Activity and Thermal Aging Stability of La1−xSrxMnO3 (x = 0.0, 0.3, 0.5, 0.7) and Ir/La1−xSrxMnO3 Catalysts for CO Oxidation with Excess O2
Abstract
1. Introduction
2. Materials and Methods
2.1. LSMx and Ir/LSMx Synthesis
2.2. Catalysts Characterization Methods
2.3. Catalytic Activity and Durability Evaluation Experiments
3. Results and Discussion
3.1. Textural, Structural and Physicochemmical Properties of the Matarials
3.2. Catalytic Activity Evaluation on CO Oxidation
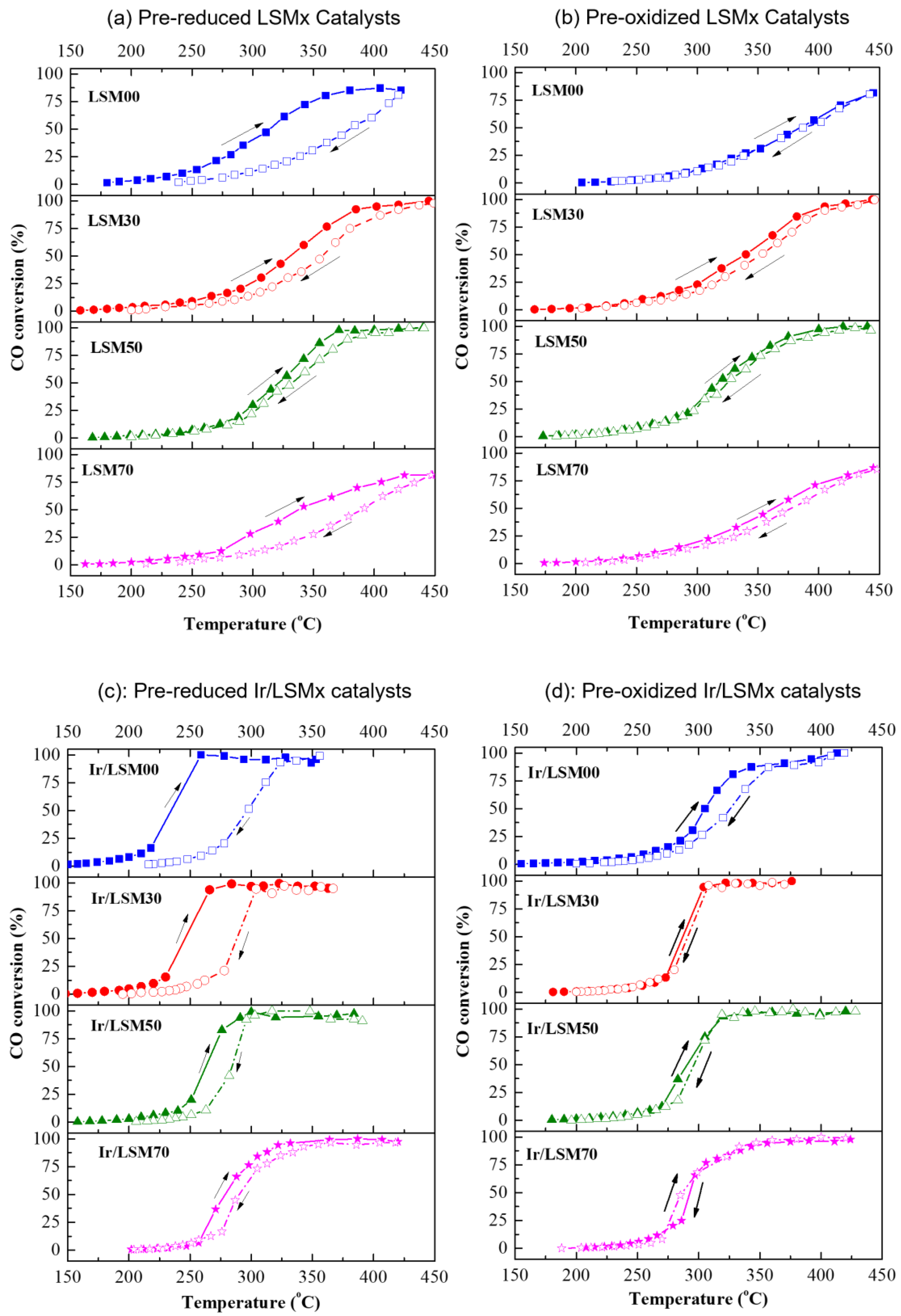
3.2.1. Light-Off Performance of Prereduced and Preoxidized Catalysts
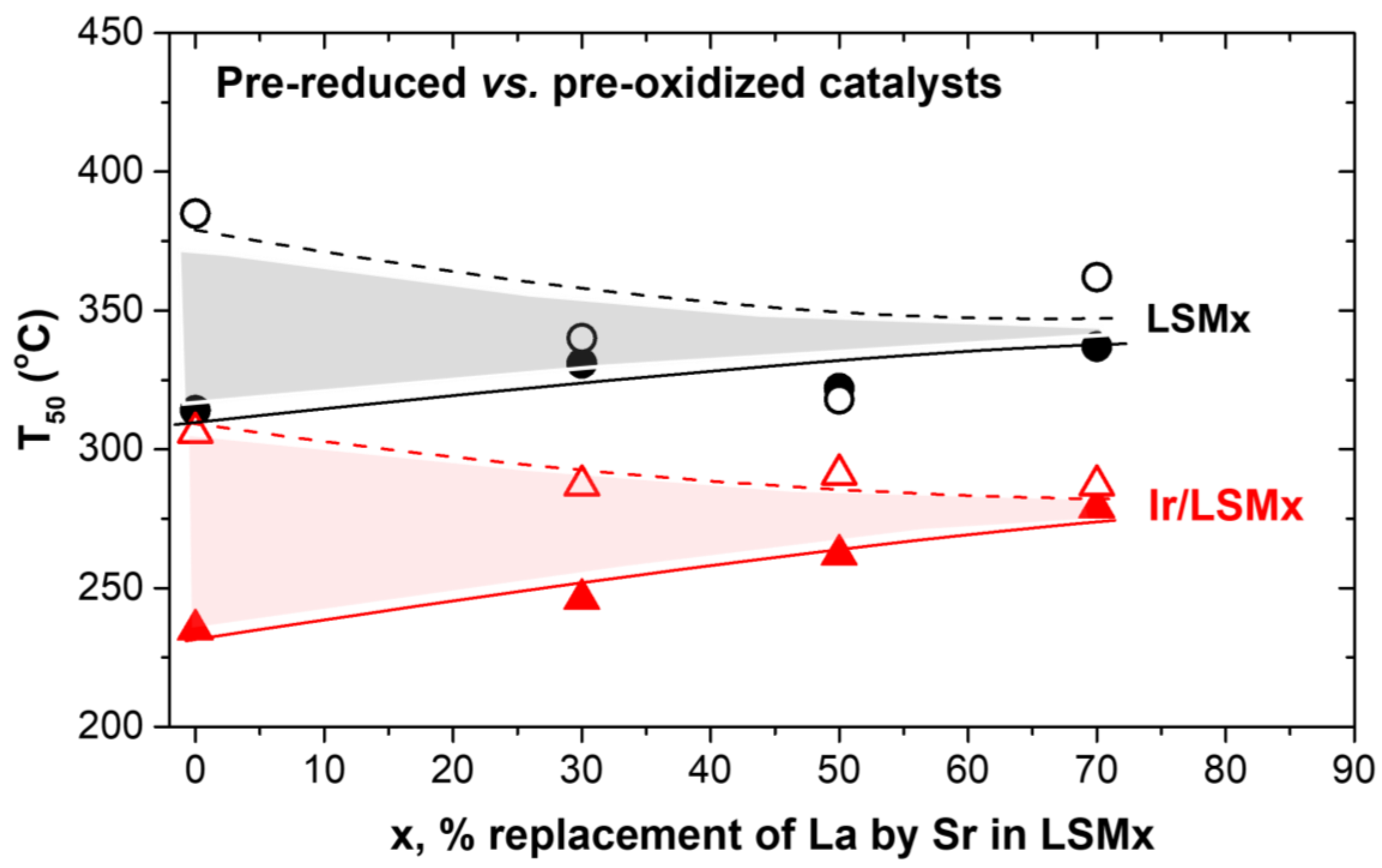
3.2.2. Heating (Light-Off)/Cooling (Light-Out) Cycles and Hysteresis Phenomena
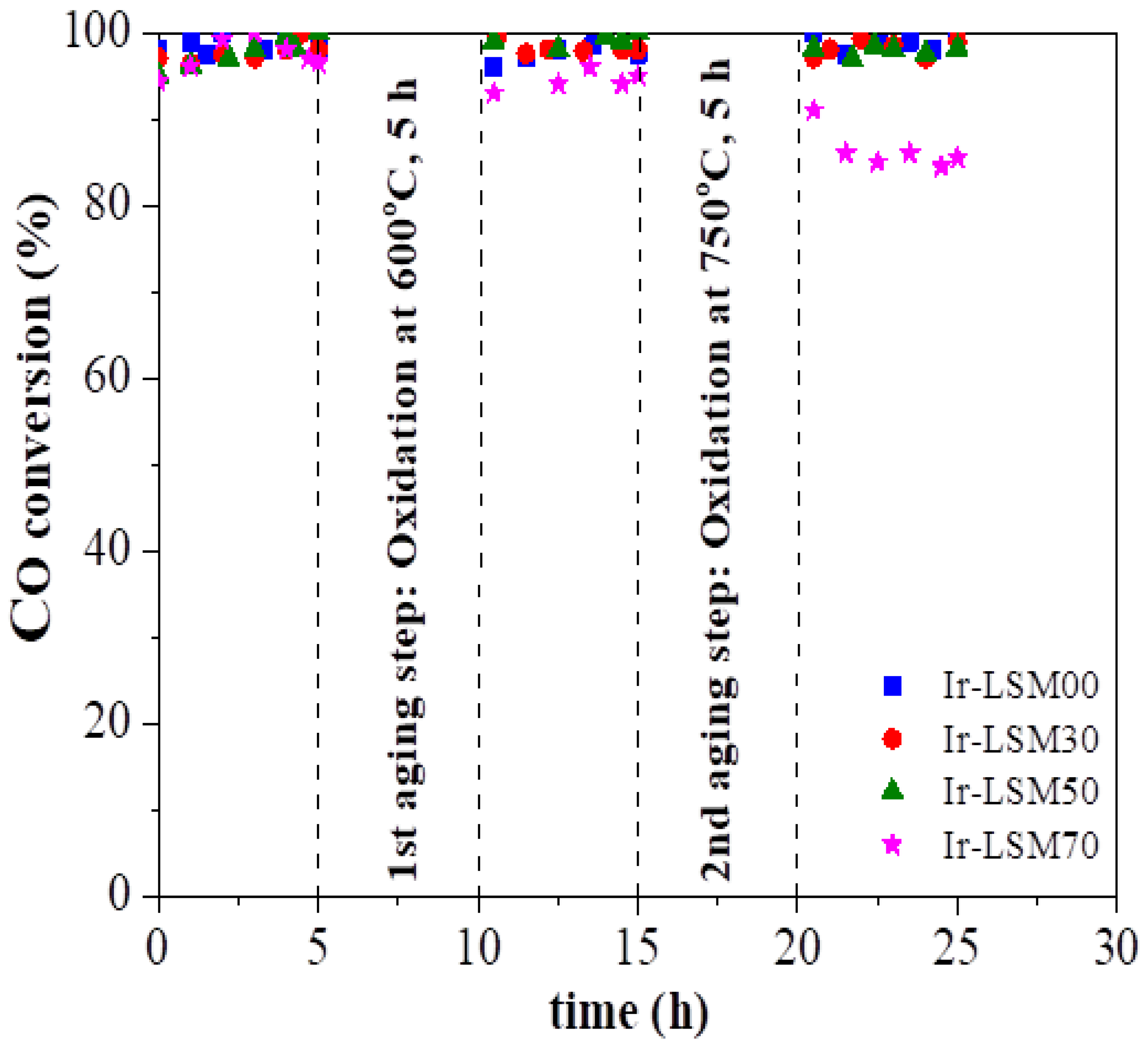
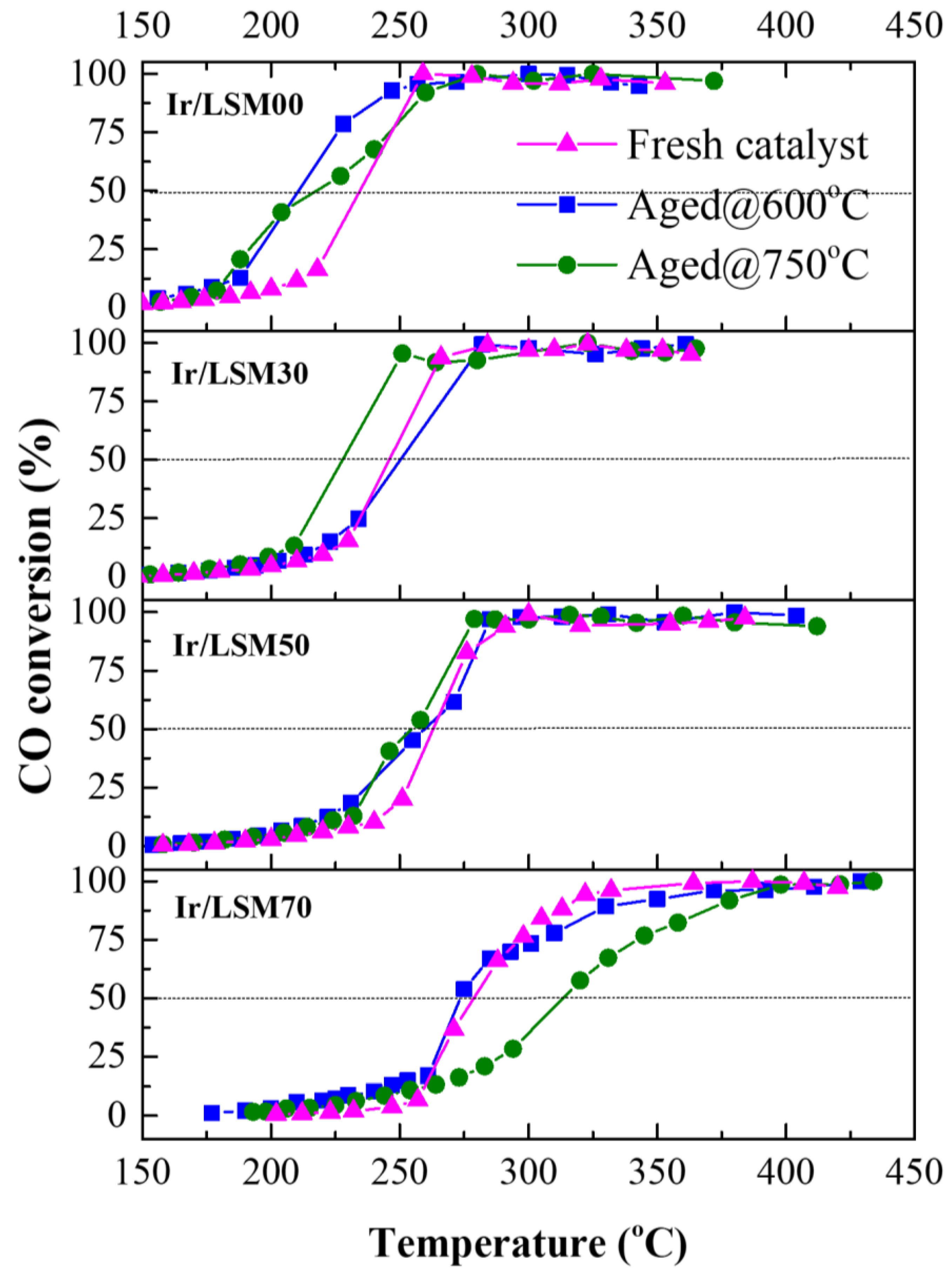
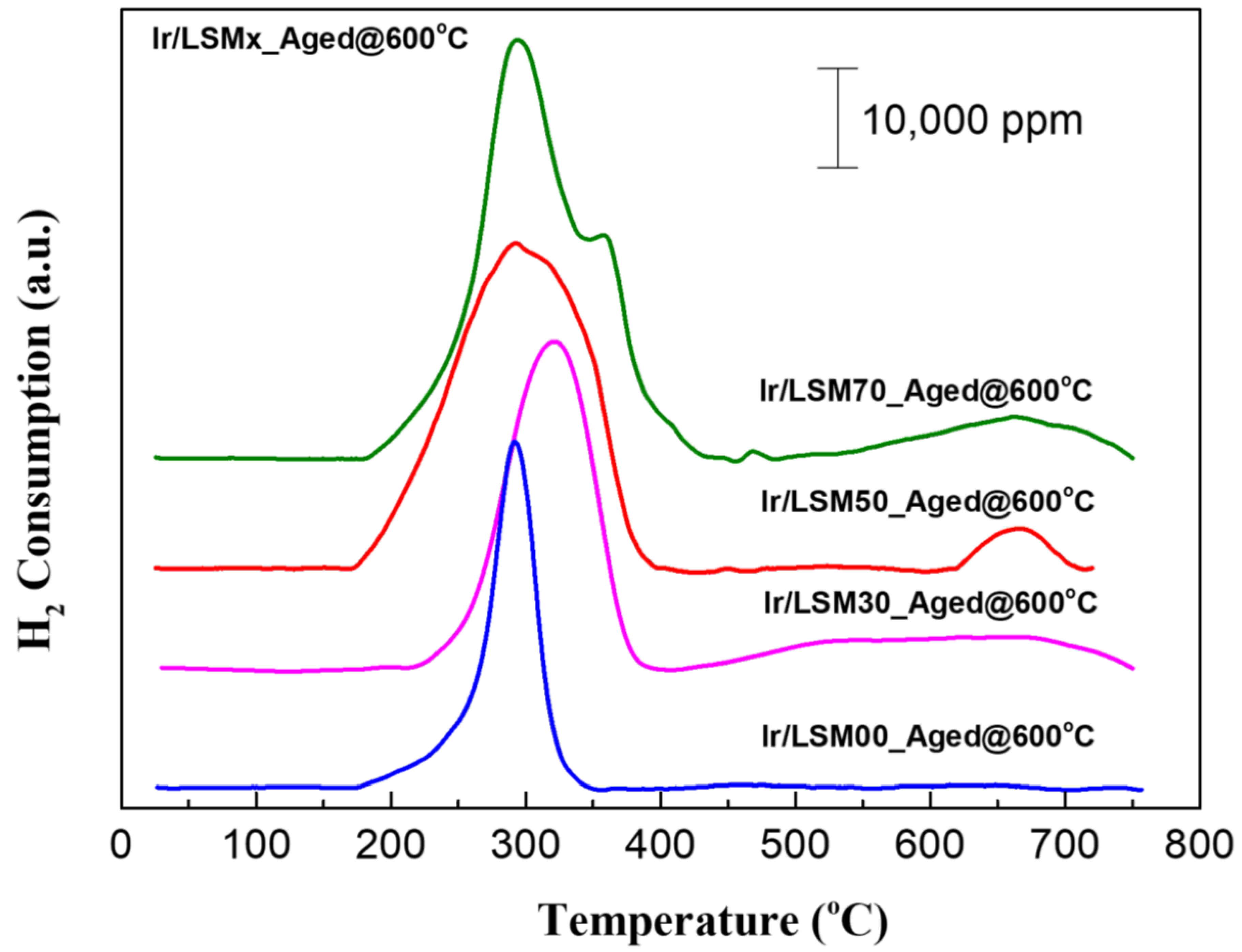
3.3. Ir/LSMx Performance Stability after Oxidative Thermal Aging Treatments
4. Conclusions
- -
- The amplitude of hysteresis appears extended in prereduced LSMx and Ir/LSMx, while significantly limited in preoxidized ones, and also decreases with increasing x;
- -
- The upper and lower limits of the hysteresis loop are bounded in between the behaviors of the prereduced and preoxidized catalysts.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al Soubaihi, R.M.; Saoud, K.M.; Dutta, J. Critical review of low-temperature CO oxidation and hysteresis phenomenon on heterogeneous catalysts. Catalysts 2018, 8, 660. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Vernoux, P.; Goula, G.; Caravaca, A. Electropositive Promotion by Alkalis or Alkaline Earths of Pt-Group Metals in Emissions Control Catalysis: A Status Report. Catalysts 2019, 9, 157. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Dong, F. Grand Challenges for Catalytic remediation in Environmental and Energy Applications toward a Cleaner and Sustainable Future. Front. Environ. Chem. 2020, 1, 5. [Google Scholar] [CrossRef]
- Soliman, N.K. Factors affecting CO oxidation reaction over nanosized materials: A review. J. Mater Res. Technol. 2019, 2, 2395–2407. [Google Scholar] [CrossRef]
- Gatla, S.; Aubert, D.; Agostini, G.; Mathon, O.; Pascarelli, S.; Lunkenbein, T.; Willinger, M.G.; Kaper, H. Room-Temperature CO Oxidation Catalyst: Low-Temperature Metal–Support Interaction between Platinum Nanoparticles and Nanosized Ceria. ACS Catal. 2016, 6, 6151–6155. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T.; Papadopoulou, C.; Batista, J.; Hocevar, S.; Matralis, H.K. A comparative study of Pt/γ-Al2O3, Au/α-Fe2O3 and CuO–CeO2 catalysts for the selective oxidation of carbon monoxide in excess hydrogen. Catal. Today 2002, 75, 157–167. [Google Scholar] [CrossRef]
- Konsolakis, M.; Macleod, N.; Isaac, J.; Yentekakis, I.V.; Lambert, R.M. Strong Promotion by Na of Pt/γ-Al2O3 Catalysts Operated under Simulated Exhaust Conditions. J. Catal. 2000, 193, 330–337. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Moggridge, G.; Vayenas, C.G.; Lambert, R.M. In Situ Controlled Promotion of Catalyst Surfaces via NEMCA: The Effect of Na on the Pt-Catalyzed CO Oxidation. J. Catal. 1994, 146, 292–305. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Vayenas, C.G. In Situ Controlled Promotion of Pt for CO Oxidation via NEMCA Using CaF2, as the Solid Electrolyte. J. Catal. 1994, 149, 238–242. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Freund, H.-J.; Meijer, G.; Scheffler, M.; Schlögl, R.; Wolf, M. CO Oxidation as a Prototypical Reaction for Heterogeneous Processes. Angew. Chem. Int. Ed. 2011, 50, 10064–10094. [Google Scholar] [CrossRef]
- Hatchings, G.J. Catalysis by Gold. Catal. Today 2005, 100, 55–61. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Neophytides, S.; Vayenas, C.G. Solid electrolyte aided study of the mechanism of CO oxidation on polycrystalline platinum. J. Catal. 1988, 111, 152–169. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Vayenas, C.G. The effect of electrochemical oxygen pumping on the steady-state and oscillatory behavior of CO oxidation on polycrystalline Pt. J. Catal. 1988, 111, 170–188. [Google Scholar] [CrossRef]
- Lashina, E.A.; Slavinskaya, E.M.; Chumakova, N.A.; Stadnichenko, A.I.; Salanov, A.N.; Chumakov, G.A.; Boronin, A.I. Inverse temperature hysteresis and self-sustained oscillations in CO oxidation over Pd at elevated pressures of reaction mixture: Experiment and mathematical modeling. Chem. Eng. Sci. 2020, 212, 115312. [Google Scholar] [CrossRef]
- Ladas, S.; Imbihl, R.; Ertl, G. Kinetic oscillations during the catalytic CO oxidation on Pd(110): The role of subsurface oxygen. Surf. Sci. 1989, 219, 88–106. [Google Scholar] [CrossRef]
- Lindstrom, T.H.; Tsotsis, T.T. Reaction rate oscillations during CO oxidation over Pt/γ-Al2O3; experimental observations and mechanistic causes. Surf. Sci. 1985, 150, 487–502. [Google Scholar] [CrossRef]
- Johanek, V.; Laurin, M.; Grant, A.W.; Kasemo, B.; Henry, C.R.; Libuda, J. Fluctuations and Bistabilities on Catalyst Nanoparticles. Science 2004, 304, 1639–1644. [Google Scholar] [CrossRef]
- Passos, A.R.; Rochet, A.; Manente, L.M.; Suzana, A.F.; Cha, W.; Meneau, F. Three-dimensional strain dynamics govern the hysteresis in heterogeneous catalysis. Nat. Commun. 2020, 11, 4733. [Google Scholar] [CrossRef]
- Al Soubaihi, R.M.; Saoud, K.M.; Myint, M.T.Z.; Gothelid, M.A.; Dutta, J. CO Oxidation Efficiency and Hysteresis Behavior over Mesoporous Pd/SiO2 Catalyst. Catalysts 2021, 11, 131. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef] [PubMed]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as substitutes of noble metals for heterogeneous catalysis: Dream or reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Georgiadis, A.G.; Drosou, C.; Charisiou, N.D.; Goula, M.A. Selective Catalytic Reduction of NOx over Perovskite-Based Catalysts Using CxHy(Oz), H2 and CO as Reducing Agents—A Review of the Latest Developments. Nanomaterials 2022, 12, 1042. [Google Scholar] [CrossRef]
- Zhang, M.; Sui, X.; Zhang, X.; Niu, M.; Li, C.; Wan, H.; Qiao, Z.-A.; Xie, H.; Li, X. Multi-fefects engineering of NiCo2O4 for catalytic propane oxidation. Appl. Surf. Sci. 2022, 600, 154040. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, C.; Li, X.; Li, Z.; Wang, L.; Zhang, Q.; Wu, G.; Li, Z. Engineering an effective MnO2 catalyst from LaMnO3 for catalytic methane combustion. Fuel 2019, 239, 1240–1245. [Google Scholar] [CrossRef]
- Si, W.; Wang, Y.; Peng, Y.; Li, J. Selective dissolution of A-site cations in ABO3 perovskites: A new path to high-performance catalysts. Angew. Chem. Int. Ed. 2015, 54, 7954–7957. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Hatzisymeon, M.; Betsi-Argyropoulou, I.; Botzolaki, G.; Kousi, K.; Kondarides, D.I.; Taylor, M.J.; Parlett, C.M.A.; Osatiashtiani, A.; et al. Effect of support oxygen storage capacity on the catalytic performance of Rh nanoparticles for CO2 reforming of methane. Appl. Catal. Environ. 2019, 243, 490–501. [Google Scholar] [CrossRef]
- Nikolaraki, E.; Goula, G.; Panagiotopoulou, P.; Taylor, M.J.; Kousi, K.; Kyriakou, G.; Kondarides, D.I.; Lambert, R.M.; Yentekakis, I.V. Support Induced Effects on the Ir Nanoparticles Activity, Selectivity and Stability Performance under CO2 Reforming of Methane. Nanomaterials 2021, 11, 2880. [Google Scholar] [CrossRef]
- Gálvez, M.E.; Jacot, R.; Scheffe, J.; Cooper, T.; Patzke, G.; Steinfeld, A. Physico-chemical changes in Ca, Sr and Al-doped La–Mn–O perovskites upon thermochemical splitting of CO2 via redox cycling. Phys. Chem. Chem. Phys. 2015, 17, 6629–6634. [Google Scholar] [CrossRef]
- Santos, V.P.; Carabineiro, S.A.C.; Tavares, P.B.; Pereira, M.F.R.; Orfao, J.J.M.; Figueirdo, J.L. Oxidation of CO, ethanol and toluene over TiO2 supported noble metal catalysts. Appl. Catal. 2010, 99, 198–205. [Google Scholar] [CrossRef]
- Okumura, M.; Konishi, E.; Ichikawa, S.; Akita, T. Preparation of iridium catalysts by deposition precipitation: Room temperature oxidation of CO. Stud. Surf. Sci. Catal. 2000, 143, 345–352. [Google Scholar] [CrossRef]
- Tomska-Foralewska, I.; Zielinski, M.; Pietrowski, M.; Przystajko, W.; Wojciechowska, M. Iridium supported on MgF2–MgO as catalyst for CO oxidation. Catal. Today 2011, 176, 263–266. [Google Scholar] [CrossRef]
- Schick, L.; Sanchis, R.; Gonzalez-Alfaro, V.; Agouram, S.; Lopez, J.M.; Torrente-Murciano, L.; Garcia, T.; Solsona, B. Size-activity relationship of iridium particles supported on silica for the total oxidation of volatile organic compounds (VOCs). Chem. Eng. J. 2019, 366, 100–111. [Google Scholar] [CrossRef]
- Ashcroft, A.T.; Cheetham, A.K.; Green, M.L.H.; Vernon, P.D.F. Partial oxidation of methane to synthesis gas using carbon dioxide. Nature 1991, 352, 225–226. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Panagiotopoulou, P.; Katsoni, A.; Diamadopoulos, E.; Mantzavinos, D.; Delimitis, A. Dry Reforming of Methane: Catalytic Performance and Stability of Ir Catalysts Supported on γ-Al2O3, Zr0.92Y0.08O2−δ (YSZ) or Ce0.9Gd0.1O2−δ (GDC) Supports. Top. Catal. 2015, 58, 1228–1241. [Google Scholar] [CrossRef]
- Pachatouridou, E.; Papista, E.; Iliopoulou, E.F.; Delimitis, A.; Goula, G.; Yentekakis, I.V.; Marnellos, G.E.; Konsolakis, M. Nitrous oxide decomposition over Al2O3 supported noble metals (Pt, Pd, Ir): Effect of metal loading and feed composition. J. Environ. Chem. Eng. 2015, 3, 815–821. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Panagiotopoulou, P.; Kampouri, S.; Taylor, M.J.; Kyriakou, G.; Lambert, R.M. Stabilization of catalyst particles against sintering on oxide supports with high oxygen ion lability exemplified by Ir-catalysed decomposition of N2O. Appl. Catal. Environ. 2016, 192, 357–364. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Kampouri, S.; Betsi-Argyropoulou, I.; Panagiotopoulou, P.; Taylor, M.J.; Kyriakou, G.; Lambert, R.M. Ir-catalyzed nitrous oxide (N2O) decomposition: Effect of the Ir particle size and metal-support interactions. Catal. Lett. 2018, 148, 341–347. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Papageridis, K.N.; Delimitis, A.; Papista, E.; Pachatouridou, E.; Iliopoulou, E.F.; Marnellos, G.; Konsolakis, M.; Yentekakis, I.V. A comparative study of the H2-assisted selective catalytic reduction of nitric oxide by propene over noble metal (Pt, Pd, Ir)/γ-Al2O3 catalysts. J. Environ. Chem. Eng. 2016, 4, 1629–1641. [Google Scholar] [CrossRef]
- Goula, G.; Botzolaki, G.; Osatiashtiani, A.; Parlett, C.M.A.; Kyriakou, G.; Lambert, R.M.; Yentekakis, I.V. Oxidative Thermal Sintering and Redispersion of Rh Nanoparticles on Supports with High Oxygen Ion Lability. Catalysts 2019, 9, 541. [Google Scholar] [CrossRef]
- Yentekakis, I.V. The effective-double-layer as an efficient tool for the design of sinter-resistant catalysts. In Recent Advances in Electrochemical Promotion of Catalysis; Vayenas, C.G., Vernoux, P., Eds.; Springer-Nature: Berlin/Heidelberg, Germany, 2023; pp. 117–149. Available online: https://link.springer.com/chapter/10.1007/978-3-031-13893-5_4 (accessed on 10 January 2023).
- Matsouka, C.; Zaspalis, V.; Nalbandian, L. Perovskites as oxygen carriers in chemical looping reforming process—Preparation of dense perovskite membranes and ionic conductivity measurement. Mater. Today Proc. 2018, 5, 27543–27552. [Google Scholar] [CrossRef]
- Nalbandian, L.; Evdou, A.; Matsouka, C.; Zaspalis, V. Assessment of (La1−xSrx)MnO3±δ perovskites as oxygen-carrier materials in chemical-looping processes. Fuel Proc. Technol. 2022, 226, 107086. [Google Scholar] [CrossRef]
- Evdou, A.; Georgitsis, T.; Matsouka, C.; Pachatouridou, E.; Iliopoulou, E.; Zaspalis, V. Defect Chemistry and Chemical Looping Performance of La1−xMxMnO3 (M = Sr, Ca, (x = 0–0.5)) Perovskites. Nanomaterials 2022, 12, 3461. [Google Scholar] [CrossRef] [PubMed]
- Nishihata, Y.; Mizuki, J.; Akao, T.; Tanaka, H.; Uenishi, M.; Kimura, M.; Okamoto, T.; Hamada, N. Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 2002, 418, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Yentekakis, I.V.; Konsolakis, M. Three-way Catalysis. In Perovskites and Related Mixed Oxides; Wiley-VCH, Vergal GmbH & Co., KGaA: Weinheim, Germany, 2016; pp. 559–586. [Google Scholar] [CrossRef]
- Haron, W.; Wisitsoraat, A.; Wongnawa, S. Comparison of monocrystalline LaMO3 (M = Co, Al) Perovskite Oxide Prepared by Co-Precipitation Method. Int. J. Chem. Eng. Appl. 2014, 5, 123–126. [Google Scholar] [CrossRef]
- Kröger, F.A.; Vink, H.J. Relations between the Concentrations of Imperfections in Crystalline Solids. J. Phys. Chem. Sol. 1958, 5, 208–223. [Google Scholar] [CrossRef]
- Nowotny, J.; Rekas, M. Defect Chemistry of (La,Sr)MnO3. J. Am. Ceram. Soc. 1998, 81, 67–80. [Google Scholar] [CrossRef]
- Fornasiero, P.; Di Monte, R.; Ranga Rao, G.; Kaspar, J.; Meriani, S.; Trovarelli, A.; Graziani, M. Rh-Loaded CeO2-ZrO2 Solid-Solutions as Highly Efficient Oxygen Exchangers: Dependence of the Reduction Behavior and the Oxygen Storage Capacity on the Structural-Properties. J. Catal. 1995, 151, 168–177. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, R.; Chen, B.; Bion, N.; Duprez, D.; Royer, S. Activity of perovskite-type mixed oxides for the low-temperature CO oxidation: Evidence of oxygen species participation from the solid. J. Catal. 2012, 295, 45–58. [Google Scholar] [CrossRef]
- Petrolekas, P.D.; Metcalfe, I.S. Solid Electrolyte Potentiometric Study of La(Sr)MnO3 Catalyst During Carbon-Monoxide Oxidation. J. Catal. 1995, 152, 147–163. [Google Scholar] [CrossRef]
- Petrolekas, P.D.; Metcalfe, I.S. Ionic Redox Behavior of La(Sr)MnO3 Catalyst During Transient CO Oxidation. J. Catal. 1995, 157, 545–549. [Google Scholar] [CrossRef]
- Ruh, T.; Buchinger, R.; Lindenthal, L.; Schrenk, F.; Rameshan, C. CO Oxidation Capabilities of La- and Nd-Based Perovskites. Fuels 2022, 3, 31–43. [Google Scholar] [CrossRef]
- Yang, J.; Hu, S.Y.; Fang, Y.R.; Hoang, S.; Li, L.; Yang, W.W.; Liang, Z.F.; Wu, J.; Hu, J.P.; Xiao, W.; et al. Oxygen Vacancy Promoted O2 Activation over Perovskite Oxide for Low-Temperature CO Oxidation. ACS Catal. 2019, 9, 9751–9763. [Google Scholar] [CrossRef]
- Hauptmann, W.; Votsmeier, M.; Gieshoff, J.; Drochner, A.; Vogel, H. Inverse hysteresis during the NO oxidation on Pt under lean conditions. Appl. Catal. 2009, 93, 22–29. [Google Scholar] [CrossRef]
- Ghosh, T.; Arce-Ramos, J.M.; Li, W.-Q.; Yan, H.; Chee, S.W.; Genest, A.; Mirsaidov, U. Periodic structural changes in Pd nanoparticles during oscillatory CO oxidation reaction. Nat. Commun. 2022, 13, 6176. [Google Scholar] [CrossRef]
- Hendriksen, B.; Ackermann, M.; van Rijn, R.; Stoltz, D.; Popa, I.; Balmes, O.; Resta, A.; Wermeille, D.; Felici, R.; Ferrer, S.; et al. The role of steps in surface catalysis and reaction oscillations. Nat. Chem. 2010, 2, 730–734. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Fiedorow, R.M.J.; Chahar, B.S.; Wanke, S.E. The sintering of supported metal catalysts. II. Comparison of sintering rates of supported Pt, Ir, and Rh Catalysts in hydrogen and oxygen. J. Catal. 1978, 51, 193–202. [Google Scholar] [CrossRef]
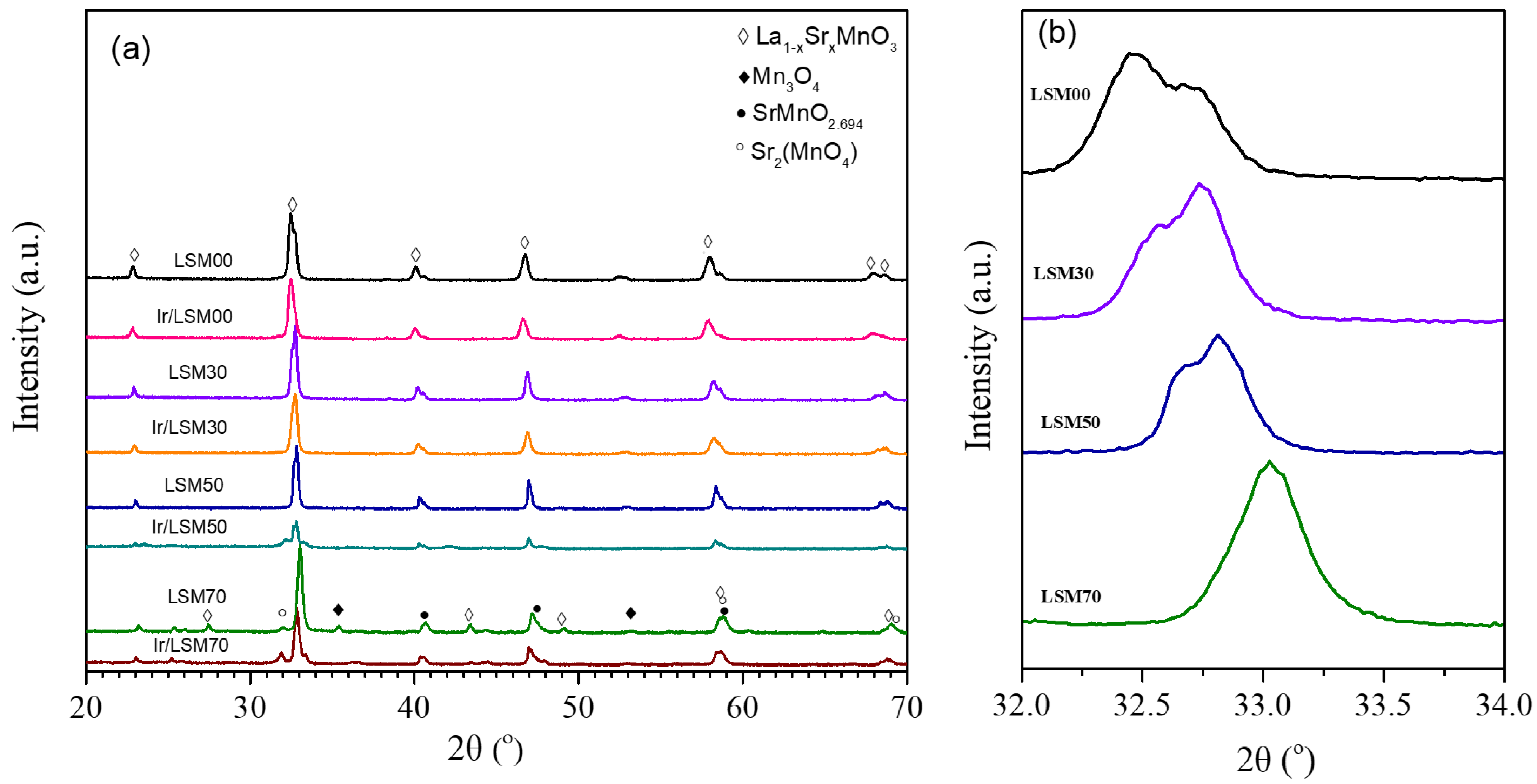
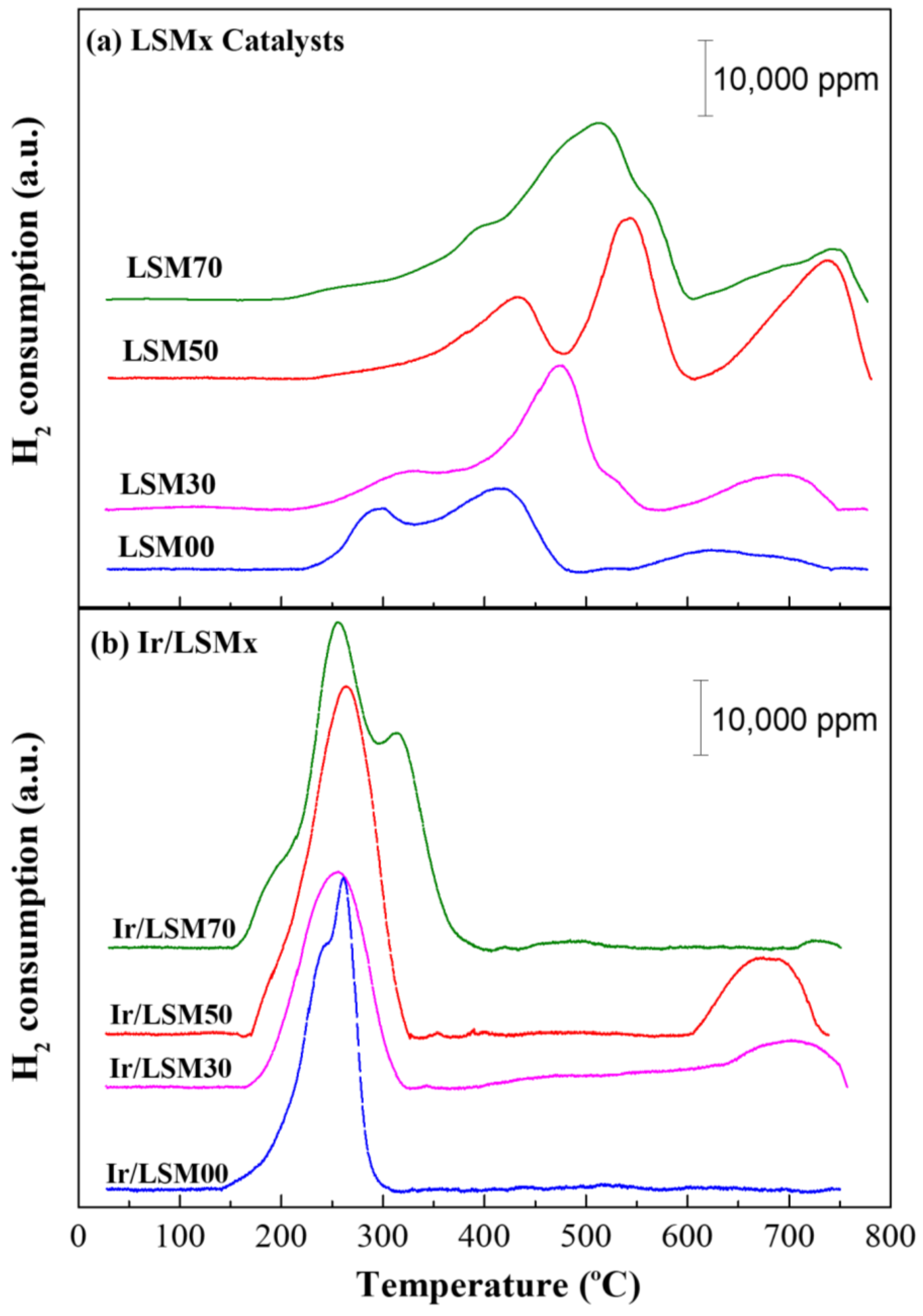
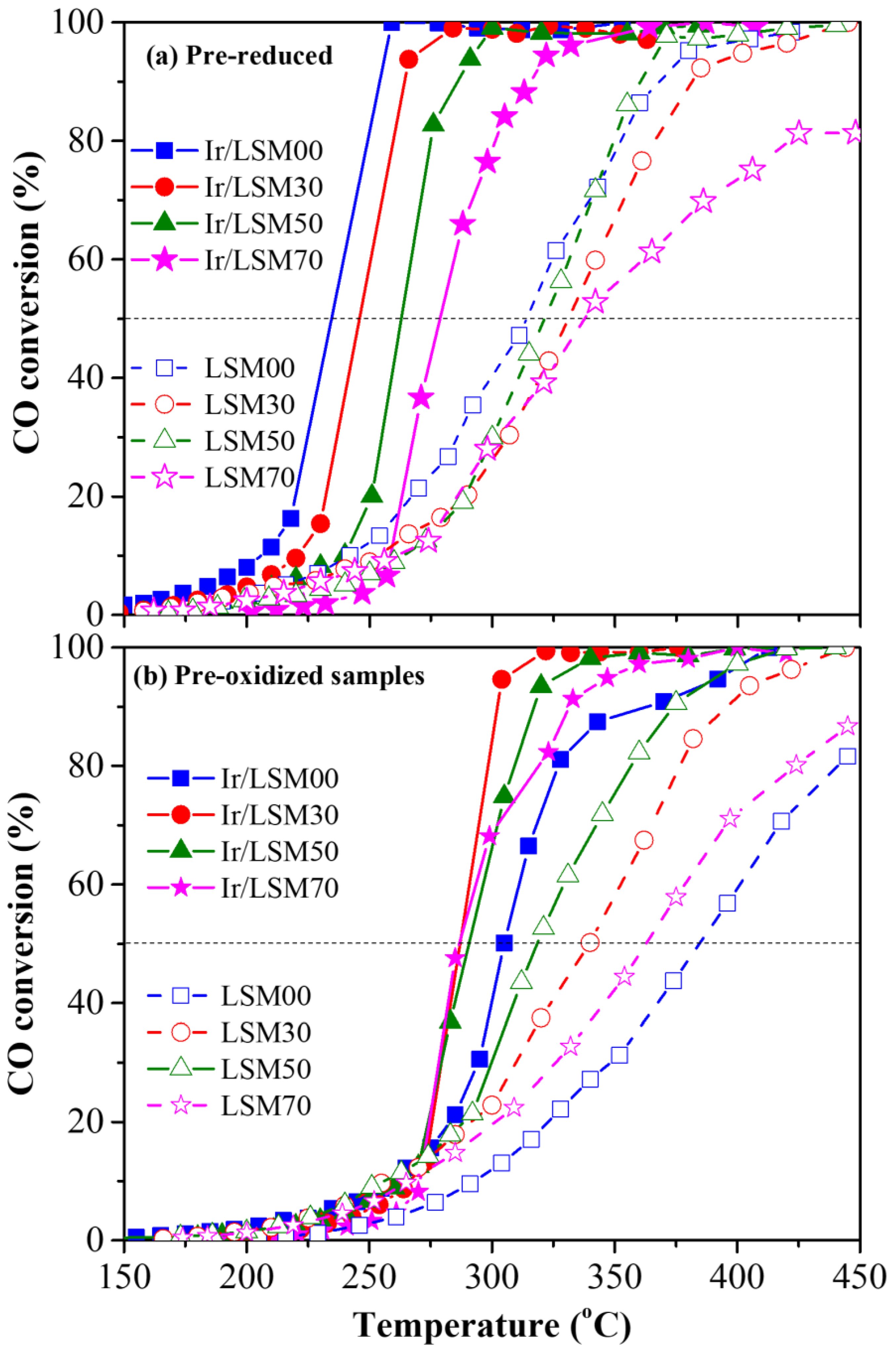
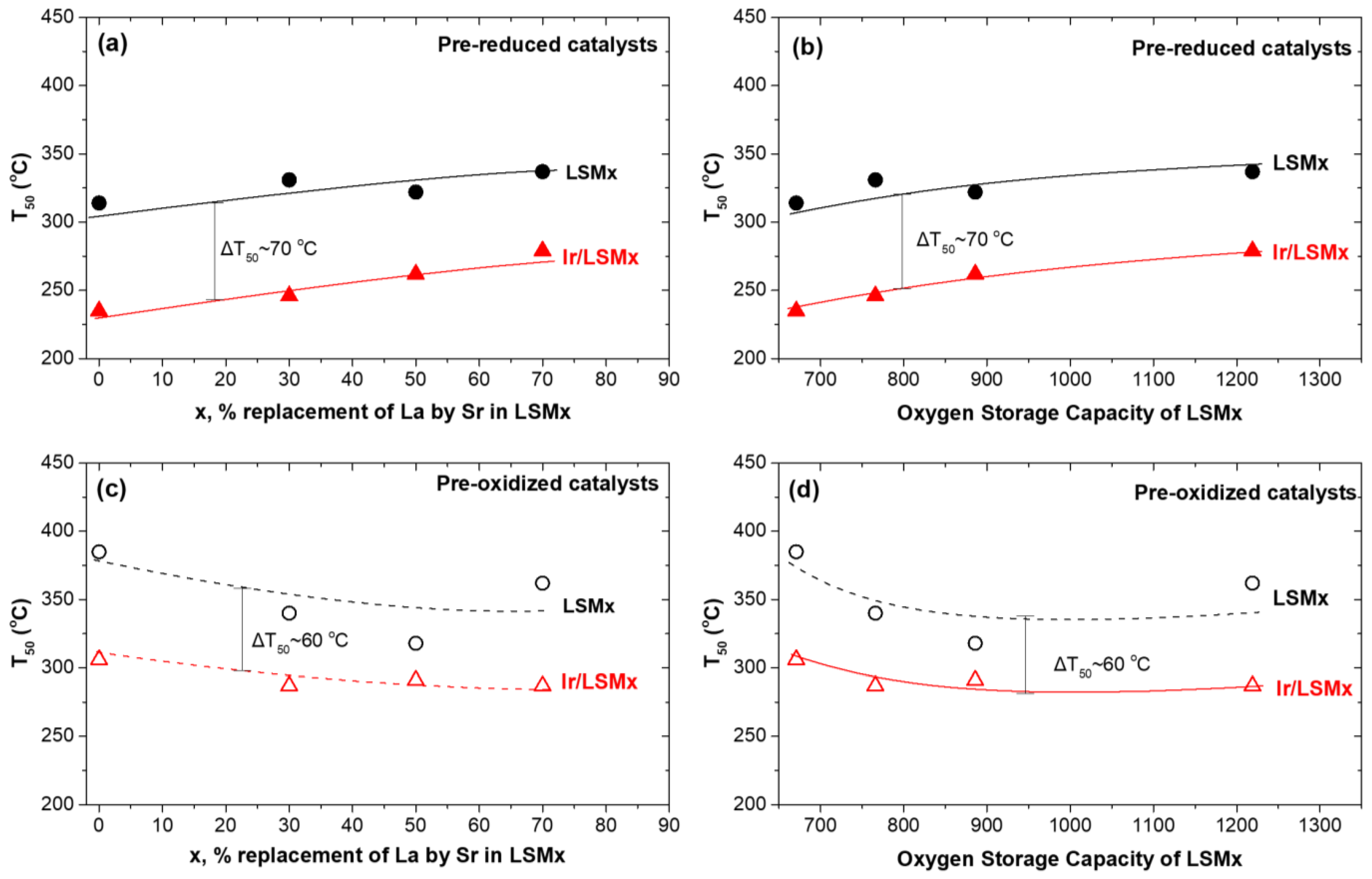
| Catalyst Code | Chemical Formula | SBET (m2/g) | Mean Pores Diameter (nm) | Total OSC (μmol O2/g) | H2 Uptake VChem.H2 (cm3/g) | Average Ir Particle Size (nm) | Ir Dispersion (H−Ir) |
|---|---|---|---|---|---|---|---|
| LSM00 | LaMnO3 | 12.0 | 10.9 | 671 | - | - | - |
| LSM30 | La0.7Sr0.3MnO3 | 10.4 | 9.84 | 766 | - | - | - |
| LSM50 | La0.5Sr0.5MnO3 | 6.8 | 8.91 | 886 | - | - | - |
| LSM70 | La0.3Sr0.7MnO3 | 11.3 | 8.79 | 1219 | - | - | - |
| Ir/LSM00 | Ir/LaMnO3 | 9.7 | 11.9 | 753 | 0.80 | 1.1 | 0.63 |
| Ir/LSM30 | Ir/La0.7Sr0.3MnO3 | 10.5 | 9.96 | 981 | 0.79 | 1.1 | 0.62 |
| Ir/LSM50 | Ir/La0.5Sr0.5MnO3 | 6.2 | 8.11 | 1203 | 0.92 | 1.0 | 0.73 |
| Ir/LSM70 | Ir/La0.3Sr0.7MnO3 | 11.0 | 13.7 | 1348 | 0.78 | 1.2 | 0.61 |
| Catalyst Code | SBET (m2/g) | H2 Uptake VChem.H2 (mL/g) | Average Ir Particle Size (nm) | Ir Dispersion (H−Ir) |
|---|---|---|---|---|
| Ir/LSM00-Aged@600°C | 14.1 | 0.82 | 1.1 | 0.64 |
| Ir/LSM30-Aged@600°C | 15.6 | 0.81 | 1.1 | 0.64 |
| Ir/LSM50-Aged@600°C | 9.1 | 0.99 | 0.9 | 0.78 |
| Ir/LSM70-Aged@600°C | 10.7 | 0.62 | 1.5 | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drosou, C.; Nikolaraki, E.; Nikolaou, V.; Koilia, E.; Artemakis, G.; Stratakis, A.; Evdou, A.; Charisiou, N.D.; Goula, M.A.; Zaspalis, V.; et al. Activity and Thermal Aging Stability of La1−xSrxMnO3 (x = 0.0, 0.3, 0.5, 0.7) and Ir/La1−xSrxMnO3 Catalysts for CO Oxidation with Excess O2. Nanomaterials 2023, 13, 663. https://doi.org/10.3390/nano13040663
Drosou C, Nikolaraki E, Nikolaou V, Koilia E, Artemakis G, Stratakis A, Evdou A, Charisiou ND, Goula MA, Zaspalis V, et al. Activity and Thermal Aging Stability of La1−xSrxMnO3 (x = 0.0, 0.3, 0.5, 0.7) and Ir/La1−xSrxMnO3 Catalysts for CO Oxidation with Excess O2. Nanomaterials. 2023; 13(4):663. https://doi.org/10.3390/nano13040663
Chicago/Turabian StyleDrosou, Catherine, Ersi Nikolaraki, Vasilios Nikolaou, Evangelia Koilia, Georgios Artemakis, Antonios Stratakis, Antigoni Evdou, Nikolaos D. Charisiou, Maria A. Goula, Vasilios Zaspalis, and et al. 2023. "Activity and Thermal Aging Stability of La1−xSrxMnO3 (x = 0.0, 0.3, 0.5, 0.7) and Ir/La1−xSrxMnO3 Catalysts for CO Oxidation with Excess O2" Nanomaterials 13, no. 4: 663. https://doi.org/10.3390/nano13040663
APA StyleDrosou, C., Nikolaraki, E., Nikolaou, V., Koilia, E., Artemakis, G., Stratakis, A., Evdou, A., Charisiou, N. D., Goula, M. A., Zaspalis, V., & Yentekakis, I. V. (2023). Activity and Thermal Aging Stability of La1−xSrxMnO3 (x = 0.0, 0.3, 0.5, 0.7) and Ir/La1−xSrxMnO3 Catalysts for CO Oxidation with Excess O2. Nanomaterials, 13(4), 663. https://doi.org/10.3390/nano13040663










