
3. Results and Discussion
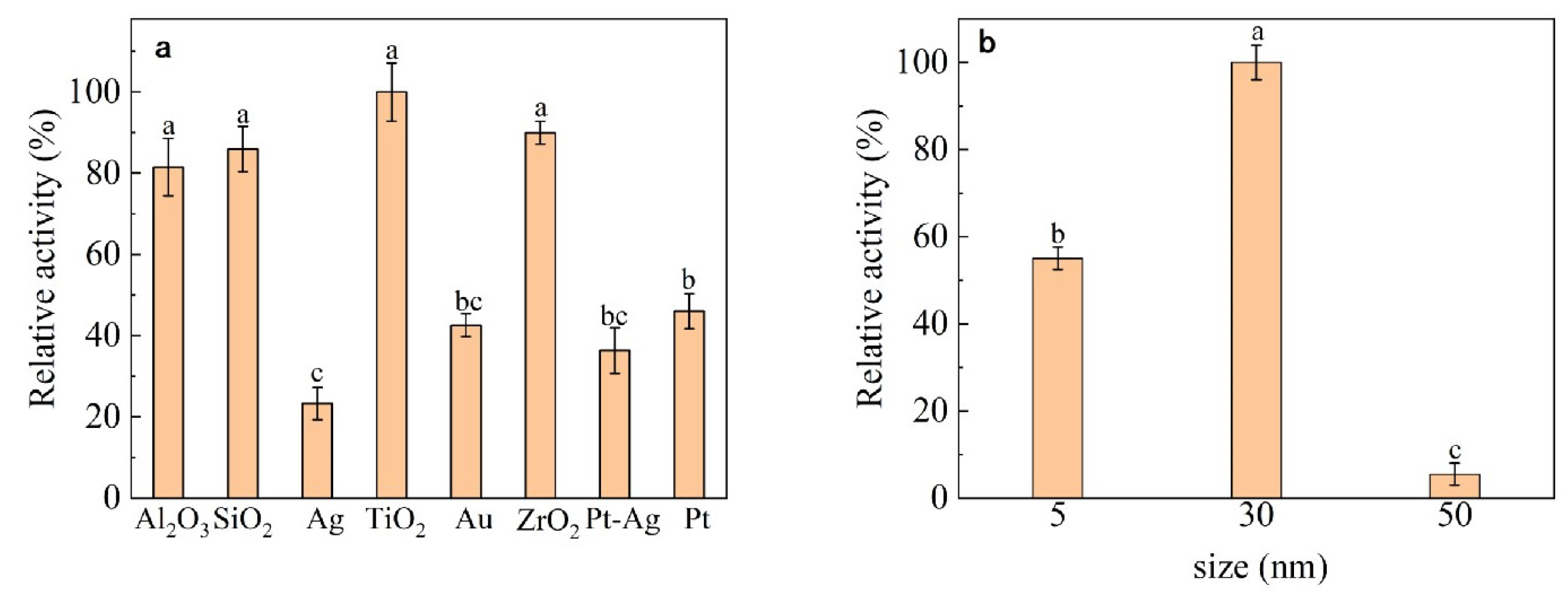
3.1. Nanomaterial Selection
3.2. Immobilization Conditions
3.3. Enzymatic Properties of Immobilized Dextranase
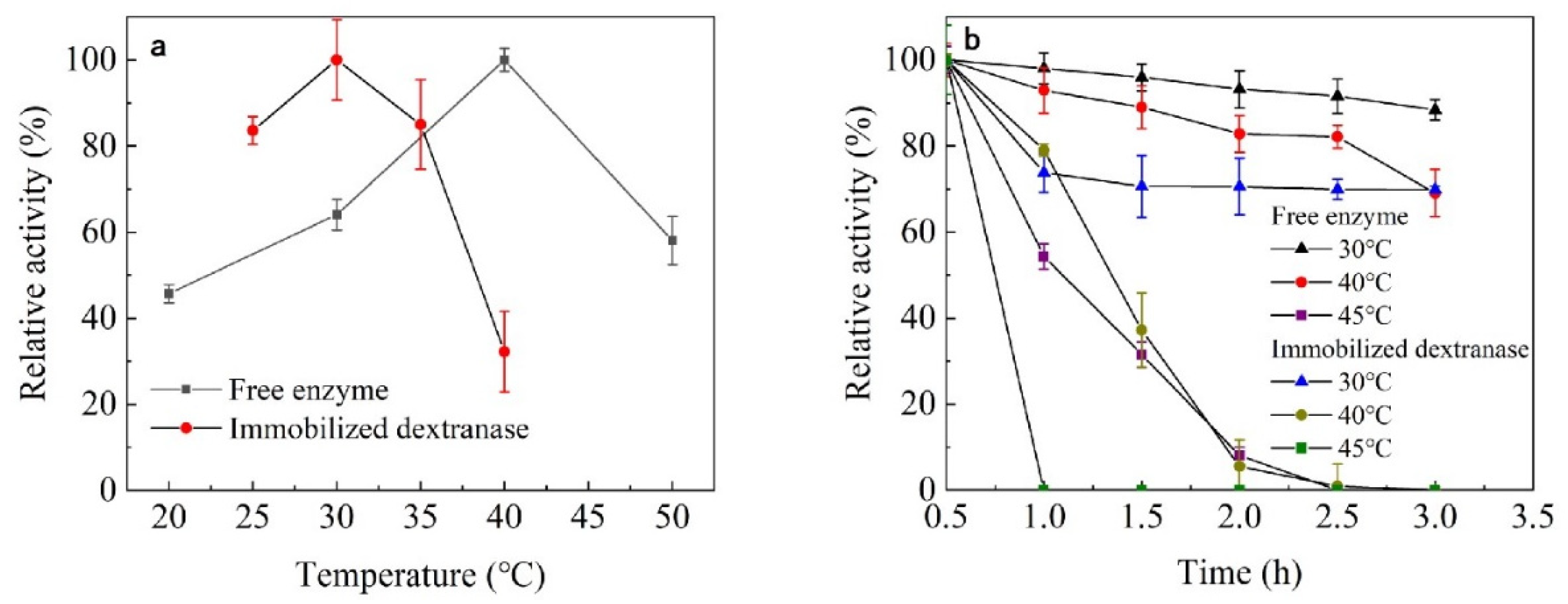
3.3.1. The Effect of Temperature on Enzyme Activity and Stability
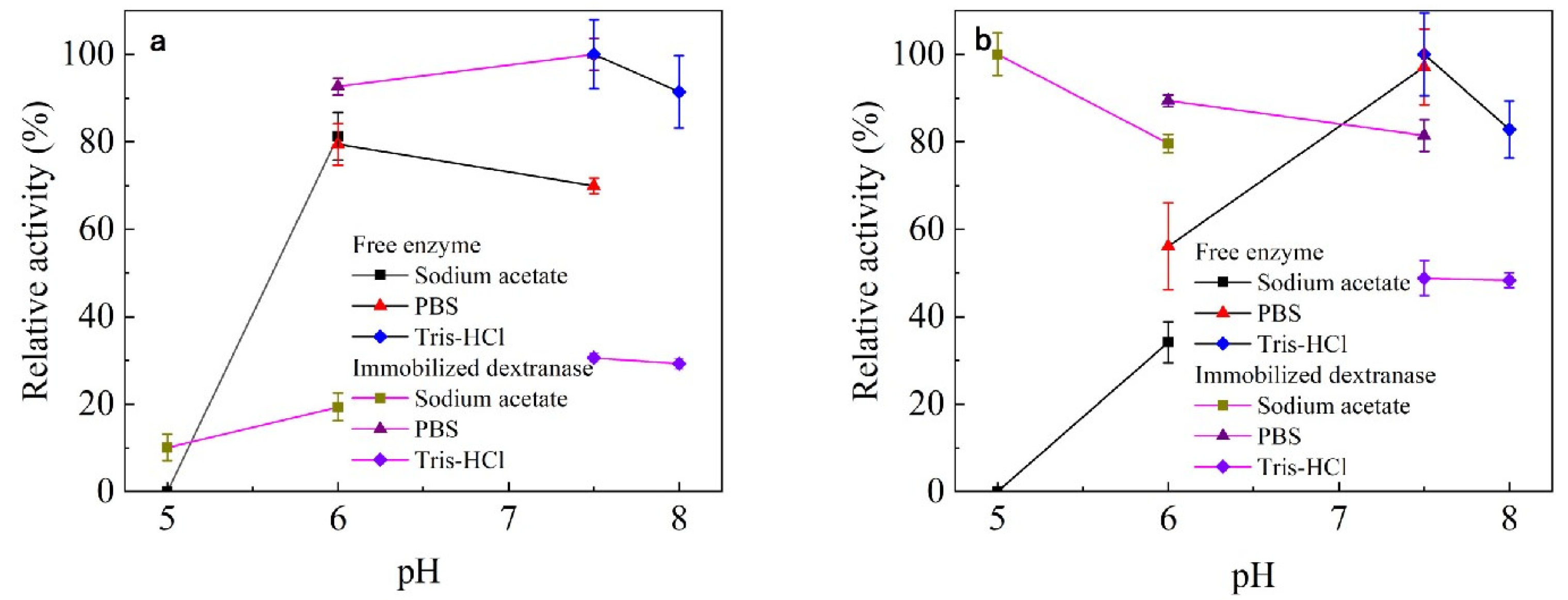
3.3.2. Effect of pH on Enzyme Activity and Stability
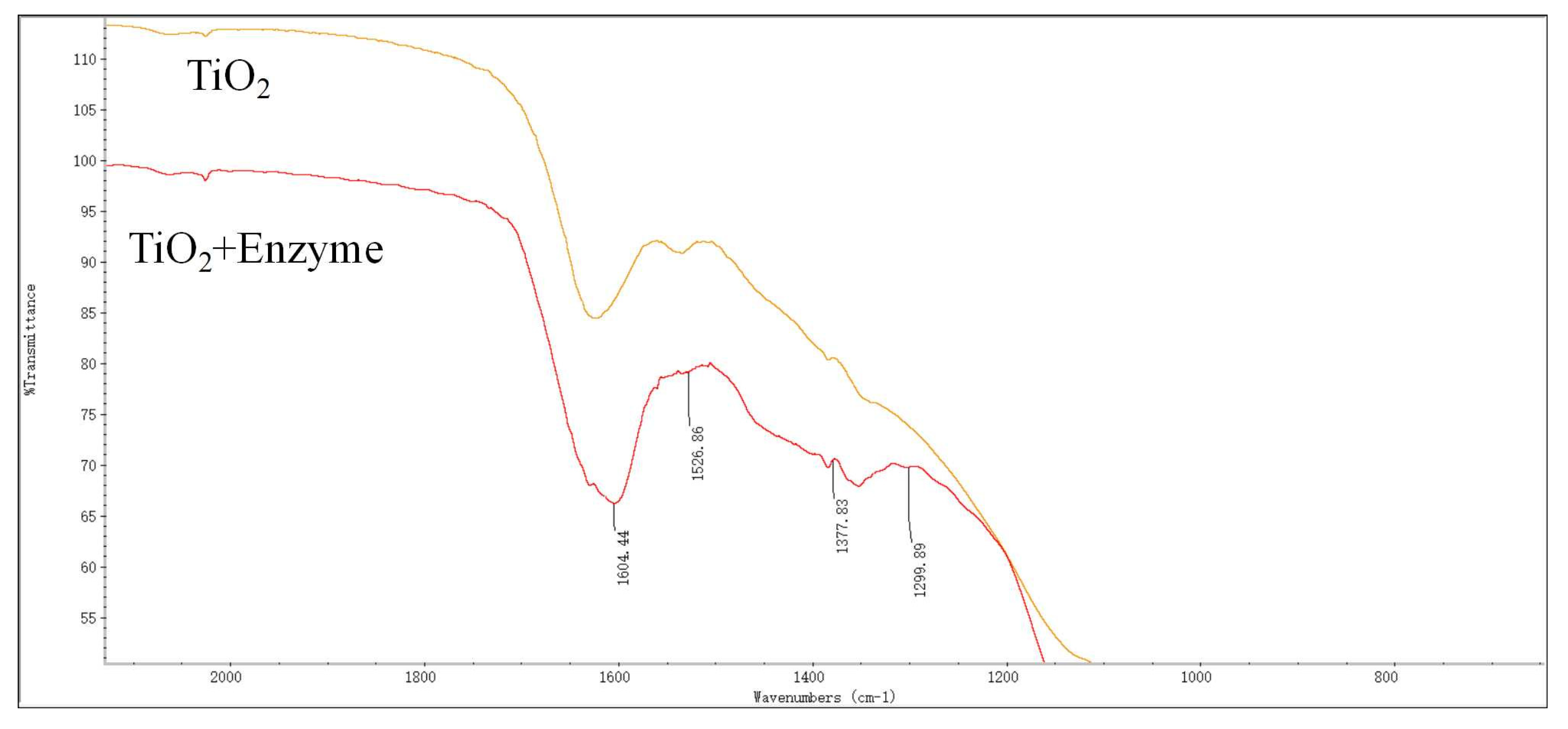
3.4. Characterization of Immobilized Dextranase
3.5. Analysis of the Hydrolysates
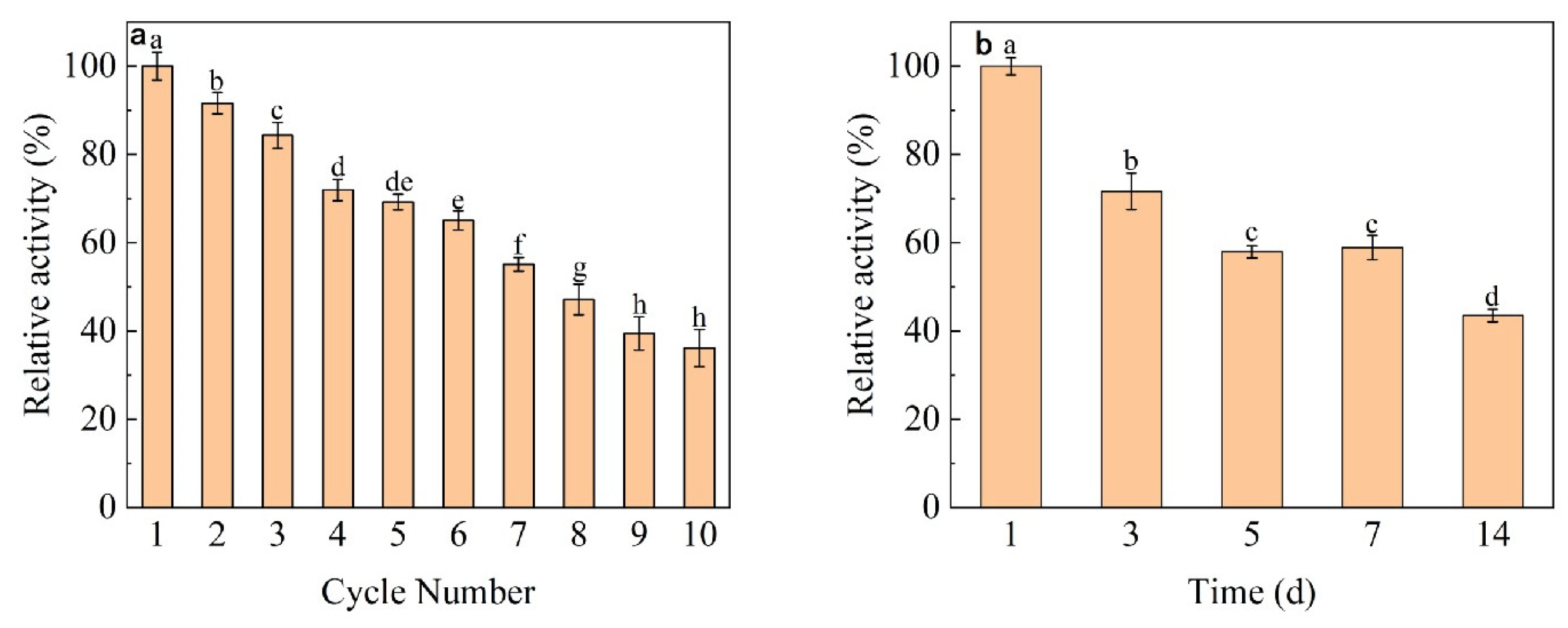
3.6. Reproducibility and Storage of Immobilized Dextranase
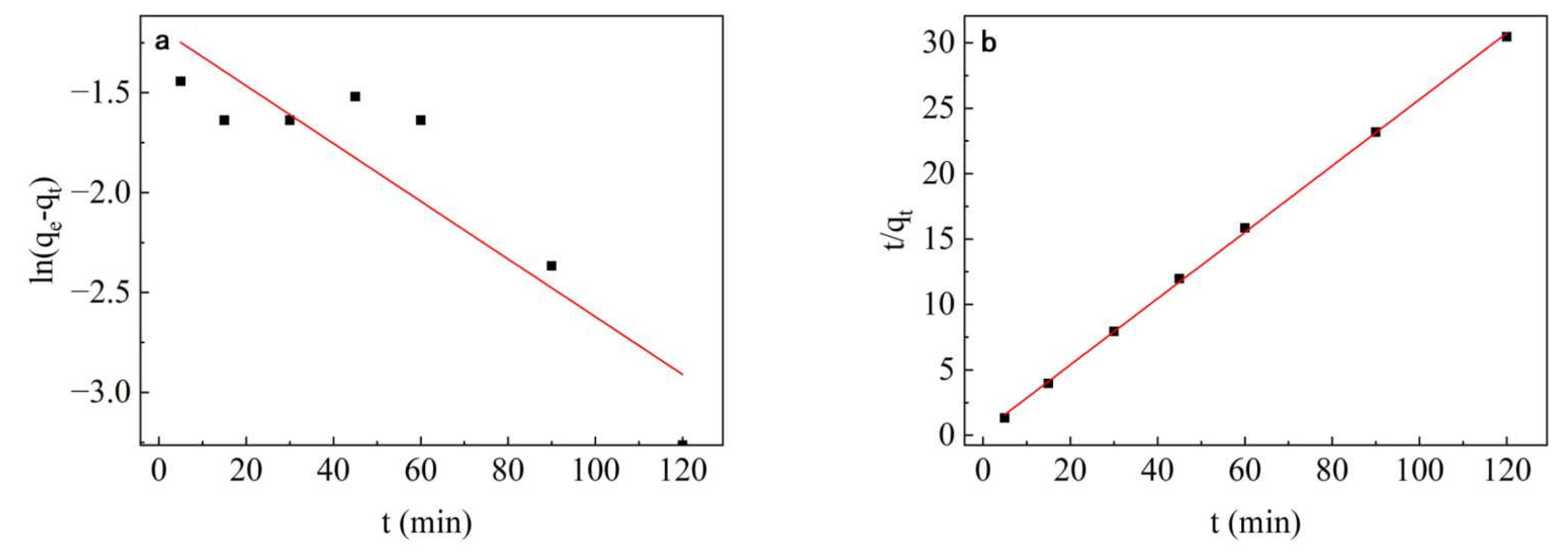
3.7. Adsorption Kinetic Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Da Silva, R.M.; Souza, P.M.P.; Fernandes, F.A.; Gonçalves, L.R.; Rodrigues, S. Co-immobilization of dextransucrase and dextranase in epoxy-agarose- tailoring oligosaccharides synthesis. Process. Biochem. 2019, 78, 71–81. [Google Scholar] [CrossRef]
- Wang, X.; Lu, M.; Wang, S.; Fang, Y.; Wang, D.; Ren, W.; Zhao, G. The atmospheric and room-temperature plasma (ARTP) method on the dextranase activity and structure. Int. J. Biol. Macromol. 2014, 70, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.C.T.; Soccol, C.R.; Spier, M.R.; Junior, N.L.; de Souza Vandenberghe, L.P. Potential application of dextranase produced by Penicillium aculeatum in solid-state fermentation from brewer’s spent grain in sugarcane process factories. Biocatal. Agric. Biotechnol. 2021, 35, 102086–102094. [Google Scholar] [CrossRef]
- Khalikova, E.; Susi, P.; Korpela, T. Microbial Dextran-Hydrolyzing Enzymes: Fundamentals and Applications. Microbiol. Mol. Biol. Rev. 2005, 69, 306–325. [Google Scholar] [CrossRef]
- Zohra, R.R.; Aman, A.; Zohra, R.R.; Ansari, A.; Ghani, M.; Qader, S.A.U. Dextranase: Hyper production of dextran degrading enzyme from newly isolated strain of Bacillus licheniformis. Carbohydr. Polym. 2013, 92, 2149–2153. [Google Scholar] [CrossRef]
- Goulas, A.K.; Fisher, D.A.; Grimble, G.K.; Grandison, A.S.; Rastall, R.A. Synthesis of isomaltooligosaccharides and oligodextrans by the combined use of dextransucrase and dextranase. Enzym. Microb. Technol. 2004, 35, 327–338. [Google Scholar] [CrossRef]
- Davidović, S.; Miljković, M.; Lazić, V.; Jović, D.; Jokić, B.; Dimitrijević, S.; Radetić, M. Impregnation of cotton fabric with silver nanoparticles synthesized by dextran isolated from bacterial species Leuconostoc mesenteroides T3. Carbohydr. Polym. 2015, 131, 331–336. [Google Scholar] [CrossRef]
- Miljković, M.G.; Lazić, V.; Banjanac, K.; Davidović, S.Z.; Bezbradica, D.I.; Marinković, A.D.; Sredojević, D.; Nedeljković, J.M.; Branković, S.I.D. Immobilization of dextransucrase on functionalized TiO2 supports. Int. J. Biol. Macromol. 2018, 114, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Song, X.; Cai, J. Hydrophilic polyethylenimine modified magnetic graphene oxide composite as an efficient support for dextranase immobilization with improved stability and recyclable performance. Biochem. Eng. J. 2019, 141, 163–172. [Google Scholar] [CrossRef]
- Ölçer, Z.; Tanriseven, A. Co-immobilization of dextransucrase and dextranase in alginate. Process Biochem. 2010, 45, 1645–1651. [Google Scholar] [CrossRef]
- Shahid, F.; Ansari, A.; Aman, A.; Qader, S.A.U. A Comparative Study Among Different Protocols of Immobilization of Dextranase Using Chitin as a Matrix. Catal. Lett. 2019, 150, 613–622. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, H.; Wang, X.; Zu, H.; Wang, C.; Dong, D.; Lyu, M.; Wang, S. Immobilization of Dextranase on Nano-Hydroxyapatite as a Recyclable Catalyst. Materials 2020, 14, 130. [Google Scholar] [CrossRef]
- Sanjana, S.; Medha, M.; Meghna, M.; Shruthi, T.; Srinivas, S.; Madhyastha, H.; Navya, P.; Daima, H.K. Enzyme immobilization on quercetin capped gold and silver nanoparticles for improved performance. Mater. Today Proc. 2019, 10, 92–99. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, W. Preparation of chitosan-based nanoparticles for enzyme immobilization. Int. J. Biol. Macromol. 2019, 126, 1125–1132. [Google Scholar] [CrossRef]
- Aldhahri, M.; Almulaiky, Y.Q.; El-Shishtawy, R.M.; Al-Shawafi, W.M.; Salah, N.; Alshahrie, A.; Alzahrani, H.A.H. Ultra-Thin 2D CuO Nanosheet for HRP Immobilization Supported by Encapsulation in a Polymer Matrix: Characterization and Dye Degradation. Catal. Lett. 2020, 151, 232–246. [Google Scholar] [CrossRef]
- Al-Harbi, S.A.; Almulaiky, Y.Q. Purification and biochemical characterization of Arabian balsam α-amylase and enhancing the retention and reusability via encapsulation onto calcium alginate/Fe2O3 nanocomposite beads. Int. J. Biol. Macromol. 2020, 160, 944–952. [Google Scholar] [CrossRef]
- Austin, L.A.; Mackey, M.A.; Dreaden, E.C.; El-Sayed, M.A. The optical, photothermal, and facile surface chemical properties of gold and silver nanoparticles in biodiagnostics, therapy, and drug delivery. Arch. Toxicol. 2014, 88, 1391–1417. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Peng, Q. Protein-gold nanoparticle interactions and their possible impact on biomedical applications. Acta Biomater. 2017, 55, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Pudlarz, A.M.; Ranoszek-Soliwoda, K.; Karbownik, M.S.; Czechowska, E.; Tomaszewska, E.; Celichowski, G.; Grobelny, J.; Chabielska, E.; Gromotowicz-Popławska, A.; Szemraj, J. Antioxidant enzymes immobilized on gold and silver nanoparticles enhance DNA repairing systems of rat skin after exposure to ultraviolet radiation. Nanomed. Nanotechnol. Biol. Med. 2022, 43, 102558. [Google Scholar] [CrossRef] [PubMed]
- Köller, M.; Bellova, P.; Javid, S.M.; Motemani, Y.; Khare, C.; Sengstock, C.; Tschulik, K.; Schildhauer, T.A.; Ludwig, A. Antibacterial activity of microstructured sacrificial anode thin films by combination of silver with platinum group elements (platinum, palladium, iridium). Mater. Sci. Eng. C 2017, 74, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, J.; Kang, X.; Wang, C.; Wang, D.; Liu, J.; Aksay, I.A.; Lin, Y. Glucose biosensor based on immobilization of glucose oxidase in platinum nanoparticles/graphene/chitosan nanocomposite film. Talanta 2009, 80, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Fortes, C.C.; Daniel-Da-Silva, A.L.; Xavier, A.M.; Tavares, A.P. Optimization of enzyme immobilization on functionalized magnetic nanoparticles for laccase biocatalytic reactions. Chem. Eng. Process. Process. Intensif. 2017, 117, 1–8. [Google Scholar] [CrossRef]
- Isanapong, J.; Lohawet, K.; Kumnorkaew, P. Optimization and characterization of immobilized laccase on titanium dioxide nanostructure and its application in removal of Remazol Brilliant Blue R. Biocatal. Agric. Biotechnol. 2021, 37, 102186–102196. [Google Scholar] [CrossRef]
- Shafi, A.; Husain, Q. Immobilization of β-galactosidase on concanavalin A modified silica-coated titanium dioxide nanocomposite and its interaction with monovalent and divalent cations. Mater. Today Commun. 2022, 32, 103828–103840. [Google Scholar] [CrossRef]
- Ahmad, R.; Sardar, M. Immobilization of cellulase on TiO2 nanoparticles by physical and covalent methods: A comparative study. Indian J. Biochem. Biophys. 2014, 51, 314–320. [Google Scholar]
- Nagar, S.; Mittal, A.; Kumar, D.; Kumar, L.; Gupta, V.K. Immobilization of xylanase on glutaraldehyde activated aluminum oxide pellets for increasing digestibility of poultry feed. Process. Biochem. 2012, 47, 1402–1410. [Google Scholar] [CrossRef]
- Bruera, F.A.; Kramer, G.R.; Velázquez, J.E.; Sadañoski, M.A.; Fonseca, M.I.; Ares, A.E.; Zapata, P.D. Laccase immobilization on nanoporous aluminum oxide for black liquor treatment. Surf. Interfaces 2022, 30, 101879. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, W.; Zhao, L.; Gao, S.; Liu, T.; Yu, D. Immobilization of lipase in chitosan-mesoporous silica material and pore size adjustment. Int. J. Biol. Macromol. 2023, 235, 123789. [Google Scholar] [CrossRef]
- You, H.H.P.; Wright, P.A. Enzymes supported on ordered mesoporous solids: A special case of an inorganic-organic hybrid. J. Mater. Chem. 2005, 15, 3690–3700. [Google Scholar] [CrossRef]
- Ispas, I.S.C.; Andreescu, S. Enzyme-functionalized mesoporous silica for bioanalytical applications. J. Bioanal Chem. 2009, 393, 543–554. [Google Scholar] [CrossRef]
- Cai, L.; YGao YChu YLin, L.; Zhang, G. Green synthesis of silica-coated magnetic nanocarriers for simultaneous purification-immobilization of beta-1,3-xylanase. Int. J. Biol. Macromol. 2023, 233, 123223. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, C.H.C.R.; Lin, Y.S. Thermal stability improvement on pore and phase structure of sol–gel derived zirconia. J. Mater. Sci. 1995, 30, 3075–3081. [Google Scholar] [CrossRef]
- Hisbergues, S.V.M.; Vendeville, P. Zirconia established facts and perspectives for a biomaterial in dental implantology. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Reshmi, R.; Sanjay, G.; Sugunan, S. Immobilization of α-amylase on zirconia: A heterogeneous biocatalyst for starch hydrolysis. Catal. Commun. 2007, 8, 393–399. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, J.; Xin, X.; Wang, L.; Li, H.; Zheng, X.; Jiang, Y. Immobilization laccase on heterophase TiO2 microsphere as a photo-enzyme integrated catalyst for emerging contaminants degradation under visible light. Appl. Mater. Today 2020, 21, 100810. [Google Scholar] [CrossRef]
- Romero-Arcos, M.; Garnica-Romo, M.G.; Martinez-Flores, H.E.; Vázquez-Marrufo, G.; Ramírez-Bon, R.; González-Hernández, J.; Barbosa-Cánovas, G.V. Enzyme Immobilization by Amperometric Biosensors with TiO2 Nanoparticles Used to Detect Phenol Compounds. Food Eng. Rev. 2015, 8, 235–250. [Google Scholar] [CrossRef]
- Wang, H.Q.; Yao, Z.; Sun, Y.; Zhou, Z.; Xiong, Q.; Zhong, Z.X. Immobilization of γ-glutamyltranspeptidase on silylated mesoporous TiO2 whiskers. Biotechnol. Bioprocess Eng. 2014, 19, 304–310. [Google Scholar] [CrossRef]
- Li, G.; Nandgaonkar, A.G.; Wang, Q.; Zhang, J.; Krause, W.E.; Wei, Q.; Lucian, A. Laccase-immobilized bacterial cellulose/TiO2 functionalized composite membranes: Evaluation for photo- and bio-catalytic dye degradation. J. Membr. Sci. 2017, 525, 89–98. [Google Scholar] [CrossRef]
- Milićević, B.; Đorđević, V.; Lončarević, D.; Ahrenkiel, S.P.; Dramićanin, M.D.; Nedeljković, J.M. Visible light absorption of surface modified TiO2 powders with bidentate benzene derivatives. Microporous Mesoporous Mater. 2015, 217, 184–189. [Google Scholar] [CrossRef]
- Mihailović, D.; Šaponjić, Z.; Molina, R.; Puač, N.; Jovančić, P.; Nedeljković, J.; Radetić, M. Improved Properties of Oxygen and Argon RF Plasma-Activated Polyester Fabrics Loaded with TiO2 Nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.H.; Jang, A.; Yoon, J.; Kim, P.; Kim, J.S.; Kim, Y.-H.; Min, J. Evaluation of whole lysosomal enzymes directly immobilized on titanium (IV) oxide used in the development of antimicrobial agents. Enzym. Microb. Technol. 2011, 49, 260–265. [Google Scholar] [CrossRef]
- Zhuang, W.; Zhang, Y.; He, L.; An, R.; Li, B.; Ying, H.; Wu, J.; Chen, Y.; Zhou, J.; Lu, X. Facile synthesis of amino-functionalized mesoporous TiO2 microparticles for adenosine deaminase immobilization. Microporous Mesoporous Mater. 2017, 239, 158–166. [Google Scholar] [CrossRef]
- Ji, C.; Nguyen, L.N.; Hou, J.; Hai, F.I.; Chen, V. Direct immobilization of laccase on titania nanoparticles from crude enzyme extracts of P. ostreatus culture for micro-pollutant degradation. Sep. Purif. Technol. 2017, 178, 215–223. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Lagergren, S. About the Theory of So-Called Adsorption of Soluble Substances. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ritchie, A.G. Alternative to the Elovich equation for kinetics of adsorption of gases on solids. J. Chem. Soc. Faraday Trans. 1977, 73, 1650–1653. [Google Scholar] [CrossRef]
- Zeinabad, H.A.; Kachooei, E.; Saboury, A.A.; Kostova, I.; Attar, F.; Vaezzadeh, M.; Falahati, M. Thermodynamic and conformational changes of protein toward interaction with nanoparticles: A spectroscopic overview. RSC Adv. 2016, 6, 105903–105919. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Suksatan, W.; Kazemzadeh, P.; Afzali, D.; Moghaddam-Manesh, M.; Chauhan, N.P.S.; Sargazi, G. A controllable study on ultrasound assisted synthesis of a novel Ni/Zn based hybrid MOF nanostructures for Dextranase immobilization. Inorg. Chem. Commun. 2022, 139, 109410. [Google Scholar] [CrossRef]
- Han, Y.; Watson, J.T.; Stucky, G.D.; Butler, A. Catalytic activity of mesoporous silicate-immobilized chloroperoxidase. J. Mol. Catal. B Enzym. 2002, 17, 1–8. [Google Scholar] [CrossRef]
- Wu, L.; Wu, S.; Xu, Z.; Qiu, Y.; Li, S.; Xu, H. Modified nanoporous titanium dioxide as a novel carrier for enzyme immobilization. Biosens. Bioelectron. 2016, 80, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, C.; Vera, C.; Araya, E.; Conejeros, R.; Illanes, A. Repeated-batch operation for the synthesis of lactulose with b-galactosidase immobilized by aggregation and crosslinking. Bioresour. Technol. 2015, 190, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Li, M.; Qin, Q.; Xie, T. Purification and characterization of dextranase from Penicillium cyclopium CICC-4022 and its degradation of dextran. Int. J. Biol. Macromol. 2022, 204, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Feng, Y.; Xu, L.; Tian, X.; Lai, X.; Lyu, M.; Wang, S. Expression, purification and characterization of a cold-adapted dextranase from marine bacteria and its ability to remove dental plaque. Protein Expr. Purif. 2020, 174, 105678. [Google Scholar] [CrossRef] [PubMed]
- Subhan, F.B.; Hashemi, Z.; Herrera, M.; Turner, K.; Chan, C.B. Ingestion of isomalto-oligosaccharides stimulates insulin and incretin hormone secretion in healthy adults. J. Funct. Food 2019, 65, 103730. [Google Scholar] [CrossRef]
- Yao, L.W.; Khan, F.S.A.; Mubarak, N.M.; Karri, R.R.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Mazari, S.A.; Ahmad, A.; Dehghani, M.H. Insight into immobilization efficiency of Lipase enzyme as a biocatalyst on the graphene oxide for adsorption of Azo dyes from industrial wastewater effluent. J. Mol. Liq. 2022, 354, 118849. [Google Scholar] [CrossRef]


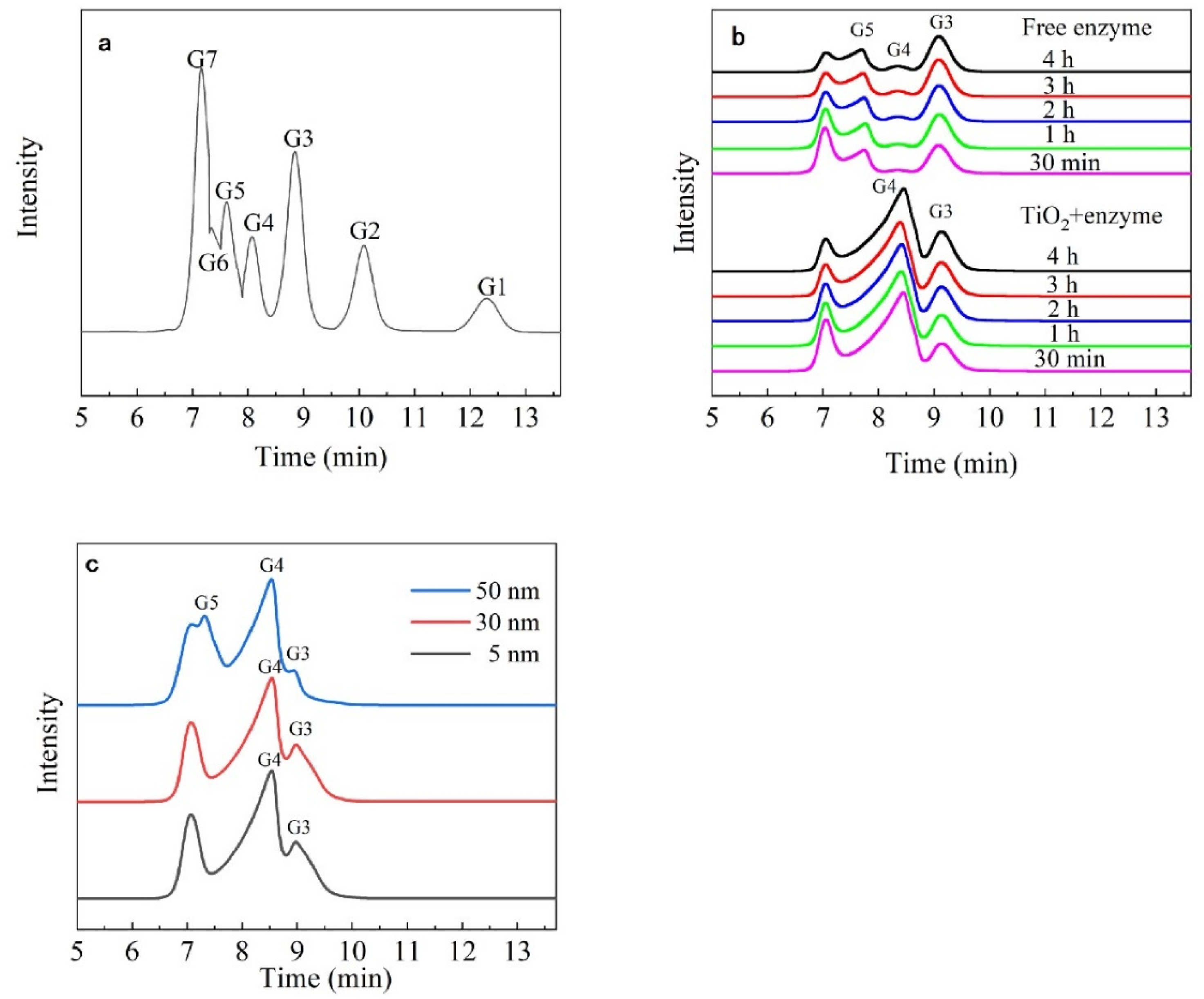
| Hydrolysis Time (h) | Hydrolysates (%) | ||
|---|---|---|---|
| Isomaltotriose | Isomaltotetraose | Isomaltopentaose | |
| Pure enzyme | |||
| 0.5 | 58.12 ± 1.94 | 6.03 ± 0.75 | 35.85 ± 5.71 |
| 1 | 61.95 ± 3.31 | 4.65 ± 1.60 | 33.40 ± 5.54 |
| 2 | 62.98 ± 4.96 | 5.4 ± 1.57 | 31.62 ± 4.31 |
| 3 | 62.34 ± 5.76 | 5.75 ± 1.55 | 31.91 ± 3.41 |
| 4 | 62.06 ± 5.45 | 5.93 ± 1.66 | 32.01 ± 3.42 |
| Immobilized enzymes | |||
| 0.5 | 21.31 ± 2.05 | 78.69 ± 4.95 | - |
| 1 | 23.23 ± 1.58 | 76.77 ± 4.59 | - |
| 2 | 25.91 ± 3.07 | 74.09 ± 4.13 | - |
| 3 | 26.66 ± 3.43 | 73.34 ± 3.66 | - |
| 4 | 26.88 ± 3.25 | 73.12 ± 3.39 | - |
| Qe,ecp (mg/g) | Pseudo First-Order Dynamics | Pseudo-Second Order Dynamics | ||||
|---|---|---|---|---|---|---|
| K1 (min−1) | Qe,1 (mg/g) | R2 | K2 (g·mg−1·min−1) | Qe,2 (mg/g) | R2 | |
| 3.99 | 0.03 | 0.308 | 0.81 | 0.21 | 3.94 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Wang, H.; Lin, Q.; Miao, Q.; Liu, M.; Ni, H.; Zhang, L.; Lyu, M.; Wang, S. Immobilization of Dextranase Obtained from the Marine Cellulosimicrobium sp. Y1 on Nanoparticles: Nano-TiO2 Improving Hydrolysate Properties and Enhancing Reuse. Nanomaterials 2023, 13, 1065. https://doi.org/10.3390/nano13061065
Xu Y, Wang H, Lin Q, Miao Q, Liu M, Ni H, Zhang L, Lyu M, Wang S. Immobilization of Dextranase Obtained from the Marine Cellulosimicrobium sp. Y1 on Nanoparticles: Nano-TiO2 Improving Hydrolysate Properties and Enhancing Reuse. Nanomaterials. 2023; 13(6):1065. https://doi.org/10.3390/nano13061065
Chicago/Turabian StyleXu, Yingying, Huanyu Wang, Qianru Lin, Qingzhen Miao, Mingwang Liu, Hao Ni, Lei Zhang, Mingsheng Lyu, and Shujun Wang. 2023. "Immobilization of Dextranase Obtained from the Marine Cellulosimicrobium sp. Y1 on Nanoparticles: Nano-TiO2 Improving Hydrolysate Properties and Enhancing Reuse" Nanomaterials 13, no. 6: 1065. https://doi.org/10.3390/nano13061065
APA StyleXu, Y., Wang, H., Lin, Q., Miao, Q., Liu, M., Ni, H., Zhang, L., Lyu, M., & Wang, S. (2023). Immobilization of Dextranase Obtained from the Marine Cellulosimicrobium sp. Y1 on Nanoparticles: Nano-TiO2 Improving Hydrolysate Properties and Enhancing Reuse. Nanomaterials, 13(6), 1065. https://doi.org/10.3390/nano13061065






