One-Step Solvothermal Synthesis by Ethylene Glycol to Produce N-rGO for Supercapacitor Applications
Abstract
1. Introduction
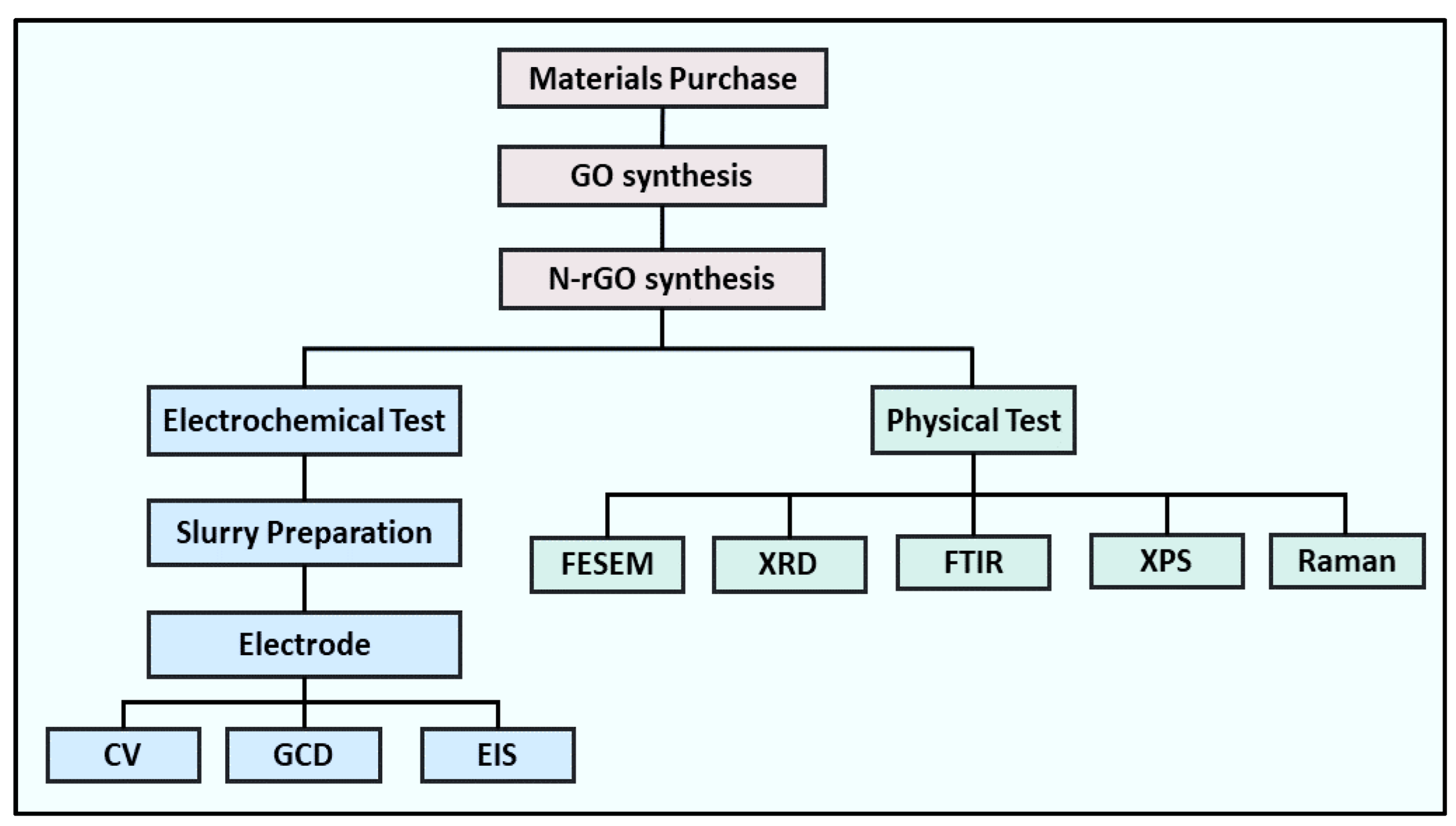
2. Experimental Section
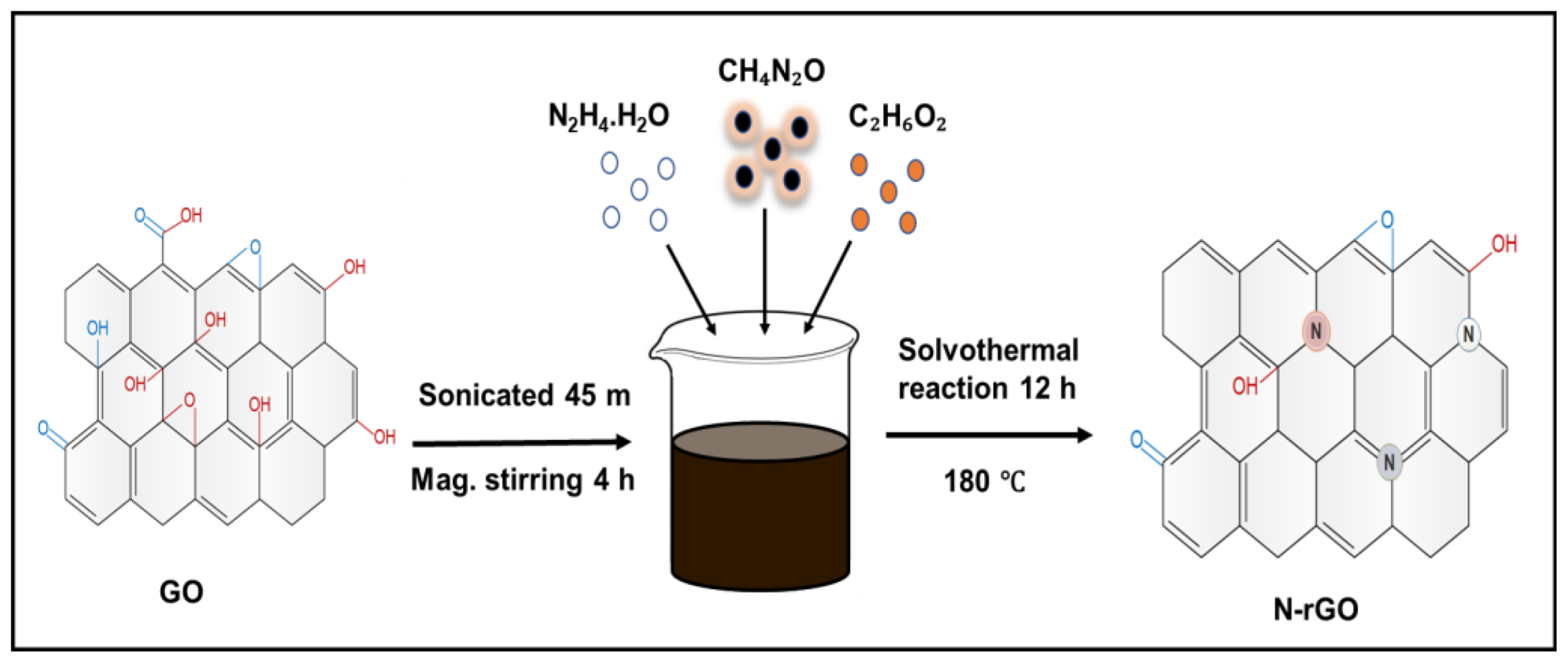
2.1. G.O. Synthesis
2.2. Synthesis of N-rGOs
2.3. Electrodes Fabrication and Performance Measurement
3. Results and Discussion
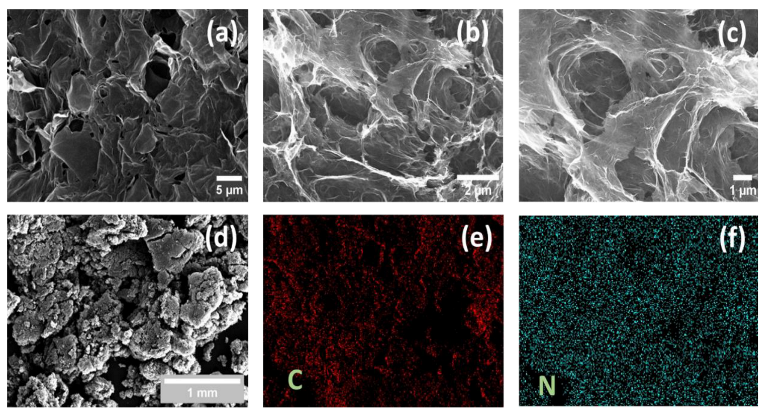
3.1. Structural Features
3.2. XRD
3.3. FTIR Spectroscopy
3.4. XPS
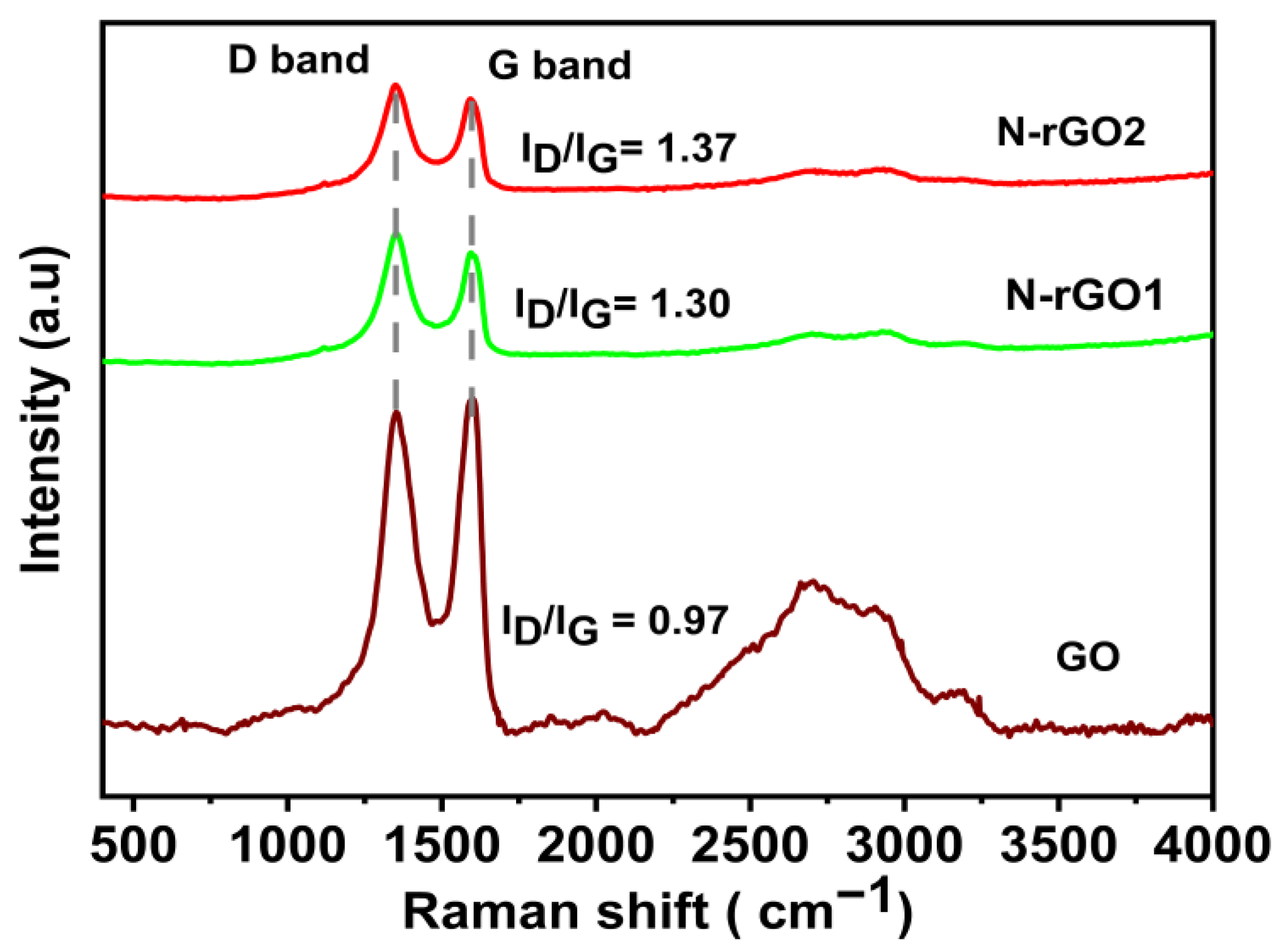
3.5. Raman Spectroscopy
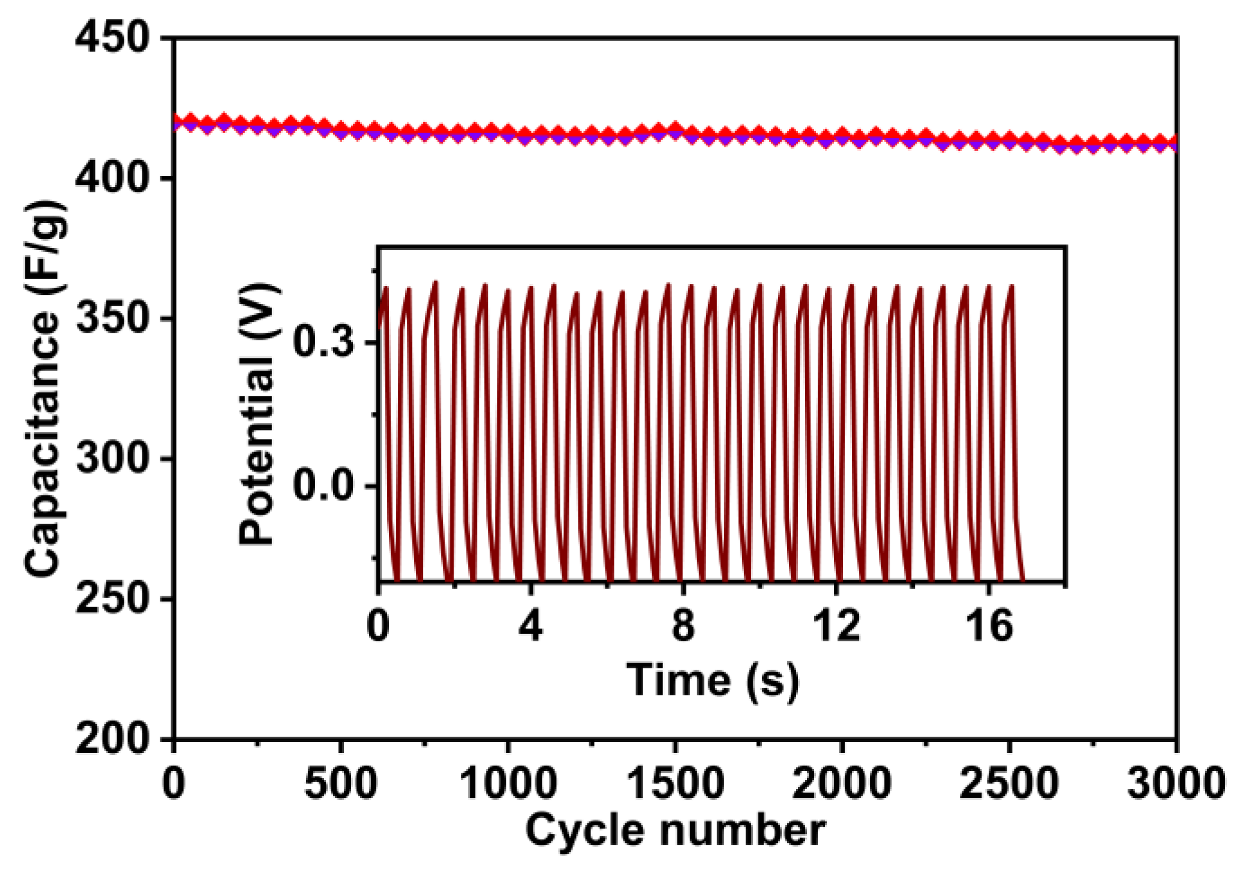
3.6. Electrochemical Performances
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, Y.; Huang, C.; Wang, L.; Hulicova-Jurcakova, D. Heteroatom-doped graphene for electrochemical energy storage. Chin. Sci. Bull. 2014, 59, 2102–2121. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Zhang, F.; Long, G.; Zhang, T.; Leng, K.; Zhang, Y.; Huang, Y.; Ma, Y.; Zhang, M. Controlling the effective surface area and pore size distribution of sp2 carbon materials and their impact on the capacitance performance of these materials. J. Am. Chem. Soc. 2013, 135, 5921–5929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, T.; Yang, X.; Zhang, L.; Leng, K.; Huang, Y.; Chen, Y. A high-performance supercapacitor-battery hybrid energy storage device based on graphene-enhanced electrode materials with ultrahigh energy density. Energy Environ. Sci. 2013, 6, 1623–1632. [Google Scholar] [CrossRef]
- Elessawy, N.A.; El Nady, J.; Wazeer, W.; Kashyout, A. Development of high-performance supercapacitor based on a novel controllable green synthesis for 3D nitrogen doped graphene. Sci. Rep. 2019, 9, 1129. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, H.; Zhang, H.; He, S.; Zhang, Q.; Liu, K.; Jiang, S.; Duan, G.; Zhang, K. Nanocellulose and its derived composite electrodes toward supercapacitors: Fabrication, properties, and challenges. J. Bioresour. Bioprod. 2022, 7, 245–269. [Google Scholar] [CrossRef]
- Yang, L.; Guo, X.; Jin, Z.; Guo, W.; Duan, G.; Liu, X.; Li, Y. Emergence of melanin-inspired supercapacitors. Nano Today 2021, 37, 101075. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Li, H.; Duan, G.; He, S.; Zhang, L.; Zhang, G.; Zhou, Z.; Jiang, S. N-doped honeycomb-like porous carbon towards high-performance supercapacitor. Chin. Chem. Lett. 2020, 31, 1986–1990. [Google Scholar] [CrossRef]
- Sharma, P.; Bhatti, T. A review on electrochemical double-layer capacitors. Energy Convers. Manag. 2010, 51, 2901–2912. [Google Scholar] [CrossRef]
- Han, X.; Xiao, G.; Wang, Y.; Chen, X.; Duan, G.; Wu, Y.; Gong, X.; Wang, H. Design and fabrication of conductive polymer hydrogels and their applications in flexible supercapacitors. J. Mater. Chem. A 2020, 8, 23059–23095. [Google Scholar] [CrossRef]
- Sinha, P.; Kar, K.K. Introduction to supercapacitors. In Handbook of Nanocomposite Supercapacitor Materials II; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–28. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Luo, X.-y.; Chen, Y.; Mo, Y. A review of charge storage in porous carbon-based supercapacitors. New Carbon Mater. 2021, 36, 49–68. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, J.G.; Xiao, J.Q. Nanostructured electrodes for high-performance pseudocapacitors. Angew. Chem. Int. Ed. 2013, 52, 1882–1889. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Mandal, B.; Saha, S.; Das, D.; Panda, J.; Das, S.; Sarkar, R.; Tudu, B. Supercapacitor performance of nitrogen doped graphene synthesized via DMF assisted single-step solvothermal method. FlatChem 2022, 34, 100400. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Cao, L.; Li, H.; Liu, X.; Liu, S.; Zhang, L.; Xu, W.; Yang, H.; Hou, H.; He, S.; Zhao, Y.; et al. Nitrogen, sulfur co-doped hierarchical carbon encapsulated in graphene with “sphere-in-layer” interconnection for high-performance supercapacitor. J. Colloid Interface Sci. 2021, 599, 443–452. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, M.O.; Ansari, S.A.; Cho, M.H. Simultaneous sulfur doping and exfoliation of graphene from graphite using an electrochemical method for supercapacitor electrode materials. J. Mater. Chem. A 2016, 4, 233–240. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.; Dubonos, S.; Firsov, A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Mao, S.; Pu, H.; Chen, J. Graphene oxide and its reduction: Modeling and experimental progress. RSC Adv. 2012, 2, 2643–2662. [Google Scholar] [CrossRef]
- Lee, J.W.; Ko, J.M.; Kim, J.-D. Hydrothermal preparation of nitrogen-doped graphene sheets via hexamethylenetetramine for application as supercapacitor electrodes. Electrochim. Acta 2012, 85, 459–466. [Google Scholar] [CrossRef]
- Fang, Y.; Luo, B.; Jia, Y.; Li, X.; Wang, B.; Song, Q.; Kang, F.; Zhi, L. Renewing functionalized graphene as electrodes for high-performance supercapacitors. Adv. Mater. 2012, 24, 6348–6355. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Chen, Z.; Yang, D.; Lu, H. Hierarchically structured graphene-based supercapacitor electrodes. RSC Adv. 2013, 3, 21183–21191. [Google Scholar] [CrossRef]
- Qiao, M.; Titirici, M.M. Engineering the interface of carbon electrocatalysts at the triple point for enhanced oxygen reduction reaction. Chem. A Eur. J. 2018, 24, 18374–18384. [Google Scholar] [CrossRef] [PubMed]
- Joucken, F.; Tison, Y.; Le Fèvre, P.; Tejeda, A.; Taleb-Ibrahimi, A.; Conrad, E.; Repain, V.; Chacon, C.; Bellec, A.; Girard, Y.; et al. Charge transfer and electronic doping in nitrogen-doped graphene. Sci. Rep. 2015, 5, 14564. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; Ma, C.; Li, G.; Wang, Y.; Zhang, K.; Li, F.; Liu, C.; Cheng, H.M.; Du, Y. Nitrogen-superdoped 3D graphene networks for high-performance supercapacitors. Adv. Mater. 2017, 29, 1701677. [Google Scholar] [CrossRef]
- Mortazavi, B.; Ahzi, S.; Toniazzo, V.; Rémond, Y. Nitrogen doping and vacancy effects on the mechanical properties of graphene: A molecular dynamics study. Phys. Lett. A 2012, 376, 1146–1153. [Google Scholar] [CrossRef]
- Tung, V.C.; Allen, M.J.; Yang, Y.; Kaner, R.B. High-throughput solution processing of large-scale graphene. Nat. Nanotechnol. 2009, 4, 25–29. [Google Scholar] [CrossRef]
- Ren, L.; Xu, H. Effect of ethylene glycol as solvent on the composition and morphology of nickel phosphide. Micro Nano Lett. 2018, 13, 1646–1648. [Google Scholar] [CrossRef]
- Coros, M.; Varodi, C.; Pogacean, F.; Gal, E.; Pruneanu, S.M. Nitrogen-Doped Graphene: The Influence of Doping Level on the Charge-Transfer Resistance and Apparent Heterogeneous Electron Transfer Rate. Sensors 2020, 20, 1815. [Google Scholar] [CrossRef]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and thermal reduction of graphene oxide: Reaction mechanisms, product structures, and reaction design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-efficient Synthesis of Graphene Oxide Based on Improved Hummers Method. Sci. Rep. 2016, 6, 36143. [Google Scholar] [CrossRef]
- Junaid, M.; Khir, M.M.; Witjaksono, G.; Tansu, N.; Saheed, M.S.M.; Kumar, P.; Ullah, Z.; Yar, A.; Usman, F. Boron-doped reduced graphene oxide with tunable bandgap and enhanced surface plasmon resonance. Molecules 2020, 25, 3646. [Google Scholar] [CrossRef]
- Zou, X.; Wu, D.; Mu, Y.; Xing, L.; Zhang, W.; Gao, Z.; Xu, F.; Jiang, K. Boron and nitrogen Co-doped holey graphene aerogels with rich B–N motifs for flexible supercapacitors. Carbon 2020, 159, 94–101. [Google Scholar] [CrossRef]
- Zhou, Q.; Ju, W.; Yong, Y.; Zhang, Q.; Liu, Y.; Li, J. Effect of the N/P/S and transition-metal co-doping on the quantum capacitance of supercapacitor electrodes based on mono- and multilayer graphene. Carbon 2020, 170, 368–379. [Google Scholar] [CrossRef]
- Prakash, D.; Manivannan, S. N, B co-doped and Crumpled Graphene Oxide Pseudocapacitive Electrode for High Energy Supercapacitor. Surf. Interfaces 2021, 23, 101025. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Sikiru, S.; Yahya, N.; Soleimani, H. Photon–phonon interaction of surface ionic adsorption within electric double layer in reservoir sandstone. J. Mater. Res. Technol. 2020, 9, 10957–10969. [Google Scholar] [CrossRef]
- Hassan, Y.M.; Guan, B.H.; Chuan, L.K.; Hamza, M.F.; Khandaker, M.U.; Sikiru, S.; Adam, A.A.; Sani, S.F.A.; Abdulkadir, B.A.; Ayub, S. The Influence of ZnO/SiO2 nanocomposite concentration on rheology, interfacial tension, and wettability for enhanced oil recovery. Chem. Eng. Res. Des. 2022, 179, 452–461. [Google Scholar] [CrossRef]
- Witjaksono, G.; Junaid, M.; Khir, M.H.; Ullah, Z.; Tansu, N.; Saheed, M.S.B.M.; Siddiqui, M.A.; Ba-Hashwan, S.S.; Algamili, A.S.; Magsi, S.A. Effect of Nitrogen Doping on the Optical Bandgap and Electrical Conductivity of Nitrogen-Doped Reduced Graphene Oxide. Molecules 2021, 26, 6424. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Acik, M.; Lee, G.; Mattevi, C.; Pirkle, A.; Wallace, R.M.; Chhowalla, M.; Cho, K.; Chabal, Y. The role of oxygen during thermal reduction of graphene oxide studied by infrared absorption spectroscopy. J. Phys. Chem. C 2011, 115, 19761–19781. [Google Scholar] [CrossRef]
- Page, A.J.; Chou, C.-P.; Pham, B.Q.; Witek, H.A.; Irle, S.; Morokuma, K. Quantum chemical investigation of epoxide and ether groups in graphene oxide and their vibrational spectra. Phys. Chem. Chem. Phys. 2013, 15, 3725–3735. [Google Scholar] [CrossRef] [PubMed]
- Van Khai, T.; Na, H.G.; Kwak, D.S.; Kwon, Y.J.; Ham, H.; Shim, K.B.; Kim, H.W. Comparison study of structural and optical properties of boron-doped and undoped graphene oxide films. Chem. Eng. J. 2012, 211, 369–377. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Uddin, M.E.; Kim, N.H.; Lau, A.K.; Lee, J.H. In-situ synthesis and characterization of electrically conductive polypyrrole/graphene nanocomposites. Polymer 2010, 51, 5921–5928. [Google Scholar] [CrossRef]
- Zhang, H.; Kuila, T.; Kim, N.H.; Yu, D.S.; Lee, J.H. Simultaneous reduction, exfoliation, and nitrogen doping of graphene oxide via a hydrothermal reaction for energy storage electrode materials. Carbon 2014, 69, 66–78. [Google Scholar] [CrossRef]
- Yadav, R.; Dixit, C. Synthesis, characterization and prospective applications of nitrogen-doped graphene: A short review. J. Sci. Adv. Mater. Devices 2017, 2, 141–149. [Google Scholar] [CrossRef]
- Yan, Y.; Kuila, T.; Kim, N.H.; Ku, B.-C.; Lee, J.H. Effects of reduction and polystyrene sulfate functionalization on the capacitive behaviour of thermally exfoliated graphene. J. Mater. Chem. A 2013, 1, 5892–5901. [Google Scholar] [CrossRef]
- Dai, S.; Liu, Z.; Zhao, B.; Zeng, J.; Hu, H.; Zhang, Q.; Chen, D.; Qu, C.; Dang, D.; Liu, M. A high-performance supercapacitor electrode based on N-doped porous graphene. J. Power Sources 2018, 387, 43–48. [Google Scholar] [CrossRef]
- Qin, Y.; Yuan, J.; Li, J.; Chen, D.; Kong, Y.; Chu, F.; Tao, Y.; Liu, M. Crosslinking graphene oxide into robust 3D porous N-doped graphene. Adv. Mater. 2015, 27, 5171–5175. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, S.; Xia, M.; Rehman, S.; Mu, S.; Kou, Z.; Zhang, Z.; Chen, Z.; Gao, F.; Hou, Y. N-P-O co-doped high performance 3D graphene prepared through red phosphorous-assisted “cutting-thin” technique: A universal synthesis and multifunctional applications. Nano Energy 2016, 28, 346–355. [Google Scholar] [CrossRef]
- Wang, K.; Xu, M.; Gu, Y.; Gu, Z.; Liu, J.; Fan, Q.H. Low-temperature plasma exfoliated n-doped graphene for symmetrical electrode supercapacitors. Nano Energy 2017, 31, 486–494. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Z.; Wang, H.; Wang, Y.; Liang, M.; Xue, S. Nitrogen-doped graphene as a cathode material for dye-sensitized solar cells: Effects of hydrothermal reaction and annealing on electrocatalytic performance. RSC Adv. 2015, 5, 10430–10439. [Google Scholar] [CrossRef]
- Ngidi, N.P.; Ollengo, M.A.; Nyamori, V.O. Effect of doping temperatures and nitrogen precursors on the physicochemical, optical, and electrical conductivity properties of nitrogen-doped reduced graphene oxide. Materials 2019, 12, 3376. [Google Scholar] [CrossRef]
- Muzyka, R.; Drewniak, S.; Pustelny, T.; Chrubasik, M.; Gryglewicz, G. Characterization of Graphite Oxide and Reduced Graphene Oxide Obtained from Different Graphite Precursors and Oxidized by Different Methods Using Raman Spectroscopy. Materials 2018, 11, 1050. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, F.; Zhang, T.; Leng, K.; Zhang, L.; Yang, X.; Ma, Y.; Huang, Y.; Zhang, M.; Chen, Y. Synthesis and supercapacitor performance studies of N-doped graphene materials using o-phenylenediamine as the double-N precursor. Carbon 2013, 63, 508–516. [Google Scholar] [CrossRef]
- Sahu, V.; Grover, S.; Tulachan, B.; Sharma, M.; Srivastava, G.; Roy, M.; Saxena, M.; Sethy, N.; Bhargava, K.; Philip, D.; et al. Heavily nitrogen doped, graphene supercapacitor from silk cocoon. Electrochim. Acta 2015, 160, 244–253. [Google Scholar] [CrossRef]
- Pham, V.H.; Nguyen-Phan, T.-D.; Jang, J.; Tuyet Vu, T.D.; Lee, Y.J.; Song, I.K.; Shin, E.W.; Chung, J.S. Nitrogen-doped mesoporous reduced graphene oxide for high-performance supercapacitors. RSC Adv. 2014, 4, 22455–22462. [Google Scholar] [CrossRef]
- Das, T.K.; Banerjee, S.; Kumar, A.; Debnath, A.K.; Sudarsan, V. Electrochemical performance of hydrothermally synthesized N-Doped reduced graphene oxide electrodes for supercapacitor application. Solid State Sci. 2019, 96, 105952. [Google Scholar] [CrossRef]
- Sui, Z.-Y.; Meng, Y.-N.; Xiao, P.-W.; Zhao, Z.-Q.; Wei, Z.-X.; Han, B.-H. Nitrogen-Doped Graphene Aerogels as Efficient Supercapacitor Electrodes and Gas Adsorbents. ACS Appl. Mater. Interfaces 2015, 7, 1431–1438. [Google Scholar] [CrossRef]
- Singh, S.K.; Dhavale, V.M.; Boukherroub, R.; Kurungot, S.; Szunerits, S. N-doped porous reduced graphene oxide as an efficient electrode material for high performance flexible solid-state supercapacitor. Appl. Mater. Today 2017, 8, 141–149. [Google Scholar] [CrossRef]
- Thalji, M.R.; Ali, G.A.; Lee, S.P.; Chong, K.F. Solvothermal synthesis of reduced graphene oxide as electrode material for supercapacitor application. Chem. Adv. Mater. 2019, 4, 17–26. [Google Scholar]
- Gao, Y.; Wang, L.; Li, Z.; Zhang, Y.; Xing, B.; Zhang, C.; Zhou, A. Electrochemical performance of Ti3C2 supercapacitors in KOH electrolyte. J. Adv. Ceram. 2015, 4, 130–134. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.; Ji, H.; Stoller, M.D.; Lai, L.; Murali, S.; Mcdonnell, S.; Cleveger, B.; Wallace, R.M.; Ruoff, R.S. Nitrogen doping of graphene and its effect on quantum capacitance, and a new insight on the enhanced capacitance of N-doped carbon. Energy Environ. Sci. 2012, 5, 9618–9625. [Google Scholar] [CrossRef]


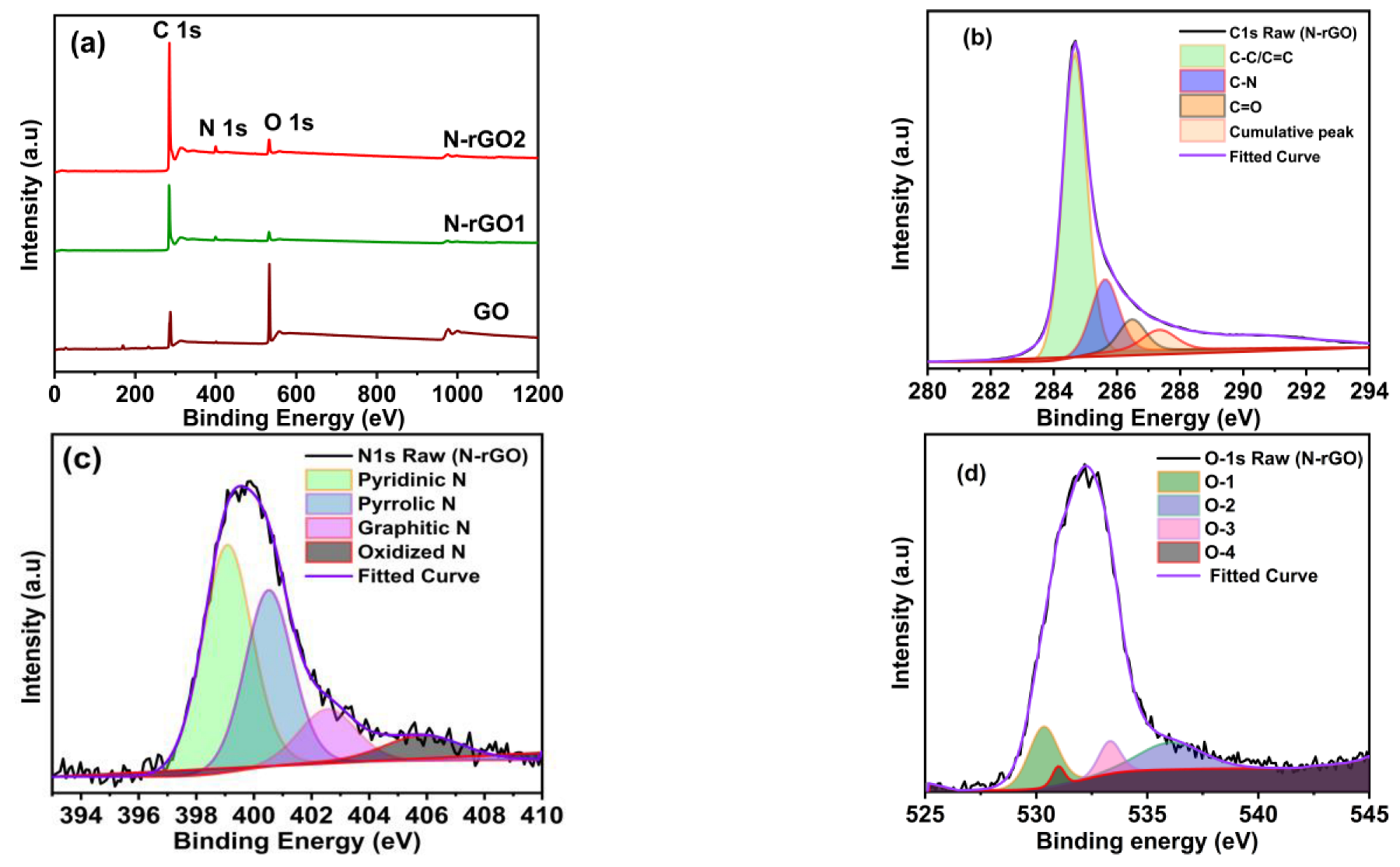

| Samples | C (%) | O (%) | N (%) |
|---|---|---|---|
| GO | 64.74 | 35.26 | - |
| N-rGO-1 | 90.70 | 5.89 | 3.41 |
| N-rGO-2 | 90.27 | 6.42 | 3.31 |
| Material | Synthesis Process | Electrolyte | Current Density (A/g) | Specific Capacitance (F/g) | Ref. |
|---|---|---|---|---|---|
| NG | Solvothermal | 6 M K.O.H. | 0.1 | 301 | [57] |
| N-rGO | Hydrothermal | BMIMBF4 | 1 | 390 | [50] |
| NG sheet | Hydrothermal | 6 M K.O.H. | 5 | 295 | [47] |
| NDG | Pyrolysis | 1 M H2SO4 | 0.8 | 220.5 | [58] |
| TRGO | Pyrolysis | EMIMBF4 | 1 | 290 | [59] |
| NRGO | Hydrothermal | 1 M H2SO4 | 0.1 | 199 | [60] |
| NGA | Hydrothermal | 1 M H2SO4 | 0.2 | 223 | [61] |
| NGS-HMT | Hydrothermal | 6 M K.O.H. | 0.5 | 161 | [22] |
| N-pGr | Hydrothermal | P.V.A.:H2SO4 | 1 | 230 | [62] |
| rGO | Solvothermal | 5 M KOH | 0.25 | 183 | [63] |
| Ti3C2 | Solvothermal | 3 M K.O.H. | 2.5 | 119 F/cm3 | [64] |
| S-GN | Electrochemical | 3 M K.O.H. | 3 | 320 | [18] |
| N-rGO | Solvothermal (EG) | 3 M K.O.H. | 1 | 420 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.O.; Nor, N.B.M.; Sawaran Singh, N.S.; Sikiru, S.; Dennis, J.O.; Shukur, M.F.b.A.; Junaid, M.; Abro, G.E.M.; Siddiqui, M.A.; Al-Amin, M. One-Step Solvothermal Synthesis by Ethylene Glycol to Produce N-rGO for Supercapacitor Applications. Nanomaterials 2023, 13, 666. https://doi.org/10.3390/nano13040666
Rahman MO, Nor NBM, Sawaran Singh NS, Sikiru S, Dennis JO, Shukur MFbA, Junaid M, Abro GEM, Siddiqui MA, Al-Amin M. One-Step Solvothermal Synthesis by Ethylene Glycol to Produce N-rGO for Supercapacitor Applications. Nanomaterials. 2023; 13(4):666. https://doi.org/10.3390/nano13040666
Chicago/Turabian StyleRahman, Mohammad Obaidur, Nursyarizal Bin Mohd Nor, Narinderjit Singh Sawaran Singh, Surajudeen Sikiru, John Ojur Dennis, Muhammad Fadhlullah bin Abd. Shukur, Muhammad Junaid, Ghulam E. Mustafa Abro, Muhammad Aadil Siddiqui, and Md Al-Amin. 2023. "One-Step Solvothermal Synthesis by Ethylene Glycol to Produce N-rGO for Supercapacitor Applications" Nanomaterials 13, no. 4: 666. https://doi.org/10.3390/nano13040666
APA StyleRahman, M. O., Nor, N. B. M., Sawaran Singh, N. S., Sikiru, S., Dennis, J. O., Shukur, M. F. b. A., Junaid, M., Abro, G. E. M., Siddiqui, M. A., & Al-Amin, M. (2023). One-Step Solvothermal Synthesis by Ethylene Glycol to Produce N-rGO for Supercapacitor Applications. Nanomaterials, 13(4), 666. https://doi.org/10.3390/nano13040666









