Antimicrobial Activity of Gelatin Nanofibers Enriched by Essential Oils against Cutibacterium acnes and Staphylococcus epidermidis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Composition of Essential Oils
2.1.1. Antioxidant Activity
2.1.2. Determination of Allergens Content by HPLC
2.1.3. Composition Determined by GC/MS
2.2. Antimicrobial Susceptibility Tests
2.2.1. Preliminary Broth Microdilution Tests Minimal Inhibition Concentration (MIC) Assay
2.2.2. Resazurin Reduction Assay
2.2.3. Minimal Bactericidal Concentration Test (MBC)
2.2.4. Agar Diffusion Tests
2.3. Preparation of Gelatin Nanofibers
2.4. Nanofiber Characterization
2.5. Cytotoxicity Assays
2.6. MTT Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization of Essential Oils
3.1.1. Antioxidant Activity
3.1.2. Quantitative and Qualitative Analysis of Essential Oils
3.2. Antimicrobial Activity Testing
3.2.1. Preliminary Broth Dilution Tests—Minimal Inhibition Concentration (MIC) Assay
3.2.2. Discs Diffusion Test of Pure and Diluted EOs
3.2.3. Antimicrobial Activity of Gelatin Nanofibers Analyzed by Diffusion Test
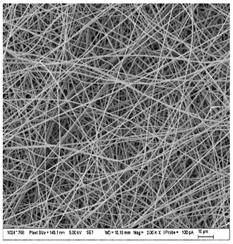
3.3. Morphology of Gelatin Nanofibers (Electrospun Meshes)
3.4. MTT Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woo, T.E.; Sibley, C.D. The Emerging Utility of the Cutaneous Microbiome in the Treatment of Acne and Atopic Dermatitis. J. Am. Acad. Derm. 2020, 82, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Chiller, K.; Selkin, B.A.; Murakawa, G.J. Skin Microflora and Bacterial Infections of the Skin. J. Investig. Dermatol. Symp. Proc. 2001, 6, 170–174. [Google Scholar] [CrossRef]
- Sarkar, S.; Patra, P.; Mridha, K.; Ghosh, S.; Mukhopadhyay, A.; Thakurta, R. Personality Disorders and Its Association with Anxiety and Depression among Patients of Severe Acne: A Cross-Sectional Study from Eastern India. Indian J. Psychiatry 2016, 58, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Castellanos Lorduy, H.J.; Pérez Cely, H.C.; Casadiego Rincón, E.J.; Henao Riveros, S.C.; Colorado, C.L. Cutibacterium Acnes Tetracycline Resistance Profile in Patients with Acne Vulgaris, in a Colombian Dermatologic Center. Actas Dermo-Sifiliográficas (Engl. Ed.) 2021, 112, 873–880. [Google Scholar] [CrossRef]
- Penso, L.; Touvier, M.; Deschasaux, M.; Szabo De Edelenyi, F.; Hercberg, S.; Ezzedine, K.; Sbidian, E. Association between Adult Acne and Dietary Behaviors: Findings from the NutriNet-Santé Prospective Cohort Study. JAMA Derm. 2020, 156, 854–862. [Google Scholar] [CrossRef]
- Burkhart, C.; Burkhart, C.; Lehmann, P. Acne: A Review of Immunologic and Microbiologic Factors. Postgrad. Med. J. 1999, 75, 328. [Google Scholar] [CrossRef] [PubMed]
- Fitz-Gibbon, S.; Tomida, S.; Chiu, B.H.; Nguyen, L.; Du, C.; Liu, M.; Elashoff, D.; Erfe, M.C.; Loncaric, A.; Kim, J.; et al. Propionibacterium Acnes Strain Populations in the Human Skin Microbiome Associated with Acne. J. Investig. Dermatol. 2013, 133, 2152–2160. [Google Scholar] [CrossRef]
- Scholz, C.F.P.; Kilian, M. The Natural History of Cutaneous Propionibacteria, and Reclassification of Selected Species within the Genus Propionibacterium to the Proposed Novel Genera Acidipropionibacterium Gen. Nov., Cutibacterium Gen. Nov. and Pseudopropionibacterium Gen. Nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 4422–4432. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B. What Is New in the Pathophysiology of Acne, an Overview. J. Eur. Acad. Derm. Venereol. 2017, 31 (Suppl. 5), 8–12. [Google Scholar] [CrossRef]
- Dalgard, F.; Gieler, U.; Holm, J.Ø.; Bjertness, E.; Hauser, S. Self-Esteem and Body Satisfaction among Late Adolescents with Acne: Results from a Population Survey. J. Am. Acad. Derm. 2008, 59, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Pécastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium Acnes (Propionibacterium acnes) and Acne Vulgaris: A Brief Look at the Latest Updates. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Aubin, G.G.; Portillo, M.E.; Trampuz, A.; Corvec, S. Propionibacterium Acnes, an Emerging Pathogen: From Acne to Implant-Infections, from Phylotype to Resistance. Med. Mal. Infect. 2014, 44, 241–250. [Google Scholar] [CrossRef]
- Pineau, R.M.; Hanson, S.E.; Lyles, J.T.; Quave, C.L. Growth Inhibitory Activity of Callicarpa Americana Leaf Extracts against Cutibacterium acnes. Front. Pharm. 2019, 10, 1206. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; del Rosso, J.Q.; Mancini, A.J.; Cook-Bolden, F.; Gold, L.S.; Desai, S.; Weiss, J.; Pariser, D.; Zeichner, J.; Bhatia, N.; et al. Evolving Perspectives on the Etiology and Pathogenesis of Acne Vulgaris. J. Drugs Derm. 2015, 14, 263–272. [Google Scholar]
- Thiboutot, D.; Gollnick, H.; Bettoli, V.; Dréno, B.; Kang, S.; Leyden, J.J.; Shalita, A.R.; Lozada, V.T.; Berson, D.; Finlay, A.; et al. New Insights into the Management of Acne: An Update from the Global Alliance to Improve Outcomes in Acne Group. J. Am. Acad. Derm. 2009, 60, S1–S50. [Google Scholar] [CrossRef]
- Sardana, K.; Gupta, T.; Garg, V.K.; Ghunawat, S. Antibiotic Resistance to Propionobacterium acnes: Worldwide Scenario, Diagnosis and Management. Expert Rev. Anti-Infect. Ther. 2015, 13, 883–896. [Google Scholar] [CrossRef]
- Esmael, A.; Hassan, M.G.; Amer, M.M.; Abdelrahman, S.; Hamed, A.M.; Abd-raboh, H.A.; Foda, M.F. Antimicrobial Activity of Certain Natural-Based Plant Oils against the Antibiotic-Resistant Acne Bacteria. Saudi J. Biol. Sci. 2020, 27, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential Oils: From Extraction to Encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Cossetin, L.F.; Garlet, Q.I.; Velho, M.C.; Gündel, S.; Ourique, A.F.; Heinzmann, B.M.; Monteiro, S.G. Development of Nanoemulsions Containing Lavandula dentata or Myristica fragrans Essential Oils: Influence of Temperature and Storage Period on Physical-Chemical Properties and Chemical Stability. Ind. Crops Prod. 2021, 160, 113115. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P. Chemical Composition, Seasonal Variability, and Antifungal Activity of Lavandula stoechas L. Ssp. Stoechas Essential Oils from Stem/Leaves and Flowers. J. Agric. Food Chem. 2006, 54, 4364–4370. [Google Scholar] [CrossRef]
- Smith, R.L.; Cohen, S.M.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Portoghese, P.S.; Waddell, W.J.; Wagner, B.M.; Hall, R.L.; et al. A Procedure for the Safety Evaluation of Natural Flavor Complexes Used as Ingredients in Food: Essential Oils. Food Chem. Toxicol. 2005, 43, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Rather, A.H.; Wani, T.U.; Khan, R.S.; Pant, B.; Park, M.; Sheikh, F.A. Prospects of Polymeric Nanofibers Loaded with Essential Oils for Biomedical and Food-Packaging Applications. Int. J. Mol. Sci. 2021, 22, 4017. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Cui, H. Moringa Oil/Chitosan Nanoparticles Embedded Gelatin Nanofibers for Food Packaging against Listeria monocytogenes and Staphylococcus aureus on Cheese. Food Packag. Shelf. Life 2019, 19, 86–93. [Google Scholar] [CrossRef]
- Hadipour-Goudarzi, E.; Montazer, M.; Latifi, M.; Aghaji, A.A.G. Electrospinning of Chitosan/Sericin/PVA Nanofibers Incorporated with in Situ Synthesis of Nano Silver. Carbohydr. Polym. 2014, 113, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Meziani, J.; Li, Z.; Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef]
- Tsai, R.Y.; Hung, S.C.; Lai, J.Y.; Wang, D.M.; Hsieh, H.J. Electrospun Chitosan–Gelatin–Polyvinyl Alcohol Hybrid Nanofibrous Mats: Production and Characterization. J. Taiwan Inst. Chem. Eng. 2014, 45, 1975–1981. [Google Scholar] [CrossRef]
- Bhattarai, R.S.; Bachu, R.D.; Boddu, S.H.S.; Bhaduri, S. Biomedical Applications of Electrospun Nanofibers: Drug and Nanoparticle Delivery. Pharmaceutics 2018, 11, 5. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Fathi, M.; Kadivar, M. Production and Characterization of Chitosan-Gelatin Nanofibers by Nozzle-Less Electrospinning and Their Application to Enhance Edible Film’s Properties. Food Packag. Shelf. Life 2019, 22, 100387. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, C.; Wang, Q.; Zhou, F.; Li, T.; Wang, B.; Su, W.; Shang, D.; Wu, S. A Simple, Quick, and Cost-Effective Strategy to Fabricate Polycaprolactone/Silk Fibroin Nanofiber Yarns for Biotextile-Based Tissue Scaffold Application. Eur. Polym. J. 2023, 186, 111863. [Google Scholar] [CrossRef]
- Li, M.; Qiu, W.; Wang, Q.; Li, N.; Liu, L.; Wang, X.; Yu, J.; Li, X.; Li, F.; Wu, D. Nitric Oxide-Releasing Tryptophan-Based Poly(Ester Urea)s Electrospun Composite Nanofiber Mats with Antibacterial and Antibiofilm Activities for Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 15911–15926. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, E.S.; Glenn, G.M.; Klamczynski, A.P.; Orts, W.J.; Mattoso, L.H.C. Solution Blow Spinning: A New Method to Produce Micro- and Nanofibers from Polymer Solutions. J. Appl. Polym. Sci. 2009, 113, 2322–2330. [Google Scholar] [CrossRef]
- Li, M.; Yu, H.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Fabrication of Eugenol Loaded Gelatin Nanofibers by Electrospinning Technique as Active Packaging Material. LWT 2021, 139, 110800. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Villa, C.; Gambaro, R.; Mariani, E.; Dorato, S. High-Performance Liquid Chromatographic Method for the Simultaneous Determination of 24 Fragrance Allergens to Study Scented Products. J. Pharm. Biomed. Anal. 2007, 44, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.K.; Hospenthal, D.R. Broth Microdilution Susceptibility Testing for Leptospira Spp. Antimicrob. Agents Chemother. 2004, 48, 1548. [Google Scholar] [CrossRef]
- Lavogina, D.; Lust, H.; Tahk, M.J.; Laasfeld, T.; Vellama, H.; Nasirova, N.; Vardja, M.; Eskla, K.L.; Salumets, A.; Rinken, A.; et al. Revisiting the Resazurin-Based Sensing of Cellular Viability: Widening the Application Horizon. Biosensors 2022, 12, 196. [Google Scholar] [CrossRef]
- Palomino, J.C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin Microtiter Assay Plate: Simple and Inexpensive Method for Detection of Drug Resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 2002, 46, 2720. [Google Scholar] [CrossRef]
- Blažeković, B.; Yang, W.; Wang, Y.; Li, C.; Kindl, M.; Pepeljnjak, S.; Vladimir-Knežević, S. Chemical Composition, Antimicrobial and Antioxidant Activities of Essential Oils of Lavandula × intermedia ‘Budrovka’ and L. Angustifolia Cultivated in Croatia. Ind. Crops Prod. 2018, 123, 173–182. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, Y.; Liu, Y.; Cui, B. Physicochemical Characterization and Antibacterial Activity Assessment of Lavender Essential Oil Encapsulated in Hydroxypropyl-Beta-Cyclodextrin. Ind. Crops Prod. 2019, 130, 104–110. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Zhang, C.; Feng, F.; Zhang, H. Sequential Electrospinning of Multilayer Ethylcellulose/Gelatin/Ethylcellulose Nanofibrous Film for Sustained Release of Curcumin. Food Chem. 2020, 308, 125599. [Google Scholar] [CrossRef] [PubMed]
- Bokrova, J.; Marova, I.; Matouskova, P.; Pavelkova, R. Fabrication of Novel PHB-Liposome Nanoparticles and Study of Their Toxicity in Vitro. J. Nanoparticle Res. 2019, 21, 1–12. [Google Scholar] [CrossRef]
- Li, X.; Turánek, J.; Knötigová, P.; Kudláčková, H.; Mašek, J.; Parkin, S.; Rankin, S.E.; Knutson, B.L.; Lehmler, H.J. Hydrophobic Tail Length, Degree of Fluorination and Headgroup Stereochemistry Are Determinants of the Biocompatibility of (Fluorinated) Carbohydrate Surfactants. Colloids Surf. Biointerfaces 2009, 73, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, Ì.; Şat, I.G.; Beydemir, Ş.; Elmastaş, M.; Küfrevioǧlu, Ö.I. Comparison of Antioxidant Activity of Clove (Eugenia caryophylata Thunb) Buds and Lavender (Lavandula stoechas L.). Food Chem. 2004, 87, 393–400. [Google Scholar] [CrossRef]
- Hou, T.; Sana, S.S.; Li, H.; Xing, Y.; Nanda, A.; Netala, V.R.; Zhang, Z. Essential Oils and Its Antibacterial, Antifungal and Anti-Oxidant Activity Applications: A Review. Food Biosci. 2022, 101716. [Google Scholar] [CrossRef]
- Badr, M.M.; Badawy, M.E.I.; Taktak, N.E.M. Characterization, Antimicrobial Activity, and Antioxidant Activity of the Nanoemulsions of Lavandula spica Essential Oil and Its Main Monoterpenes. J. Drug Deliv. Sci. Technol. 2021, 65, 102732. [Google Scholar] [CrossRef]
- Kıvrak, Ş. Essential Oil Composition and Antioxidant Activities of Eight Cultivars of Lavender and Lavandin from Western Anatolia. Ind. Crops Prod. 2018, 117, 88–96. [Google Scholar] [CrossRef]
- Al-Ansari, M.M.; Andeejani, A.M.I.; Alnahmi, E.; AlMalki, R.H.; Masood, A.; Vijayaraghavan, P.; Rahman, A.A.; Choi, K.C. Insecticidal, Antimicrobial and Antioxidant Activities of Essential Oil from Lavandula latifolia L. and Its Deterrent Effects on Euphoria leucographa. Ind. Crops Prod. 2021, 170, 113740. [Google Scholar] [CrossRef]
- Dammak, I.; Hamdi, Z.; Kammoun El Euch, S.; Zemni, H.; Mliki, A.; Hassouna, M.; Lasram, S. Evaluation of Antifungal and Anti-Ochratoxigenic Activities of Salvia Officinalis, Lavandula Dentata and Laurus Nobilis Essential Oils and a Major Monoterpene Constituent 1,8-Cineole against Aspergillus carbonarius. Ind. Crops Prod. 2019, 128, 85–93. [Google Scholar] [CrossRef]
- El Hamdaoui, A.; Msanda, F.; Boubaker, H.; Leach, D.; Bombarda, I.; Vanloot, P.; El Aouad, N.; Abbad, A.; Boudyach, E.H.; Achemchem, F.; et al. Essential Oil Composition, Antioxidant and Antibacterial Activities of Wild and Cultivated Lavandula mairei Humbert. Biochem. Syst. Ecol. 2018, 76, 1–7. [Google Scholar] [CrossRef]
- Cherrat, L.; Espina, L.; Bakkali, M.; Pagán, R.; Laglaoui, A. Chemical Composition, Antioxidant and Antimicrobial Properties of Mentha pulegium, Lavandula stoechas and Satureja calamintha Scheele Essential Oils and an Evaluation of Their Bactericidal Effect in Combined Processes. Innov. Food Sci. Emerg. Technol. 2014, 22, 221–229. [Google Scholar] [CrossRef]
- Mollaei, S.; Ebadi, M.; Hazrati, S.; Habibi, B.; Gholami, F.; Sourestani, M.M. Essential Oil Variation and Antioxidant Capacity of Mentha pulegium Populations and Their Relation to Ecological Factors. Biochem. Syst. Ecol. 2020, 91, 104084. [Google Scholar] [CrossRef]
- Marincaş, O.; Feher, I. A New Cost-Effective Approach for Lavender Essential Oils Quality Assessment. Ind. Crops Prod. 2018, 125, 241–247. [Google Scholar] [CrossRef]
- Martucci, J.F.; Gende, L.B.; Neira, L.M.; Ruseckaite, R.A. Oregano and Lavender Essential Oils as Antioxidant and Antimicrobial Additives of Biogenic Gelatin Films. Ind. Crops Prod. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Virgiliou, C.; Zisi, C.; Kontogiannopoulos, K.N.; Nakas, A.; Iakovakis, A.; Varsamis, V.; Gika, H.G.; Assimopoulou, A.N. Headspace Gas Chromatography-Mass Spectrometry in the Analysis of Lavender’s Essential Oil: Optimization by Response Surface Methodology. J. Chromatogr. 2021, 1179, 122852. [Google Scholar] [CrossRef] [PubMed]
- Ilić, Z.S.; Milenković, L.; Tmušić, N.; Stanojević, L.; Stanojević, J.; Cvetković, D. Essential Oils Content, Composition and Antioxidant Activity of Lemon Balm, Mint and Sweet Basil from Serbia. LWT 2022, 153, 112210. [Google Scholar] [CrossRef]
- Desam, N.R.; Al-Rajab, A.J.; Sharma, M.; Mylabathula, M.M.; Gowkanapalli, R.R.; Albratty, M. Chemical Constituents, in Vitro Antibacterial and Antifungal Activity of Mentha × piperita L. (Peppermint) Essential Oils. J. King Saud. Univ. Sci. 2019, 31, 528–533. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential Oils as Antimicrobials in Food Systems—A Review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Botsaris, G.; Tzortzakis, N. Antioxidant and Antibacterial Activities, Mineral and Essential Oil Composition of Spearmint (Mentha spicata L.) Affected by the Potassium Levels. Ind. Crops Prod. 2017, 103, 202–212. [Google Scholar] [CrossRef]
- Jamróz, E.; Juszczak, L.; Kucharek, M. Investigation of the Physical Properties, Antioxidant and Antimicrobial Activity of Ternary Potato Starch-Furcellaran-Gelatin Films Incorporated with Lavender Essential Oil. Int. J. Biol. Macromol. 2018, 114, 1094–1101. [Google Scholar] [CrossRef]
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils Against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Sharma, A.; Baek, K.H. Antibacterial Mode of Action of Cudrania tricuspidata Fruit Essential Oil, Affecting Membrane Permeability and Surface Characteristics of Food-Borne Pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Xiang, F.; Bai, J.; Tan, X.; Chen, T.; Yang, W.; He, F. Antimicrobial Activities and Mechanism of the Essential Oil from Artemisia argyi Levl. et Van. Var. Argyi Cv. Qiai. Ind. Crops Prod. 2018, 125, 582–587. [Google Scholar] [CrossRef]
- Maddheshiya, S.; Ahmad, A.; Ahmad, W.; Zakir, F.; Aggarwal, G. Essential Oils for the Treatment of Skin Anomalies: Scope and Potential. S. Afr. J. Bot. 2022, 151, 187–197. [Google Scholar] [CrossRef]
- Zu, Y.; Yu, H.; Liang, L.; Fu, Y.; Efferth, T.; Liu, X.; Wu, N. Activities of Ten Essential Oils towards Propionibacterium acnes and PC-3, A-549 and MCF-7 Cancer Cells. Molecules 2010, 15, 3200–3210. [Google Scholar] [CrossRef]
- Kozics, K.; Bučková, M.; Puškárová, A.; Kalászová, V.; Cabicarová, T.; Pangallo, D. The Effect of Ten Essential Oils on Several Cutaneous Drug-Resistant Microorganisms and Their Cyto/Genotoxic and Antioxidant Properties. Molecules 2019, 24, 4570. [Google Scholar] [CrossRef]
- Alexa, V.T.; Galuscan, A.; Soica, C.M.; Cozma, A.; Coricovac, D.; Borcan, F.; Popescu, I.; Mioc, A.; Szuhanek, C.; Dehelean, C.A.; et al. In Vitro Assessment of the Cytotoxic and Antiproliferative Profile of Natural Preparations Containing Bergamot, Orange and Clove Essential Oils. Molecules 2022, 27, 990. [Google Scholar] [CrossRef]
| Compound | Lavender F Bulgaria | Lavender S Provence |
|---|---|---|
| Linalool | 31.26 | 36.98 |
| Linalyl Acetate | 31.63 | 42.13 |
| beta-Caryophyllene | 3.96 | 2.37 |
| 3-Octanone | 1.48 | 0.54 |
| Bornyl Acetate | 0.1 | ND |
| Borneol | ND | 1.52 |
| beta-Farnesene | 3.96 | ND |
| Lavandulyl Acetate | 3.9 | ND |
| Terpinen-4-ol | 3.74 | ND |
| Lavandulol | 1.77 | ND |
| Ocimene | 5.13 | ND |
| Limonene | 0.761 | ND |
| Total number of components | 65 | 59 |
| Compound | Mint S India | Mint F Spain |
|---|---|---|
| Menthol | 48.52 | 40 |
| Menthone | 19.61 | 10–25 |
| Isomenthone | ND | 10–25 |
| Neomenthol/Menthofuran/Isomenthone | 8.83 | ND |
| alpha-Pinen | 0.27 | 1–10 |
| beta-Pinen | 0.26 | 1–10 |
| Limonene | 1.71 | 1–10 |
| Pulegone | 2.21 | 5 |
| Methyl acetate | 4.4 | ND |
| 1,8-Cineol | 4.04 | ND |
| beta-Caryophyllene | 2.16 | ND |
| Linalool | 0.33 | ND |
| Total number of components | 50 | 62 |
| Sample | Antioxidant Activity (mmolTE) | SC50 (mg/mL) |
|---|---|---|
| Mint F Spain | 0.415 ± 0.004 * | 1.90 ± 0.02 * |
| Mint S India | 0.217 ± 0.016 * | 3.64 ± 0.27 * |
| Lavender F Bulgaria | 0.033 ± 0.026 | 15.37 ± 0.86 |
| Lavender S Provence | 0.037 ± 0.021 | 16.37 ± 1.12 |
| Sample | Lavender F Bulgaria | Lavender S Provence | Mint F Spain | Mint S India |
|---|---|---|---|---|
| Allergen | (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) |
| Coumarin | ND Q | 2.36 Q | ND | ND |
| Linalool | 441.41 Q | 479.05 Q | 17.32 Q | 76.29 |
| Citral | 18.42 Q | ND Q | 6.81 | 39.04 |
| Limonene | 21.14 Q | 2.39 Q | 20.08 Q | 47.64 Q |
| Geraniol | 15.43 Q | 5.55 Q | ND | ND |
| Eugenol | ND | ND | 1.89 Q | 1.16 Q |
| Myrcene | Q | ND | ND | ND |
| Eucalyptole | Q | Q | Q | Q |
| (Z)-beta-Ocimene | Q | ND | ND | ND |
| Octenyl Acetate | Q | ND | ND | ND |
| Linalyl Butyrate | ND | Q | Q | Q |
| Bornanone | ND | Q | ND | ND |
| beta-Caryophyllene | ND | Q | Q | ND |
| Menthone | ND | ND | Q | Q |
| Menthol | ND | ND | Q | Q |
| S. epidermidis | C. acnes | |||
|---|---|---|---|---|
| Sample | MIC (μL/mL) | MBC (μL/mL) | MIC (μL/mL) | MBC (μL/mL) |
| Lavender F Bulgaria | 8.3 ± 2.2 | 16.7 ± 7.2 | 9.4 ± 3.6 | 25.0 ± 2.1 |
| Lavender S Provence | 6.3 ± 1.0 | 12.5 ± 1.0 | 6.3 ± 1.0 | 25.0 ± 1.0 |
| Mint F Spain | 6.3 ± 3.0 | 9.4 ± 3.1 | 6.3 ± 3.4 | 20.8 ±7.2 |
| Mint S India | 7.3 ± 2.1 | 12.5 ± 1.1 | 5.7 ± 0.9 | 14.0 ± 5.6 |
| S. epidermidis | C. acnes | |||
|---|---|---|---|---|
| Sample | Pure EO | 20% in Almond Oil | Pure EO | 20% in Almond Oil |
| Inhibition Zones (mm) | Inhibition Zones (mm) | |||
| Lavender F Bulgaria | 14.0 ± 2.0 | 6.0 ± 0.5 | +,+,+ | +,+,+ |
| Lavender S Provence | 16.5 ± 0.5 | 6.5 ± 0.5 | +,+,+ | +,+,+ |
| Mint F Spain | 28.0 ± 1.0 | 8.5 ± 1.5 | ++,++,++ | +,+,+ |
| Mint S India | 26.0 ± 4.0 | 6.5 ± 0.5 | ++,++,++ | +,+,− |
| C. acnes—control agar plate | C. acnes—lavender F Bulgaria |
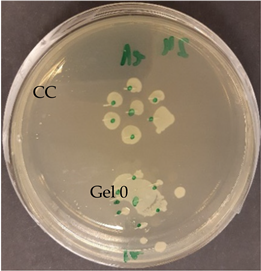 | 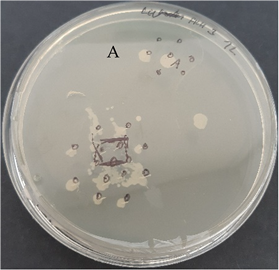 |
| S. epidermidis—control agar plate | S. epidermidis—lavender F Bulgaria |
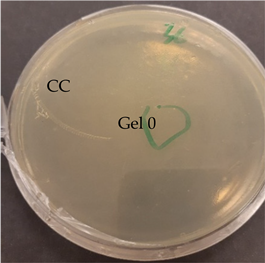 | 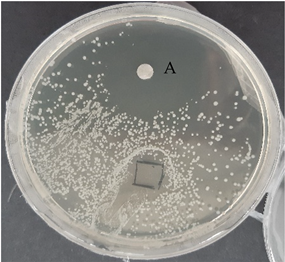 |
| A: Gelatin | B: Gelatin with 2% EO | C: Gelatin with 20%EO |
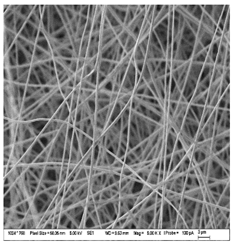 | 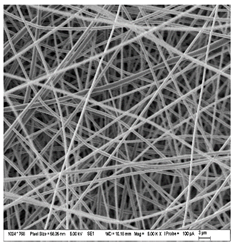 | 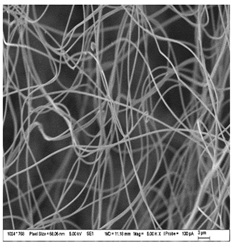 |
| A1: 5000x | B1: 5000x | C1: 5000x |
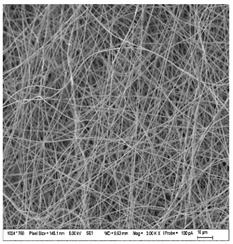 |  |  |
| A2: 2000x | B2: 2000x | C2: 2000x |
| Sample | Fiber Diameter (nm) | Treatment Pairs | Tukey HSD p-Value | Tukey HSD Inference |
|---|---|---|---|---|
| Gelatin (A) | 430.8 ± 20.6 a | A vs. B | 0.082 8 | n.s. |
| Gelatin 2% EO (B) | 476.6 ± 51.2 b | A vs. C | 0.317 1 | n.s. |
| Gelatin 20% EO (C) | 402.6 ± 37.7 ab | B vs. C | 0.001 1 | ** p < 0.01 |
| Sample | LC10 (μL/mL) | LC50 (μL/mL) | LC90 (μL/mL) |
|---|---|---|---|
| Lavender F Bulgaria | 0.70 ± 0.09 | 1.48 ± 0.16 | 2.26 ± 0.22 |
| Lavender S Provence | 0.27 ± 0.08 | 0.95 ± 0.29 | 1.63 ± 0.50 |
| Mint F Spain | 0.45 ± 0.15 | 2.02 ± 0.06 | 3.59 ± 0.03 |
| Mint S India | 0.40 ± 0.06 | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uhlířová, R.; Langová, D.; Bendová, A.; Gross, M.; Skoumalová, P.; Márová, I. Antimicrobial Activity of Gelatin Nanofibers Enriched by Essential Oils against Cutibacterium acnes and Staphylococcus epidermidis. Nanomaterials 2023, 13, 844. https://doi.org/10.3390/nano13050844
Uhlířová R, Langová D, Bendová A, Gross M, Skoumalová P, Márová I. Antimicrobial Activity of Gelatin Nanofibers Enriched by Essential Oils against Cutibacterium acnes and Staphylococcus epidermidis. Nanomaterials. 2023; 13(5):844. https://doi.org/10.3390/nano13050844
Chicago/Turabian StyleUhlířová, Renata, Denisa Langová, Agáta Bendová, Michal Gross, Petra Skoumalová, and Ivana Márová. 2023. "Antimicrobial Activity of Gelatin Nanofibers Enriched by Essential Oils against Cutibacterium acnes and Staphylococcus epidermidis" Nanomaterials 13, no. 5: 844. https://doi.org/10.3390/nano13050844
APA StyleUhlířová, R., Langová, D., Bendová, A., Gross, M., Skoumalová, P., & Márová, I. (2023). Antimicrobial Activity of Gelatin Nanofibers Enriched by Essential Oils against Cutibacterium acnes and Staphylococcus epidermidis. Nanomaterials, 13(5), 844. https://doi.org/10.3390/nano13050844






