Concave Ni(OH)2 Nanocube Synthesis and Its Application in High-Performance Hybrid Capacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Ni-Co PBA Nanocubes
2.2. Synthesis of Concave Ni(OH)2 Nanocubes
2.3. Material Characterizations
2.4. Electrochemical Measurements
3. Results
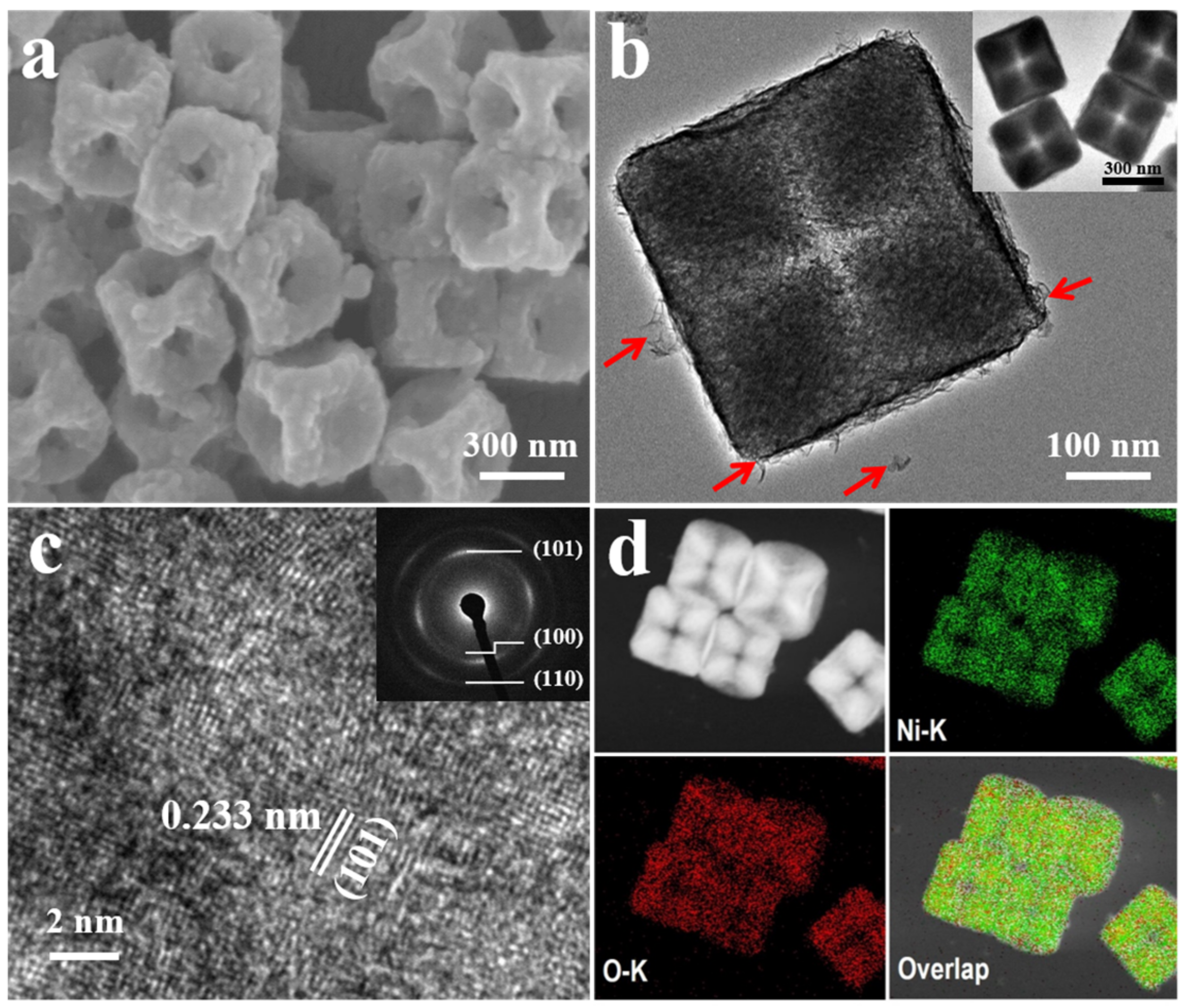
3.1. Characterization of Concave Ni(OH)2 Nanocubes
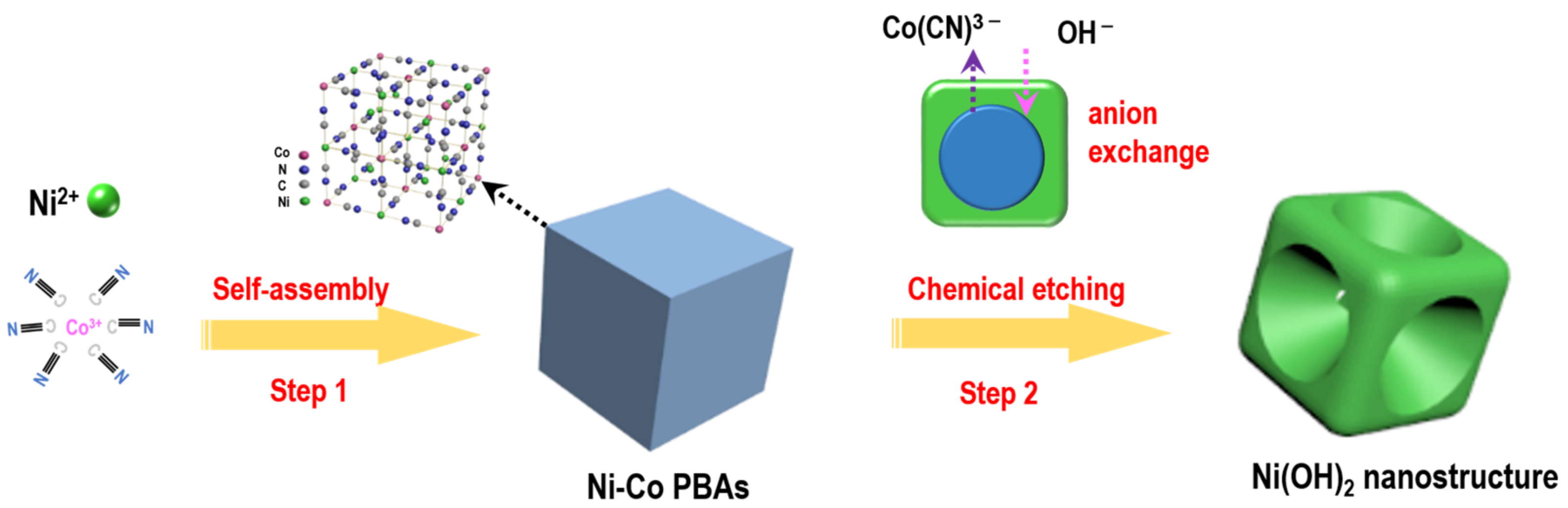
3.2. Formation Mechanism of Concave Ni(OH)2 Nanocubes
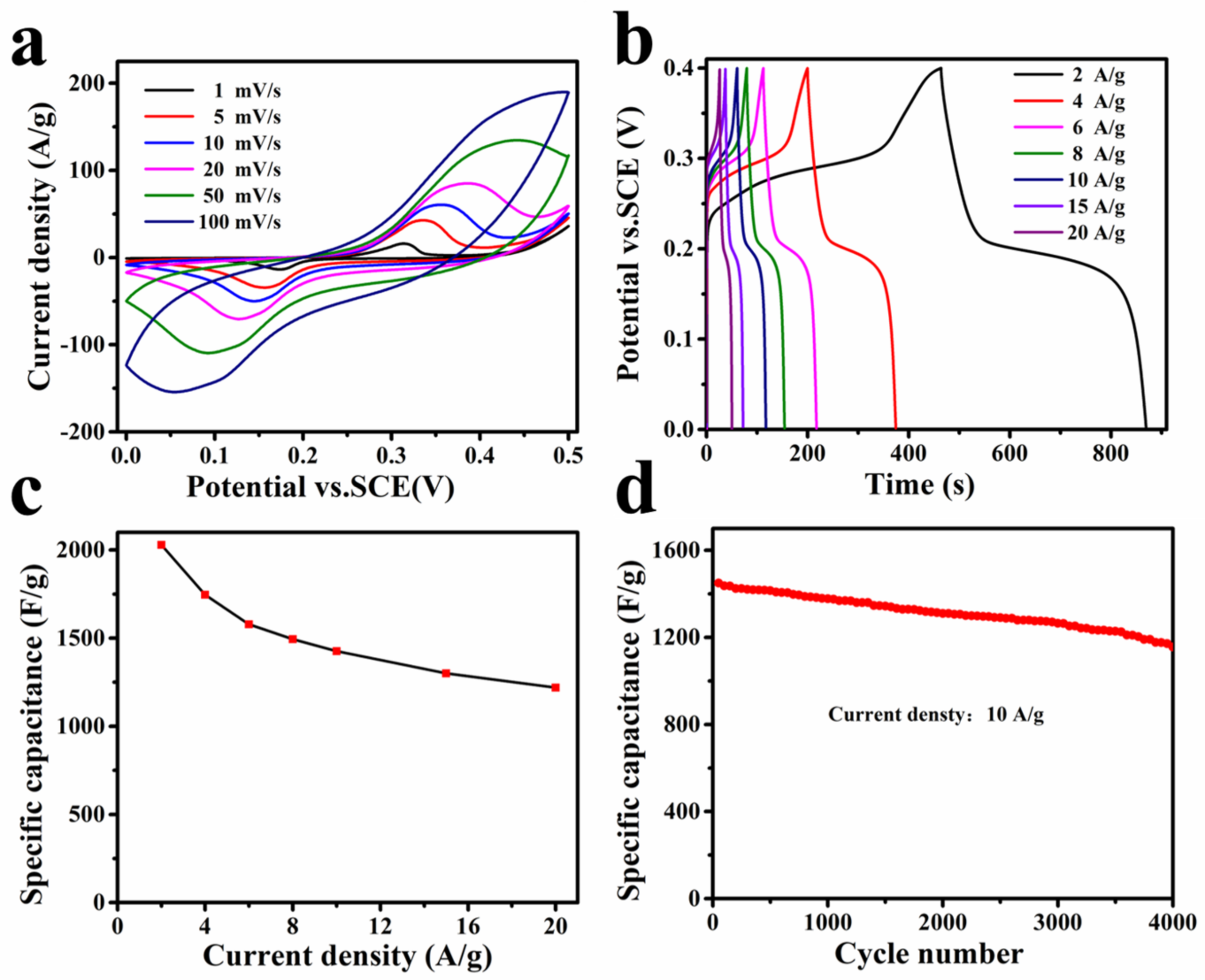
3.3. Electrochemical Property of Concave Ni(OH)2 Nanocubes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [PubMed]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [PubMed]
- Lu, X.; Yu, M.; Wang, G.; Tong, Y.; Li, Y. Flexible solid-state supercapacitors: Design, fabrication and applications. Energy Environ. Sci. 2014, 7, 2160–2181. [Google Scholar]
- Dubal, D.P.; Ayyad, O.; Ruiz, V.; Gomez-Romero, P. Hybrid energy storage: The merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 2015, 44, 1777–1790. [Google Scholar] [CrossRef] [PubMed]
- Cherusseri, J.; Sambath Kumar, K.; Choudhary, N.; Nagaiah, N.; Jung, Y.; Roy, T.; Thomas, J. Novel mesoporous electrode materials for symmetric, asymmetric and hybrid supercapacitors. Nanotechnology 2019, 30, 202001–202019. [Google Scholar] [PubMed]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar]
- Qin, Q.; Ou, D.; Ye, C.; Chen, L.; Lan, B.; Yan, J.; Wu, Y. Systematic study on hybrid supercapacitor of Ni-Co layered double hydroxide//activated carbons. Electrochim. Acta 2019, 305, 403–415. [Google Scholar] [CrossRef]
- Liang, Z.; Qu, C.; Zhou, W.; Zhao, R.; Zhang, H.; Zhu, B.; Guo, W.; Meng, W.; Wu, Y.; Aftab, W.; et al. Synergistic Effect of Co-Ni Hybrid Phosphide Nanocages for Ultrahigh Capacity Fast Energy Storage. Adv. Sci. 2019, 6, 1802005–1802011. [Google Scholar] [CrossRef]
- Yang, X.; Fu, K.; Mao, L.; Peng, W.; Jin, J.; Yang, S.; Li, G. Bio-mediated synthesis of α-Ni(OH)2 nanobristles on hollow porous carbon nanofibers for rechargeable alkaline batteries. Chem. Eng. Sci. 2019, 205, 269–277. [Google Scholar]
- Nai, J.; Wang, S.; Bai, Y.; Guo, L. Amorphous Ni(OH)2 nanoboxes: Fast fabrication and enhanced sensing for glucose. Small 2013, 9, 3147–3152. [Google Scholar]
- Hu, B.; Qin, X.; Asiri, A.M.; Alamry, K.A.; Al-Youbi, A.O.; Sun, X. Fabrication of Ni(OH)2 nanoflakes array on Ni foam as a binder-free electrode material for high performance supercapacitors. Electrochim. Acta 2013, 107, 339–342. [Google Scholar]
- Xie, M.; Xu, Z.; Duan, S.; Tian, Z.; Zhang, Y.; Xiang, K.; Lin, M.; Guo, X.; Ding, W. Facile growth of homogeneous Ni(OH)2 coating on carbon nanosheets for high-performance asymmetric supercapacitor applications. Nano Res. 2017, 11, 216–224. [Google Scholar]
- Li, Y.; Shi, J. Hollow-structured mesoporous materials: Chemical synthesis, functionalization and applications. Adv. Mater. 2014, 26, 3176–3205. [Google Scholar] [PubMed]
- Li, L.; Tan, L.; Li, G.; Zhang, Y.; Liu, L. Self-Templated Synthesis of Porous Ni(OH)2 Nanocube and Its High Electrochemical Performance for Supercapacitor. Langmuir 2017, 33, 12087–12094. [Google Scholar]
- Qian, J.F.; Wu, C.; Cao, Y.L.; Ma, Z.F.; Huang, Y.H.; Ai, X.P.; Yang, H.X. Prussian Blue Cathode Materials for Sodium-Ion Batteries and Other Ion Batteries. Adv. Energy Mater. 2018, 8, 1702619–1702643. [Google Scholar]
- Ma, F.; Li, Q.; Wang, T.Y.; Zhang, H.G.; Wu, G. Energy storage materials derived from Prussian blue analogues. Sci. Bull. 2017, 62, 358–368. [Google Scholar]
- Karyakin, A.A. Prussian Blue and its analogues: Electrochemistry and analytical applications. Electroanalysis 2001, 13, 813–819. [Google Scholar]
- Nai, J.; Zhang, J.; Lou, X.W. Construction of Single-Crystalline Prussian Blue Analog Hollow Nanostructures with Tailorable Topologies. Chem 2018, 4, 1967–1982. [Google Scholar]
- Feng, Y.; Yu, X.Y.; Paik, U. Nickel cobalt phosphides quasi-hollow nanocubes as an efficient electrocatalyst for hydrogen evolution in alkaline solution. Chem. Commun. 2016, 52, 1633–1636. [Google Scholar]
- Han, L.; Yu, X.-Y.; Lou, X.W.D. Formation of Prussian-Blue-Analog Nanocages via a Direct Etching Method and their Conversion into Ni-Co-Mixed Oxide for Enhanced Oxygen Evolution. Adv. Mater. 2016, 28, 4601–4605. [Google Scholar] [CrossRef]
- Yu, X.Y.; Yu, L.; Wu, H.B.; Lou, X.W. Formation of nickel sulfide nanoframes from metal-organic frameworks with enhanced pseudocapacitive and electrocatalytic properties. Angew. Chem. Int. Ed. Engl. 2015, 54, 5331–5335. [Google Scholar]
- Zhang, L.; Shi, L.; Huang, L.; Zhang, J.; Gao, R.; Zhang, D. Rational Design of High-Performance DeNOx Catalysts Based on MnxCo3−xO4 Nanocages Derived from Metal–Organic Frameworks. ACS Catal. 2014, 4, 1753–1763. [Google Scholar]
- Wang, D.; Qi, X.; Gao, H.; Yu, J.; Zhao, Y.; Zhou, G.; Li, G. Fabricating hierarchical porous ZnCo2O4 microspheres as high-performance anode material for lithium-ion batteries. Mater. Lett. 2016, 164, 93–96. [Google Scholar]
- Zhang, J.; Luan, Y.; Lyu, Z.; Wang, L.; Xu, L.; Yuan, K.; Pan, F.; Lai, M.; Liu, Z.; Chen, W. Synthesis of hierarchical porous delta-MnO2 nanoboxes as an efficient catalyst for rechargeable Li–O2 batteries. Nanoscale 2015, 7, 14881–14888. [Google Scholar]
- Zhang, J.; Lyu, Z.; Zhang, F.; Wang, L.; Xiao, P.; Yuan, K.; Lai, M.; Chen, W. Facile synthesis of hierarchical porous Co3O4 nanoboxes as efficient cathode catalysts for Li–O2 batteries. J. Mater. Chem. A 2016, 4, 6350–6356. [Google Scholar]
- Nai, J.; Yin, H.; You, T.; Zheng, L.; Zhang, J.; Wang, P.; Jin, Z.; Tian, Y.; Liu, J.; Tang, Z.; et al. Efficient Electrocatalytic Water Oxidation by Using Amorphous Ni-Co Double Hydroxides Nanocages. Adv. Energy Mater. 2015, 5, 1401880–1401886. [Google Scholar]
- Tian, L.; Yang, T.; Pu, W.; Zhang, J. Synthesis of Cubic Ni(OH)2 Nanocages Through Coordinating Etching and Precipitating Route for High-Performance Supercapacitors. Nanoscale Res. Lett. 2019, 14, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Cao, C.; Chang, R.; Hou, J.; Zhai, H. Hierarchical mesoporous NiCo2O4 hollow nanocubes for supercapacitors. Phys. Chem. Chem. Phys. 2016, 18, 6268–6274. [Google Scholar]
- Wang, G.; Yan, Z.; Ding, Y.; Xu, Z.; Li, Z. Hierarchical core-shell nickel hydroxide@nitrogen-doped hollow carbon spheres composite for high-performance hybrid supercapacitor. J. Colloid Interface Sci. 2022, 628, 286–296. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, L.L.; Ji, H.; Li, Y.; Zhao, X.; Bai, X.; Fan, X.; Zhang, F.; Ruoff, R.S. Nanoporous Ni(OH)2 Thin Film on 3D Ultrathin-Graphite Foam for Asymmetric Supercapacitor. ACS Nano 2013, 7, 6237–6243. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, H.; Ji, S.; Linkov, V.; Wang, R. Core-shell structured Ni3S2@Co(OH)2 nano-wires grown on Ni foam as binder-free electrode for asymmetric supercapacitors. Chem. Eng. J. 2018, 345, 48–57. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, L.; Zhang, Q.; Chen, D.; Cheng, Y.; Deng, X.; Chen, Y.; Murphy, R.; Xiong, X.; Song, B.; et al. Rational Design of Nickel Hydroxide-Based Nanocrystals on Graphene for Ultrafast Energy Storage. Adv. Energy Mater. 2018, 8, 1702247. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, Z.; Li, X.; Guo, H.; Zhang, D.; Wang, Z. Smartly tailored Co(OH)2-Ni(OH)2 heterostucture on nickel foam as binder-free electrode for high-energy hybrid capacitors. Electrochim. Acta 2019, 309, 140–147. [Google Scholar] [CrossRef]
- Albohani, S.; Minakshi Sundaram, M.; Laird, D.W. Egg shell membrane template stabilises formation of β-NiMoO4 nanowires and enhances hybrid supercapacitor behaviour. Mater. Lett. 2019, 236, 64–68. [Google Scholar] [CrossRef]
- Barmi, M.J.; Minakshi, M. Tuning the Redox Properties of the Nanostructured CoMoO4 Electrode: Effects of Surfactant Content and Synthesis Temperature. ChemPlusChem 2016, 81, 964–977. [Google Scholar] [CrossRef]
- Verma, M.L.; Minakshi, M.; Singh, N.K. Synthesis and Characterization of Solid Polymer Electrolyte based on Activated Carbon for Solid State Capacitor. Electrochim. Acta 2014, 137, 497–503. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Minakshi, M. Status on electrodeposited manganese dioxide and biowaste carbon for hybrid capacitors: The case of high-quality oxide composites, mechanisms, and prospects. J. Energy Storage 2022, 56, 106099–106126. [Google Scholar] [CrossRef]
- Mullangi, D.; Dhavale, V.; Shalini, S.; Nandi, S.; Collins, S.; Woo, T.; Kurungot, S.; Vaidhyanathan, R. Low-Overpotential Electrocatalytic Water Splitting with Noble-Metal-Free Nanoparticles Supported in a sp3 N-Rich Flexible COF. Adv. Energy Mater. 2016, 6, 1600110–1600116. [Google Scholar] [CrossRef]
- Nandi, S.; Singh, S.K.; Mullangi, D.; Illathvalappil, R.; George, L.; Vinod, C.P.; Kurungot, S.; Vaidhyanathan, R. Low Band Gap Benzimidazole COF Supported Ni3N as Highly Active OER Catalyst. Adv. Energy Mater. 2016, 6, 1601189–1601199. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Y.; Yu, S.; Zhao, Y.; Mu, S.; Gao, F. Hydrothermal synthesis of a flower-like nano-nickel hydroxide for high performance supercapacitors. Electrochim. Acta 2014, 123, 158–166. [Google Scholar] [CrossRef]
- Huang, J.; Xu, P.; Cao, D.; Zhou, X.; Yang, S.; Li, Y.; Wang, G. Asymmetric supercapacitors based on β-Ni(OH)2 nanosheets and activated carbon with high energy density. J. Power Sources 2014, 246, 371–376. [Google Scholar] [CrossRef]
- Kazemi, S.H.; Kazemi, H.; Kabiri-Samani, M.; Kiani, M.A. Highly stable asymmetric supercapacitors based on Ni(OH)2 deposited on electro-etched carbon paper. J. Iran. Chem. Soc. 2018, 15, 2117–2122. [Google Scholar] [CrossRef]
- Wang, X.; Sumboja, A.; Lin, M.; Yan, J.; Lee, P.S. Enhancing electrochemical reaction sites in nickel-cobalt layered double hydroxides on zinc tin oxide nanowires: A hybrid material for an asymmetric supercapacitor device. Nanoscale 2012, 4, 7266–7272. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Mirfakhrai, T.; Chen, Z.; Casalongue, H.S.; Dai, H. Advanced asymmetrical supercapacitors based on graphene hybrid materials. Nano Res. 2011, 4, 729–736. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, N.; Li, P.; Guo, X.; Chen, X. Concave Ni(OH)2 Nanocube Synthesis and Its Application in High-Performance Hybrid Capacitors. Nanomaterials 2023, 13, 2538. https://doi.org/10.3390/nano13182538
Cong N, Li P, Guo X, Chen X. Concave Ni(OH)2 Nanocube Synthesis and Its Application in High-Performance Hybrid Capacitors. Nanomaterials. 2023; 13(18):2538. https://doi.org/10.3390/nano13182538
Chicago/Turabian StyleCong, Nan, Pan Li, Xuyun Guo, and Xiaojuan Chen. 2023. "Concave Ni(OH)2 Nanocube Synthesis and Its Application in High-Performance Hybrid Capacitors" Nanomaterials 13, no. 18: 2538. https://doi.org/10.3390/nano13182538
APA StyleCong, N., Li, P., Guo, X., & Chen, X. (2023). Concave Ni(OH)2 Nanocube Synthesis and Its Application in High-Performance Hybrid Capacitors. Nanomaterials, 13(18), 2538. https://doi.org/10.3390/nano13182538





