Exploring Pt-Pd Alloy Nanoparticle Cluster Formation through Conventional Sizing Techniques and Single-Particle Inductively Coupled Plasma—Sector Field Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of Bimetallic Pd-Pt Alloy NCs
2.3. Characterization of Bimetallic Pd-Pt Alloy NCs
2.3.1. SEM
2.3.2. DLS
2.3.3. spICP-SFMS
3. Results and Discussion
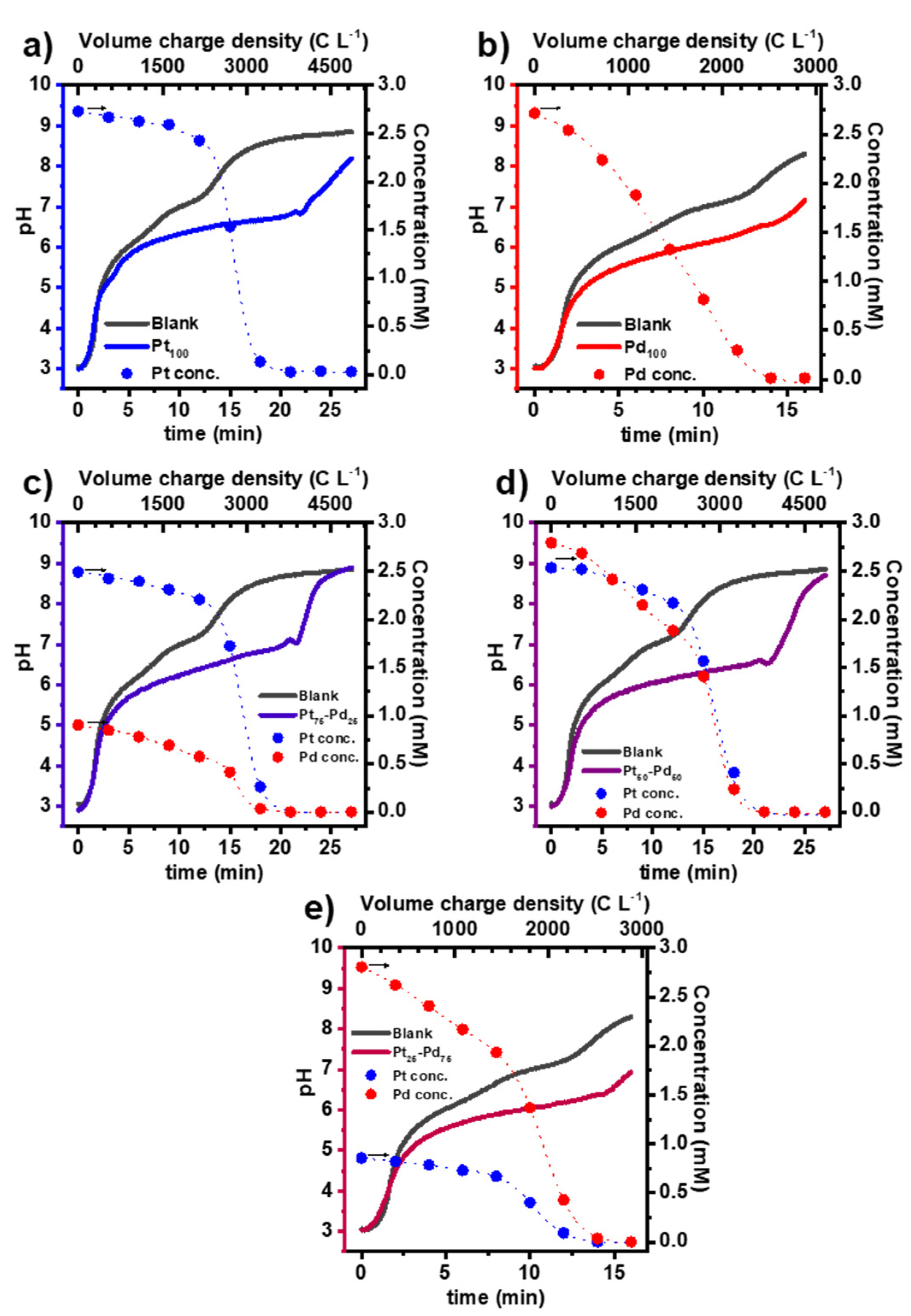
3.1. Synthesis of the Pt NCs, Pd NCs, and Pt-Pd Alloy NCs
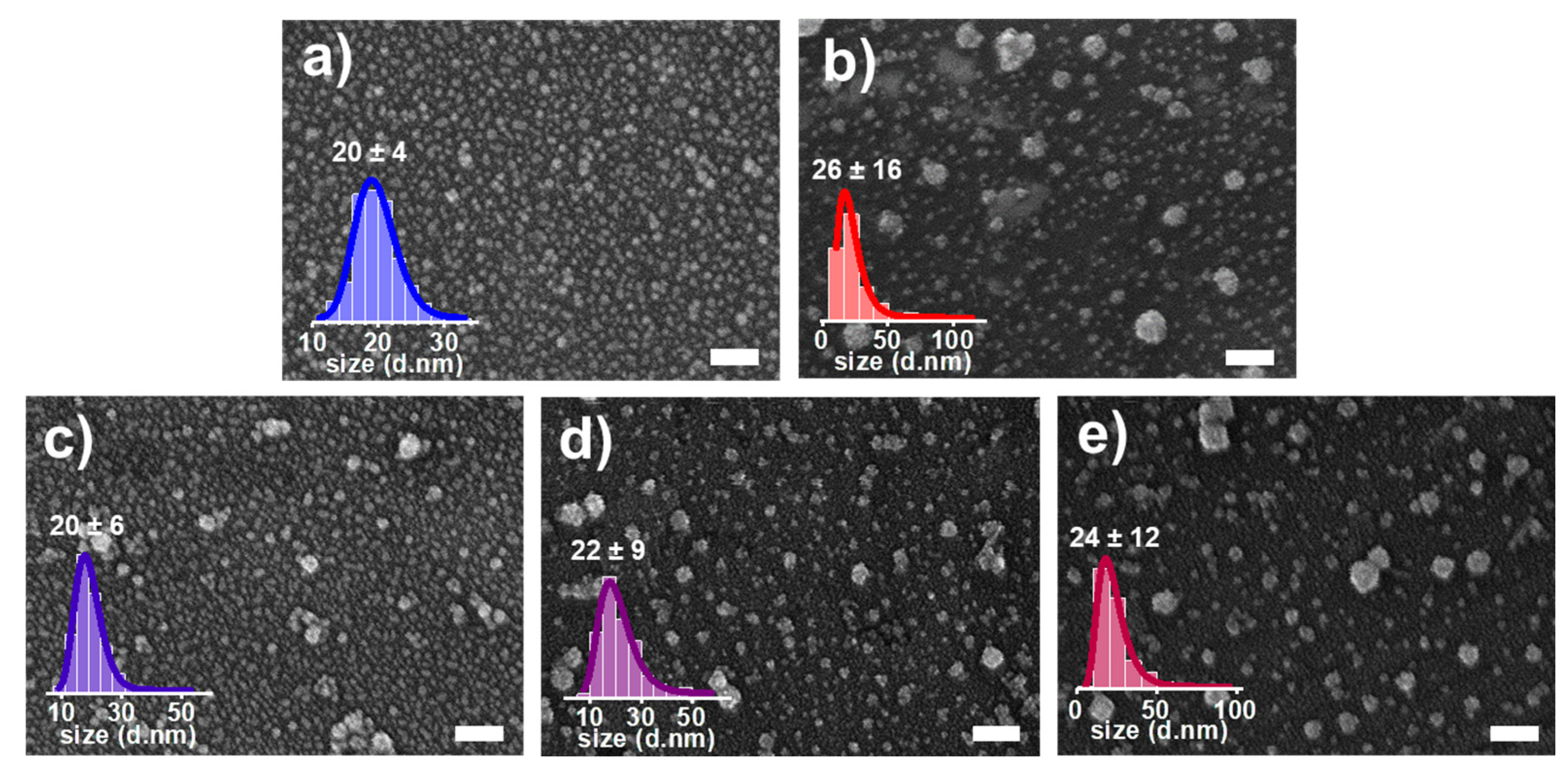
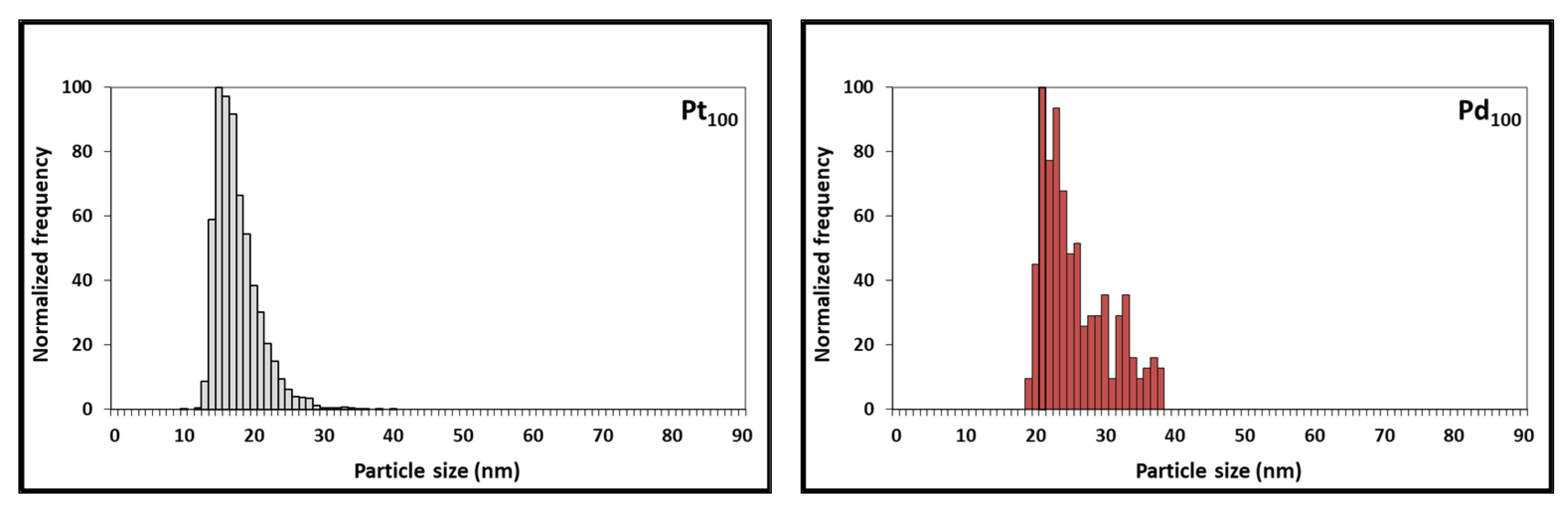
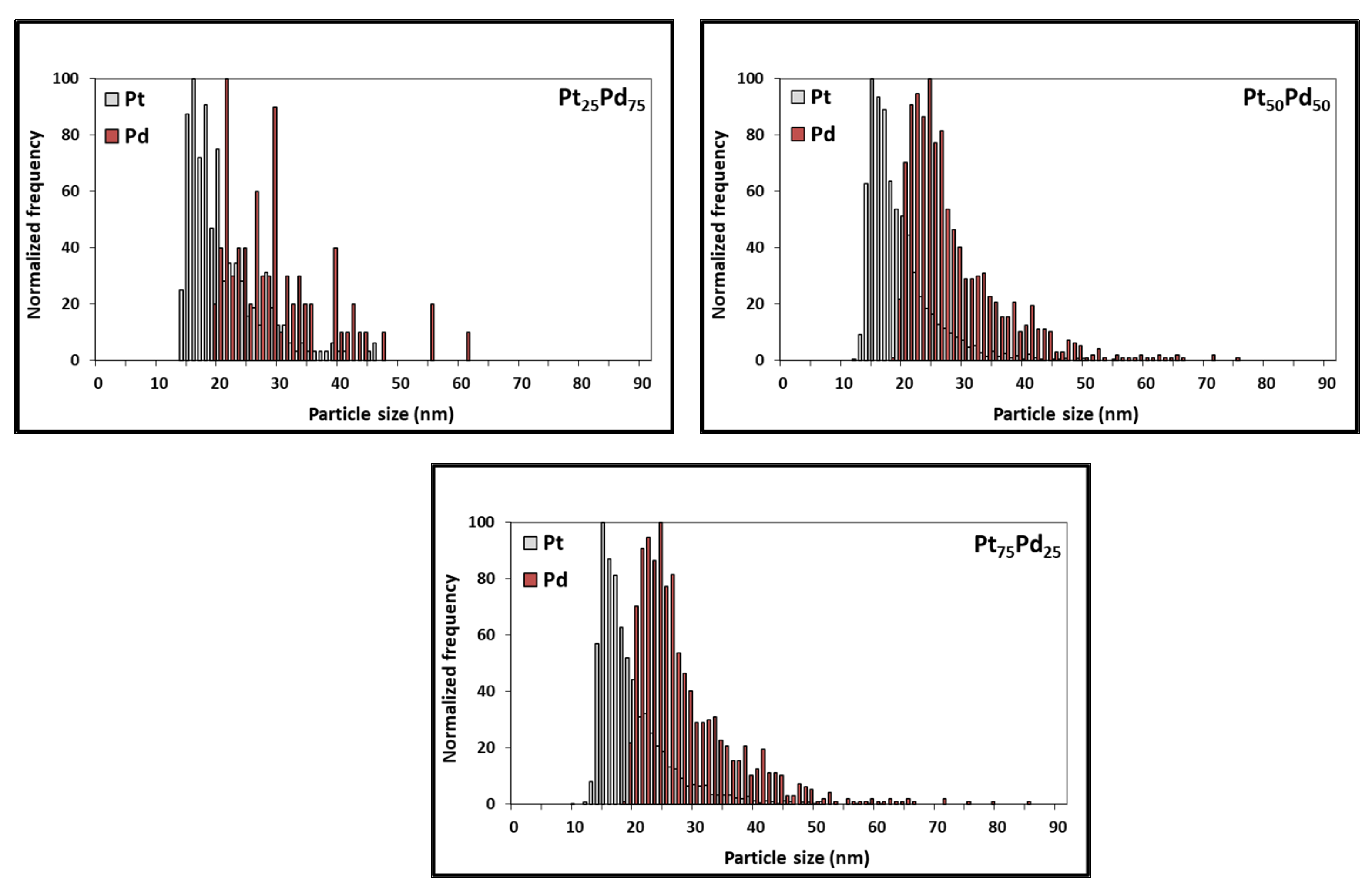
3.2. Characterization of Pt NCs, Pd NCs, and Pt-Pd Alloy NCs Using SEM
3.3. Characterization of Pt NCs, Pd NCs, and Pt-Pd Alloy NCs Using DLS
3.4. Feasibility of Using spICP-SFMS as a Complementary Technique to DLS and SEM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, T.; Ding, L.; Yao, Z.C.; Pan, H.R.; Hu, J.S.; Wan, L.J. Synergistic electrocatalysts for alkaline hydrogen oxidation and evolution reactions. Adv. Funct. Mater. 2022, 32, 2107479. [Google Scholar] [CrossRef]
- Li, Z.; Ge, R.; Su, J.; Chen, L. Recent progress in low Pt content electrocatalysts for hydrogen evolution reaction. Adv. Mater. Interfaces 2020, 7, 2000396. [Google Scholar] [CrossRef]
- Mahata, A.; Nair, A.S.; Pathak, B. Recent advancements in Pt-nanostructure-based electrocatalysts for the oxygen reduction reaction. Catal. Sci. Technol. 2019, 9, 4835–4863. [Google Scholar] [CrossRef]
- Tian, H.; Yu, Y.; Wang, Q.; Li, J.; Rao, P.; Li, R.; Du, Y.; Jia, C.; Luo, J.; Tian, X.; et al. Recent advances in two-dimensional Pt based electro-catalysts for methanol oxidation reaction. Int. J. Hydrogen Energy 2021, 46, 31202–31215. [Google Scholar] [CrossRef]
- Ren, X.; Wang, Y.; Liu, A.; Zhang, Z.; Lv, Q.; Liu, B. Current progress and performance improvement of Pt/C catalysts for fuel cells. J. Mater. Chem. A 2020, 8, 24284–24306. [Google Scholar] [CrossRef]
- Yousaf, A.B.; Imran, M.; Uwitonze, N.; Zeb, A.; Zaidi, S.J.; Ansari, T.M.; Manzoor, S. Enhanced electrocatalytic performance of Pt3Pd1 alloys supported on CeO2/C for methanol oxidation and oxygen reduction reactions. J. Phys. Chem. C 2017, 121, 2069–2079. [Google Scholar] [CrossRef]
- Loza, K.; Heggen, M.; Epple, M. Synthesis, structure, properties, and applications of bimetallic nanoparticles of noble metals. Adv. Funct. Mater. 2020, 30, 1909260. [Google Scholar] [CrossRef]
- Martinez-Mora, O.; Pozo, G.; Leon-Fernandez, L.F.; Fransaer, J.; Dominguez-Benetton, X. Synthesis of platinum group metal nanoparticles assisted by CO2 reduction and H2 cogeneration at gas-diffusion electrodes. RSC Sustain. 2023, 1, 454–458. [Google Scholar] [CrossRef]
- Leon-Fernandez, L.F.; Caballero-Ortiz, A.; Martinez-Mora, O.; Fransaer, J.; Dominguez-Benetton, X. Mechanism and kinetics of gold recovery and Au nanoparticle synthesis by Gas-Diffusion Electrocrystallization (GDEx). Electrochim. Acta 2023, 460, 142592. [Google Scholar] [CrossRef]
- Martinez-Mora, O.; Leon-Fernandez, L.F.; Velimirovic, M.; Vanhaecke, F.; Tirez, K.; Fransaer, J.; Dominguez-Benetton, X. Platinum nanoclusters made by gas-diffusion electrocrystallization (GDEx) as electrocatalysts for methanol oxidation. Mater. Adv. 2023. [Google Scholar] [CrossRef]
- Hendriks, L.; Brünjes, R.; Taskula, S.; Kocic, J.; Hattendorf, B.; Bland, G.; Lowry, G.; Bolea-Fernandez, E.; Vanhaecke, F.; Wang, J.; et al. Results of an interlaboratory comparison for characterization of Pt nanoparticles using single-particle ICP-TOFMS. Nanoscale 2023, 15, 11268–11279. [Google Scholar] [CrossRef] [PubMed]
- Heetpat, N.; Sumranjit, J.; Siripinyanond, A. Use of single particle inductively coupled plasma mass spectrometry for understanding the formation of bimetallic nanoparticles. Talanta 2022, 236, 122871. [Google Scholar] [CrossRef] [PubMed]
- Kéri, A.; Kálomista, I.; Ungor, D.; Bélteki, Á.; Csapó, E.; Dékány, I.; Prohaska, T.; Galbács, G. Determination of the structure and composition of Au-Ag bimetallic spherical nanoparticles using single particle ICP-MS measurements performed with normal and high temporal resolution. Talanta 2018, 179, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Tharaud, M.; Schlatt, L.; Shaw, P.; Benedetti, M.F. Nanoparticle identification using single particle ICP-ToF-MS acquisition coupled to cluster analysis. From engineered to natural nanoparticles. J. Anal. At. Spectrom. 2022, 37, 2042–2052. [Google Scholar] [CrossRef]
- Resano, M.; Aramendía, M.; García-Ruiz, E.; Bazo, A.; Bolea-Fernandez, E.; Vanhaecke, F. Living in a transient world: ICP-MS reinvented via time-resolved analysis for monitoring single events. Chem. Sci. 2022, 13, 4436. [Google Scholar] [CrossRef]
- Montaño, M.D.; Olesik, J.W.; Barber, A.G.; Challis, K.; Ranville, J.F. Single particle ICP-MS: Advances toward routine analysis of nanomaterials. Anal. Bioanal. Chem. 2016, 408, 5053–5074. [Google Scholar] [CrossRef]
- Shaw, P.; Donard, A. Nano-particle analysis using dwell times between 10 μs and 70 μs with an upper counting limit of greater than 3 × 107 cps and a gold nanoparticle detection limit of less than 10 nm diameter. J. Anal. At. Spectrom. 2016, 31, 1234–1242. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST). Report of Investigation, Reference Material 8012, Gold Nanoparticles, Nominal 30 nm Diameter. Available online: https://www-s.nist.gov/srmors/certificates/8012.pdf (accessed on 22 June 2021).
- National Institute of Standards and Technology (NIST). Report of Investigation, Reference Material 8013, Gold Nanoparticles, Nominal 60 nm Diameter. Available online: https://www-s.nist.gov/srmors/certificates/8013.pdf (accessed on 22 June 2021).
- Pace, H.E.; Rogers, N.J.; Jarolimek, C.; Coleman, V.A.; Higgins, C.P.; Ranville, J.F. Determining transport efficiency for the purpose of counting and sizing nanoparticles via single particle inductively coupled plasma mass spectrometry. Anal. Chem. 2011, 83, 9361–9369. [Google Scholar] [CrossRef]
- Ooka, H.; Figueiredo, M.C.; Koper, M.T.M. Competition between Hydrogen Evolution and Carbon Dioxide Reduction on Copper Electrodes in Mildly Acidic Media. Langmuir 2017, 33, 9307–9313. [Google Scholar] [CrossRef]
- Zapata-Fernández, J.R.; Gochi-Ponce, Y.; Salazar-Gastélum, M.I.; Reynoso-Soto, E.A.; Paraguay-Delgado, F.; Lin, S.W.; Félix-Navarro, R.M. Ultrasonic-assisted galvanic displacement synthesis of Pt–Pd/MWCNT for enhanced oxygen reduction reaction: Effect of Pt concentration. Int. J. Hydrogen Energy 2017, 42, 9806–9815. [Google Scholar] [CrossRef]
- Chaudhuri, R.G.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Modena, M.M.; Rühle, B.; Burg, T.P.; Wuttke, S. Nanoparticle characterization: What to measure? Adv. Mater. 2019, 31, 1901556. [Google Scholar] [CrossRef] [PubMed]
- Bolea, E.; Jimenez, M.S.; Perez-Arantegui, J.; Vidal, J.C.; Bakir, M.; Ben-Jeddou, K.; Gimenez-Ingalaturre, A.C.; Ojeda, D.; Trujillo, C.; Laborda, F. Analytical applications of single particle inductively coupled plasma mass spectrometry: A comprehensive and critical review. Anal. Methods 2021, 13, 2742–2795. [Google Scholar] [CrossRef] [PubMed]


| Solution | Feeding | Composition | ||
|---|---|---|---|---|
| Pt(IV) | Pd(II) | Empirical | As Synthesized * | |
| 1 | 3 mM | -- | Pt100 | Pt100 |
| 2 | 3 mM | 1 mM | Pt75-Pd25 | Pt74-Pd26 |
| 3 | 3 mM | 3 mM | Pt50-Pd50 | Pt54-Pd46 |
| 4 | 1 mM | 3 mM | Pt25-Pd75 | Pt26-Pt74 |
| 5 | -- | 3 mM | Pd100 | Pd100 |
| Parameter | |
|---|---|
| Radio frequency power | 1300 W |
| Plasma gas (Ar) flow rate | 13 L min−1 |
| Carrier gas flow rate | 0.93 L min−1 |
| Measurement mode | TRA |
| Nuclide monitored | 195Pt, 105Pd |
| Type of detection for multiple elements | sequential |
| Dwell time | 50 µs |
| Acquisition time | 60 s |
| Nebulizer | MicroMist |
| Spray chamber | Cyclonic |
| Spherical Equivalent Diameter (nm) Normal Distribution * | Spherical Equivalent Diameter (nm) Log Normal Distribution * | Spherical Equivalent Diameter (nm) Cubic Spline * | Particle Number Concentration (Particles per mL) ** | |
|---|---|---|---|---|
| Pt NCs (Pt100) | 17.2 | 16.4 | 16.0 | 3.70 × 105 |
| Pd NCs (Pd100) | 37.4 | 26.0 | 22.2 | 2.84 × 104 |
| Pt75-Pd25 alloy NCs | 30.7 | 27.5 | 24.9 | 2.68 × 105 (Pt-based) 8.99 × 104 (Pd-based) |
| Pt50-Pd50 alloy NCs | 29.0 | 26.6 | 24.4 | 1.73 × 105 (Pt-based) 2.54 × 104 (Pd-based) |
| Pt25-Pd75 alloy NCs | 35.4 | 29.5 | 27.0 | 2.13 × 104 (Pt-based) 2.44 × 104 (Pd-based) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Mora, O.; Tirez, K.; Beutels, F.; Brusten, W.; Leon-Fernandez, L.F.; Fransaer, J.; Dominguez-Benetton, X.; Velimirovic, M. Exploring Pt-Pd Alloy Nanoparticle Cluster Formation through Conventional Sizing Techniques and Single-Particle Inductively Coupled Plasma—Sector Field Mass Spectrometry. Nanomaterials 2023, 13, 2610. https://doi.org/10.3390/nano13182610
Martinez-Mora O, Tirez K, Beutels F, Brusten W, Leon-Fernandez LF, Fransaer J, Dominguez-Benetton X, Velimirovic M. Exploring Pt-Pd Alloy Nanoparticle Cluster Formation through Conventional Sizing Techniques and Single-Particle Inductively Coupled Plasma—Sector Field Mass Spectrometry. Nanomaterials. 2023; 13(18):2610. https://doi.org/10.3390/nano13182610
Chicago/Turabian StyleMartinez-Mora, Omar, Kristof Tirez, Filip Beutels, Wilfried Brusten, Luis F. Leon-Fernandez, Jan Fransaer, Xochitl Dominguez-Benetton, and Milica Velimirovic. 2023. "Exploring Pt-Pd Alloy Nanoparticle Cluster Formation through Conventional Sizing Techniques and Single-Particle Inductively Coupled Plasma—Sector Field Mass Spectrometry" Nanomaterials 13, no. 18: 2610. https://doi.org/10.3390/nano13182610
APA StyleMartinez-Mora, O., Tirez, K., Beutels, F., Brusten, W., Leon-Fernandez, L. F., Fransaer, J., Dominguez-Benetton, X., & Velimirovic, M. (2023). Exploring Pt-Pd Alloy Nanoparticle Cluster Formation through Conventional Sizing Techniques and Single-Particle Inductively Coupled Plasma—Sector Field Mass Spectrometry. Nanomaterials, 13(18), 2610. https://doi.org/10.3390/nano13182610








