Sensor to Electronics Applications of Graphene Oxide through AZO Grafting
Abstract
1. Introduction
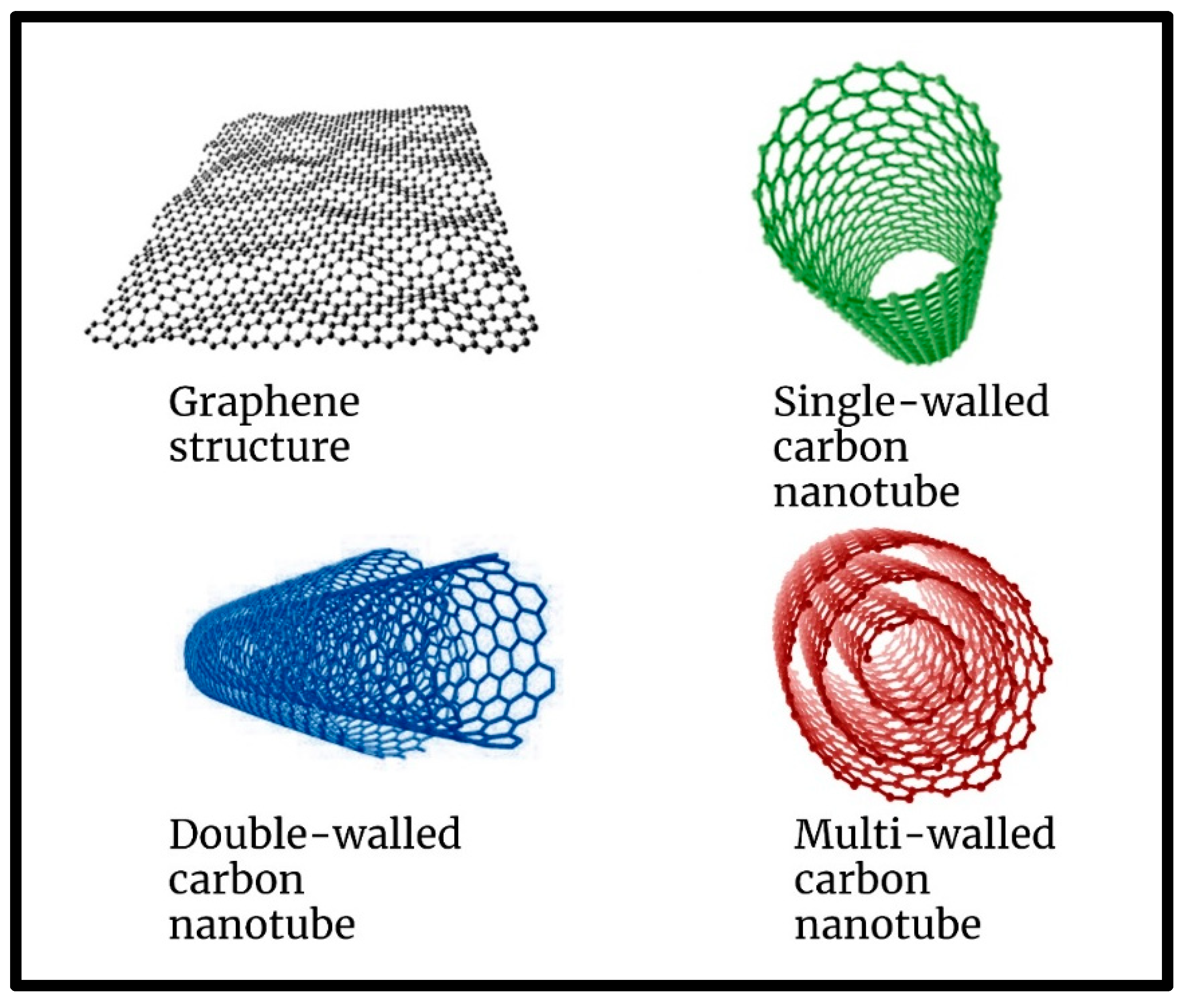
1.1. Graphene-Related 2D Material (Gr2Ms) Foundation to All Carbon Materials
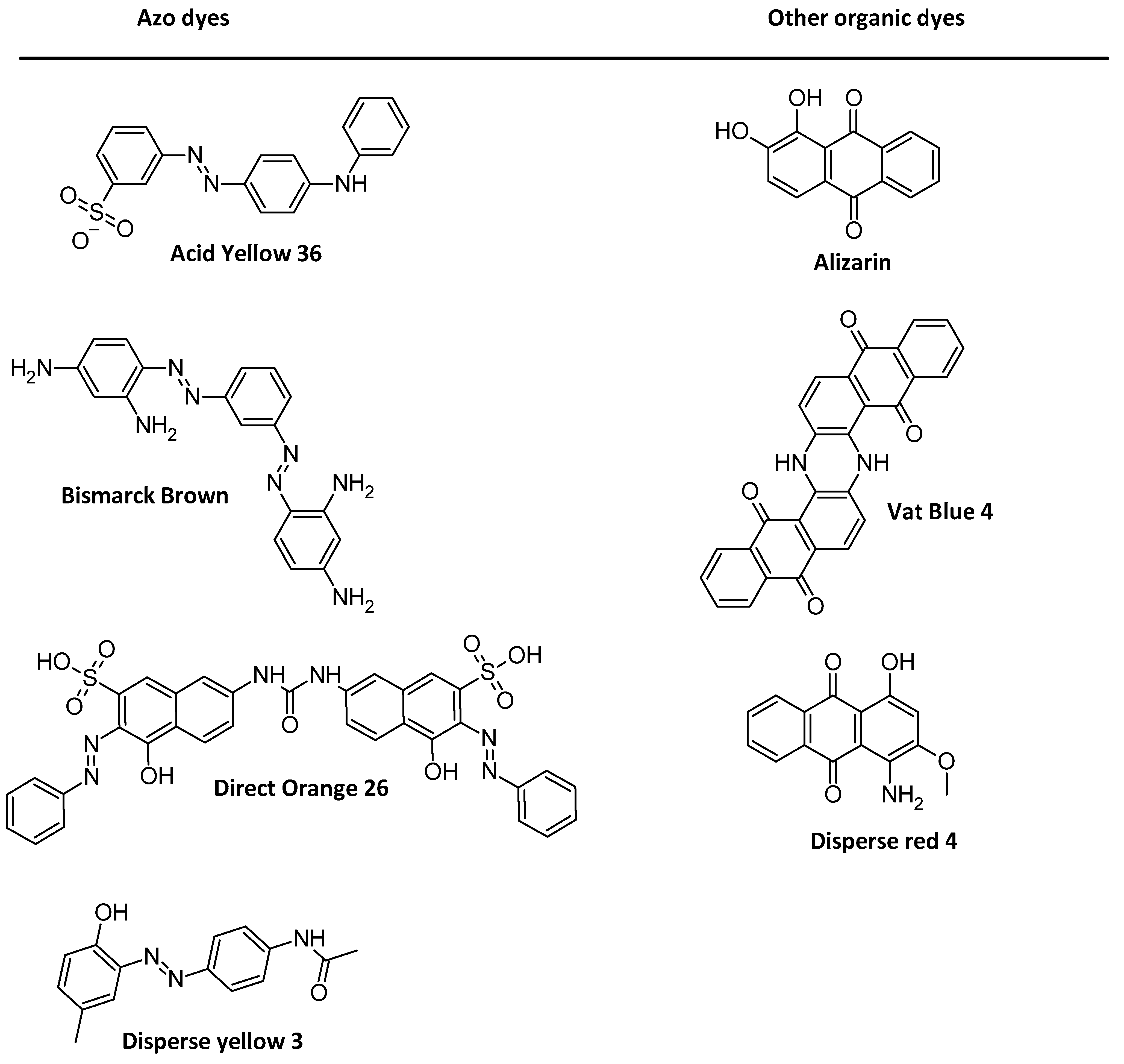
1.2. AZO Dyes
2. General Review of Graphene-Based Materials and GO-AZO Syntheses
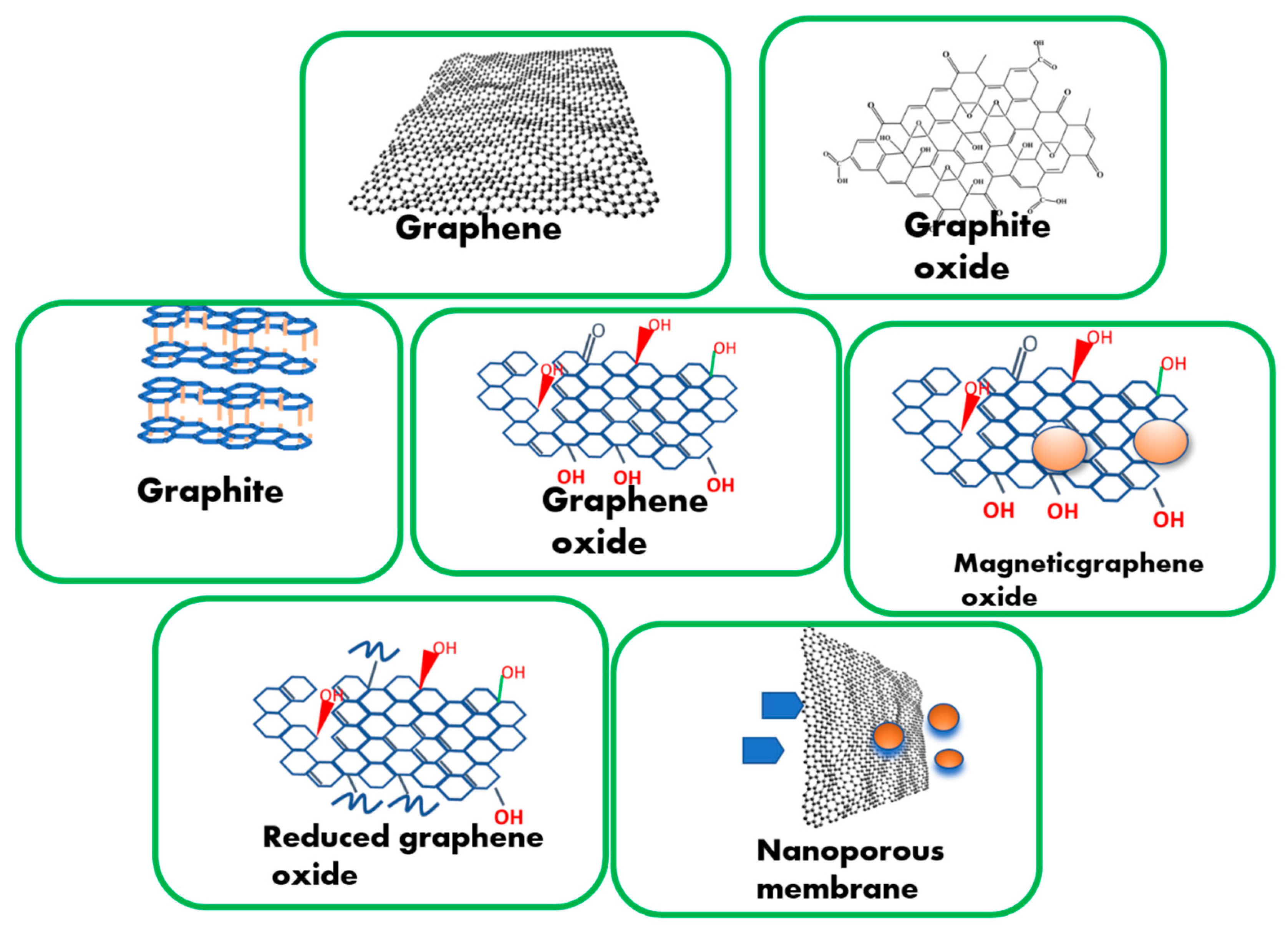
2.1. Existing Graphene-Based Materials Available for Hybridization
2.2. GO Synthesis
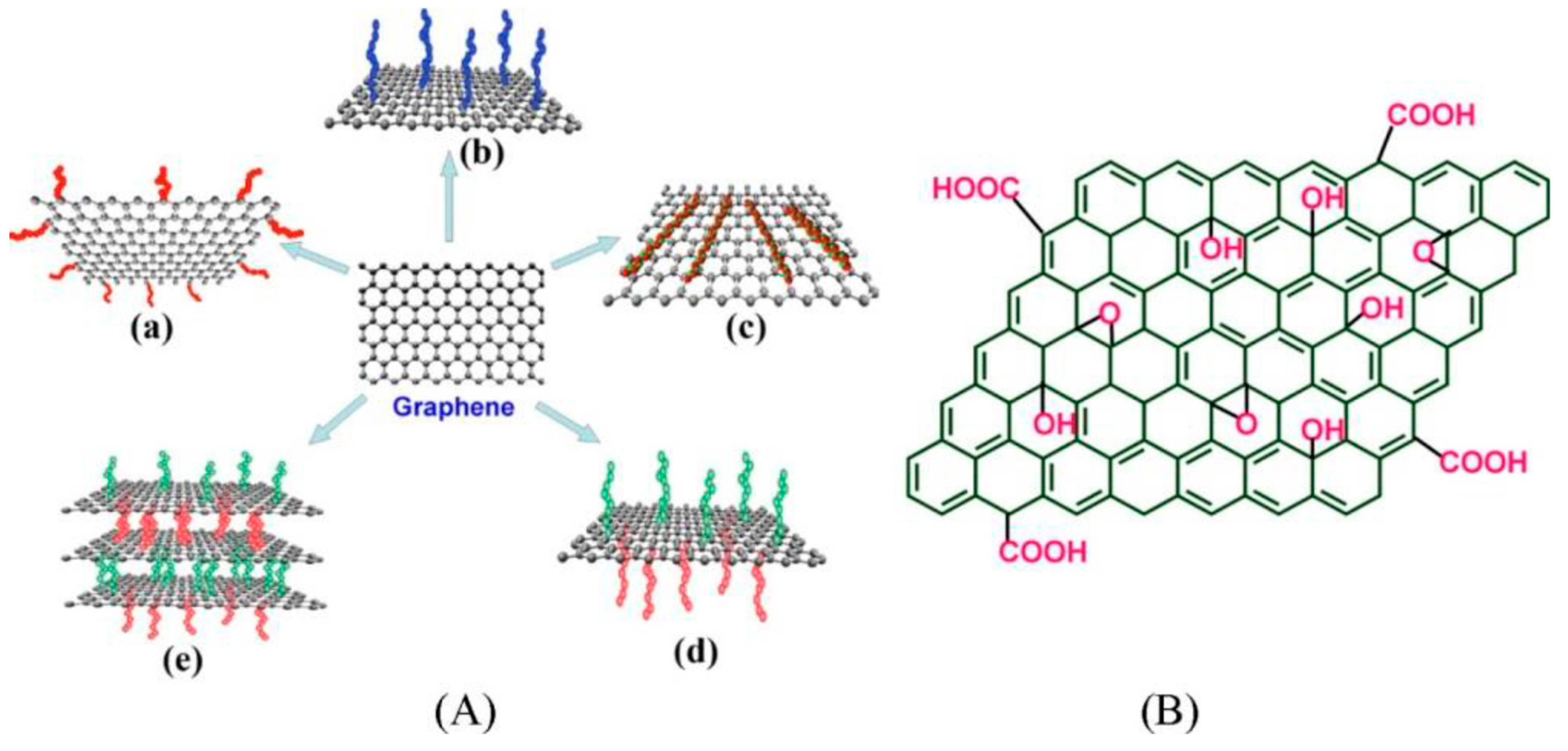
2.3. Functionalization of GR2Ms
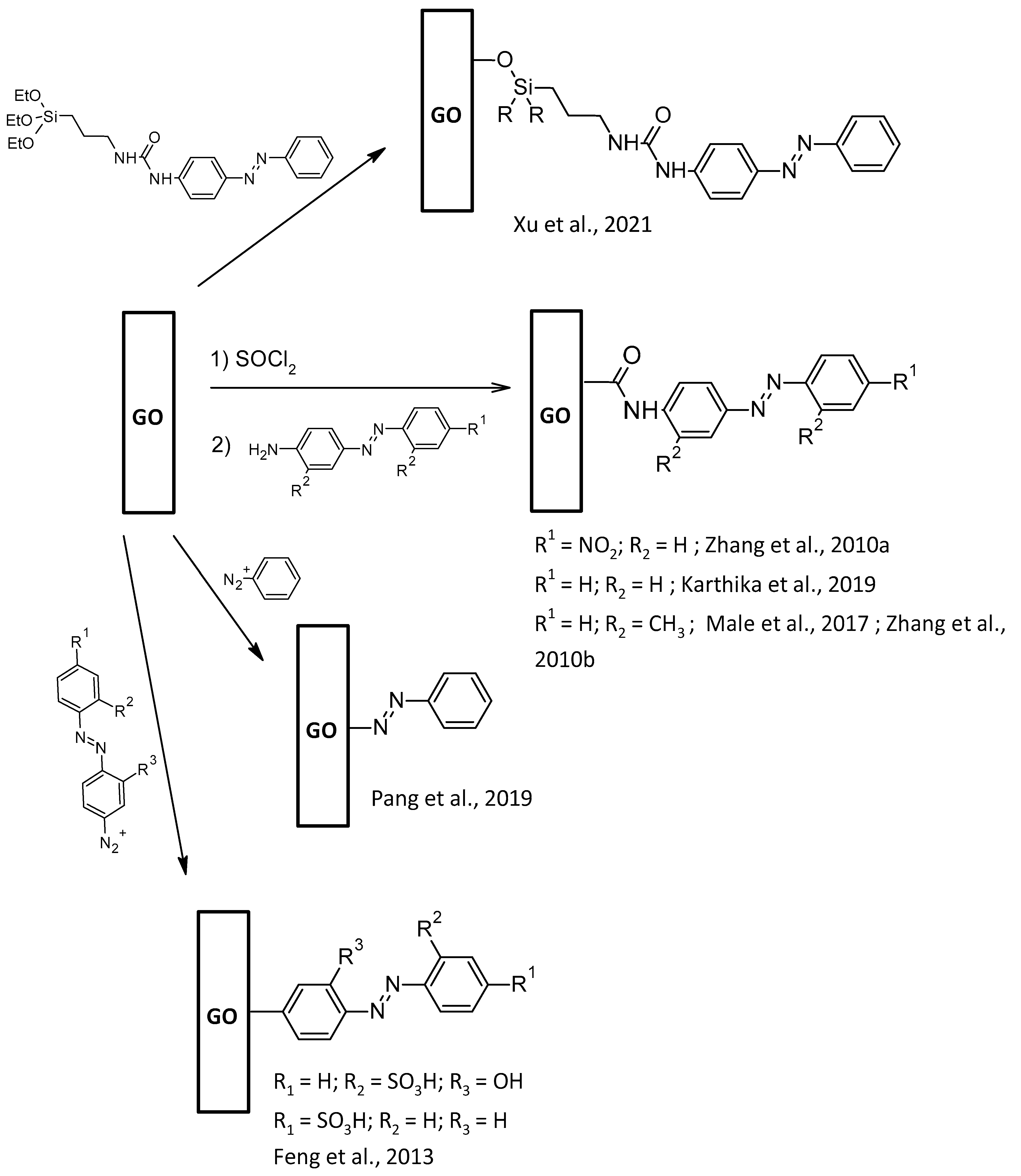
2.4. AZO-Functionalization of Gr2Ms GO
3. Applications of AZO-Functionalized GO
Sensors for Depollution
4. Applications of AZO-Gr2Ms Hybrids
4.1. Photoswitches
4.2. Solar Thermal Storage
4.3. Memory
4.4. Other Applications
4.5. AZO-Gr2Ms Polymers Composites
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Rao, C.E.N.E.R.; Sood, A.E.K.; Subrahmanyam, K.E.S.; Govindaraj, A. Graphene: The new two-dimensional nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.J.; Koduru, J.R.; Malek, N.I.; Hussain, C.M.; Kailasa, S.K. Surface modifications and analytical applications of graphene oxide: A review. TrAC Trends Anal. Chem. 2021, 144, 116448. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial properties of graphene-based nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.W.; Baek, J.-B. Nitrogen-Doped Graphene for Photocatalytic Hydrogen Generation. Chem.—Asian J. 2016, 11, 1125–1137. [Google Scholar] [CrossRef]
- Chen, L.; He, F.; Zhao, N.; Guo, R. Fabrication of 3D quasi-hierarchical Z-scheme RGO-Fe2O3-MoS2 nanoheterostructures for highly enhanced visible-light-driven photocatalytic degradation. Appl. Surf. Sci. 2017, 420, 669–680. [Google Scholar] [CrossRef]
- Sagadevan, S.; Chowdhury, Z.Z.; Johan, M.R.B.; Khan, A.A.; Aziz, F.A.; FRafique, R.; Hoque, M.E. A facile hydrothermal approach for catalytic and optical behavior of tin oxide-graphene (SnO2/G) nanocomposite. PLoS ONE 2018, 13, e0202694. [Google Scholar] [CrossRef]
- Ma, M.; Li, H.; Xiong, Y.; Dong, F. Rational design, synthesis, and application of silica/graphene-based nanocomposite: A review. Mater. Des. 2021, 198, 109367. [Google Scholar] [CrossRef]
- Ma, R.; Zhou, Y.; Bi, H.; Yang, M.; Wang, J.; Liu, Q.; Huang, F. Multidimensional graphene structures and beyond: Unique properties, syntheses and applications. Prog. Mater. Sci. 2020, 113, 100665. [Google Scholar] [CrossRef]
- Mamba, G.; Gangashe, G.; Moss, L.; Hariganesh, S.; Thakur, S.; Vadivel, S.; Mishra, A.; Vilakati, G.; Muthuraj, V.; Nkambule, T. State of the art on the photocatalytic applications of graphene based nanostructures: From elimination of hazardous pollutants to disinfection and fuel generation. J. Environ. Chem. Eng. 2020, 8, 103505. [Google Scholar] [CrossRef]
- Hussain, S.Z.; Ihrar, M.; Hussain, S.B.; Oh, W.C.; Ullah, K. A review on graphene-based transition metal oxide composites and its application towards supercapacitor electrodes. SN Appl. Sci. 2020, 2, 1–23. [Google Scholar] [CrossRef]
- Passaretti, P. Graphene oxide and biomolecules for the production of functional 3D graphene-based materials. Front. Mol. Biosci. 2022, 9, 774097. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L.; Sukriti; Dhanya, B.S.; Saini, R.; Das, A.; Varma, R.S. Synthesis and application of graphene-based sensors in biology: A review. Environ. Chem. Lett. 2022, 20, 2189–2212. [Google Scholar] [CrossRef]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Oh, W.-C.; Lim, C.S.; Liu, Y.; Sagadevan, S.; Jang, W.K.; Biswas, R.U.D. Quaternary nanorod-type BaInSbSe5 semiconductor combined graphene-based conducting polymer (PPy) nanocomposite and highly sensing performance of H2O2 & H2S gases. J. Mater. Sci. Mater. Electron. 2021, 32, 15944–15963. [Google Scholar]
- Fatema, K.N.; Sagadevan, S.; Liu, Y.; Cho, K.Y.; Jung, C.-H.; Oh, W.-C. New design of mesoporous SiO2 combined In2O3-graphene semiconductor nanocomposite for highly effective and selective gas detection. J. Mater. Sci. 2020, 55, 13085–13101. [Google Scholar] [CrossRef]
- Song, Y.; Luo, Y.; Zhu, C.; Li, H.; Du, D.; Lin, Y. Recent advances in electrochemical biosensors based on graphene two-dimensional nanomaterials. Biosens. Bioelectron. 2016, 76, 195–212. [Google Scholar] [CrossRef]
- Oh, W.-C.; Fatema, K.N.; Liu, Y.; Jung, C.H.; Sagadevan, S.; Biswas, R.U.D. Polypyrrole-bonded quaternary semiconductor LiCuMo2O11–graphene nanocomposite for a narrow band gap energy effect and its gas-sensing performance. ACS Omega 2020, 5, 17337–17346. [Google Scholar] [CrossRef]
- Yildiz, G.; Bolton-Warberg, M.; Awaja, F. Graphene and graphene oxide for bio-sensing: General properties and the effects of graphene ripples. Acta Biomater. 2021, 131, 62–79. [Google Scholar] [CrossRef]
- Oh, W.C.; Liu, Y.; Sagadevan, S.; Fatema, K.N.; Biswas, M.R. Polymer bonded Graphene-LaNiSbWO4 nanocomposite (G-LaNiSbWO4-PPy) for CO2 sensing performance under normal temperature condition. Inorg. Nano-Met. Chem. 2021, 51, 1803–1812. [Google Scholar] [CrossRef]
- Pantwalawalkar, J.; Chandankar, S.; Tade, R.; Khan, Z.; Shaikh, M.; Powar, T.; Patil, P.; Sugandhi, V.; Nangare, S. Graphene quantum dot based ultrasensitive probe for biosensing of prostate cancer biomarkers: Current updates and future challenges. Adv. Nat. Sci. Nanosci. Nanotechnol. 2022, 13, 013001. [Google Scholar] [CrossRef]
- Fatema, K.N.; Jung, C.H.; Liu, Y.; Sagadevan, S.; Cho, K.Y.; Oh, W.C. New design of active material based on YInWO4-G-SiO2 for a urea sensor and high performance for nonenzymatic electrical sensitivity. ACS Biomater. Sci. Eng. 2020, 6, 6981–6994. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, G.; Aziz, A.; Iftikhar, T.; Zhong, Z.T.; Asif, M.; Chen, W. The Roadmap of Graphene-Based Sensors: Electrochemical Methods for Bioanalytical Applications. Biosensors 2022, 12, 1183. [Google Scholar] [CrossRef]
- Fatema, K.N.; Lim, C.S.; Liu, Y.; Cho, K.Y.; Jung, C.H.; Oh, W.C. 3D Modeling of silver doped ZrO2 coupled graphene-based mesoporous silica quaternary nanocomposite for a nonenzymatic glucose sensing effects. Nanomaterials 2022, 12, 193. [Google Scholar] [CrossRef]
- Otero, F.; Mandal, T.; Leech, D.; Magner, E. An electrochemical NADH biosensor based on a nanoporous gold electrode modified with diaphorase and an osmium polymer. Sens. Actuators Rep. 2022, 4, 100117. [Google Scholar] [CrossRef]
- Cho, I.-H.; Kim, D.H.; Park, S. Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis. Biomater. Res. 2020, 24, 6. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, Y.; Ramasamy, R. Non-covalent functionalization of carbon nanotubes for electrochemical biosensor development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Yang, Y.; Yuan, Q. Aptamer-functionalized carbon nanomaterials electrochemical sensors for detecting cancer relevant biomolecules. Carbon 2018, 129, 380–395. [Google Scholar] [CrossRef]
- Huang, B.; Li, Z.; Liu, Z.; Zhou, G.; Hao, S.; Wu, J.; Gu, B.-L.; Duan, W. Adsorption of Gas Molecules on Graphene Nanoribbons and Its Implication for Nanoscale Molecule Sensor. J. Phys. Chem. C 2008, 112, 13442–13446. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B Condens. Matter Mater. Phys. 2008, 77, 125416. [Google Scholar] [CrossRef]
- Quercia, L.; Loffredo, F.; Di Francia, G. Influence of Filler Dispersion on Thin Film Composites Sensing Properties. Sens. Actuators B 2005, 109, 153–158. [Google Scholar] [CrossRef]
- Persaud, K.C. Polymers for Chemical Sensing. Mater. Today 2005, 8, 38–44. [Google Scholar] [CrossRef]
- Miao, J.; Miyauchi, M.; Simmons, T.J.; Dordick, J.S.; Linhardt, R.J. Electrospinning of Nanomaterials and Applications in Electronic Components and Devices. J. Nanosci. Nanotechnol. 2010, 10, 5507–5519. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Ding, B.; Wang, M.; Yu, J.; Sun, G. Gas Sensors Based on Electrospun Nanofibers. Sensors 2009, 9, 1609–1624. [Google Scholar] [CrossRef]
- Wu, H.; Xia, Y.; Zhang, C.; Xie, S.; Wu, S.; Cui, H. Adsorptions of C5F10O decomposed compounds on the Cu-decorated NiS2 monolayer: A first-principles theory. Mol. Phys. 2023, e2163715. [Google Scholar] [CrossRef]
- Speranza, G. The Role of Functionalization in the Applications of Carbon Materials: An Overview. C 2019, 5, 84. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad family of carbon nanoallotropes: Classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Randriamahazaka, H.; Ghilane, J. Electrografting and controlled surface functionalization of carbon based surfaces for electroanalysis. Electroanalysis 2016, 28, 13–26. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Janjani, H. Antibiotics adsorption from aqueous solutions using carbon nanotubes: A systematic review. Toxin Rev. 2018, 39, 87–98. [Google Scholar] [CrossRef]
- Zhang, T.; Li, C.; Wang, F.; Noori, A.; Mousavi, M.F.; Xia, X.; Zhang, Y. Recent Advances in Carbon Anodes for Sodium-Ion Batteries. Chem. Rec. 2022, 22, e202200083. [Google Scholar] [CrossRef] [PubMed]
- Maduraiveeran, G.; Jin, W. Carbon nanomaterials: Synthesis, properties and applications in electrochemical sensors and energy conversion systems. Mater. Sci. Eng. B 2021, 272, 115341. [Google Scholar] [CrossRef]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the development of advanced catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef] [PubMed]
- Kiciński, W.; Dyjak, S. Transition metal impurities in carbon-based materials: Pitfalls, artifacts and deleterious effects. Carbon 2020, 168, 748–845. [Google Scholar] [CrossRef]
- Sinha, A.; Dhanjai; Jain, R.; Zhao, H.; Karolia, P.; Jadon, N. Voltammetric sensing based on the use of advanced carbonaceous nanomaterials: A review. Microchim. Acta 2018, 185, 89. [Google Scholar] [CrossRef]
- Chadha, U.; Selvaraj, S.K.; Thanu, S.V.; Cholapadath, V.; Abraham, A.M.; Zaiyan, M.; Manikandan, M.; Paramasivam, V. A review of the function of using carbon nanomaterials in membrane filtration for contaminant removal from wastewater. Mater. Res. Express 2022, 9, 012003. [Google Scholar] [CrossRef]
- Vardharajula, S.; Ali, S.Z.; Tiwari, P.M.; Eroğlu, E.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized carbon nanotubes: Biomedical applications. Int. J. Nanomed. 2012, 7, 5361–5374. [Google Scholar]
- Lanone, S.; Andujar, P.; Kermanizadeh, A.; Boczkowski, J. Determinants of carbon nanotube toxicity. Adv. Drug Deliv. Rev. 2013, 65, 2063–2069. [Google Scholar] [CrossRef]
- De Menezes, B.R.C.; Rodrigues, K.F.; da Silva Fonseca, B.C.; Ribas, R.G.; do Amaral Montanheiro, T.L.; Thim, G.P. Recent advances in the use of carbon nanotubes as smart biomaterials. J. Mater. Chem. B 2019, 7, 1343–1360. [Google Scholar] [CrossRef] [PubMed]
- Smart, S.K.; Cassady, A.I.; Lu, G.Q.; Martin, D.J. The biocompatibility of carbon nanotubes. Carbon 2006, 44, 1034–1047. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 8, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.; Tousley, M.E.; Elimelech, M. Thin-Film Composite Polyamide Membranes Functionalized with Biocidal Graphene Oxide Nanosheets. Environ. Sci. Technol. Lett. 2014, 1, 71–76. [Google Scholar] [CrossRef]
- Cao, K.; Jiang, Z.; Zhao, J.; Zhao, C.; Gao, C.; Pan, F.; Wang, B.; Cao, X.; Yang, J. Enhanced water permeation through sodium alginate membranes by incorporating graphene oxides. J. Membr. Sci. 2014, 469, 272–283. [Google Scholar] [CrossRef]
- Wei, N.; Peng, X.; Xu, Z. Understanding Water Permeation in Graphene Oxide Membranes. ACS Appl. Mater. Interfaces 2014, 8, 5877–5883. [Google Scholar] [CrossRef]
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for carbon nanotubes synthesis—Review. J. Mater. Chem. 2011, 21, 15872–15884. [Google Scholar] [CrossRef]
- Oberlin, A.; Endo, M.; Koyama, T. Filamentous growth of carbon through benzene decomposition. J. Cryst. Growth 1976, 32, 335–349. [Google Scholar] [CrossRef]
- Iijima, S. Synthesis of Carbon Nanotubes. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Mani, S.; Chowdhary, P.; Bharagava, R.N. Textile Wastewater Dyes: Toxicity Profile and Treatment Approaches. In Emerging and Eco-Friendly Approaches for Waste Management; Bharagava, R.N., Chowdhary, P., Eds.; Springer: Singapore, 2019; pp. 219–244. [Google Scholar] [CrossRef]
- Saini, R.D. Textile organic dyes: Polluting effects and elimination methods from textile waste water. Int. J. Chem. Eng. Res. 2017, 9, 121–136. [Google Scholar]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Griffiths, J. II. Photochemistry of azobenzene and its derivatives. Chem. Soc. Rev. 1972, 1, 481–493. [Google Scholar] [CrossRef]
- Léonard, E.; Fayeulle, A. Azo-Dyes-Grafted Oligosaccharides—From Synthesis to Applications. Molecules 2021, 26, 3063. [Google Scholar] [CrossRef] [PubMed]
- Weis, P.; Wu, S. Light-Switchable Azobenzene-Containing Macromolecules: From UV to Near Infrared. Macromol. Rapid Commun. 2018, 39, 1700220. [Google Scholar] [CrossRef]
- Asghar, F.; Shakoor, B.; Fatima, S.; Munir, S.; Razzaq, H.; Naheed, S.; Butler, I.S. Fabrication and prospective applications of graphene oxide-modified nanocomposites for wastewater remediation. RSC Adv. 2022, 12, 11750–11768. [Google Scholar] [CrossRef]
- Surwade, S.P.; Smirnov, S.N.; Vlassiouk, I.V.; Unocic, R.R.; Veith, G.M.; Dai, S.; Mahurin, S.M. Water desalination using nanoporous single-layer graphene. Nat. Nanotechnol. 2015, 10, 459–464. [Google Scholar] [CrossRef]
- Ulian, G.; Moro, D.; Valdre, G.J.C.S. Electronic and optical properties of graphene/molybdenite bilayer composite. Compos. Struct. 2021, 255, 112978. [Google Scholar] [CrossRef]
- Chegel, R.; Behzad, S. Tunable electronic, optical, and thermal properties of two-dimensional germanene via an external electric field. Sci. Rep. 2020, 10, 704. [Google Scholar] [CrossRef]
- Su, H.; Hu, Y.H. Recent advances in graphene-based materials for fuel cell applications. Energy Sci. Eng. 2020, 9, 958–983. [Google Scholar] [CrossRef]
- Chen, X.; Tian, Z.; Li, Q.; Li, S.; Zhang, X.; Ouyang, C.; Gu, J.; Han, J.; Zhang, W. Recent progress in graphene terahertz modulators. Chin. Phys. B 2020, 29, 077803. [Google Scholar] [CrossRef]
- Wang, J.; Song, J.; Mu, X.; Sun, M. Optoelectronic and photoelectric properties and applications of graphene-based nanostructures. Mater. Today Phys. 2020, 13, 100196. [Google Scholar] [CrossRef]
- Lim, Y.; Lee, D.-K.; Kim, S.M.; Park, W.; Cho, S.Y.; Sim, U. Low dimensional carbon-based catalysts for efficient photocatalytic and photo/electrochemical water splitting reactions. Materials 2020, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Fadlalla, M.I.; Kumar, P.S.; Selvam, V.; Babu, S.G. Emerging energy and environmental application of graphene and their composites: A review. J. Mater. Sci. 2020, 55, 7156–7183. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
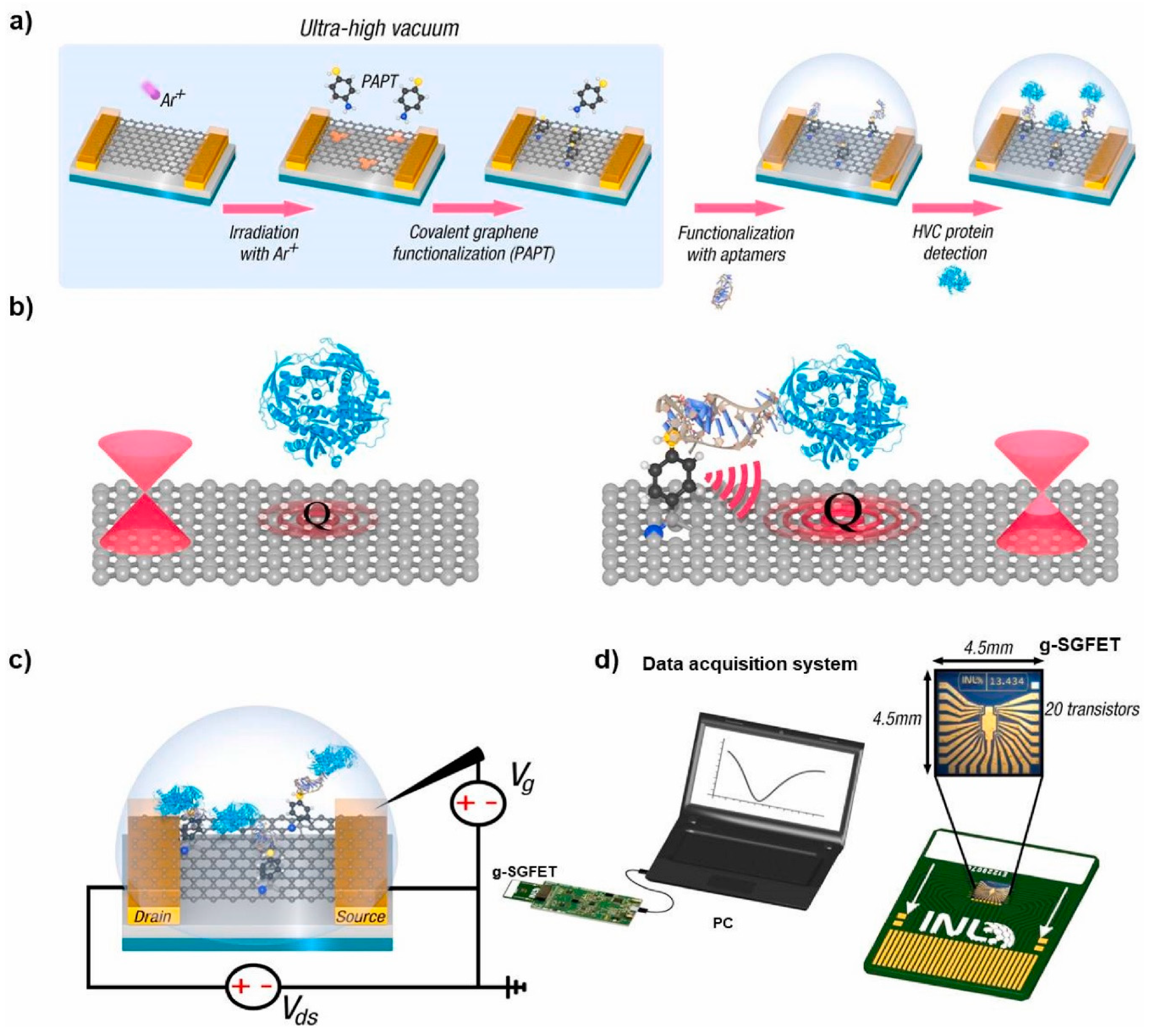
- Palacio, I.; Moreno, M.; Náñez, A.; Purwidyantri, A.; Domingues, T.; Cabral, P.D.; Borme, J.; Marciello, M.; Mendieta-Moreno, J.I.; Torres-Vázquez, B.; et al. Attomolar detection of hepatitis C virus core protein powered by molecular antenna-like effect in a graphene field-effect aptasensor. Biosens. Bioelectron. 2023, 222, 115006. [Google Scholar] [CrossRef] [PubMed]
- Compton, O.C.; Kim, S.; Pierre, C.; Torkelson, J.M.; Nguyen, S.T. Crumpled graphene nanosheets as highly effective barrier property enhancers. Adv. Mater. 2010, 22, 4759–4763. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S. Graphene Oxide, Highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Brodie, B.C. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1860, 149, 249–259. [Google Scholar]
- Staudenmaier, L. Verfahren zur darstellung der graphitsäure. Eur. J. Inorg. Chem. 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Krishnan, D.; Kim, F.; Luo, J.; Cruz-Silva, R.; Cote, L.; Jang, H.; Huang, J. Energetic graphene oxide: Challenges and opportunities. Nano Today 2012, 7, 137–152. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Ang, H.; Tadé, M. Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chem. Eng. J. 2013, 226, 336–347. [Google Scholar] [CrossRef]
- Sadegh, H. Development of graphene oxide from graphite: A review on synthesis, characterization and its application in wastewater treatment. Rev. Adv. Mater. Sci. 2017, 49, 38–43. [Google Scholar]
- Poh, H.L.; Šaněk, F.; Ambrosi, A.; Zhao, G.; Sofer, Z.; Pumera, M. Graphenes prepared by Staudenmaier, Hofmann and Hummers methods with consequent thermal exfoliation exhibit very different electrochemical properties. Nanoscale 2012, 4, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Klarich, K.L.; Pflug, N.C.; DeWald, E.M.; Hladik, M.L.; Kolpin, D.W.; Cwiertny, D.M.; Lefevre, G.H. Occurrence of Neonicotinoid Insecticides in Finished Drinking Water and Fate during Drinking Water Treatment. Environ. Sci. Technol. Lett. 2017, 4, 168–173. [Google Scholar] [CrossRef]
- Buesseler, K.; Aoyama, M.; Fukasawa, M. Impacts of the Fukushima Nuclear Power Plants on Marine Radioactivity. Environ. Sci. Technol. 2011, 45, 9931–9935. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, X.; Yang, B.; Zuo, X.; Li, G.; Feng, A.; Tang, H.; Zhang, H.; Wu, M.; Ma, Y.; et al. Controlled synthesis of CuInS2/reduced graphene oxide nanocomposites for efficient dye-sensitized solar cells. J. Power Sources 2014, 272, 639–646. [Google Scholar] [CrossRef]
- Dai, L. Functionalization of Graphene for Efficient Energy Conversion and Storage. Acc. Chem. Res. 2013, 46, 31–42. [Google Scholar] [CrossRef]
- Feng, W.; Luo, W.; Feng, Y. Photo-responsive carbon nanomaterials functionalized by azobenzene moieties: Structures, properties and application. Nanoscale 2012, 4, 6118–6134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, Y.; Huang, D.; Li, Y.; Feng, W. Investigation of optical modulated conductance effects based on a graphene oxide–azobenzene hybrid. Carbon 2010, 48, 3236–3241. [Google Scholar] [CrossRef]
- Karthika, M.; Chi, H.; Li, T.; Wang, H.; Thomas, S. Super-hydrophobic graphene oxide-azobenzene hybrids for improved hydrophobicity of polyurethane. Compos. Part B Eng. 2019, 173, 106978. [Google Scholar] [CrossRef]
- Male, U.; Modigunta, J.K.R.; Huh, D.S. Design and synthesis of polyaniline-grafted reduced graphene oxide via azobenzene pendants for high-performance supercapacitors. Polymer 2017, 110, 242–249. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, Y.; Lv, P.; Shen, Y.; Feng, W. Enhanced Reversible Photoswitching of Azobenzene-Functionalized Graphene Oxide Hybrids. Langmuir 2010, 26, 18508–18511. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Xue, J.; Pang, H. A High Energy Density Azobenzene/Graphene Oxide Hybrid with Weak Nonbonding Interactions for Solar Thermal Storage. Sci. Rep. 2019, 9, 5224. [Google Scholar] [CrossRef]
- Dong, L.; Feng, Y.; Wang, L.; Feng, W. Azobenzene-based solar thermal fuels: Design, properties, and applications. Chem. Soc. Rev. 2018, 47, 7339–7368. [Google Scholar] [CrossRef]
- Xu, T.; Guo, S.; Li, M.; Zhang, Z.; Pang, B.; Hu, Y. Multiphotoinduced Bending of Azobenzene-Modified Graphene Oxide/Poly(vinyl alcohol) Composite Films for Smart Devices. ACS Appl. Nano Mater. 2021, 4, 10209–10217. [Google Scholar] [CrossRef]
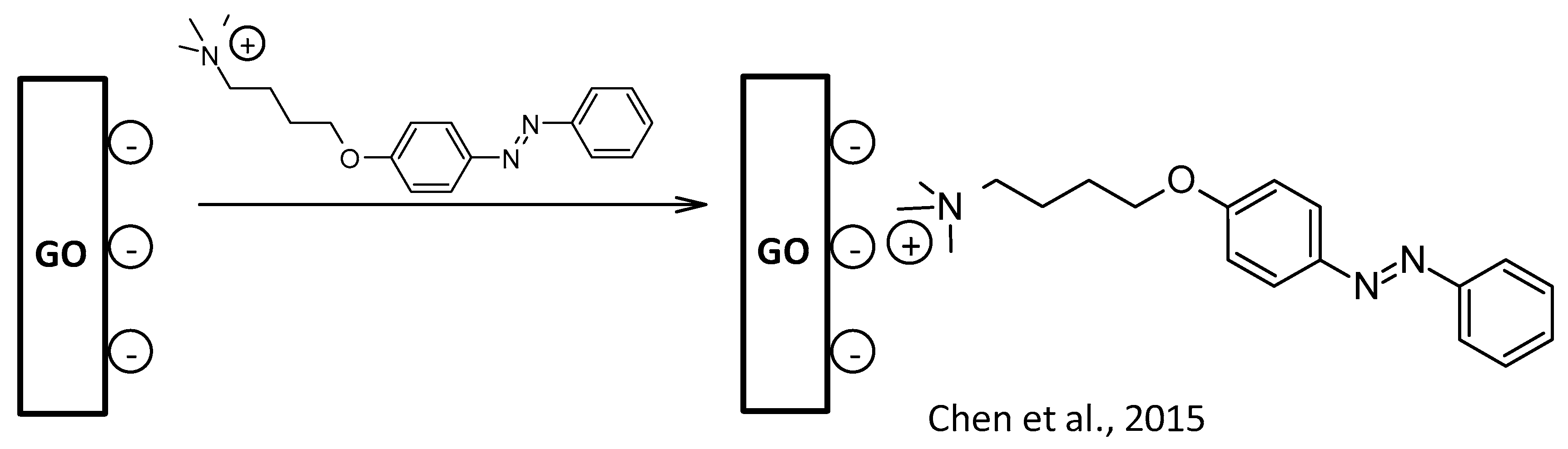
- Chen, S.; Bao, L.; Ou, E.; Peng, C.; Wang, W.; Xu, W. A cationic azobenzene-surfactant-modified graphene hybrid: Unique photoresponse and electrochemical behavior. Nanoscale 2015, 7, 19673–19686. [Google Scholar] [CrossRef]
- Li, H.; Duan, T.; Sher, O.; Han, Y.; Papadakis, R.; Grigoriev, A.; Ahuja, R.; Leifer, K. Fabrication of BP2T functionalized graphene via non-covalent π–π stacking interactions for enhanced ammonia detection. RSC Adv. 2021, 11, 35982–35987. [Google Scholar] [CrossRef]
- Shangguan, Z.; Yu, C.; Li, C.; Huang, X.; Mai, Y.; Li, T. Azobenzene-functionalized graphene nanoribbons: Bottom-up synthesis, photoisomerization behaviour and self-assembled structures. J. Mater. Chem. C 2020, 8, 10837–10843. [Google Scholar] [CrossRef]
- Surucu, O.; Bolat, G.; Abaci, S. Electrochemical behavior and voltammetric detection of fenitrothion based on a pencil graphite electrode modified with reduced graphene oxide (RGO)/poly(E)-1-(4-((4-(phenylamino)phenyl)diazenyl)phenyl)ethanone (DPA) composite film. Talanta 2017, 168, 113–120. [Google Scholar] [CrossRef] [PubMed]
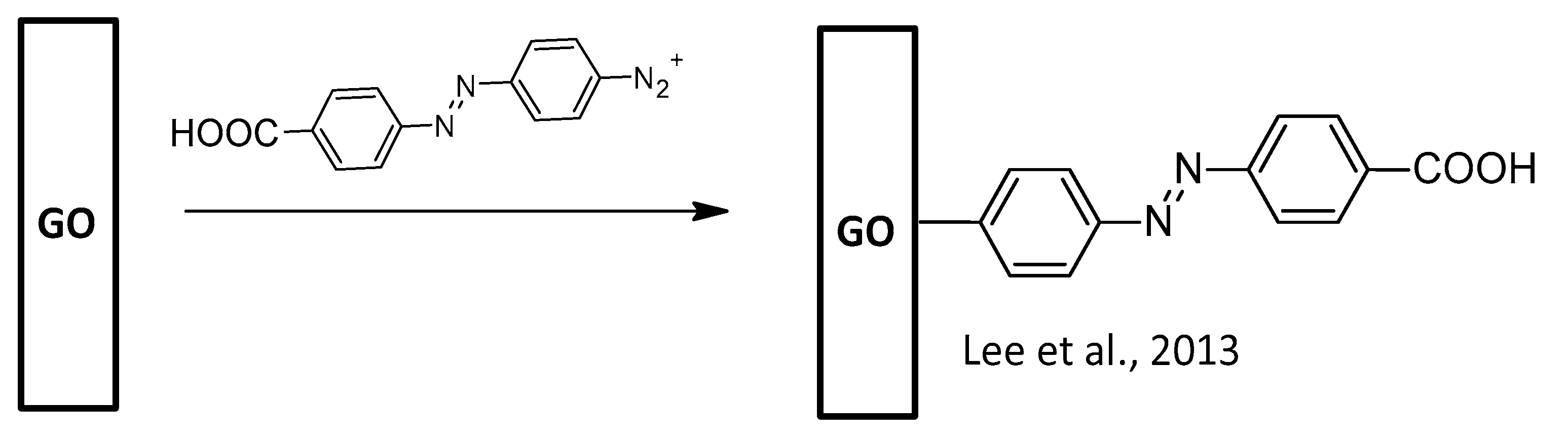
- Lee, J.H.; Jaworski, J.; Jung, J.H. Luminescent metal–organic framework-functionalized graphene oxide nanocomposites and the reversible detection of high explosives. Nanoscale 2013, 5, 8533–8540. [Google Scholar] [CrossRef] [PubMed]
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 20 December 2022).
- Omidi, S.; Kakanejadifard, A.; Azarbani, F. Non-covalent functionalization of graphene oxide and reduced graphene oxide with Schiff bases as antibacterial agents. J. Mol. Liq. 2017, 242, 812–821. [Google Scholar] [CrossRef]
- Bokare, A.; Arif, J.; Erogbogbo, F. Strategies for Incorporating Graphene Oxides and Quantum Dots into Photoresponsive Azobenzenes for Photonics and Thermal Applications. Nanomaterials 2021, 11, 2211. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, H.; Luo, W.; Liu, E.; Zhao, N.; Yoshino, K.; Feng, W. Covalent functionalization of graphene by azobenzene with molecular hydrogen bonds for long-term solar thermal storage. Sci. Rep. 2013, 3, 3260. [Google Scholar] [CrossRef]
- Park, J.Y.; Male, U.; Huh, D.S. Photo-regulated conductivity of polycaprolactone honeycomb-patterned porous films containing azobenzene-functionalized reduced graphene oxide. Macromol. Res. 2017, 25, 849–855. [Google Scholar] [CrossRef]
- Feng, W.; Li, S.; Li, M.; Qin, C.; Feng, Y. An energy-dense and thermal-stable bis-azobenzene/hybrid templated assembly for solar thermal fuel. J. Mater. Chem. A 2016, 4, 8020–8028. [Google Scholar] [CrossRef]
- Luo, W.; Feng, Y.; Qin, C.; Li, M.; Li, S.; Cao, C.; Long, P.; Liu, E.; Hu, W.; Yoshino, K.; et al. High-energy, stable and recycled molecular solar thermal storage materials using AZO/graphene hybrids by optimizing hydrogen bonds. Nanoscale 2015, 7, 16214–16221. [Google Scholar] [CrossRef]
- Wu, S.; Wang, H.; Zhao, B.; Cao, T.; Ma, J.; Liu, L.; Tong, Z. Construction of cationic polyfluorinated azobenzene/reduced graphene oxide for simultaneous determination of dopamine, uric acid and ascorbic acid. Talanta 2022, 237, 122986. [Google Scholar] [CrossRef]
- Teng, Y.; Li, S.; Xue, C.; Zhang, H.; Zhu, L.; Tang, Y. Synthesis of polyaniline/graphene oxide/azobenzene composite and its adjustable photoelectric properties. Adv. Polym. Technol. 2020, 2020, 8730852. [Google Scholar] [CrossRef]
- Li, M.; Wang, C. AAZO-Graphene composite phase change materials with Photo-thermal conversion performance. Sol. Energy 2021, 223, 11–18. [Google Scholar] [CrossRef]
- Luo, W.; Feng, Y.; Cao, C.; Li, M.; Liu, E.; Li, S.; Qin, C.; Hu, W.; Feng, W. A high energy density azobenzene/graphene hybrid: A nano-templated platform for solar thermal storage. J. Mater. Chem. A 2015, 3, 11787–11795. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, Y.; Qin, C.; Yang, W.; Si, Q.; Feng, W. Controlling Heat Release from a Close-Packed Bisazobenzene-Reduced-Graphene-Oxide Assembly Film For High-Energy Solid-State Photothermal Fuels. Chemsuschem 2017, 10, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Feng, Y.; Si, Q.; Yan, Q.; Long, P.; Dong, L.; Fu, L.; Feng, W. Efficient cycling utilization of solar-thermal energy for thermochromic displays with controllable heat output. J. Mater. Chem. A 2019, 7, 97–106. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Xu, W. A photoresponsive azobenzene-graphene-gold nanocomposite using cationic azobenzene-surfactant as stabilizers and its electrochemical behavior. Mater. Lett. 2016, 180, 196–199. [Google Scholar] [CrossRef]
- Baby, A.; Abinaya, S.; John, A.M.; Jose, S.P.; Balakrishnana, S.P. Photoresponse and Electrochemical Behaviour of Azobenzene Anchored Graphene Oxide for Energy Storage Application. SSRN J. 2022. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, L.; Wang, L.; Liu, B.; Tian, X.; Chen, Y. Covalent modification of graphene oxide with poly(N-vinylcarbazole) containing pendant azobenzene chromophores for nonvolatile ternary memories. Carbon 2018, 134, 500–506. [Google Scholar] [CrossRef]
- Cao, T.; Zhao, F.; Da, Z.; Qiu, F.; Yang, D.; Guan, Y.; Cao, G.; Zhao, Z.; Li, J.; Guo, X. Synthesis of Amino-Functionalized Graphene Oxide/Azobenzene Polyimide and Its Simulation of Optical Switches. Z. Phys. Chem. 2017, 231, 1797–1814. [Google Scholar] [CrossRef]
- Olean-Oliveira, A.; Teixeira, M.F. Development of a nanocomposite chemiresistor sensor based on π-conjugated azo polymer and graphene blend for detection of dissolved oxygen. Sensors Actuators B Chem. 2018, 271, 353–357. [Google Scholar] [CrossRef]
- Deka, M.J.; Sahoo, S.K.; Chowdhury, D. p-Type and n-type azobenzene nanocluster immobilized graphene oxide nanocomposite. J. Photochem. Photobiol. A Chem. 2019, 372, 131–139. [Google Scholar] [CrossRef]
- Ran, X.; Li, Y.; Wei, Z.; Huo, X.; He, Y.; Wang, X.; Kuang, Y.; Guo, L. Highly enhanced nonlinear optical absorption with ultrafast charge transfer of reduced graphene oxide hybridized by an azobenzene derivative. Opt. Express 2021, 29, 5213–5225. [Google Scholar] [CrossRef]
- Kizhisseri, D.R.; Venugopal, G.; Lekshmi, C.L.; Joseph, K.; Mahesh, S. Photoresponse modulation of reduced graphene oxide by surface modification with cardanol derived azobenzene. New J. Chem. 2018, 42, 18182–18188. [Google Scholar] [CrossRef]
- Siewertsen, R.; Neumann, H.; Buchheim-Stehn, B.; Herges, R.; Näther, C.; Renth, F.; Temps, F. Highly efficient reversible Z–E photoisomerization of a bridged azobenzene with visible light through resolved S1(nπ*) absorption bands. J. Am. Chem. Soc. 2009, 131, 15594–15595. [Google Scholar] [CrossRef] [PubMed]
- Döbbelin, M.; Ciesielski, A.; Haar, S.; Osella, S.; Bruna, M.; Minoia, A.; Grisanti, L.; Mosciatti, T.; Richard, F.; Prasetyanto, E.A.; et al. Light-enhanced liquid-phase exfoliation and current photoswitching in graphene–azobenzene composites. Nat. Commun. 2016, 7, 11090. [Google Scholar] [CrossRef] [PubMed]
- Min, M.; Seo, S.; Lee, S.M.; Lee, H. Voltage-controlled nonvolatile molecular memory of an azobenzene monolayer through solution-processed reduced graphene oxide contacts. Adv. Mater. 2013, 25, 7045–7050. [Google Scholar] [CrossRef]
- Di Florio, G.; Bründermann, E.; Yadavalli, N.S.; Santer, S.; Havenith, M. Graphene multilayer as nanosized optical strain gauge for polymer surface relief gratings. Nano Lett. 2014, 14, 5754–5760. [Google Scholar] [CrossRef]
- Adel, M.; Ahmed, M.A.; Elabiad, M.A.; Mohamed, A.A. Removal of heavy metals and dyes from wastewater using graphene oxide-based nanomaterials: A critical review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100719. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nakamura, D.; Liu, G.; Nishinaka, K.; Tsuda, A. Synthesis and photoisomerization of an azobenzene-containing tetrapyrrolic macrocycle. J. Photochem. Photobiol. A Chem. 2016, 331, 66–75. [Google Scholar] [CrossRef]
- Sun, S.; Liang, S.; Xu, W.-C.; Xu, G.; Wu, S. Photoresponsive polymers with multi-azobenzene groups. Polym. Chem. 2019, 10, 4389–4401. [Google Scholar] [CrossRef]
- Shang, C.; Xiong, Z.; Liu, S.; Yu, W. Molecular Dynamics of Azobenzene Polymer with Photoreversible Glass Transition. Macromolecules 2022, 55, 3711–3722. [Google Scholar] [CrossRef]
- Kumar, G.S.; Neckers, D.C. Photochemistry of Azobenzene-Containing Polymers. Chem. Rev. 1989, 89, 1915–1925. [Google Scholar] [CrossRef]
- De Martino, S.; Mauro, F.; Netti, P.A. Photonic Applications of Azobenzene Molecules Embedded in Amorphous Polymer. Riv. Nuovo Cim. 2020, 43, 599–629. [Google Scholar] [CrossRef]
- Wei, Y.-B.; Tang, Q.; Gong, C.-B.; Lam, M.H.-W. Review of the Recent Progress in Photoresponsive Molecularly Imprinted Polymers Containing Azobenzene Chromophores. Anal. Chim. Acta 2015, 900, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Yager, K.G.; Barrett, C.J. Novel Photo-Switching Using Azobenzene Functional Materials. J. Photochem. Photobiol. A Chem. 2006, 182, 250–261. [Google Scholar] [CrossRef]
- Tang, J.; Feng, Y.; Feng, W. Photothermal Storage and Controllable Release of a Phase-Change Azobenzene/aluminum Nitride Aerogel Composite. Compos. Commun. 2021, 23, 100575. [Google Scholar] [CrossRef]
- Pouliquen, J.; Wintgens, V.; Toscano, V.; Ben Jaafar, B.; Tripathi, S.; Kossanyi, J.; Valat, P. Photoisomerization of N,N′-disubstituted indigos: A search for energy storage. Can. J. Chem. 1984, 62, 2478–2486. [Google Scholar] [CrossRef]
- Bastianelli, C.; Caia, V.; Cum, G.; Gallo, R.; Mancini, V. Thermal-isomerisation of photochemically synthesized (Z)-9-styrylacridines: An unusually high enthalpy of Z→E conversion for stilbene-like compounds. J. Chem. Soc. Perkin Trans. 2 1991, 5, 679–683. [Google Scholar] [CrossRef]
- Kucharski, T.J.; Tian, Y.; Akbulatov, S.; Boulatov, R. Chemical solutions for the closed-cycle storage of solar energy. Energy Environ. Sci. 2011, 4, 4449–4472. [Google Scholar] [CrossRef]
- Maruyama, K.; Tamiaki, H.; Kawabata, S. Development of a solar energy storage process. Photoisomerization of a norbornadiene derivative to a quadricyclane derivative in an aqueous alkaline solution. J. Org. Chem. 1985, 50, 4742–4749. [Google Scholar] [CrossRef]
- Yuan, K.; Guo, Y.-J.; Zhao, X. A Novel Photo-Responsive Azobenzene-Containing Nanoring Host for Fullerene-Guest Facile Encapsulation and Release. Phys. Chem. Chem. Phys. 2014, 16, 27053–27064. [Google Scholar] [CrossRef] [PubMed]
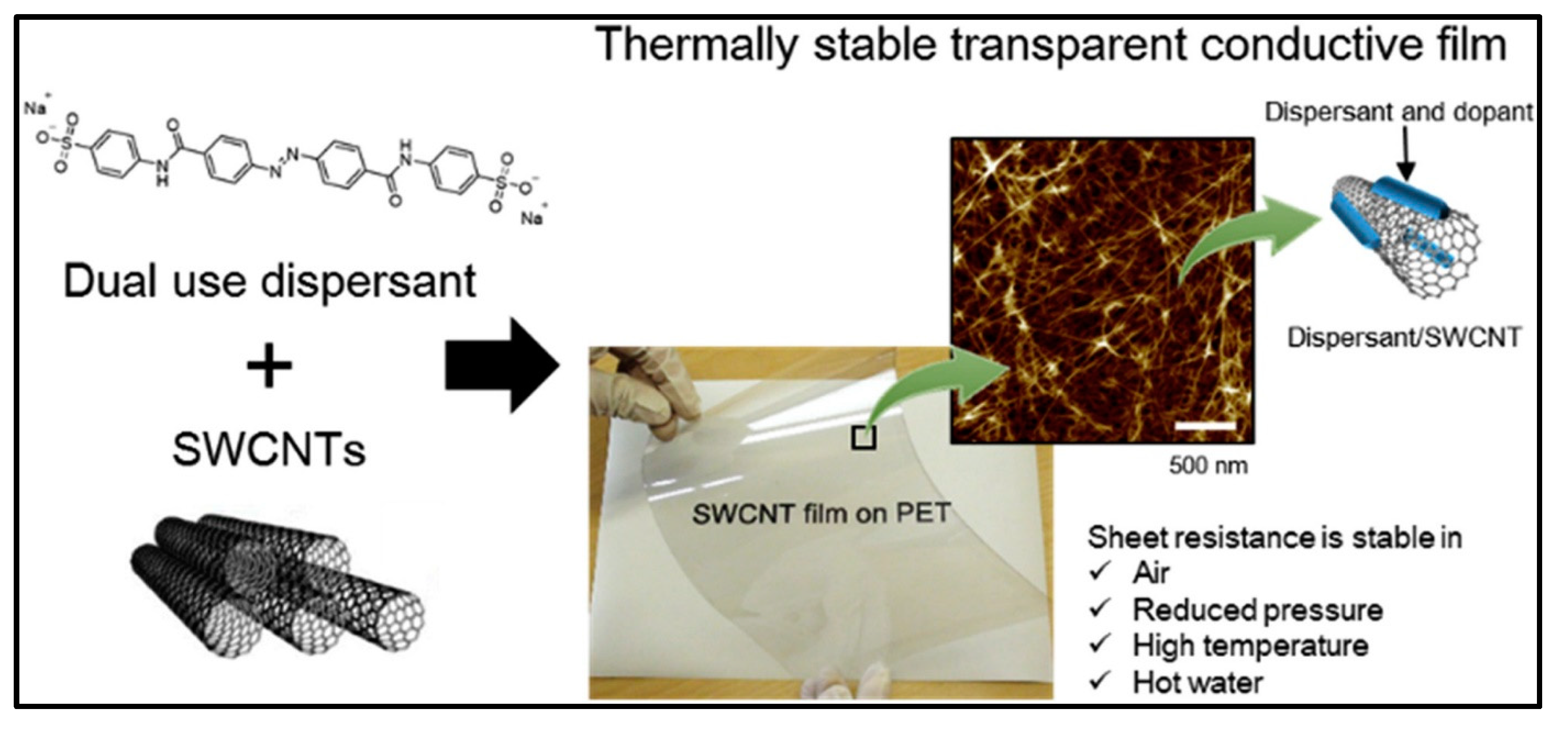
- Jintoku, H.; Matsuzawa, Y.; Yoshida, M. Dual Use of Anionic Azobenzene Derivative as Dispersant and Dopant for Carbon Nanotubes for Enhanced Thermal Stability of Transparent Conductive Films. Carbon 2019, 152, 247–254. [Google Scholar] [CrossRef]
- Romi, S.; Fanetti, S.; Alabarse, F.; Mio, A.M.; Bini, R. Synthesis of Double Core Chromophore-Functionalized Nanothreads by Compressing Azobenzene in a Diamond Anvil Cell. Chem. Sci. 2021, 12, 7048–7057. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Feng, Y.; Liu, E.; Qin, C.; Feng, W. Azobenzene/graphene Hybrid for High-Density Solar Thermal Storage by Optimizing Molecular Structure. Sci. China Technol. Sci. 2016, 59, 1383–1390. [Google Scholar] [CrossRef]


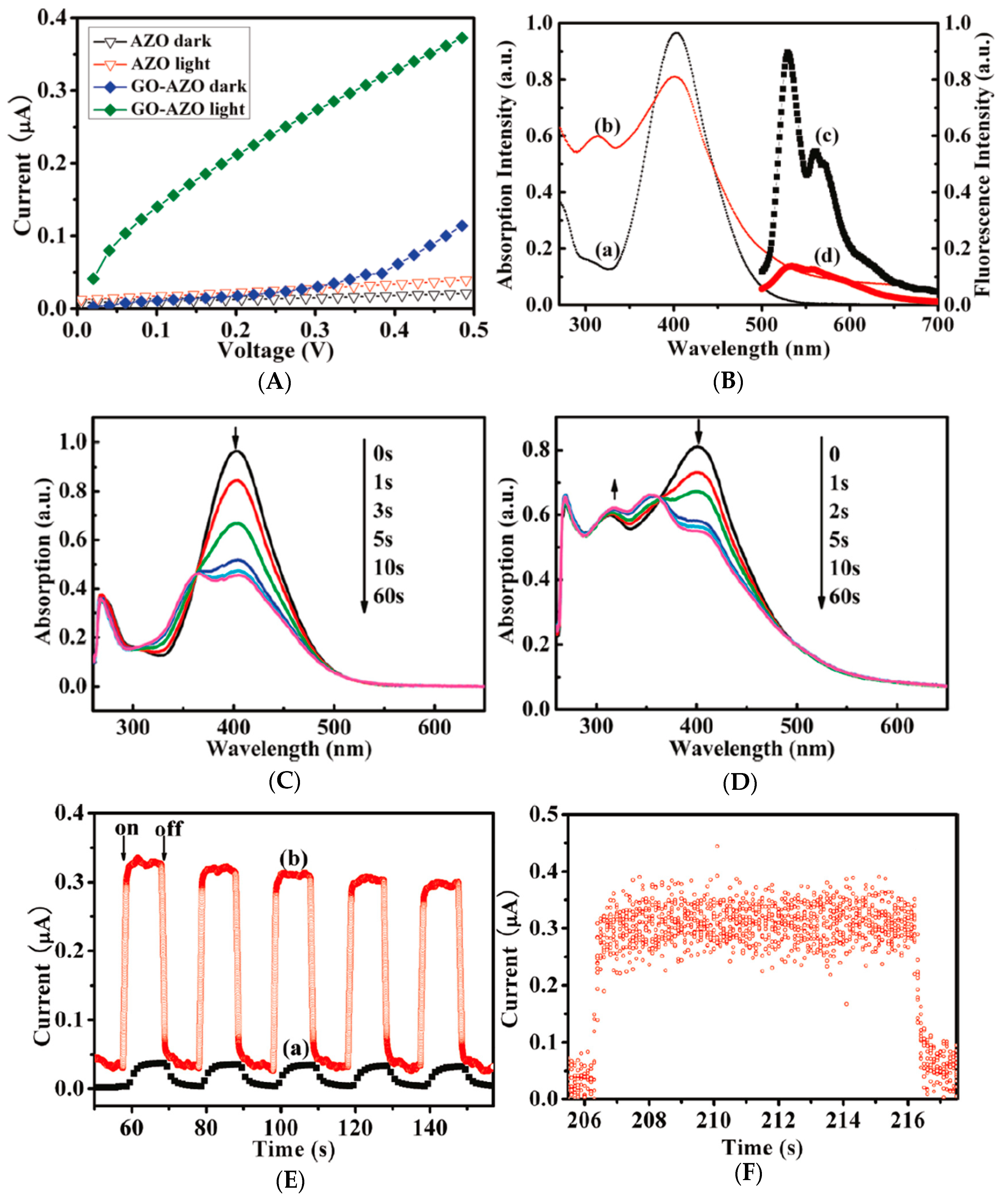
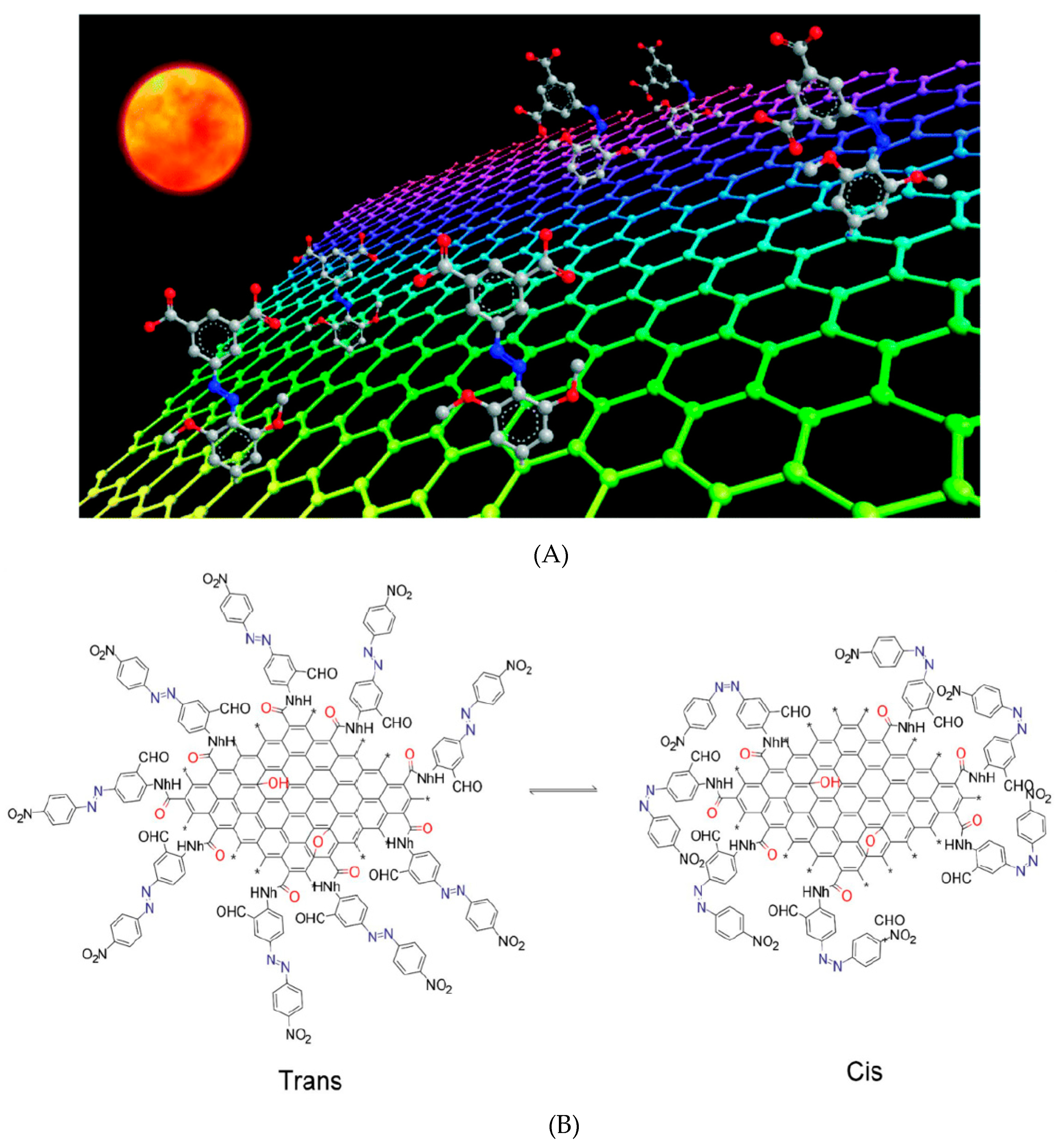
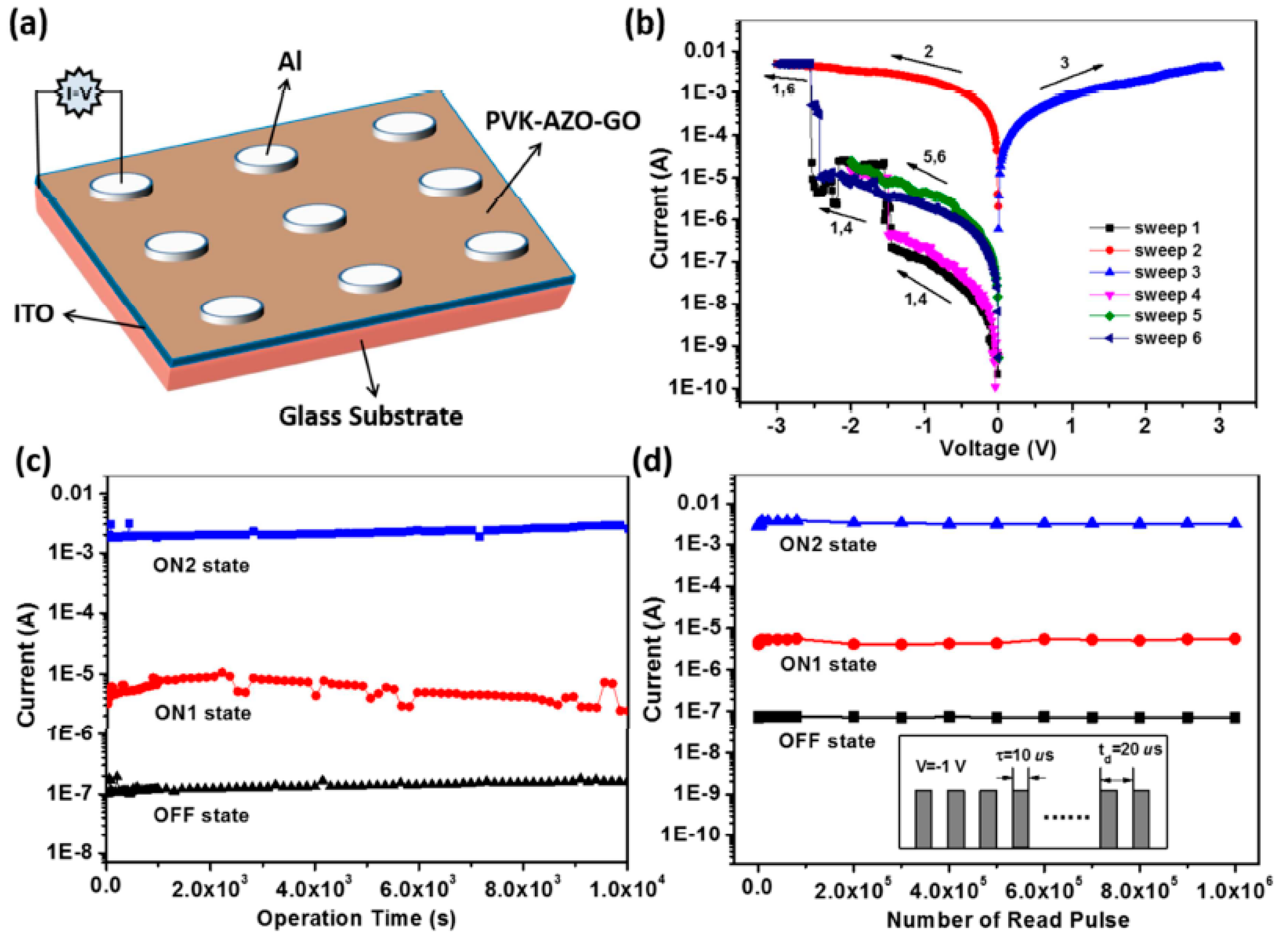
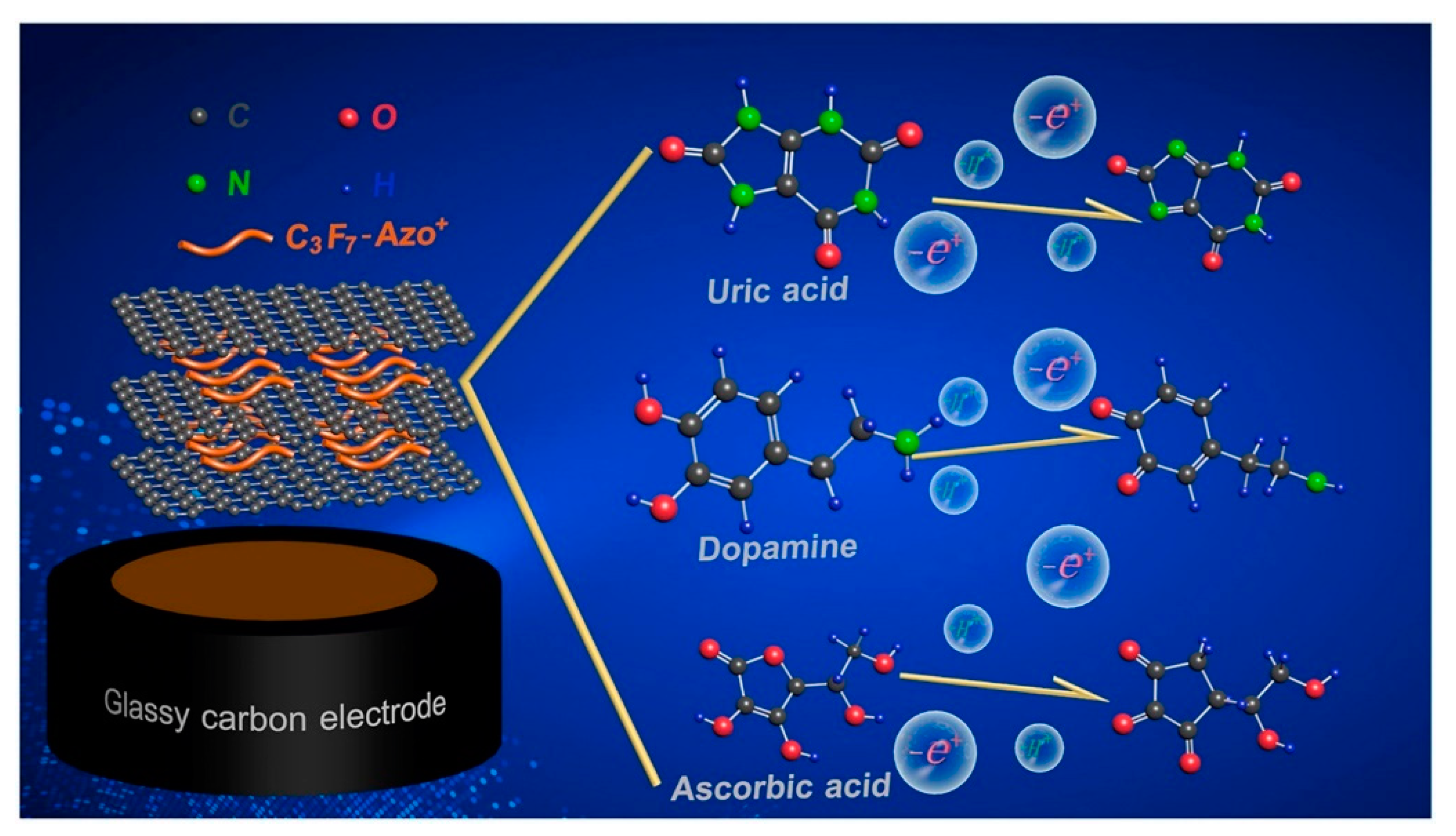
| Materials | Syntheses | Properties | Applications | References |
|---|---|---|---|---|
| RGO-AZO | Diazotization | Enhance thermal storage and trans reversion by H-bonds via para- or ortho-replacement of AZO; RGO-para-AZO has a high thermal storage density of 269.8 kJ kg−1. | Solar thermal storage | [108] |
| AZO-GO | Covalent functionalization | Reversible photoisomerization at 300–400 nm, good thermal stability, and high energy density of 240 Wh kg−1. | Solar thermal storage | [97] |
| PCL-RGO-AZO | In situ ring-opening polymerization | High conductivity, conductivity rises by UV irradiation and recovers by visible light irradiation. | Photo switches and reversible optical storage | [109] |
| RGO-bis-AZO | Covalent grafting | High energy and maximum power densities of about 80 Wh kg−1 and 2230 W kg−1, respectively. | Solar thermal storage | [110] |
| AZO-RGO | Covalent grafting | The high energy density of 138 Wh kg−1, a 52-day long storage lifespan, and 50-cycle cycling stability at 520 nm. | Solar thermal storage | [111] |
| AZO-surfactant-modified-GO | Electrostatic interactions | Light-induced reversible photoresponsivity assembly and disassembly. | Photoresponsive supercapacitors | [100] |
| AZO-RGO-GCE | Exfoliation and restacking | Excellent stability and anti-interference capability. | Determination of ascorbic acid, dopamine, and uric acid | [112] |
| AZO-GO | Amide linkage | Reversible photoisomerization. | Optic and photonic devices | [93] |
| AZO-GO-PU | Covalent grafting of the amide linkage | Improved thermal properties and high-water repellence. | Anti-biofouling, fluid transportation, sensors, self-cleanings, super-hydrophobic valves, battery, and fuel cell | [94] |
| PANI-RGO-AZO | Covalent grafting, functionalization, and aniline polymerization | A specific capacitance of 328 F g−1, 80% capacitance retention after 1500 continuous charge-discharge cycles, and high electrochemical performance. | Supercapacitors | [95] |
| PANI-GO-AZO | Diazotization | High reversibility and specific capacitance retention after 500 cycles, with a capacitance of 478.3 F g−1. Excellent photosensitive electrochemical properties under UV irradiation and capacitance change rate of 52.57%. | Photoresponsive supercapacitor | [113] |
| GO-AZO | Covalent grafting | Rapid trans-cis photoisomerization and increased reversible photoswitching with fast response time <500 ms and a high on/off ratio of 8. | Photoswitching | [96] |
| AZO-GO-PVA | Covalent grafting | Mimic the reversible grabbing-release motions of a claw upon UV/visible irradiation. | Smart devices | [99] |
| AAZO-GO-PEG | Covalent grafting | High absorbance under visible light illumination, high latent heat of 84.5 J g−1, and photothermal conversion efficiency of 91%. | Solar thermal storage | [114] |
| AZO-RGO | Covalent grafting | High solar thermal energy storage density of 112 Wh kg−1 with 32-day prolonged storage. Outstanding cycling stability for 50 visible light-irradiated cycles, suggesting more than 4.5 years of use. | Solar thermal storage | [115] |
| RGO-bisAZO-2 | Covalent grafting | High power density (2517 W kg−1), high energy density (131 Wh kg−1), good cycling performance (50 cycles), and prolonged half-life (37 days). | Photothermal fuels | [116] |
| tri-AZO-RGO | Covalent grafting | High power density (3036.9 W kg−1), high energy density (150.3 Wh kg−1), and extended half-life (1250 h). Film releases 23.6–69.7% of stored heat, raising the temperature by 2–7 °C. | Photothermal energy | [117] |
| AZO-RGO-GNP | Electrostatic interactions | Photochemical behavior du to Gemini AZO-surfactant stabilizers and electrochemical performance governed by light irradiation. | Optic and photonic devices | [118] |
| AZO-GO | Esterification reaction | Good photosensitive electrochemical properties (high energy and maximum power densities of 47 Wh Kg−1 and 156.6 W Kg−1, respectively). | Solar thermal fuels and energy storage devices | [119] |
| PVK-AZO-GO | Amidation reaction and covalent grafting | A ternary electrical switching and nonvolatile WORM (write-once-read-many-times) memory performance, with low switching threshold voltages of −1.53 (ON1) and −2.50 V (ON2) and an OFF: ON1: ON2 current ratio of 1: 101.6:104.5. | Multilevel memory devices | [120] |
| AFGO-AZO-PI | Covalent polyimide | Improved response time (i.e., 0.5 ms) with transmission loss of 0.167 dB/cm. | Photoswitches | [121] |
| PolyAZO (Bismarck brown Y)-RGO | Non-covalent π-π stacking | Good repeatability in chemiresistor response per regeneration cycle and resistance is sensitive to O2 concentration. | Chemiresistor for mitochondrial consumption | [122] |
| AZO nanocluster-RGO, AZO nanocluster-GO | Non-covalent π-π stacking and direct immobilization | RGO-AZO nanocluster functions as n-type while GO p-type. | p-type diode and n-type diode | [123] |
| AZO-BNB-t8-RGO | Non-covalent π-π stacking | Improved linear optical absorption, high nonlinear optical absorption, and saturable absorption coefficient. | Nonlinear optical material | [124] |
| AZO-RGO | Covalent and non-covalent | Phototunable conductance with light-induced AZO trans-cis isomerization. Non-covalent functionalization provides better photoconductance tuning than the covalent counterpart, which may constrain the AZO photo-isomerization activity. | Molecular electronics | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagadevan, S.; Rahman, M.Z.; Léonard, E.; Losic, D.; Hessel, V. Sensor to Electronics Applications of Graphene Oxide through AZO Grafting. Nanomaterials 2023, 13, 846. https://doi.org/10.3390/nano13050846
Sagadevan S, Rahman MZ, Léonard E, Losic D, Hessel V. Sensor to Electronics Applications of Graphene Oxide through AZO Grafting. Nanomaterials. 2023; 13(5):846. https://doi.org/10.3390/nano13050846
Chicago/Turabian StyleSagadevan, Suresh, Md Zillur Rahman, Estelle Léonard, Dusan Losic, and Volker Hessel. 2023. "Sensor to Electronics Applications of Graphene Oxide through AZO Grafting" Nanomaterials 13, no. 5: 846. https://doi.org/10.3390/nano13050846
APA StyleSagadevan, S., Rahman, M. Z., Léonard, E., Losic, D., & Hessel, V. (2023). Sensor to Electronics Applications of Graphene Oxide through AZO Grafting. Nanomaterials, 13(5), 846. https://doi.org/10.3390/nano13050846