Smart Nanofiber Mesh with Locally Sustained Drug Release Enabled Synergistic Combination Therapy for Glioblastoma
Abstract
1. Introduction
2. Materials and Methods
3. Results
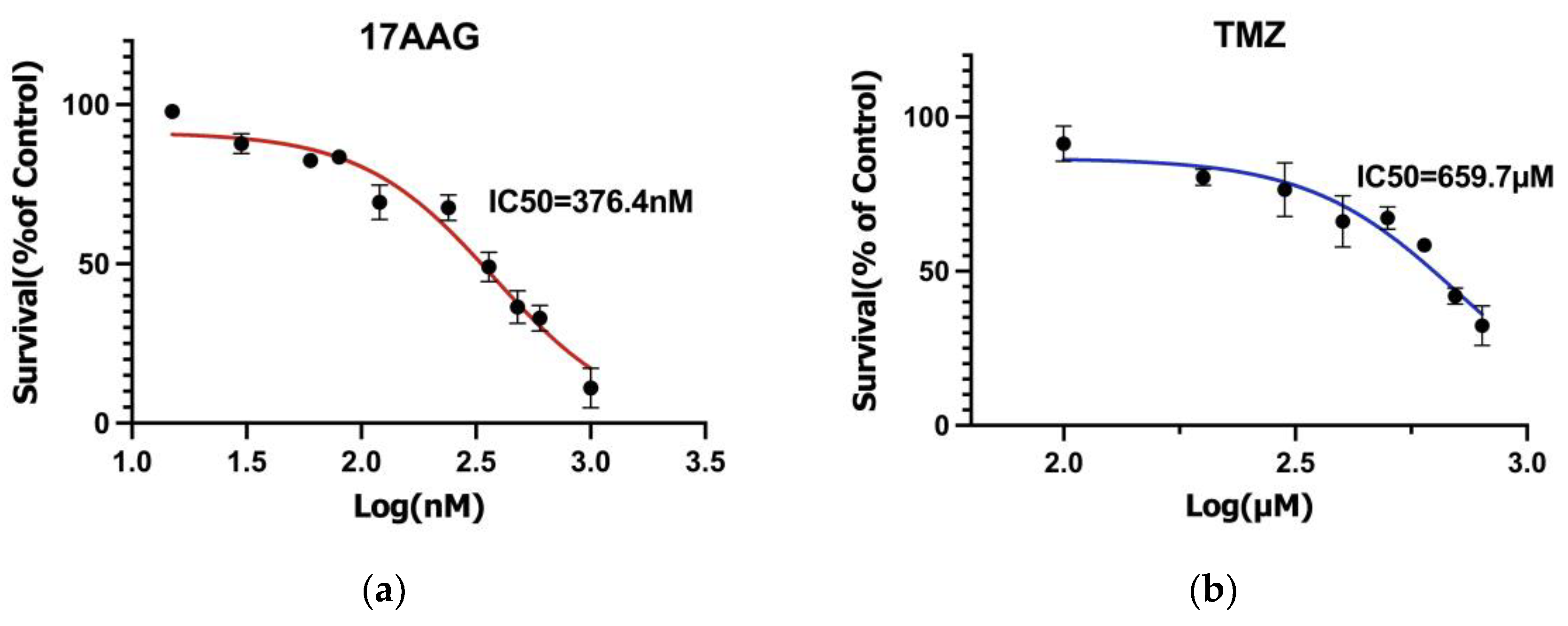
3.1. Drug Sensitivity Results
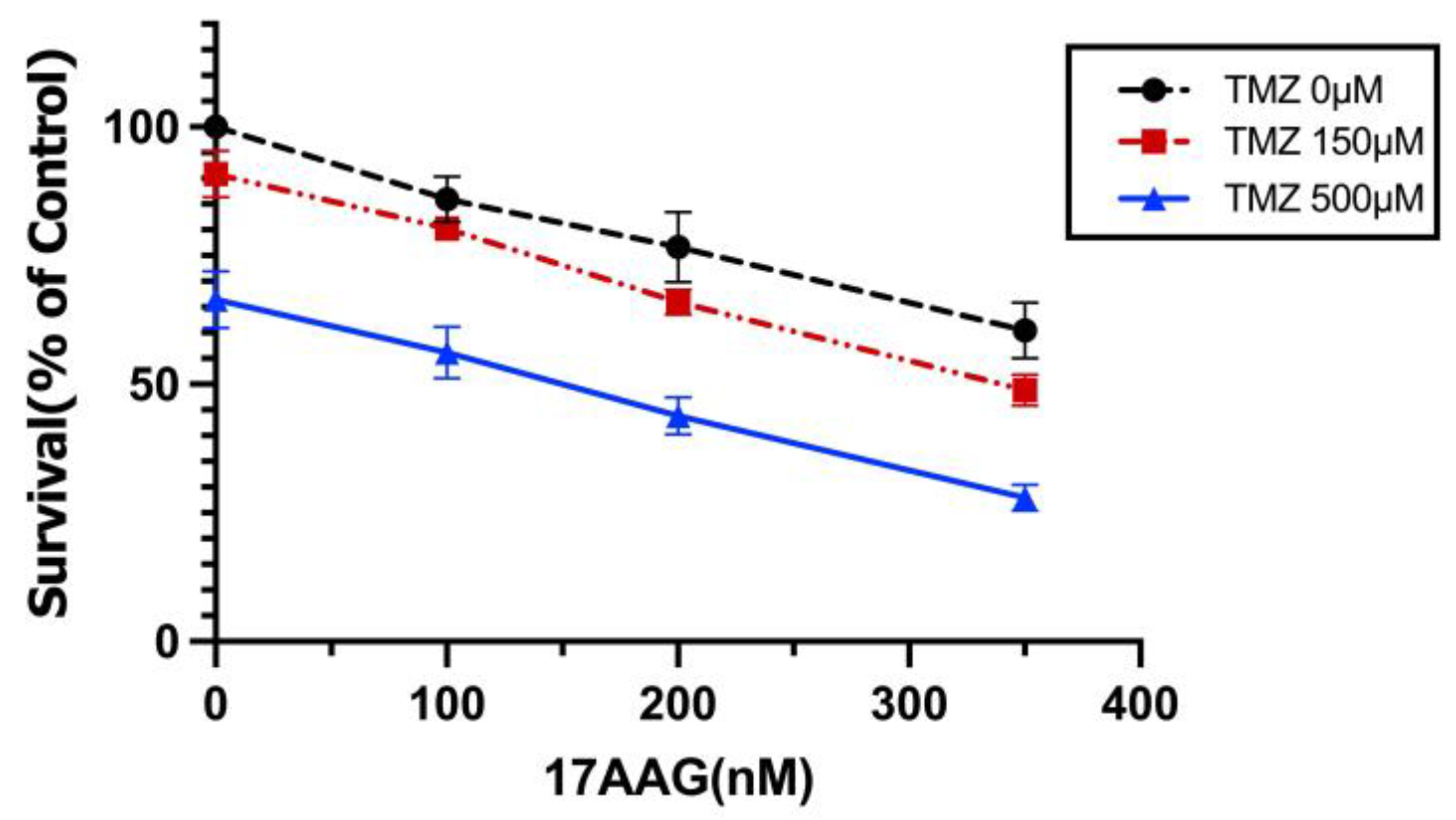
3.2. Synergistic Effects between TMZ and 17AAG
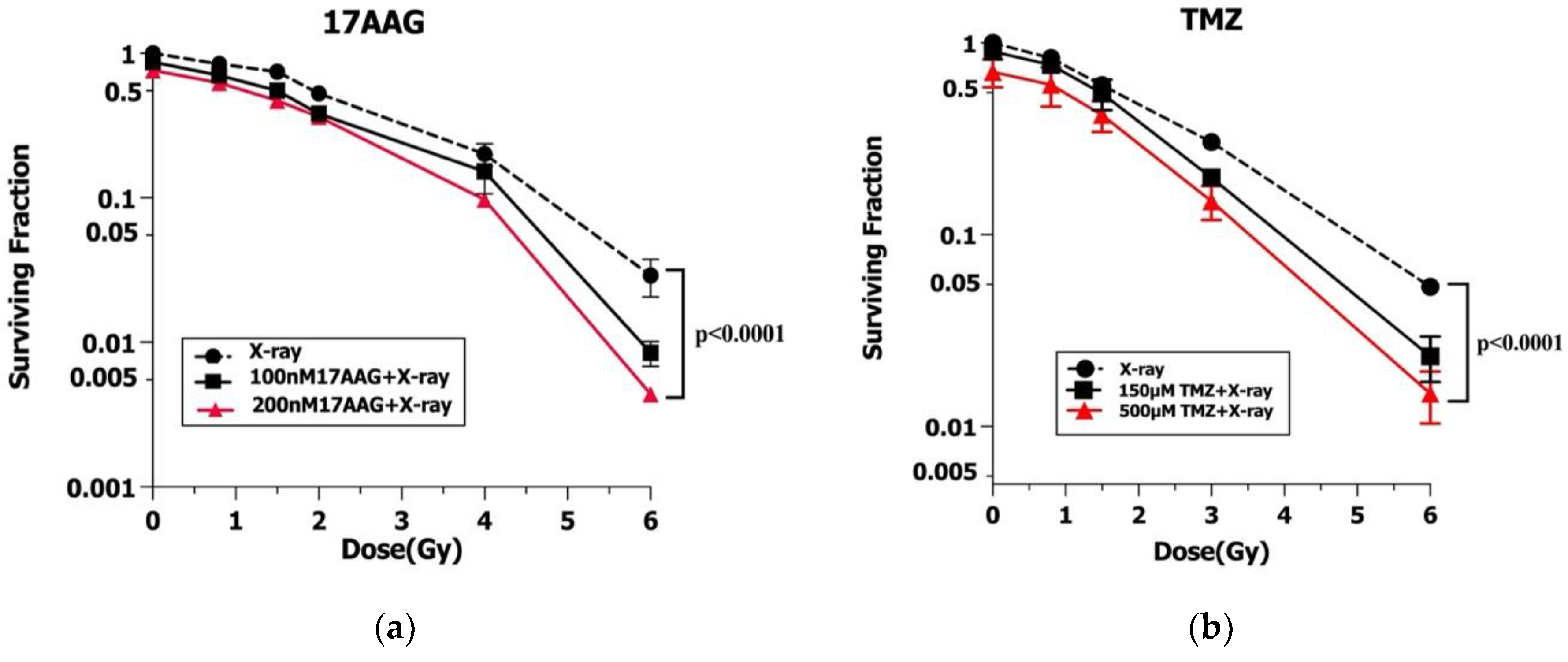
3.3. Radiosensitization of 17AAG and TMZ
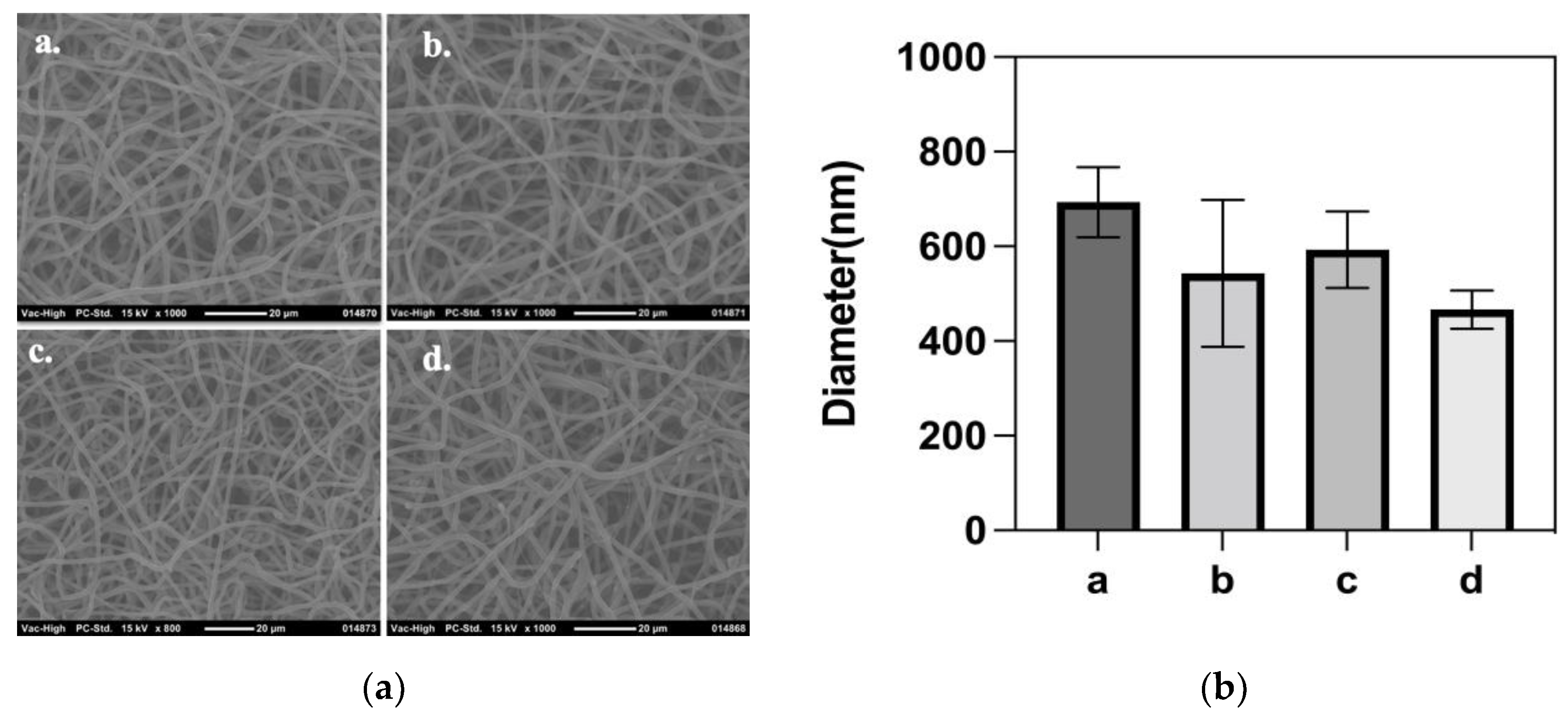
3.4. Synthesis and Characteristics of Nanofiber Mesh
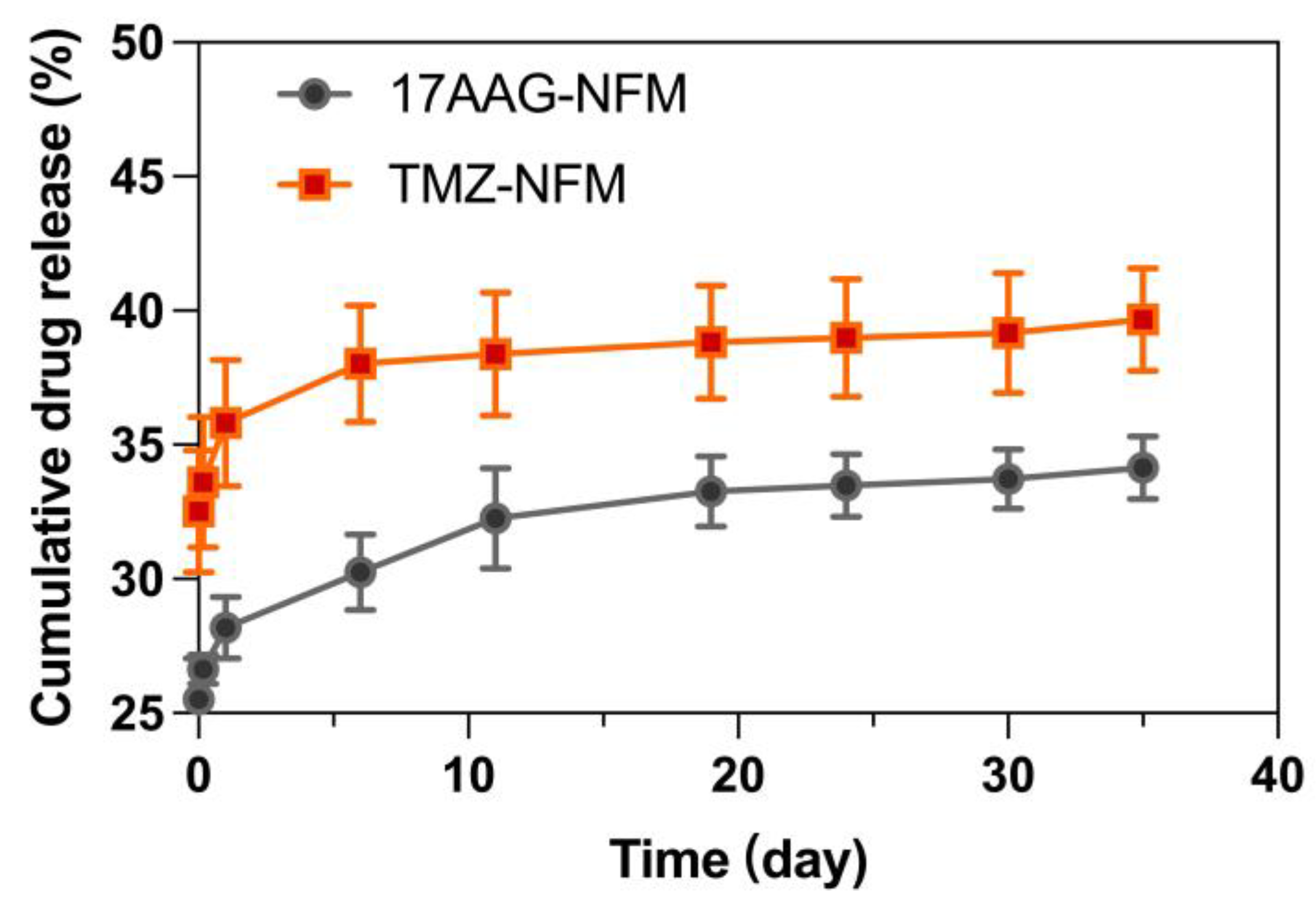
3.5. Locally Sustained Drug Release
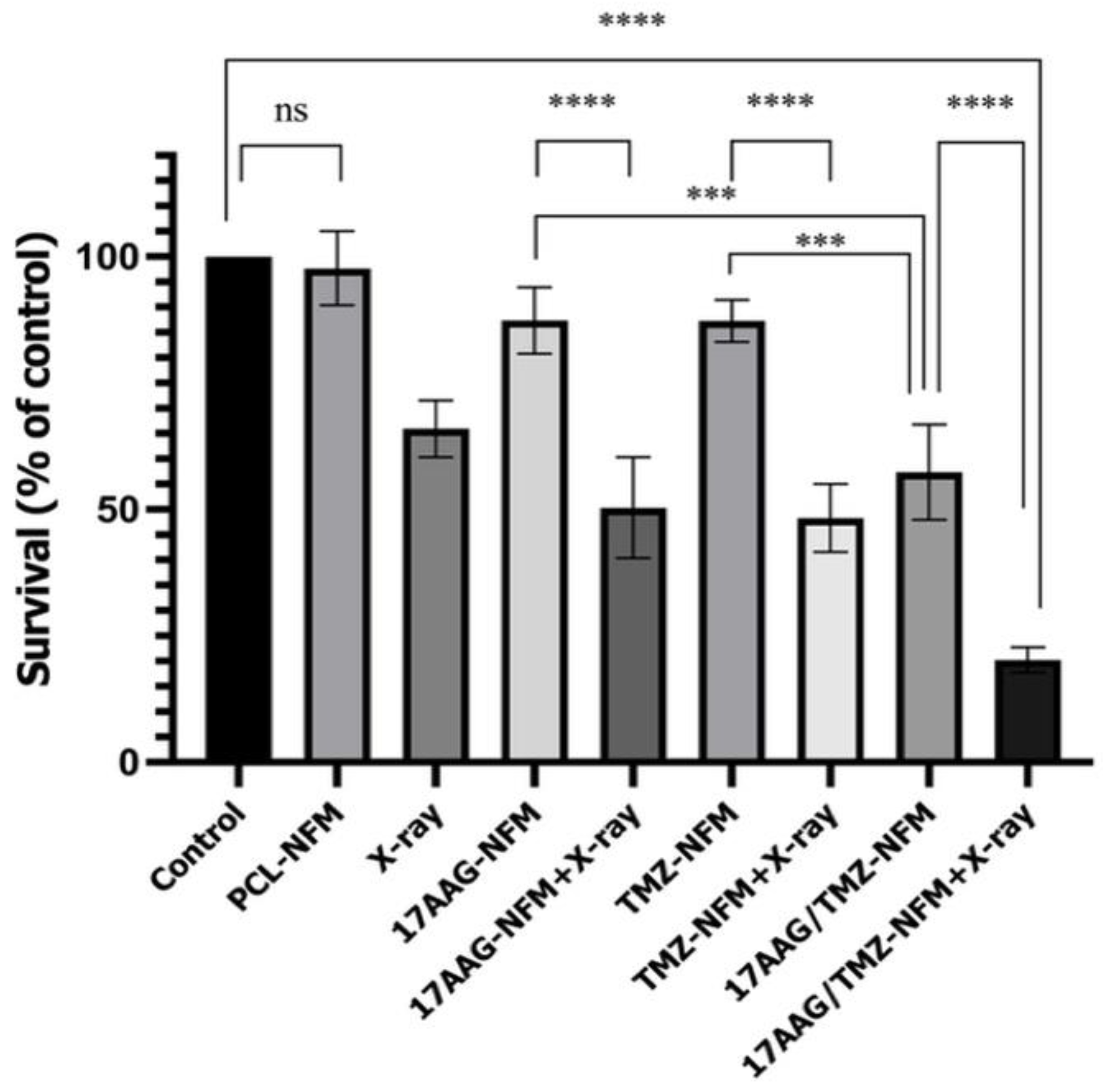
3.6. Strong Cytotoxic Effects with Nanofiber Mesh Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaoude, D.A.; Moore, J.A.; Moore, M.B.; Twumasi-Ankrah, P.; Ablah, E.; Moore, D.F., Jr. Glioblastoma and Increased Survival with Longer Chemotherapy Duration. Kans. J. Med. 2019, 12, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Djamel-Eddine, Y.C.; de Witte, O.; Mélot, C.; Lefranc, F. Recurrent Glioblastomas: Should We Operate a Second and Even a Third Time? Interdiscip. Neurosurg. 2019, 18, 100551. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of Its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Gallego, O. Nonsurgical Treatment of Recurrent Glioblastoma. Curr. Oncol. 2015, 22, e273–e281. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, S.; Neckers, L. Extracellular Heat Shock Protein 90: A Role for a Molecular Chaperone in Cell Motility and Cancer Metastasis. Cancer Sci. 2007, 98, 1536–1539. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.P.; Zakka, K.M.; Landry, J.C.; Shaib, W.L.; Lesinski, G.B.; El-Rayes, B.F. Inhibition of HSP90 Overcomes Resistance to Chemotherapy and Radiotherapy in Pancreatic Cancer. Int. J. Cancer 2019, 145, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Valbuena, J.R.; Barbhuiya, M.A.; Stein, S.; Kunkel, H.; García, P.; Bizama, C.; Riquelme, I.; Espinoza, J.A.; Kurtz, S.E.; et al. Small Molecule Inhibitor Screening Identifified HSP90 Inhibitor 17-AAG as Potential Therapeutic Agent for Gallbladder Cancer. Oncotarget 2017, 8, 26169. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, H.; Carew, J.S.; McQueen, T.J.; Andreeff, M.; Plunkett, W.; Keating, M.J.; Huang, P. Targeting Hsp90 by 17-AAG in Leukemia Cells: Mechanisms for Synergistic and Antagonistic Drug Combinations with Arsenic Trioxide and Ara-C. Leukemia 2006, 20, 610–619. [Google Scholar] [CrossRef]
- Sauvageot, C.M.E.; Weatherbee, J.L.; Kesari, S.; Winters, S.E.; Barnes, J.; Dellagatta, J.; Ramakrishna, N.R.; Stiles, C.D.; Kung, A.L.J.; Kieran, M.W.; et al. Efficacy of the HSP90 Inhibitor 17-AAG in Human Glioma Cell Lines and Tumorigenic Glioma Stem Cells. Neuro Oncol. 2009, 11, 109. [Google Scholar] [CrossRef]
- Machida, H.; Nakajima, S.; Shikano, N.; Nishio, J.; Okada, S.; Asayama, M.; Shirai, M.; Kubota, N. Heat Shock Protein 90 Inhibitor 17-Allylamino-17-Demethoxygeldanamycin Potentiates the Radiation Response of Tumor Cells Grown as Monolayer Cultures and Spheroids by Inducing Apoptosis. Cancer Sci. 2005, 96, 911–917. [Google Scholar] [CrossRef]
- Yu, D.; Sekine, E.; Fujimori, A.; Ochiya, T.; Okayasu, R. Down Regulation of BRCA2 Causes Radio-Sensitization of Human Tumor Cells in Vitro and in Vivo. Cancer Sci. 2008, 99, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Dungey, F.A.; Caldecott, K.W.; Chalmers, A.J. Enhanced Radiosensitization of Human Glioma Cells by Combining Inhibition of Poly(ADP-Ribose) Polymerase with Inhibition of Heat Shock Protein 90. Mol. Cancer Ther. 2009, 8, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Costa, A.; Osório, L.; Lago, R.C.; Linhares, P.; Carvalho, B.; Caeiro, C. Current Standards of Care in Glioblastoma Therapy. Glioblastoma 2017, Chapter 11, 197–241. [Google Scholar] [CrossRef]
- Wang, T.; Pickard, A.J.; Gallo, J.M. Histone Methylation by Temozolomide; A Classic DNA Methylating Anticancer Drug. Anticancer Res. 2016, 36, 3289. [Google Scholar] [PubMed]
- Barciszewska, A.M.; Gurda, D.; Głodowicz, P.; Nowak, S.; Naskręt-Barciszewska, M.Z. A New Epigenetic Mechanism of Temozolomide Action in Glioma Cells. PLoS ONE 2015, 10, e0136669. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Park, M.J.; Lee, M.M.; Kim, T.M.; Lee, S.H.; Cho, S.Y.; Kim, Y.H.; Kim, Y.J.; Park, C.K.; Kim, C.Y. Toxicity Profile of Temozolomide in the Treatment of 300 Malignant Glioma Patients in Korea. J. Korean Med. Sci. 2014, 29, 980. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, C.; Bafaloukos, D.; Kosmidis, P.; Samantas, E.; Bamias, A.; Papakostas, P.; Karabelis, A.; Bacoyiannis, C.; Skarlos, D.V. Phase II Study of Temozolomide in Heavily Pretreated Cancer Patients with Brain Metastases. Ann. Oncol. 2001, 12, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Ahsan, S.M.; Kumar, J.M.; Kondapi, A.K.; Rao, N.M. Overcoming Blood Brain Barrier with a Dual Purpose Temozolomide Loaded Lactoferrin Nanoparticles for Combating Glioma (SERP-17-12433). Sci. Rep. 2017, 7, 6602. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, F.; Atyabi, S.M.; Norouzian, D.; Zandi, M.; Irani, S.; Bakhshi, H. Polycaprolactone/Carboxymethyl Chitosan Nanofibrous Scaffolds for Bone Tissue Engineering Application. Int. J. Biol. Macromol. 2018, 115, 243–248. [Google Scholar] [CrossRef]
- Han, J.; Li, Z.; Sun, Y.; Cheng, F.; Zhu, L.; Zhang, Y.; Zhang, Z.; Wu, J.; Wang, J. Surface Roughness and Biocompatibility of Polycaprolactone Bone Scaffolds: An Energy-Density-Guided Parameter Optimization for Selective Laser Sintering. Front. Bioeng. Biotechnol. 2022, 10, 1013. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fujisawa, N.; Takanohashi, M.; Najmina, M.; Uto, K.; Ebara, M. A Smart Hyperthermia Nanofiber-Platform-Enabled Sustained Release of Doxorubicin and 17AAG for Synergistic Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 2542. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Chen, H.; Dong, Y.; Zhang, J.; Huang, H.; Zhu, J.; Zhang, W. Paclitaxel-Loaded Poly(Glycolide-Co-Εcaprolactone)-b-d-α-Tocopheryl Polyethylene Glycol 2000 Succinate Nanoparticles for Lung Cancer Therapy. Int. J. Nanomed. 2013, 8, 1947–1957. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Lu, W.; Wang, J.; Xu, Y.; Guo, Y. Application of Electrospinning in Antibacterial Field. Nanomaterials 2021, 11, 1822. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, T.; Li, Z.; Ni, S.; Zhou, F.; Alimi, O.A.; Chen, S.; Duan, B.; Kuss, M.; Wu, S. Review of Advances in Electrospinning-Based Strategies for Spinal Cord Regeneration. Mater. Today Chem. 2022, 24, 100944. [Google Scholar] [CrossRef]
- Chou, T.C. Preclinical versus Clinical Drug Combination Studies. Leuk. Lymphoma 2008, 49, 2059–2080. [Google Scholar] [CrossRef]
- Navarra, G.; Pagano, C.; Pacelli, R.; Crescenzi, E.; Longobardi, E.; Gazzerro, P.; Fiore, D.; Pastorino, O.; Pentimalli, F.; Laezza, C.; et al. N6-Isopentenyladenosine Enhances the Radiosensitivity of Glioblastoma Cells by Inhibiting the Homologous Recombination Repair Protein RAD51 Expression. Front. Oncol. 2020, 9, 1498. [Google Scholar] [CrossRef] [PubMed]
- Niiyama, E.; Uto, K.; Lee, C.M.; Sakura, K.; Ebara, M. Hyperthermia Nanofiber Platform Synergized by Sustained Release of Paclitaxel to Improve Antitumor Efficiency. Adv. Healthc. Mater. 2019, 8, 1900102. [Google Scholar] [CrossRef]
- Gimple, R.C.; Bhargava, S.; Dixit, D.; Rich, J.N. Glioblastoma Stem Cells: Lessons from the Tumor Hierarchy in a Lethal Cancer. Genes. Dev. 2019, 33, 591. [Google Scholar] [CrossRef]
- de Bonis, P.; Anile, C.; Pompucci, A.; Fiorentino, A.; Balducci, M.; Chiesa, S.; Lauriola, L.; Maira, G.; Mangiola, A. The Influence of Surgery on Recurrence Pattern of Glioblastoma. Clin. Neurol. Neurosurg. 2013, 115, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.V.R.; Batista, C.; Afonso, B.D.H.; Alexandre-Moreira, M.S.; Dubois, L.G.; Pontes, B.; Neto, V.M.; de Almeida Mendes, F. Obstacles to Glioblastoma Treatment Two Decades after Temozolomide. Cancers 2022, 14, 3203. [Google Scholar] [CrossRef] [PubMed]
- Pena, E.S.; Graham-Gurysh, E.G.; Bachelder, E.M.; Ainslie, K.M.; Maciaczyk, J.; Guerrero-Cazares, H.; Sharma, A. Design of Biopolymer-Based Interstitial Therapies for the Treatment of Glioblastoma. Int. J. Mol. Sci. 2021, 22, 13160. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.Z.; Wang, Z.F.; Lan, T.; Huang, W.H.; Zhao, Y.H.; Ma, C.; Li, Z.Q. Carmustine as a Supplementary Therapeutic Option for Glioblastoma: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 1036. [Google Scholar] [CrossRef] [PubMed]
- Bregy, A.; Shah, A.H.; Diaz, M.V.; Pierce, H.E.; Ames, P.L.; Diaz, D.; Komotar, R.J. The Role of Gliadel Wafers in the Treatment of High-Grade Gliomas. Expert Rev. Anticancer Ther. 2013, 13, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Maharajan, K. Feasibility of Local Administration of Chemotherapeutic Drugs as an Effective Adjuvant Therapy in Primary, Recurrent and Metastatic Extradural Tumours of the Spine-Review. J. Spine Surg. 2019, 5, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Kleinberg, L. Polifeprosan 20, 3.85% Carmustine Slow Release Wafer in Malignant Glioma: Patient Selection and Perspectives on a Low-Burden Therapy. Patient Prefer. Adherence 2016, 10, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.L.; Goebel, E.A. Cerebral Edema Associated with Gliadel Wafers: Two Case Studies. Neuro. Oncol. 2005, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, S.S.; Kirkwood, J.M. Temozolomide, a Novel Alkylating Agent with Activity in the Central Nervous System, May Improve the Treatment of Advanced Metastatic Melanoma. Oncologist 2000, 5, 144–151. [Google Scholar] [CrossRef]
- Wesolowski, J.R.; Rajdev, P.; Mukherji, S.K. Temozolomide (Temodar). Am. J. Neuroradiol. 2010, 31, 1383–1384. [Google Scholar] [CrossRef]
- Oike, T.; Suzuki, Y.; Sugawara, K.I.; Shirai, K.; Noda, S.E.; Tamaki, T.; Nagaishi, M.; Yokoo, H.; Nakazato, Y.; Nakano, T. Radiotherapy plus Concomitant Adjuvant Temozolomide for Glioblastoma: Japanese Mono-Institutional Results. PLoS ONE 2013, 8, e78943. [Google Scholar] [CrossRef]
- D’Alessandris, Q.G.; Biffoni, M.; Martini, M.; Runci, D.; Buccarelli, M.; Cenci, T.; Signore, M.; Stancato, L.; Olivi, A.; de Maria, R.; et al. The Clinical Value of Patient-Derived Glioblastoma Tumorspheres in Predicting Treatment Response. Neuro Oncol. 2017, 19, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Mahaney, B.L.; Meek, K.; Lees-Miller, S.P. Repair of Ionizing Radiation-Induced DNA Double Strand Breaks by Non-Homologous End-Joining. Biochem. J. 2009, 417, 639. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.E.; Battelli, C.; Watson, J.; Liu, J.; Curtis, J.; Morse, A.N.; Matulonis, U.A.; Chowdhury, D.; Konstantinopoulos, P.A. Sublethal Concentrations of 17-AAG Suppress Homologous Recombination DNA Repair and Enhance Sensitivity to Carboplatin and Olaparib in HR Proficient Ovarian Cancer Cells. Oncotarget 2014, 5, 2678. [Google Scholar] [CrossRef]
- Dai, N.T.; Williamson, M.R.; Khammo, N.; Adams, E.F.; Coombes, A.G.A. Composite Cell Support Membranes Based on Collagen and Polycaprolactone for Tissue Engineering of Skin. Biomaterials 2004, 25, 4263–4271. [Google Scholar] [CrossRef]
- Jirofti, N.; Mohebbi-Kalhori, D.; Masoumi, R. Enhancing Biocompatibility of PCL/PU Nano-Structures to Control the Water Wettability by NaOH Hydrolysis Treatment for Tissue Engineering Applications. J. Ind. Text. 2022, 51, 3278S–3296S. [Google Scholar] [CrossRef]
- Luo, L.; He, Y.; Chang, Q.; Xie, G.; Zhan, W.; Wang, X.; Zhou, T.; Xing, M.; Lu, F. Polycaprolactone Nanofibrous Mesh Reduces Foreign Body Reaction and Induces Adipose Flap Expansion in Tissue Engineering Chamber. Int. J. Nanomed. 2016, 11, 6471–6483. [Google Scholar] [CrossRef] [PubMed]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation Mechanisms of Polycaprolactone in the Context of Chemistry, Geometry and Environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Shahverdi, M.; Seifi, S.; Akbari, A.; Mohammadi, K.; Shamloo, A.; Movahhedy, M.R. Melt Electrowriting of PLA, PCL, and Composite PLA/PCL Scaffolds for Tissue Engineering Application. Sci. Rep. 2022, 12, 19935. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Matsumoto, Y.; Chen, L.; Sugawara, Y.; Oe, E.; Fujisawa, N.; Ebara, M.; Sakurai, H. Smart Nanofiber Mesh with Locally Sustained Drug Release Enabled Synergistic Combination Therapy for Glioblastoma. Nanomaterials 2023, 13, 414. https://doi.org/10.3390/nano13030414
Li Y, Matsumoto Y, Chen L, Sugawara Y, Oe E, Fujisawa N, Ebara M, Sakurai H. Smart Nanofiber Mesh with Locally Sustained Drug Release Enabled Synergistic Combination Therapy for Glioblastoma. Nanomaterials. 2023; 13(3):414. https://doi.org/10.3390/nano13030414
Chicago/Turabian StyleLi, Yinuo, Yoshitaka Matsumoto, Lili Chen, Yu Sugawara, Emiho Oe, Nanami Fujisawa, Mitsuhiro Ebara, and Hideyuki Sakurai. 2023. "Smart Nanofiber Mesh with Locally Sustained Drug Release Enabled Synergistic Combination Therapy for Glioblastoma" Nanomaterials 13, no. 3: 414. https://doi.org/10.3390/nano13030414
APA StyleLi, Y., Matsumoto, Y., Chen, L., Sugawara, Y., Oe, E., Fujisawa, N., Ebara, M., & Sakurai, H. (2023). Smart Nanofiber Mesh with Locally Sustained Drug Release Enabled Synergistic Combination Therapy for Glioblastoma. Nanomaterials, 13(3), 414. https://doi.org/10.3390/nano13030414






