Selective Growth of MAPbBr3 Rounded Microcrystals on Micro-Patterned Single-Layer Graphene Oxide/Graphene Platforms with Enhanced Photo-Stability
Abstract
:1. Introduction
2. Materials and Methods
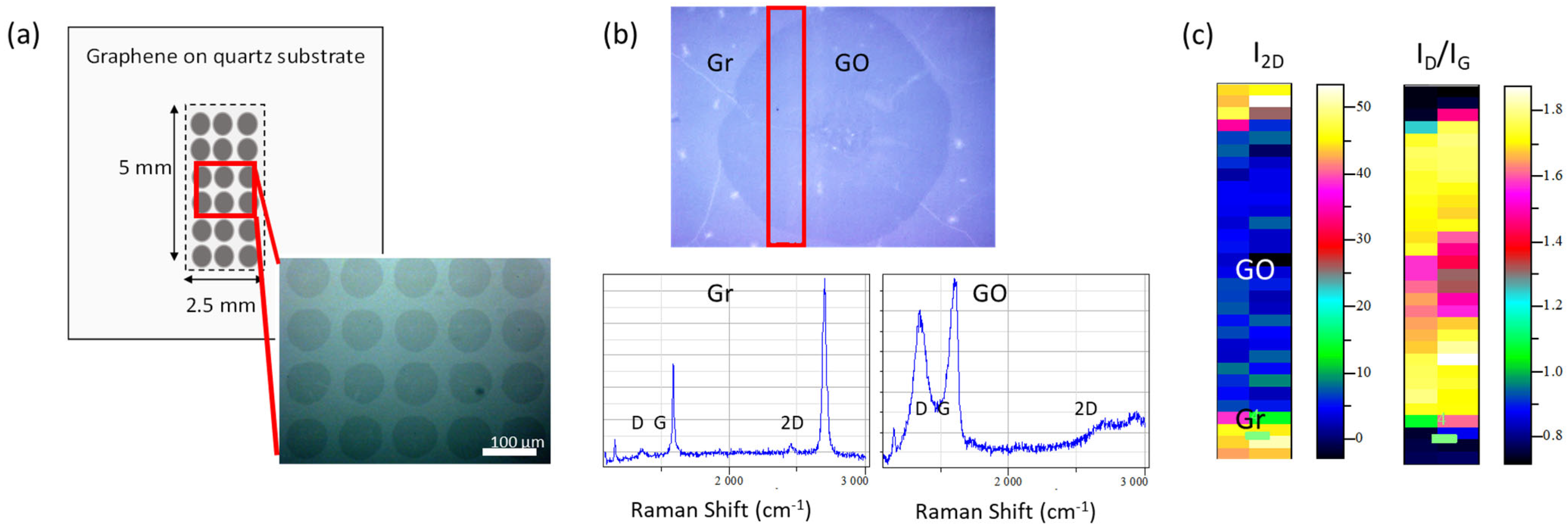
2.1. Graphene Oxide/Graphene (GO/Gr) 2D Platforms Preparation
2.2. MAPbBr3 Synthesis on GO/Gr 2D Platforms
2.3. Structural and Morphological Characterization
2.4. Absorption and Photoluminescence Measurements
3. Results
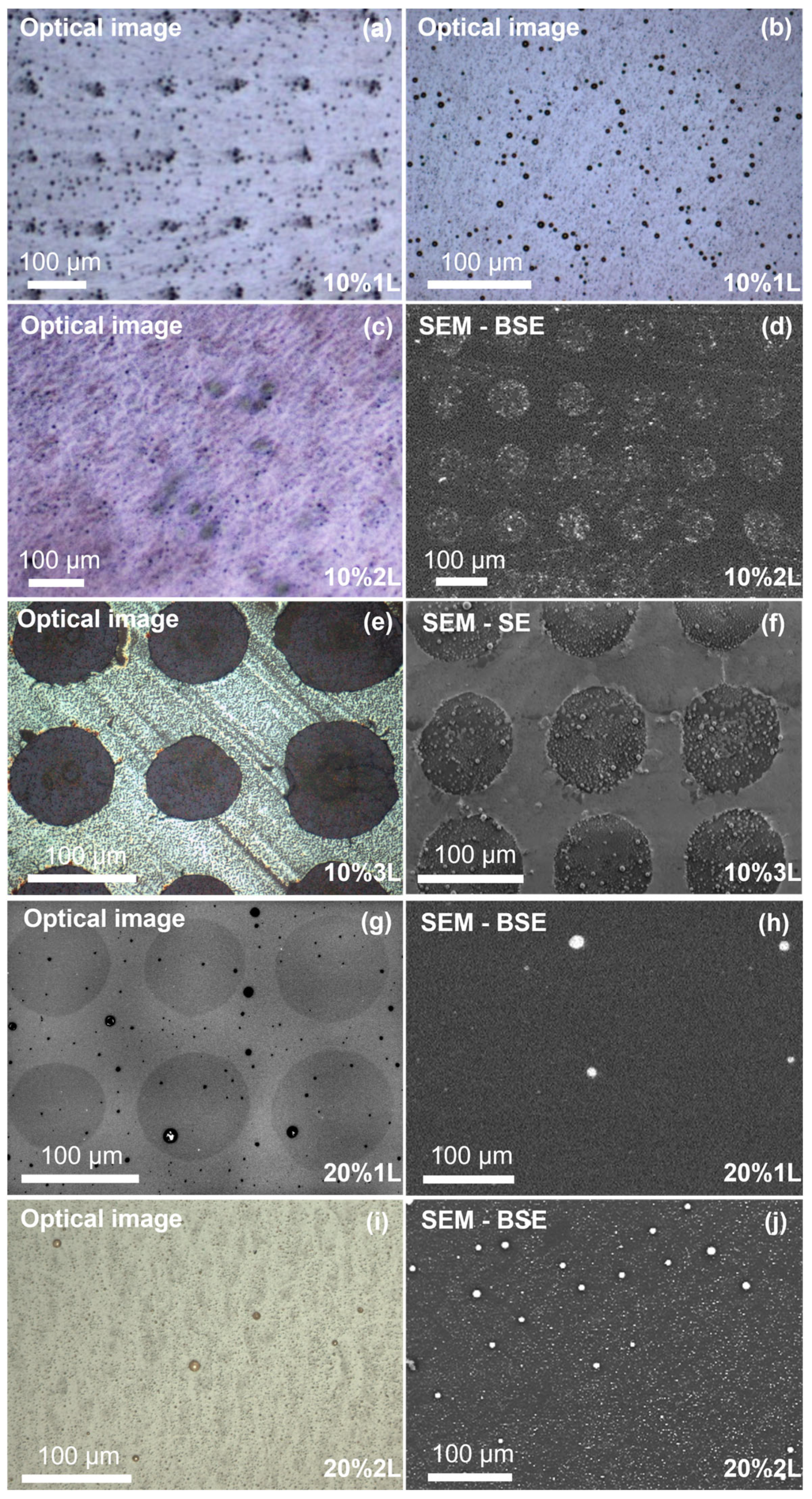
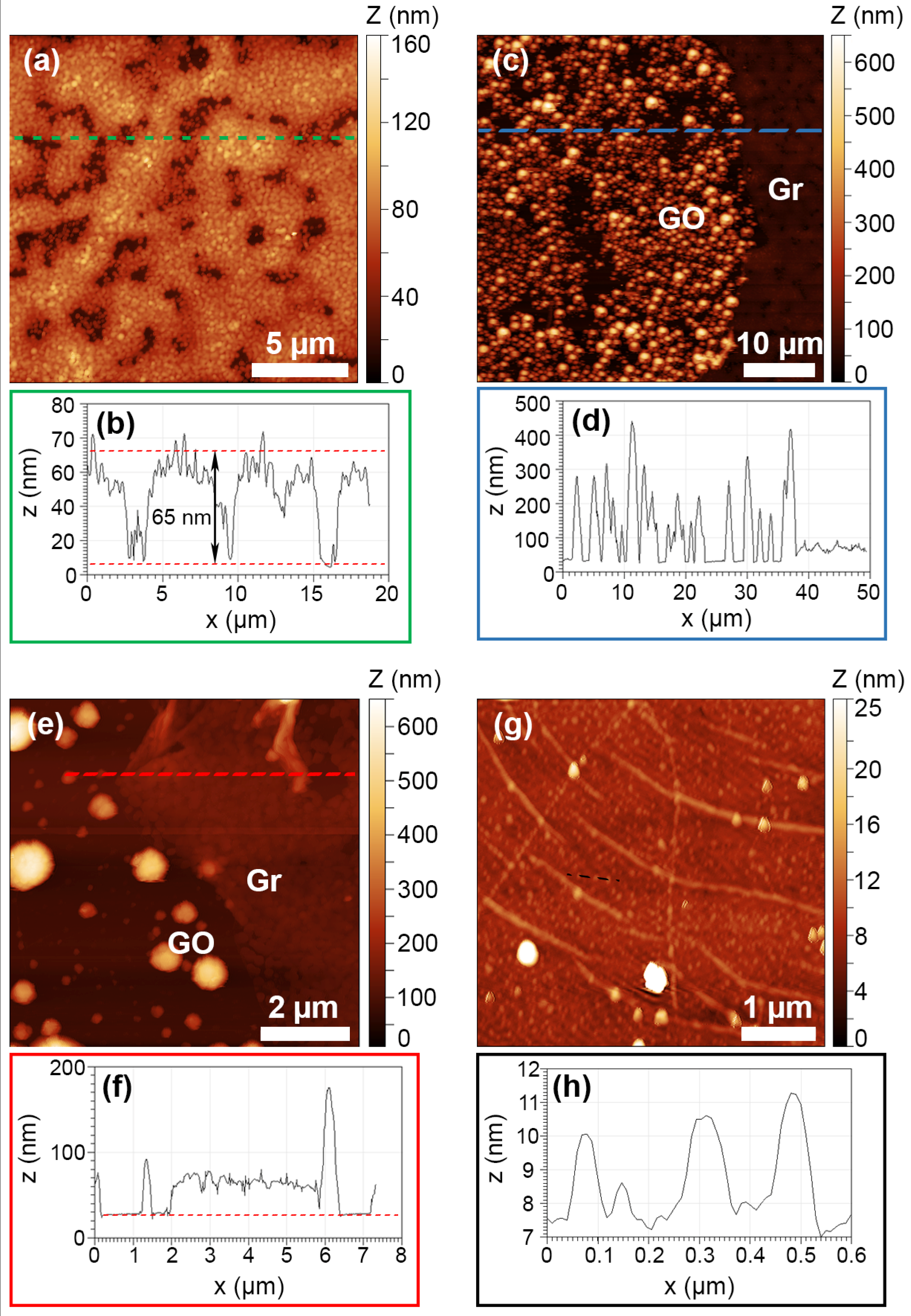
3.1. Morphological and Structural Characterization
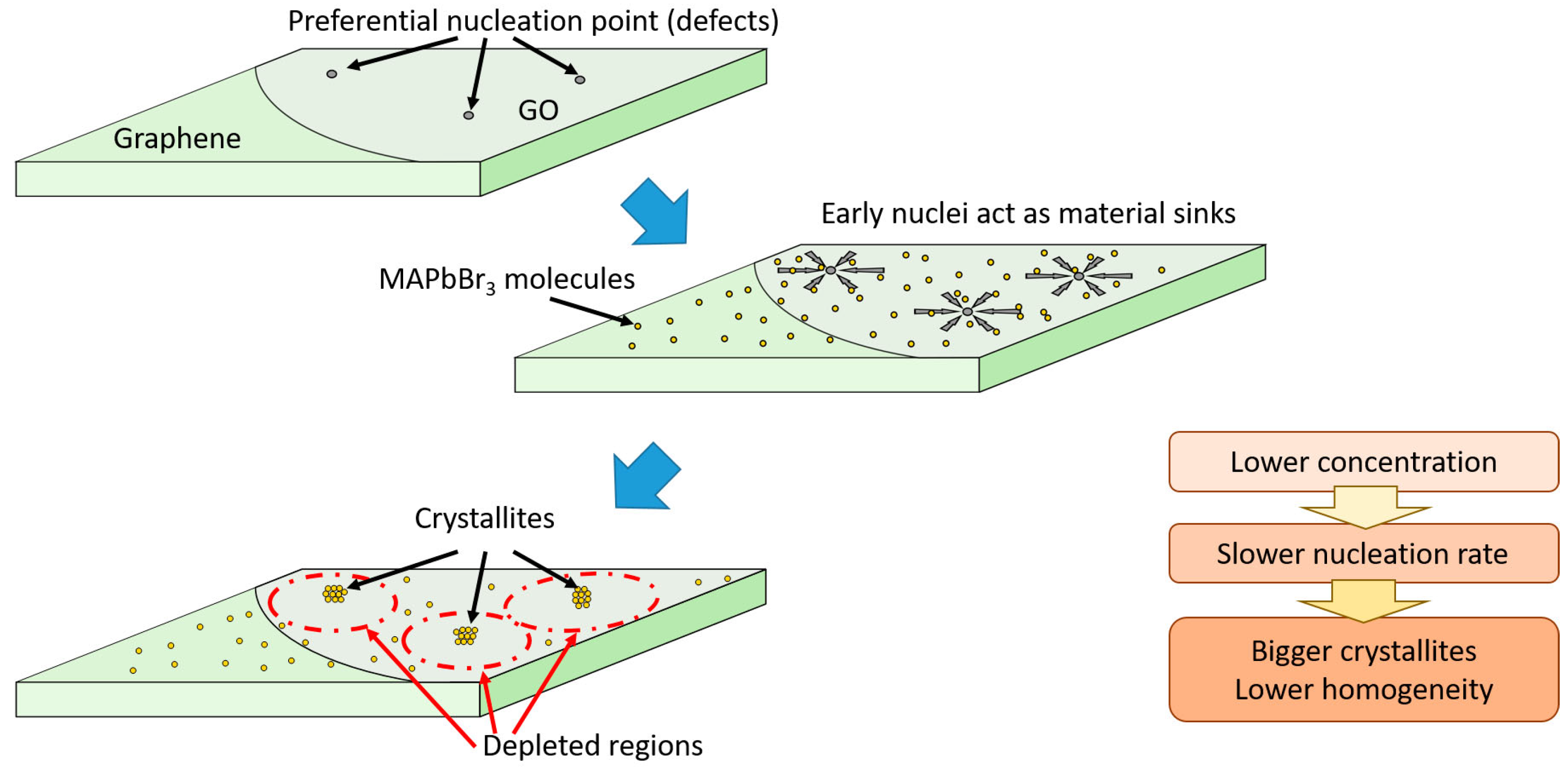
3.2. Growth Model
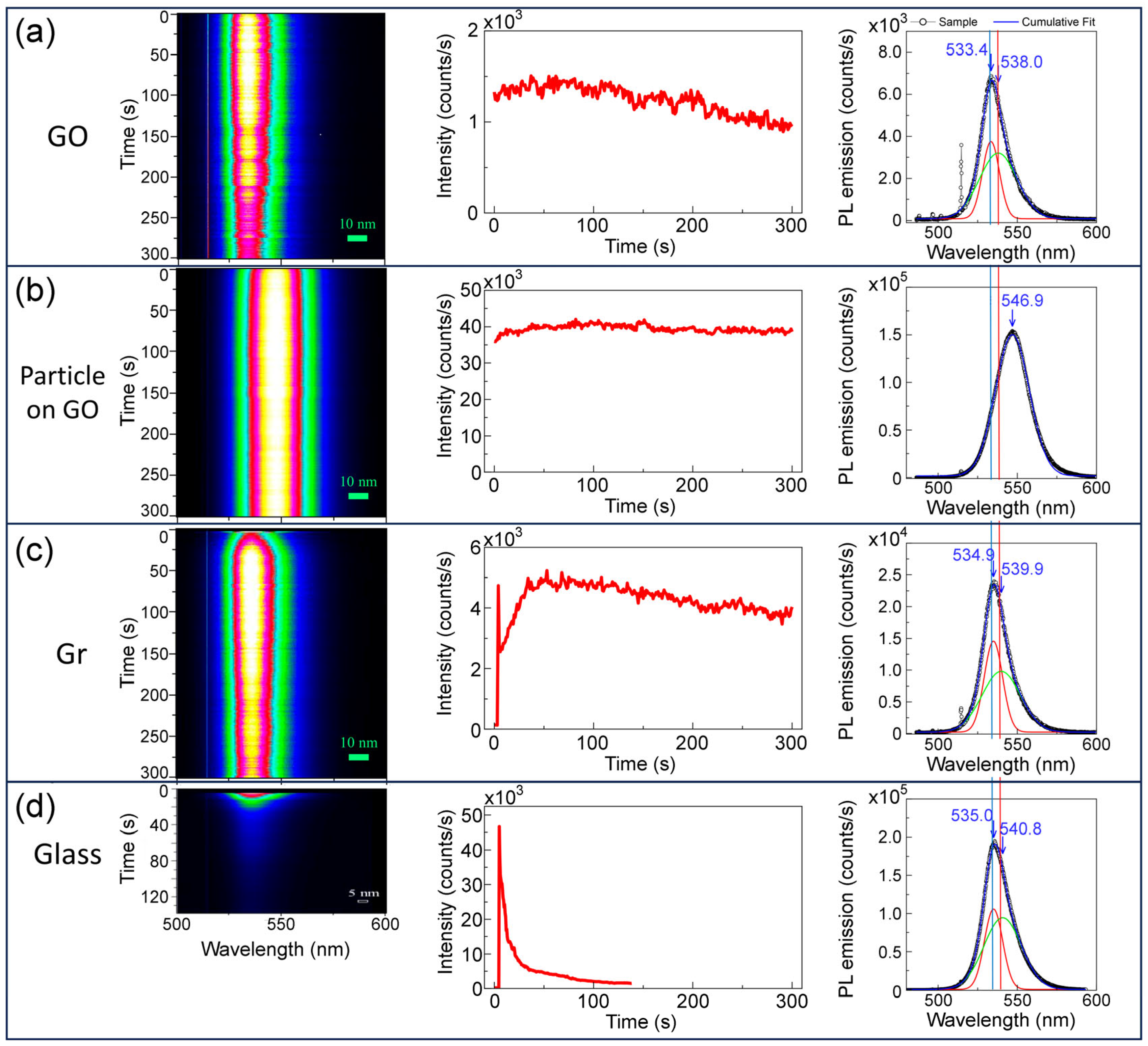
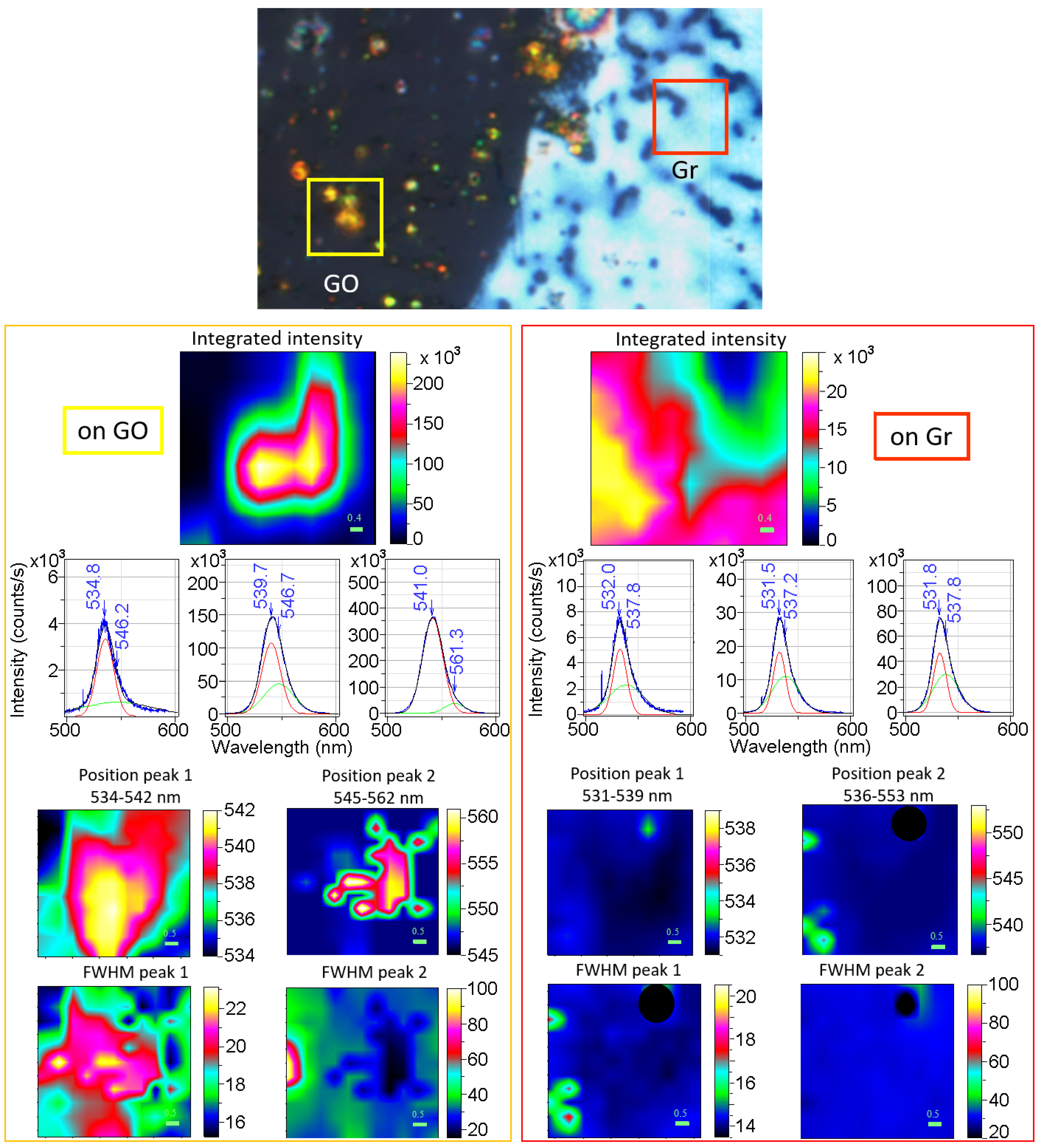
3.3. Photoluminescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schmidt-Mende, L.; Dyakonov, V.; Olthof, S.; Ünlü, F.; Lê, K.M.T.; Mathur, S.; Karabanov, A.D.; Lupascu, D.C.; Herz, L.M.; Hinderhofer, A.; et al. Roadmap on organic-inorganic hybrid perovskite semiconductors and devices. APL Mater. 2021, 9, 109202. [Google Scholar] [CrossRef]
- Shirayama, M.; Kadowaki, H.; Miyadera, T.; Sugita, T.; Tamakoshi, M.; Kato, M.; Fujiseki, T.; Murata, D.; Hara, S.; Murakami, T.-N.; et al. Optical Transitions in Hybrid Perovskite Solar Cells: Ellipsometry, Density Functional Theory, and Quantum Efficiency Analyses for CH3NH3PbI3. Phys. Rev. Appl. 2016, 5, 014012. [Google Scholar] [CrossRef]
- De Wolf, S.; Holovsky, J.; Moon, S.-J.; Löper, P.; Niesen, B.; Ledinsky, M.; Haug, F.-J.; Yum, J.-H.; Ballif, C. Organometallic Halide Perovskites: Sharp Optical Absorption Edge and Its Relation to Photovoltaic Performance. J. Phys. Chem. Lett. 2014, 5, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Stranks, D.M.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Miyata, A.; Mitioglu, A.; Plochocka, P.; Portugall, O.; Wang, J.T.W.; Stranks, S.D.; Snaith, H.J.; Nicholas, R.J. Direct measurement of the exciton binding energy and effective masses for charge carriers in organic–inorganic tri-halide perovskites. Nat. Phys. 2015, 11, 582–587. [Google Scholar] [CrossRef]
- Werner, J.; Niesen, B.; Ballif, C. Perovskite/Silicon Tandem Solar Cells: Marriage of Convenience or True Love Story?—An Oveview. Adv. Mater. Interfaces 2018, 5, 1700731. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef]
- Mohamad, D.K.; Griffin, J.; Bracher, C.; Barrows, A.T.; Lidzey, D.G. Spray-Cast Multilayer Organometal Perovskite Solar Cells Fabricated in Air. Adv. Energy Mater. 2016, 6, 1600994. [Google Scholar] [CrossRef]
- Yang, M.; Li, Z.; Reese, M.O.; Reid, O.G.; Kim, D.H.; Siol, S.; Klein, T.R.; Yan, Y.; Berry, J.J.; van Hest, M.F.A.M.; et al. Perovskite ink with wide processing window for scalable high-efficiency solar cells. Nat. Energy 2017, 2, 17038. [Google Scholar] [CrossRef]
- Ahn, N.; Son, D.-Y.; Jang, I.-H.; Kang, S.M.; Choi, M.; Park, N.-G. Highly Reproducible Perovskite Solar Cells with Average Efficiency of 18.3% and Best Efficiency of 19.7% Fabricated via Lewis Base Adduct of Lead (II) Iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. [Google Scholar] [CrossRef]
- Jiang, Y.; He, S.; Qiu, L.; Zhao, Y.; Qi, Y. Perovskite solar cells by vapor deposition based and assisted methods. Appl. Phys. Rev. 2022, 9, 021305. [Google Scholar] [CrossRef]
- Jiao, Y.; Ma, F.; Gao, G.; Wang, H.; Bell, J.; Frauenheim, T.; Du, A. Graphene-covered perovskites: An effective strategy to enhance light absorption and resist moisture degradation. RSC Adv. 2015, 5, 82346–82350. [Google Scholar] [CrossRef]
- Climent-Pascual, E.; Hames, B.C.; Moreno-Ramírez, J.S.; Álvarez, A.L.; Juarez-Perez, E.J.; Mas-Marza, E.; Mora-Seró, I.; de Andrés, A.; Coya, C. Influence of the substrate on the bulk properties of hybrid lead halide perovskite films. J. Mater. Chem. A 2016, 4, 18153–18163. [Google Scholar] [CrossRef]
- Volonakis, G.; Giustino, F. Interfaces Between Graphene-Related Materials and MAPbI3: Insights from First-Principles. Adv. Mater. Interfaces 2018, 5, 1800496. [Google Scholar] [CrossRef]
- Wu, T.; Qin, Z.; Wang, Y.; Wu, Y.; Chen, W.; Zhang, S.; Cai, M.; Dai, S.; Zhang, J.; Liu, J.; et al. The Main Progress of Perovskite Solar Cells in 2020–2021. Nano-Micro Lett. 2021, 13, 152. [Google Scholar] [CrossRef]
- Hu, Z.; Ran, C.; Zhang, H.; Chao, L.; Chen, Y.; Huang, W. The Current Status and Development Trend of Perovskite Solar Cells. Engineering 2023, 21, 15–19. [Google Scholar] [CrossRef]
- Chowdhury, T.A.; Bin Zafar, M.A.; Sajjad-Ul Islam, M.; Shahinuzzaman, M.; Islam, M.A.; Khandaker, M.U. Stability of perovskite solar cells: Issues and prospects. RSC Adv. 2023, 13, 1787–1810. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Muchuweni, E.; Martincigh, B.S.; Nyamori, V.O. Perovskite Solar Cells: Current Trends in Graphene-Based Materials for Transparent Conductive Electrodes, Active Layers, Charge Transport Layers, and Encapsulation Layers. Adv. Energy Sustain. Res. 2021, 2, 2100050. [Google Scholar] [CrossRef]
- Redondo-Obispo, C.; Ripolles, T.S.; Cortijo-Campos, S.; Álvarez, A.L.; Climent-Pascual, E.; de Andrés, A.; Coya, C. Enhanced stability and efficiency in inverted perovskite solar cells through graphene doping of PEDOT:PSS hole transport layer. Mater. Des. 2020, 191, 108587. [Google Scholar] [CrossRef]
- Redondo-Obispo, C.; Serafini, P.; Climent-Pascual, E.; Ripolles, T.S.; Mora-Seró, I.; de Andrés, A.; Coya, C. Effect of Pristine Graphene on Methylammonium Lead Iodide Films and Implications on Solar Cell Performance. ACS Appl. Energy Mater. 2021, 4, 13943–13951. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Luo, Q.; Liu, T.; Tai, M.; Lin, J.; Murugadoss, V.; Lin, H.; Wang, J.; Guo, Z.; Wang, N. Boosting Multiple Interfaces by Co-Doped Graphene Quantum Dots for High Efficiency and Durability Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 13941–13949. [Google Scholar] [CrossRef] [PubMed]
- Lou, Q.; Lou, G.; Peng, R.; Liu, Z.; Wang, W.; Ji, M.; Chen, C.; Zhang, X.; Liu, C.; Ge, Z. Synergistic Effect of Lewis Base Polymers and Graphene in Enhancing the Efficiency of Perovskite Solar Cells. ACS Appl. Energy Mater. 2021, 4, 3928–3936. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Johnson, D.W.; Dobson, B.P.; Coleman, K.S.A. Manufacturing Perspective on Graphene Dispersions. Curr. Opin. Colloid. Interface Sci. 2015, 20, 367–382. [Google Scholar] [CrossRef]
- Gidey, A.T.; Kim, J.Y. Tuning the Crystallization Process of Perovskite Active Layer Using a Functionalized Graphene Oxide for Enhanced Photovoltaic Performance. J. Mater. Sci. Mater. Electron. 2020, 31, 12257–12268. [Google Scholar] [CrossRef]
- Zibouche, N.; Volonakis, G.; Giustino, F. Graphene Oxide/Perovskite Interfaces for Photovoltaics. J. Phys. Chem. C 2018, 122, 16715–16726. [Google Scholar] [CrossRef]
- Liu, Z.; You, L.; Faraji, N.; Lin, C.H.; Xu, X.; He, J.H.; Seidel, J.; Wang, J.; Alshareef, H.N.; Wu, T. Single-Crystal Hybrid Perovskite Platelets on Graphene: A Mixed-Dimensional Van Der Waals Heterostructure with Strong Interface Coupling. Adv. Funct. Mater. 2020, 30, 1–8. [Google Scholar] [CrossRef]
- Niu, L.; Liu, X.; Cong, C.; Wu, C.; Wu, D.; Chang, T.R.; Wang, H.; Zeng, Q.; Zhou, J.; Wang, X.; et al. Controlled Synthesis of Organic/Inorganic van der Waals Solid for Tunable Light-Matter Interactions. Adv. Mater. 2015, 27, 7800–7808. [Google Scholar] [CrossRef]
- Climent-Pascual, E.; García-Vélez, M.; Álvarez, Á.L.; Coya, C.; Munuera, C.; Díez-Betriu, X.; García-Hernández, M.; de Andrés, A. Large area graphene and graphene oxide patterning and nanographene fabrication by one-step lithography. Carbon. N. Y. 2015, 90, 110–121. [Google Scholar] [CrossRef]
- Quesada, S.J.; Borrás, F.; García-Vélez, M.; Coya, C.; Climent, E.; Munuera, C.; Villar, I.; la Peña O’Shea, V.A.; Andrés, A.; Álvarez, Á.L. New Concepts for Production of Scalable Single Layer Oxidized Regions by Local Anodic Oxidation of Graphene. Small 2019, 15, 1902817. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, Á.L.; Coya, C.; García-Vélez, M. Development of electrical-erosion instrument for direct write micro-patterning on large area conductive thin films. Rev. Sci. Instrum. 2015, 86, 084704. [Google Scholar] [CrossRef]
- Díez-Betriu, X.; Álvarez-García, S.; Botas, C.; Álvarez, P.; Sánchez-Marcos, J.; Prieto, C.; Menéndez, R.; de Andrés, A. Raman spectroscopy for the study of reduction mechanisms and optimization of conductivity in graphene oxide thin films. J. Mater. Chem. C 2013, 1, 6905. [Google Scholar] [CrossRef]
- Rodriguez-Nieva, J.F.; Barros, E.B.; Saito, R.; Dresselhaus, M.S. Disorder-induced double resonant Raman process in graphene. Phys. Rev. B. 2014, 90, 235410. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Bush, K.A.; Rolston, N.; Gold-Parker, A.; Manzoor, S.; Hausele, J.; Yu, Z.J.; Raiford, J.A.; Cheacharoen, R.; Holman, Z.C.; Toney, M.F.; et al. Controlling Thin-Film Stress and Wrinkling during Perovskite Film Formation. ACS Energy Lett. 2018, 3, 1225–1232. [Google Scholar] [CrossRef]
- Muslimawati, R.M.; Manawan, M.; Takahashi, K.; Furukawa, Y.; Bahtiar, A. Single Crystal Perovskite MAPbBr 3 Prepared by Using Anti-solvent Vapor-assisted Crystallization Method. J. Phys. Conf. Ser. 2022, 2376, 012005. [Google Scholar] [CrossRef]
- Alvarez-Galván, M.C.; Alonso, J.A.; López, C.A.; López-Linares, E.; Contreras, C.; Lázaro, M.J.; Fauth, F.; Martínez-Huerta, M.V. Crystal Growth, Structural Phase Transitions, and Optical Gap Evolution of CH3NH3Pb (Br1–xClx) 3 Perovskites. Cryst. Growth Des. 2019, 19, 918–924. [Google Scholar] [CrossRef]
- Bartolomé, J.; Climent-Pascual, E.; Redondo-Obispo, C.; Zaldo, C.; Álvarez, Á.L.; de Andrés, A.; Coya, C. Huge Photostability Enhancement in Bismuth-Doped Methylammonium Lead Iodide Hybrid Perovskites by Light-Induced Transformation. Chem. Mater. 2019, 31, 3662–3671. [Google Scholar] [CrossRef]
- Ma, Y.; Hangoma, P.M.; Park, W.I.; Lim, J.H.; Jung, Y.K.; Jeong, J.H.; Park, S.H.; Kim, K.H. Controlled crystal facet of MAPbI3 perovskite for highly efficient and stable solar cell via nucleation modulation. Nanoscale 2018, 11, 170–177. [Google Scholar] [CrossRef]
- Wu, B.; Nguyen, H.T.; Ku, Z.; Han, G.; Giovanni, D.; Mathews, N.; Fan, H.J.; Sum, T.C. Discerning the Surface and Bulk Recombination Kinetics of Organic-Inorganic Halide Perovskite Single Crystals. Adv. Energy Mater. 2016, 6, 1600551. [Google Scholar] [CrossRef]
- Droseros, N.; Longo, G.; Brauer, J.C.; Sessolo, M.; Bolink, H.J.; Banerji, N. Origin of the Enhanced Photoluminescence Quantum Yield in MAPbBr 3 Perovskite with Reduced Crystal Size. ACS Energy Lett. 2018, 3, 1458–1466. [Google Scholar] [CrossRef]
- Wei, J.-H.; Wang, X.-D.; Liao, J.-F.; Kuang, D.-B. High Photoluminescence Quantum Yield (>95%) of MAPbBr3 Nanocrystals via Reprecipitation from Methylamine-MAPbBr3 Liquid. ACS Appl. Electron. Mater. 2020, 2, 2707–2715. [Google Scholar] [CrossRef]
- Grancini, G.; Marras, S.; Prato, M.; Giannini, C.; Quarti, C.; De Angelis, F.; De Bastiani, M.; Eperon, G.E.; Snaith, H.J.; Manna, L.; et al. The Impact of the Crystallization Processes on the Structural and Optical Properties of Hybrid Perovskite Films for Photovoltaics. J. Phys. Chem. Lett. 2014, 5, 3836–3842. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartolomé, J.; Vila, M.; Redondo-Obispo, C.; de Andrés, A.; Coya, C. Selective Growth of MAPbBr3 Rounded Microcrystals on Micro-Patterned Single-Layer Graphene Oxide/Graphene Platforms with Enhanced Photo-Stability. Nanomaterials 2023, 13, 2513. https://doi.org/10.3390/nano13182513
Bartolomé J, Vila M, Redondo-Obispo C, de Andrés A, Coya C. Selective Growth of MAPbBr3 Rounded Microcrystals on Micro-Patterned Single-Layer Graphene Oxide/Graphene Platforms with Enhanced Photo-Stability. Nanomaterials. 2023; 13(18):2513. https://doi.org/10.3390/nano13182513
Chicago/Turabian StyleBartolomé, Javier, María Vila, Carlos Redondo-Obispo, Alicia de Andrés, and Carmen Coya. 2023. "Selective Growth of MAPbBr3 Rounded Microcrystals on Micro-Patterned Single-Layer Graphene Oxide/Graphene Platforms with Enhanced Photo-Stability" Nanomaterials 13, no. 18: 2513. https://doi.org/10.3390/nano13182513
APA StyleBartolomé, J., Vila, M., Redondo-Obispo, C., de Andrés, A., & Coya, C. (2023). Selective Growth of MAPbBr3 Rounded Microcrystals on Micro-Patterned Single-Layer Graphene Oxide/Graphene Platforms with Enhanced Photo-Stability. Nanomaterials, 13(18), 2513. https://doi.org/10.3390/nano13182513





