Thermal Effect during Laser-Induced Plasmonic Heating of Polyelectrolyte-Coated Gold Nanorods in Well Plates
Abstract
:1. Introduction
2. Experimental Setup and Numerical Analysis
2.1. Preparation of Polyelectrolyte-Coated GNRs
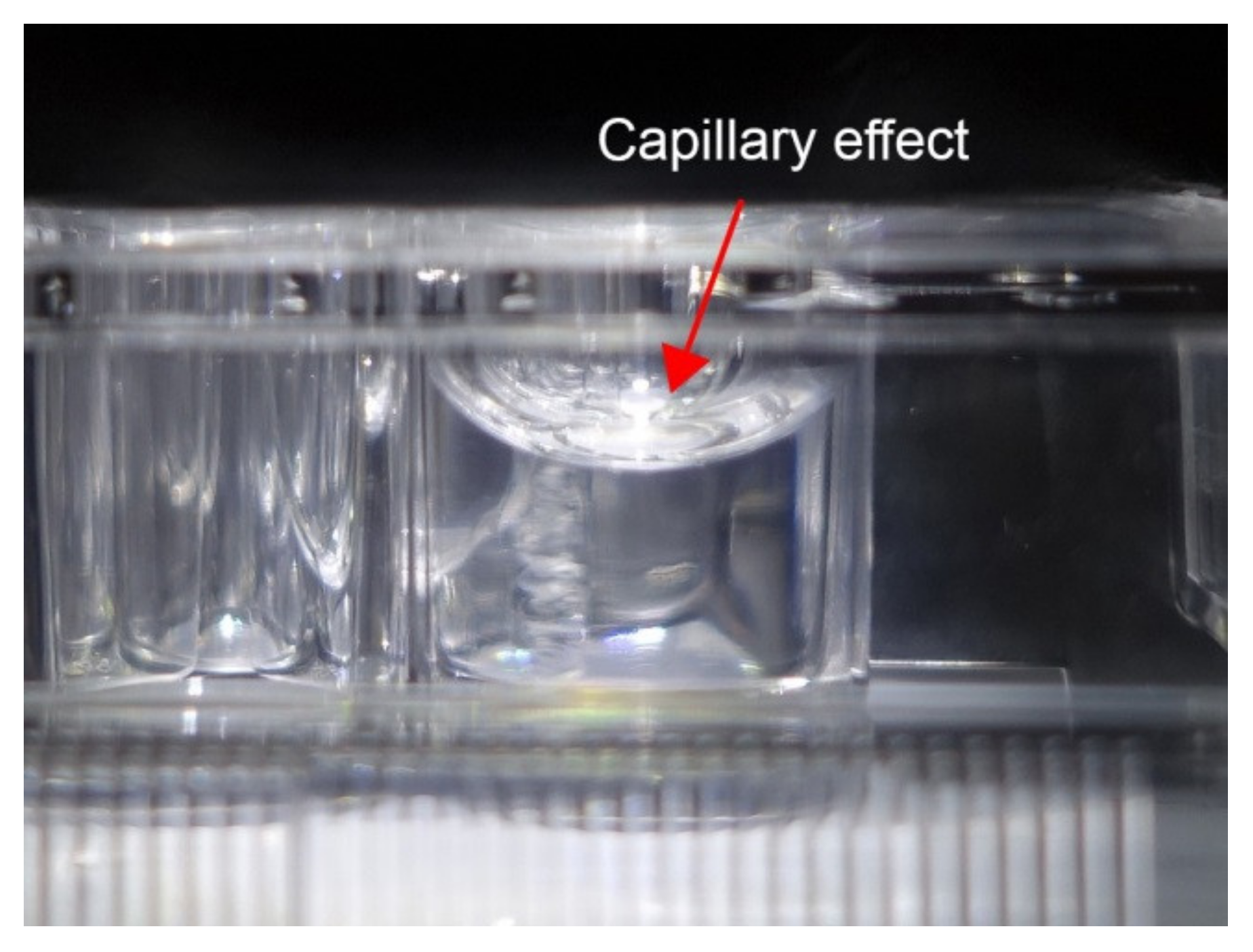
2.2. Irradiation
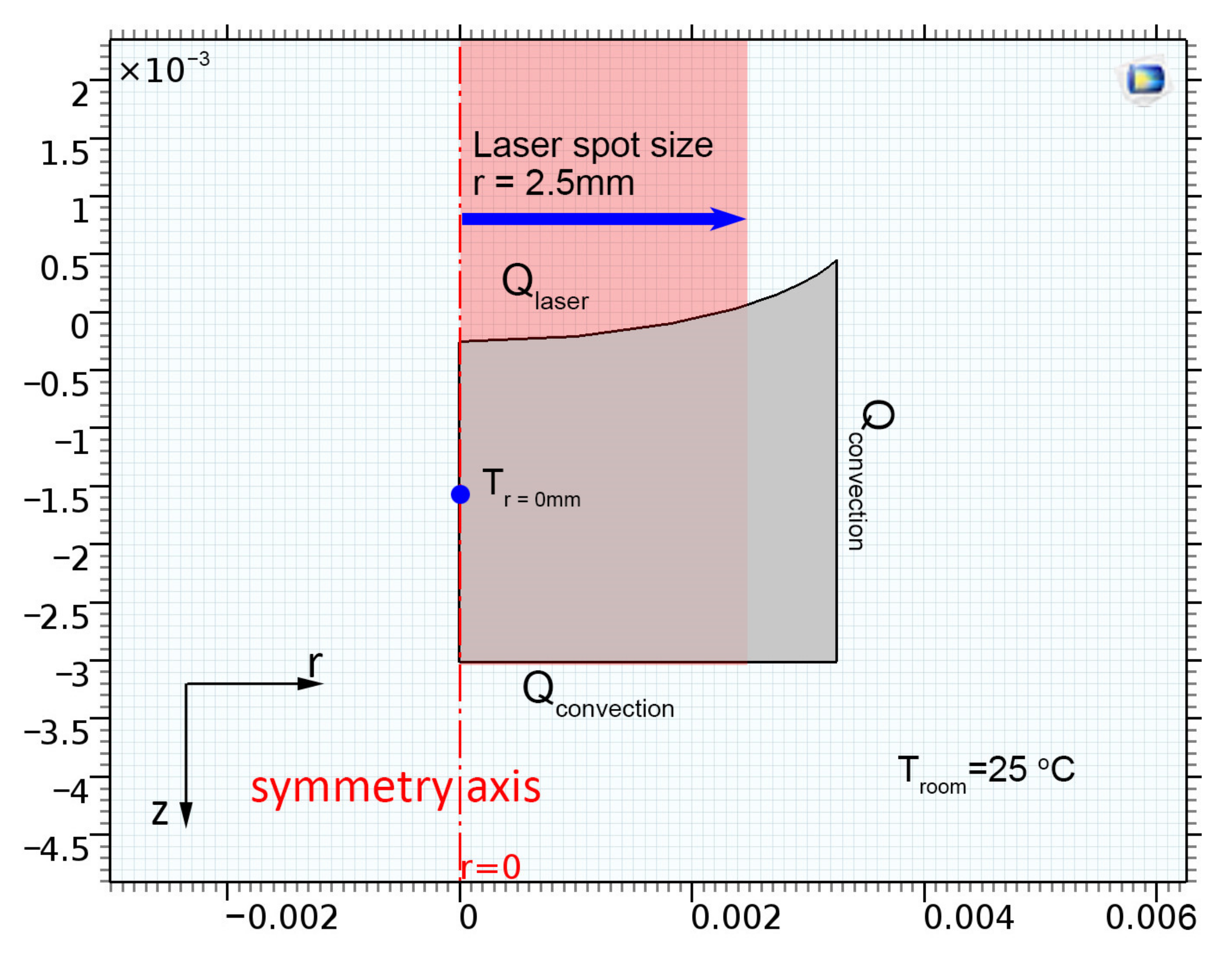
2.3. Calculation
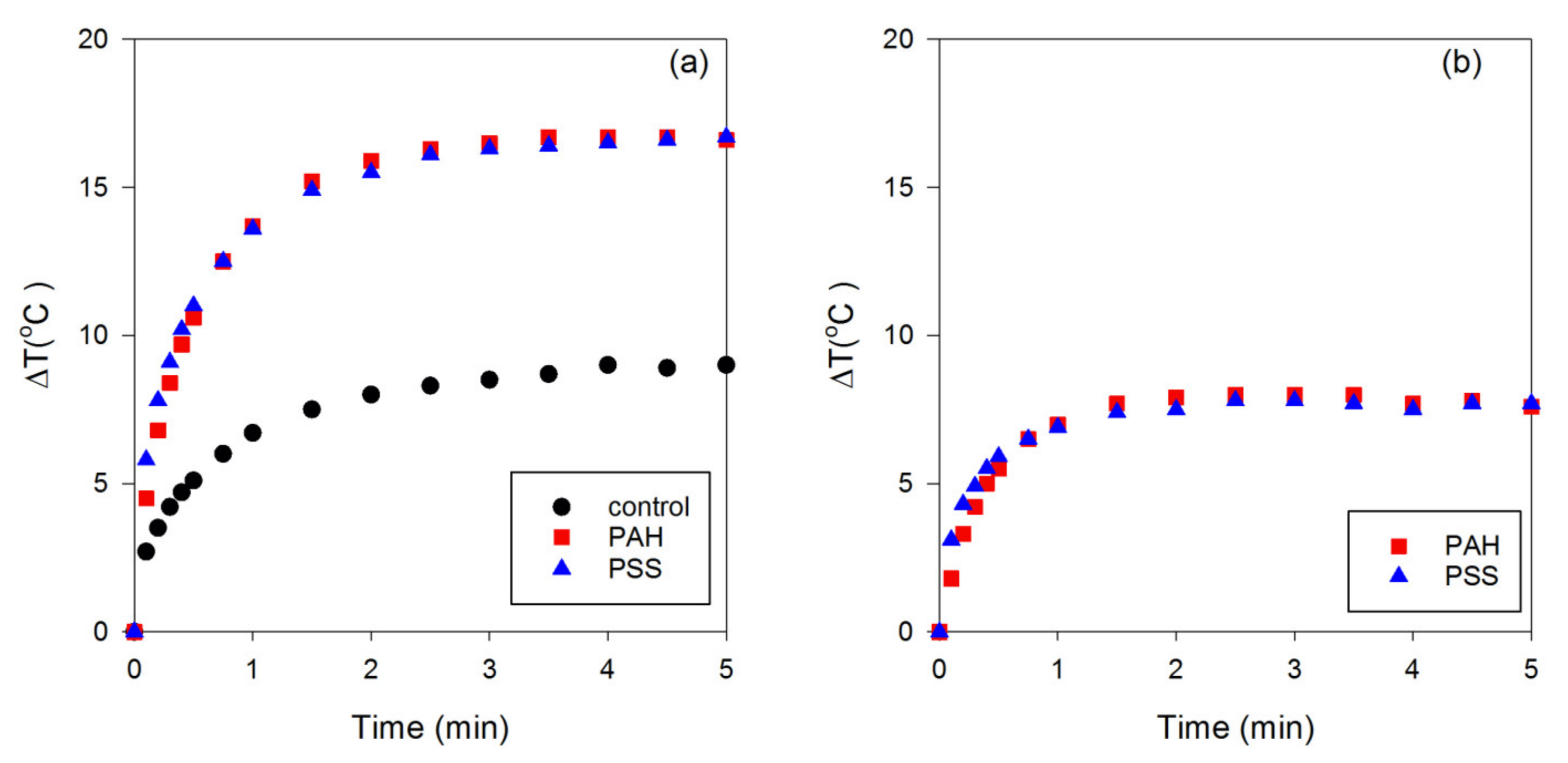
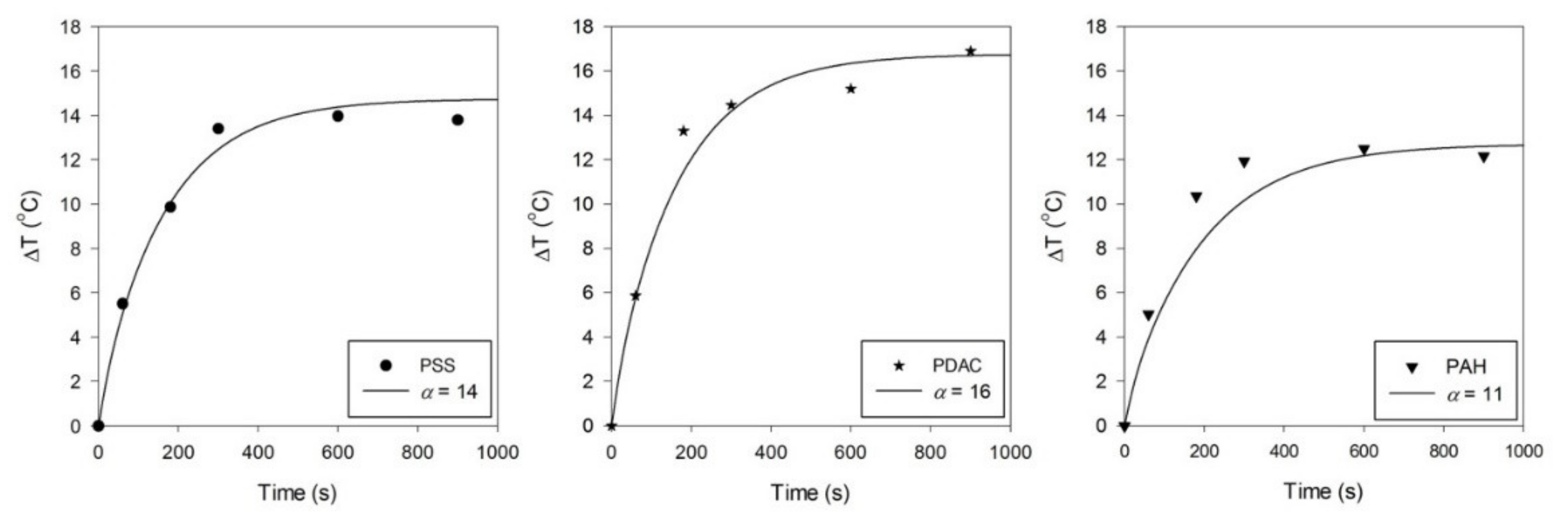
3. Results and Discussion
3.1. Polyelectrolyte-Coated GNRs
3.2. Effect of Different Surface Coatings of GNRs on Photothermal Conversion Efficiency
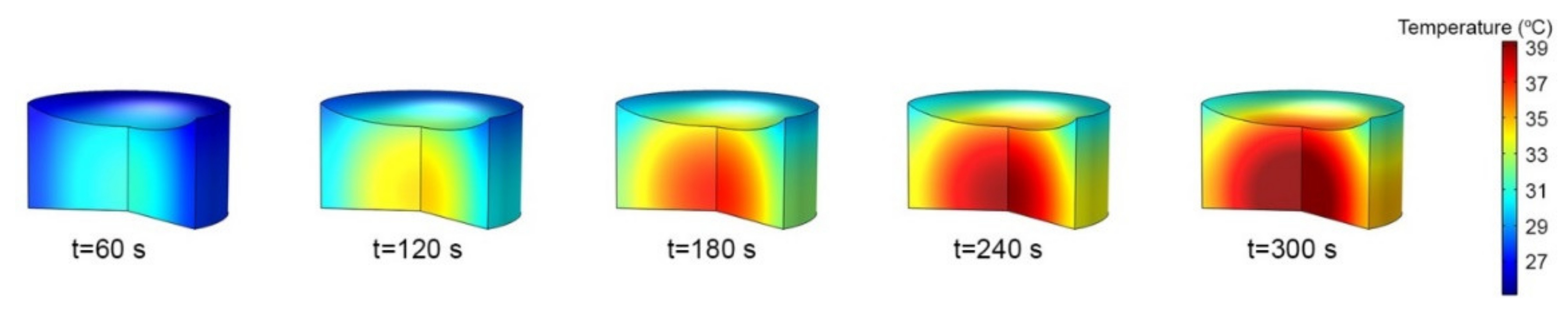
3.3. Numerical Simulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pissuwan, D.; Valenzuela, S.; Cortie, M.B. Prospects for gold nanorod particles in diagnostic and therapeutic applications. Biotechnol. Genet. Eng. Rev. 2008, 25, 93–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.N.S.; El-Sayed, M.A. Gold nanorods: From synthesis and properties to biological and biomedical applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cells assemble and align gold nanorods conjugated to antibodies to produce highly enhanced, sharp, and polarized surface Raman spectra: a potential cancer diagnostic marker. Nano Lett. 2007, 7, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [Green Version]
- Pissuwan, D.; Kumagai, Y.; Smith, N.I. Effect of surface-modified gold nanorods on the inflammatory cytokine response in macrophage cells. Part. Part. Syst. Charact. 2013, 30, 427–433. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, X.; Ji, Y.; Bai, R.; Zhao, Y.; Wu, X.; Chen, C. Surface chemistry of gold nanorods: Origin of cell membrane damage and cytotoxicity. Nanoscale 2013, 5, 8384–8391. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Pissuwan, D.; Nose, K.; Kurihara, R.; Kaneko, K.; Tahara, Y.; Kamiya, N.; Goto, M.; Katayama, Y.; Niidome, T. A solid-in-oil dispersion of gold nanorods can enhance transdermal protein delivery and skin vaccination. Small 2011, 7, 215–220. [Google Scholar] [CrossRef]
- Huang, J.; Jackson, K.S.; Murphy, C.J. Polyelectrolyte wrapping layers control rates of photothermal molecular release from gold nanorods. Nano Lett. 2012, 12, 2982–2987. [Google Scholar] [CrossRef]
- Chen, H.C.; Chi, X.; Li, B.; Zhang, M.; Ma, Y.; Achilefu, S.; Gu, Y. Drug loaded multilayered gold nanorods for combined photothermal and chemotherapy. Biomat. Sci. 2014, 2, 996–1006. [Google Scholar] [CrossRef]
- Huang, H.C.; Barua, S.; Kay, D.B.; Rege, K. Simultaneous enhancement of photothermal stability and gene delivery efficacy of gold nanorods using polyelectrolytes. ACS Nano. 2009, 3, 2941–2952. [Google Scholar] [CrossRef]
- Ekici, O.; Harrison, R.K.; Durr, N.J.; Eversole, D.S.; Lee, M.; Ben-Yakar, A. Thermal analysis of gold nanorods heated with femtosecond laser pulses. J. Phys. D Appl. Phys. 2008, 41, 185501. [Google Scholar] [CrossRef] [PubMed]
- Pallavicini, P.; Chirico, G.; Taglietti, A. Harvesting light to produce heat: Photothermal nanoparticles for technological applications and biomedical devices. Chem. Eur. J. 2021, 27, 15361. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, H.; Galbraith, K.K.; Kurisu, J.; Imahori, H.; Murakami, T.; Kengaku, M. Surface chemistry for cytosolic gene delivery and photothermal transgene expression by gold nanorods. Sci. Rep. 2017, 7, 4694. [Google Scholar] [CrossRef] [Green Version]
- Dakrong, P.; Takuro, N. Polyelectrolyte-coated gold nanorods and their biomedical applications. Nanoscale 2015, 7, 59–65. [Google Scholar]
- Yin, F.; Yang, C.; Wang, Q.; Zeng, S.; Hu, R.; Lin, G.; Tian, J.; Hu, S.; Lan, R.F.; Yoon, H.S.; et al. A light-driven therapy of pancreatic adenocarcinoma using gold nanorods-based nanocarriers for co-delivery of doxorubicin and siRNA. Theranostics 2015, 5, 818–833. [Google Scholar] [CrossRef] [Green Version]
- Ratautas, K.; Gedvilas, M.; Voisiat, B.; Račiukaitis, G.; Grigonis, A. Transformation of thin gold film to nanoparticles after nanosecond-laser irradiation. J. Laser Micro Nanoeng. 2012, 7, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef]
- Cong, B.; Kan, C.; Wang, H.; Liu, J.; Xu, H.; Ke, S. Gold Nanorods: Near-infrared plasmonic photothermal conversion and surface coating. J. Mat. Sci. Chem. Eng. 2014, 2, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Cortie, M.B.; Cortie, D.L.; Timchenko, V. Heat transfer from nanoparticles for targeted destruction of infectious organisms. Int. J. Hyperth. 2018, 34, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Brace, C. Thermal tumor ablation in clinical use. IEEE Pulse 2011, 2, 28–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, K.; Rakesh, V. An Introduction to Modeling of Transport Processes: Applications to Biomedical Systems; Cambridge University Press: Cambridge, UK, 2010; p. 303. [Google Scholar]
- Sowmya, G.; Lashin, M.M.A.; Khan, M.I.; Kumar, R.S.V.; Jagadeesha, K.C.; Prasannakumara, B.C.; Guedri, K.; Bafakeeh, O.T.; Mohamed Tag-ElDin, E.S.; Galal, A.M. Significance of convection and internal heat generation on the thermal distribution of a porous dovetail fin with radiative heat transfer by spectral collocation method. Micromachines 2022, 13, 1336. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiracheewanun, S.; Cortie, M.B.; Pissuwan, D. Thermal Effect during Laser-Induced Plasmonic Heating of Polyelectrolyte-Coated Gold Nanorods in Well Plates. Nanomaterials 2023, 13, 845. https://doi.org/10.3390/nano13050845
Jiracheewanun S, Cortie MB, Pissuwan D. Thermal Effect during Laser-Induced Plasmonic Heating of Polyelectrolyte-Coated Gold Nanorods in Well Plates. Nanomaterials. 2023; 13(5):845. https://doi.org/10.3390/nano13050845
Chicago/Turabian StyleJiracheewanun, Sujin, Michael B. Cortie, and Dakrong Pissuwan. 2023. "Thermal Effect during Laser-Induced Plasmonic Heating of Polyelectrolyte-Coated Gold Nanorods in Well Plates" Nanomaterials 13, no. 5: 845. https://doi.org/10.3390/nano13050845
APA StyleJiracheewanun, S., Cortie, M. B., & Pissuwan, D. (2023). Thermal Effect during Laser-Induced Plasmonic Heating of Polyelectrolyte-Coated Gold Nanorods in Well Plates. Nanomaterials, 13(5), 845. https://doi.org/10.3390/nano13050845





