A Review of Perovskite-Based Photodetectors and Their Applications
Abstract
1. Introduction
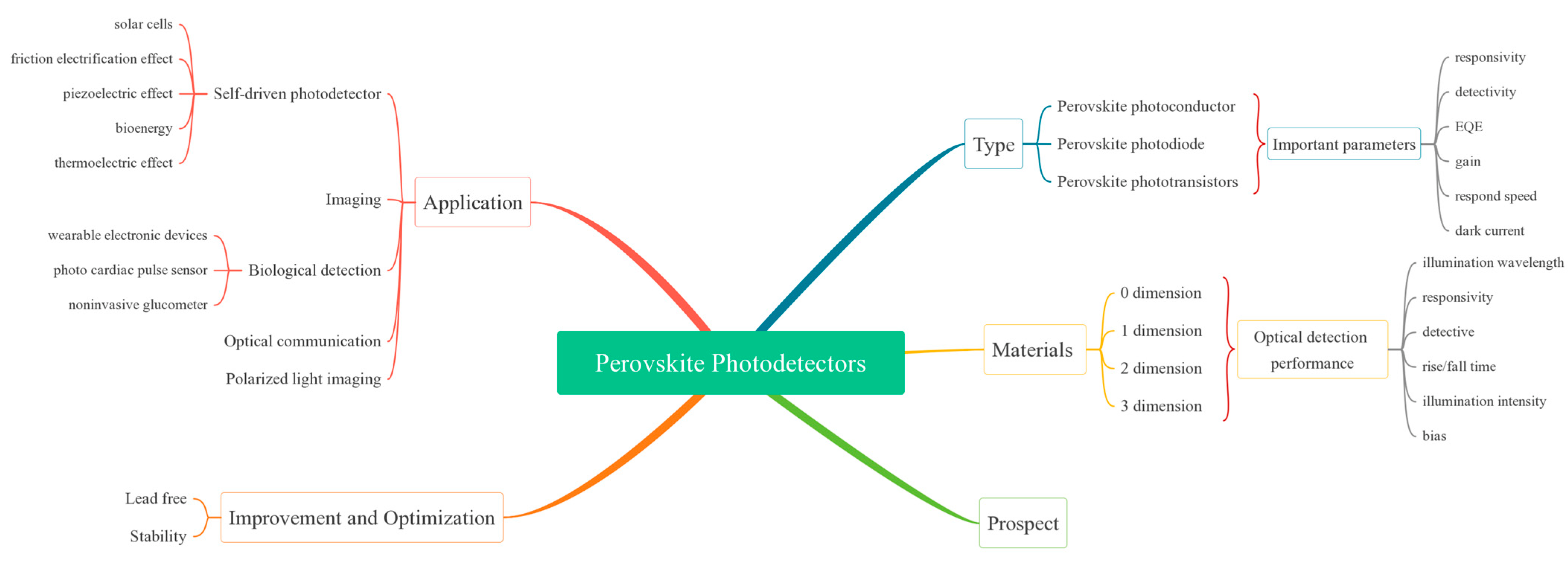
2. Perovskite Photodetector Type
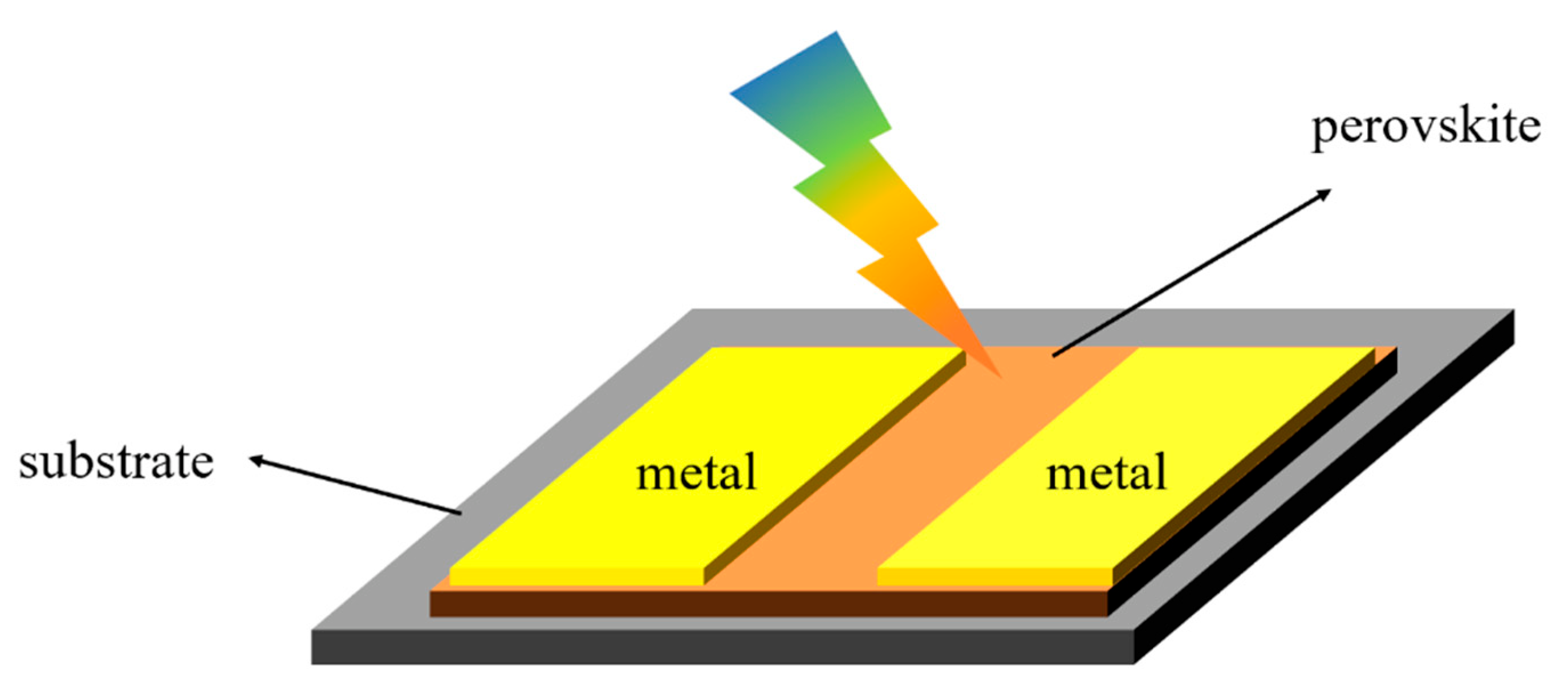
2.1. Perovskite Photoconductor
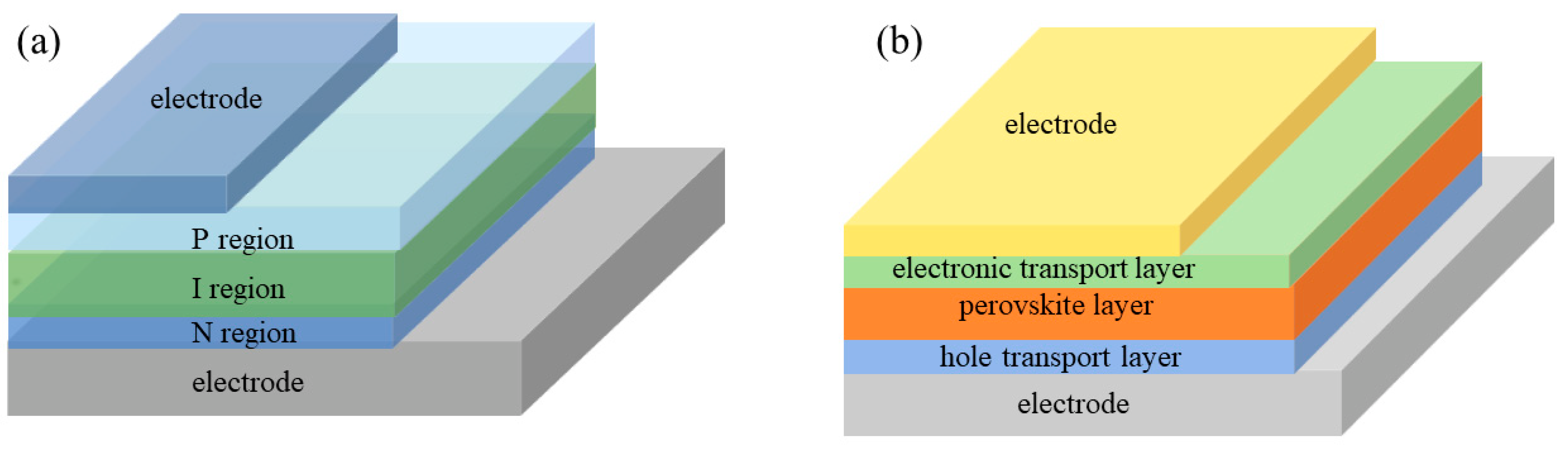
2.2. Perovskite Photodiode
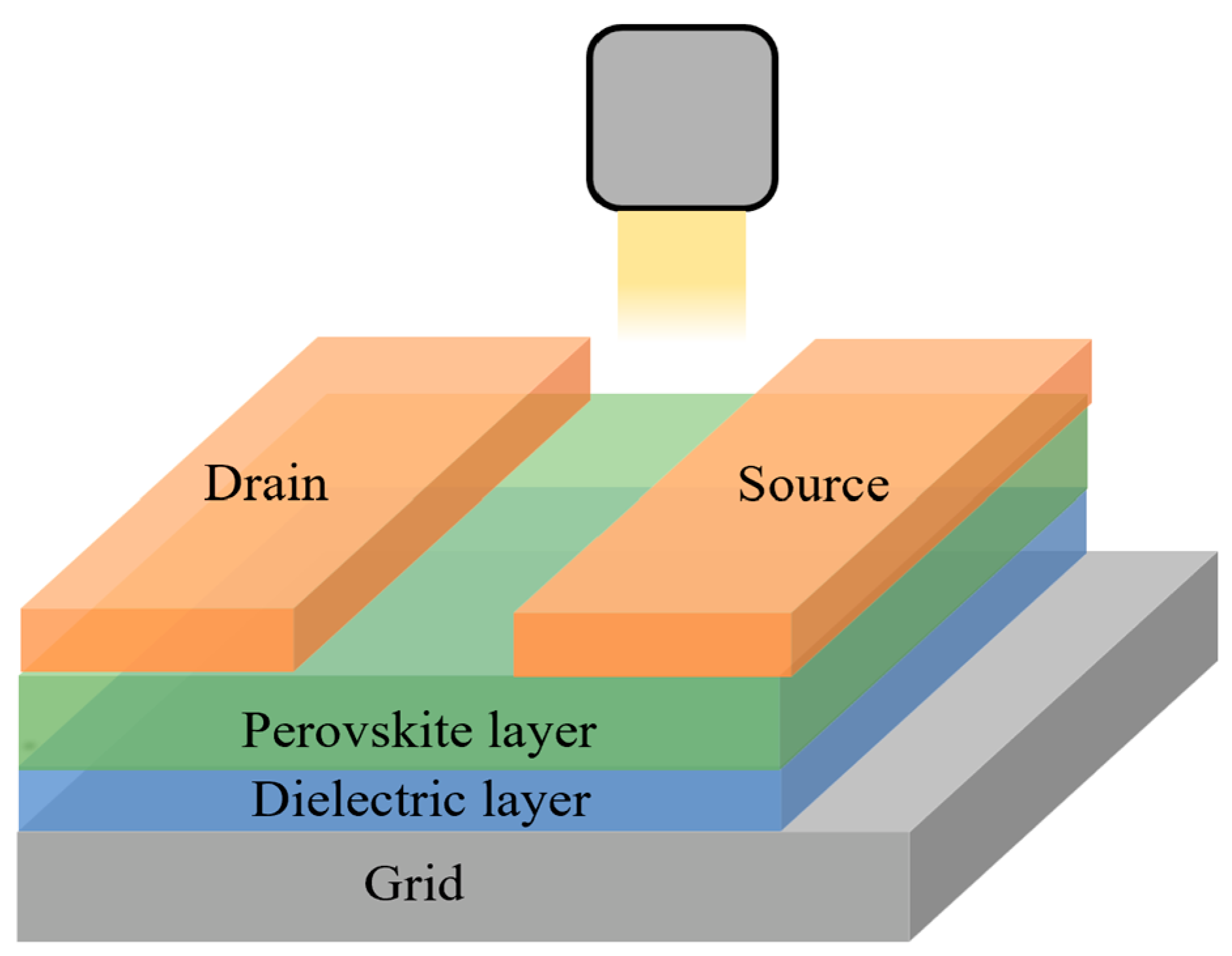
2.3. Perovskite Phototransistors
3. Perovskite Photodetectors with Low-Dimensional Materials
3.1. 0D Perovskite Photodetector
3.2. 1D Perovskite Photodetector
3.3. 2D Perovskite Photodetector
4. Application of Photodetectors
4.1. Introduction to Self-Driven Photodetector
4.2. Imaging Applications
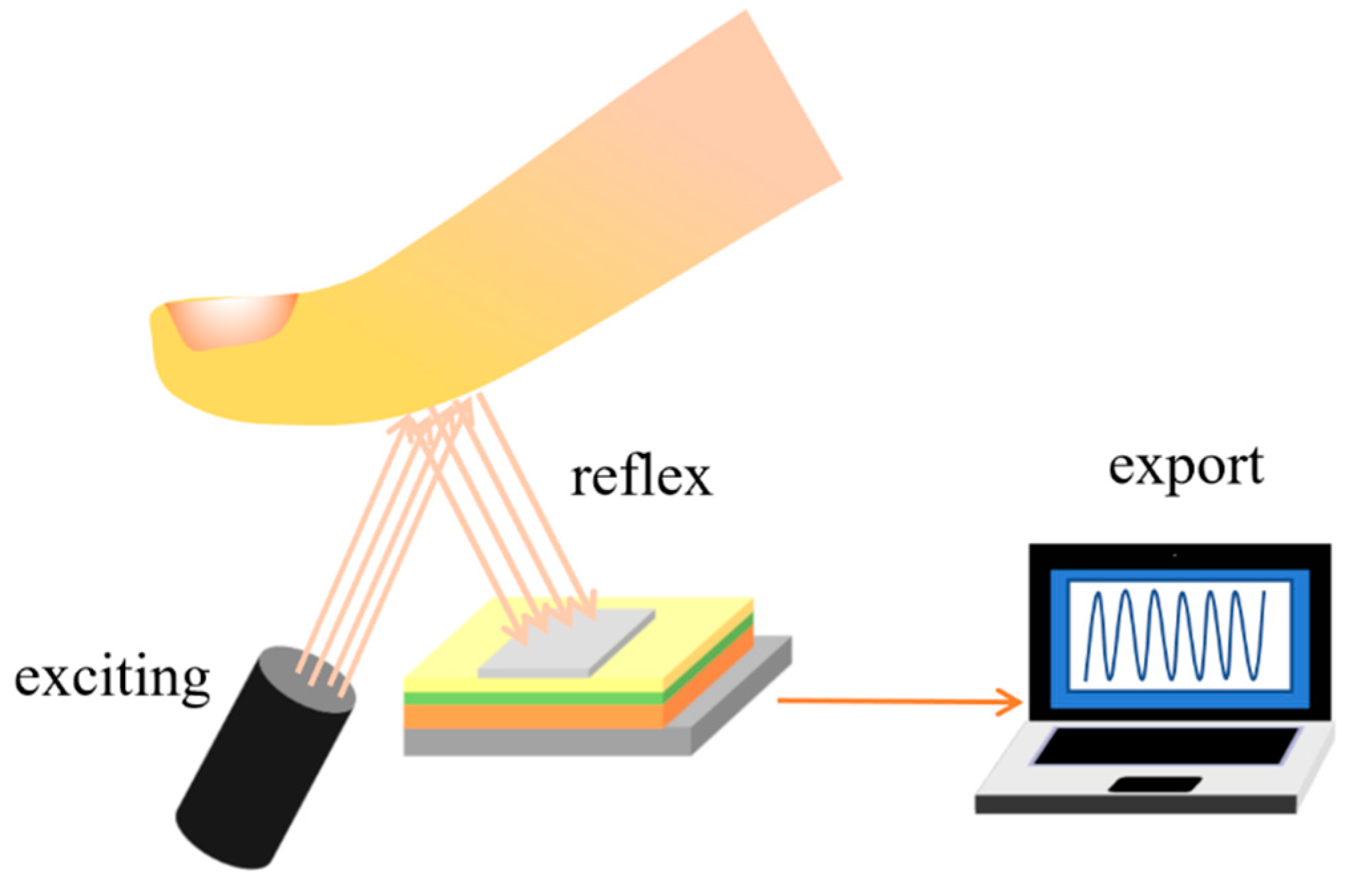
4.3. Biological Detection
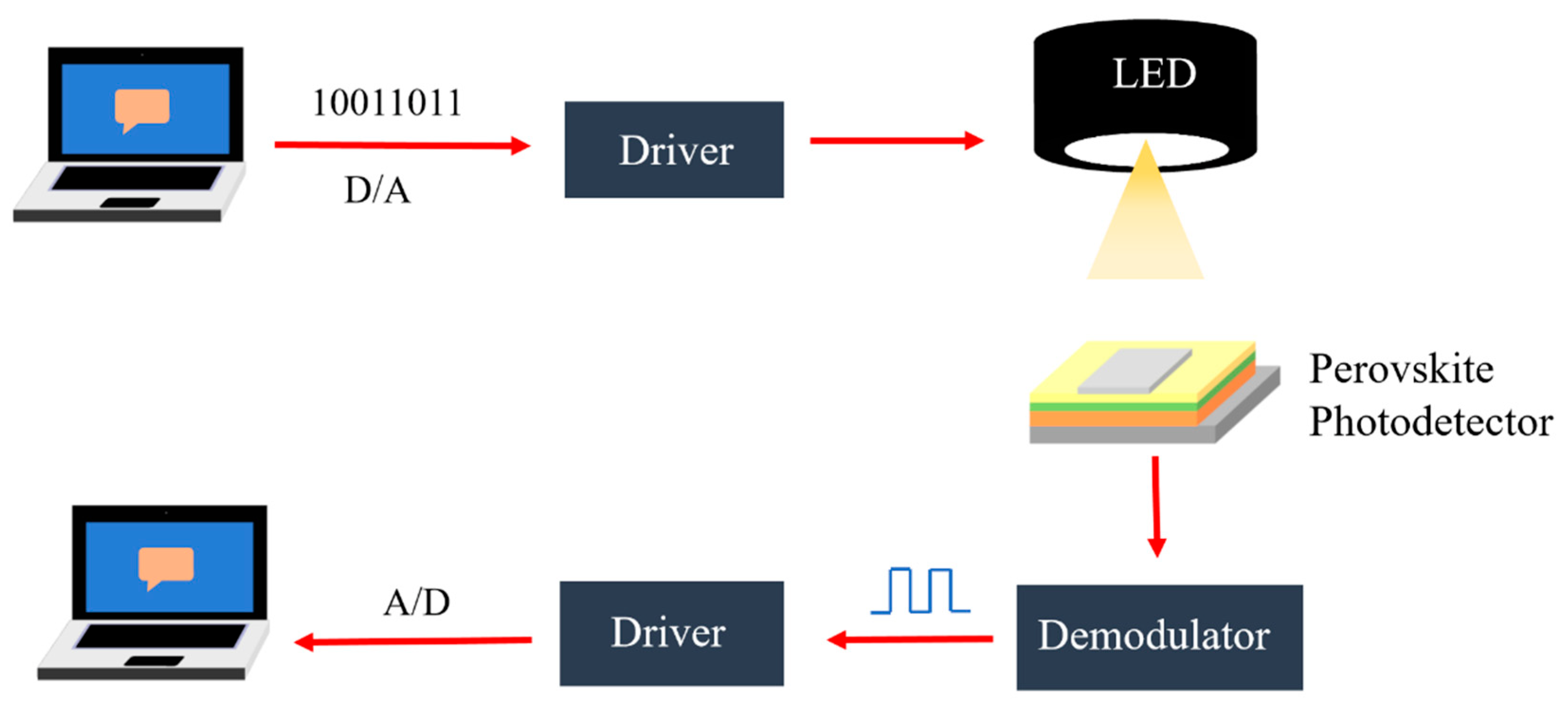
4.4. Optical Communication and Up-Conversion Systems
4.5. Polarized Light Imaging
5. Improvement and Optimization
5.1. Lead-Free Materials
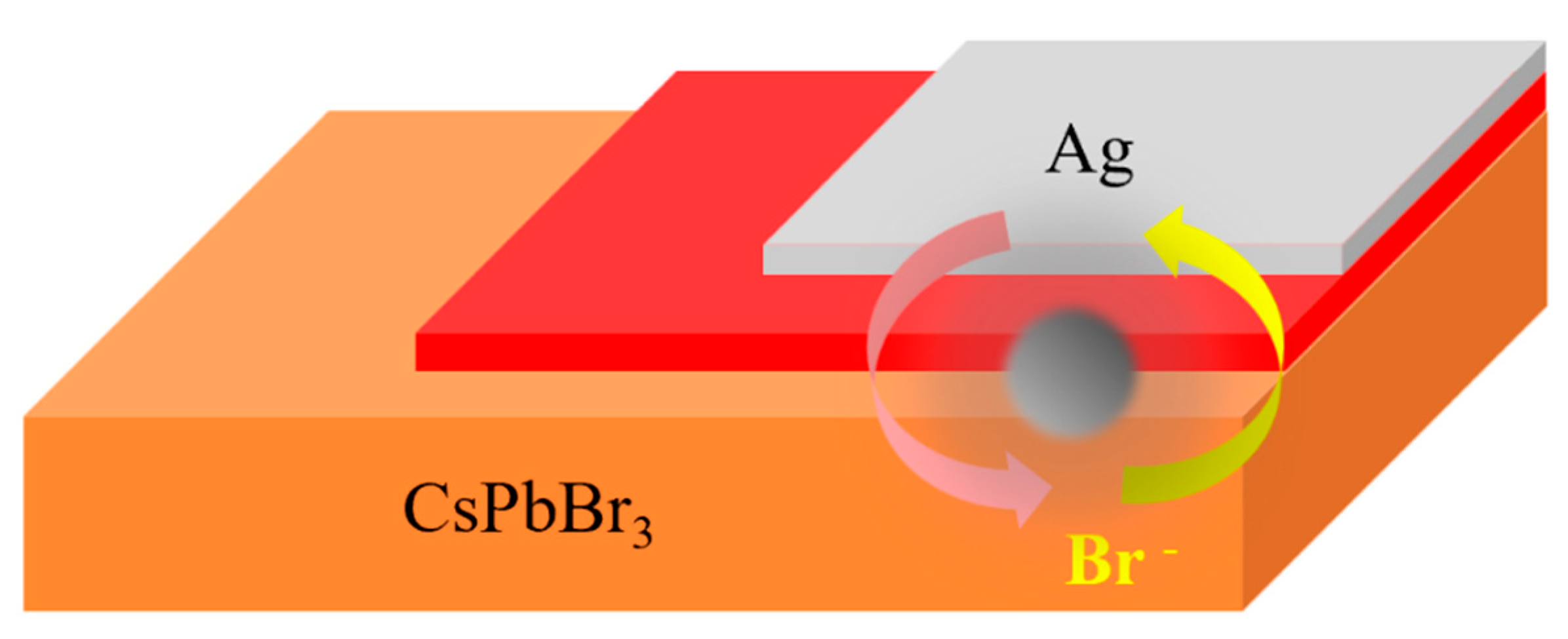
5.2. Stability
6. Prospect
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ma, Y.; Xiao, Y.; Shen, L.; Ding, L. Advances in perovskite photodetectors. InfoMat 2020, 2, 1247–1256. [Google Scholar] [CrossRef]
- Pradhan, B.; Kumar, G.S.; Sain, S.; Dalui, A.; Ghorai, U.K.; Pradhan, S.K.; Acharya, S. Size tunable cesium antimony chloride perovskite nanowires and nanorods. Chem. Mater. 2018, 30, 2135–2142. [Google Scholar] [CrossRef]
- Tian, W.; Wang, Y.; Chen, L.; Li, L. Self-powered nanoscale photodetectors. Small 2017, 13, 1701848. [Google Scholar] [CrossRef]
- Lin, Z.Q.; Lian, H.J.; Ge, B.; Zhou, Z.; Yuan, H.; Hou, Y.; Yang, S.; Yang, H.G. Mediating the local oxygen-bridge interactions of oxysalt/perovskite interface for defect passivation of perovskite photovoltaics. Nanomicro Lett. 2021, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, W.; Ma, B.; Shen, W.; Liu, L.; Cao, K.; Chen, S.; Huang, W. Lead-free perovskite materials for solar cells. Nanomicro Lett. 2021, 13, 62. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Lim, S.S.; Yantara, N.; Liu, X.; Sabba, D.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Low-temperature solution-processed wavelength-tunable perovskites for lasing. Nat. Mater. 2014, 13, 476–480. [Google Scholar] [CrossRef]
- Qin, C.; Sandanayaka, A.S.D.; Zhao, C.; Matsushima, T.; Zhang, D.; Fujihara, T.; Adachi, C. Stable room-temperature continuous-wave lasing in quasi-2D perovskite films. Nature 2020, 585, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Yang, Y.; You, J.; Hong, Z.; Chang, W.-H.; Li, G.; Yang, Y. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Armin, A.; Lyons, D.M.; Burn, P.L.; Meredith, P. Low noise, IR-Blind organohalide perovskite photodiodes for visible light detection and imaging. Adv. Mater. 2015, 27, 2060–2064. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Wang, F.; Tian, H.; Feng, Q.; Zhang, H.; Liang, R.; Shen, Y.; Ju, Z.; Gou, G.-Y.; Deng, N.; et al. Ultrafast photodetector by integrating perovskite directly on silicon wafer. ACS Nano 2020, 14, 2860–2868. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, L.; Deng, Y.; Xiao, X.; Ni, Z.; Xu, S.; Huang, J. Perovskite-filled membranes for flexible and large-area direct-conversion X-ray detector arrays. Nat. Photonics 2020, 14, 612–617. [Google Scholar] [CrossRef]
- Ahmadi, M.; Wu, T.; Hu, B. A Review on Organic–Inorganic Halide Perovskite Photodetectors: Device Engineering and Fundamental Physics. Adv. Mater 2017, 29, 1605242. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; Huang, L.; Sun, W. Research progress of high-sensitivity perovskite photodetectors: A review of photodetectors: Noise, structure, and materials. ACS Appl. Electron. Mater. 2022, 4, 1485–1505. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Shen, L. Recent research process on perovskite photodetectors: A review for photodetector—Materials, physics, and applications. Chin. Phys. B 2018, 27, 127806. [Google Scholar] [CrossRef]
- Dong, Y.; Gu, Y.; Zou, Y.; Song, J.; Xu, L.; Li, J.; Xue, J.; Li, X.; Zeng, H. Improving all-inorganic perovskite photodetectors by preferred orientation and plasmonic effect. Small 2016, 12, 5622–5632. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, P.; Zang, Z. High performance CsPbBr3 quantum dots photodetectors by using zinc oxide nanorods arrays as an electron-transport layer. Appl. Phys. Lett. 2020, 116, 162103. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, J.; Hwang, E.; Ra, C.-H.; Yoo, W.J.; Ahn, J.-H.; Park, J.H.; Cho, J.H. High-performance perovskite–graphene hybrid photodetector. Adv. Mater. 2015, 27, 41–46. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X.; Wu, G.; Li, H.; Chen, H. Visible-light ultrasensitive solution-prepared layered organic–inorganic hybrid perovskite field-effect transistor. Adv. Opt. Mater. 2017, 5, 1600539. [Google Scholar] [CrossRef]
- Sun, S.; Lu, M.; Gao, X.; Shi, Z.; Bai, X.; Yu, W.W.; Zhang, Y. 0D perovskites: Unique properties, synthesis, and their applications. Adv. Sci. 2021, 8, 2102689. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Wang, F.; Li, T.; Xu, M.; Wang, H.; Wang, Z.; Zhan, X.; Hu, W.; Shen, L. Ultrafast and broadband photodetectors based on a perovskite/organic bulk heterojunction for large-dynamic-range imaging. Light Sci. Appl. 2020, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Meng, C.; Li, X.; Wu, Y.; Liu, S.; Zhou, H.; Wang, H.; Zeng, H. Interfacial-tunneling-effect-enhanced CsPbBr3 photodetectors featuring high detectivity and stability. Adv. Funct. Mater. 2019, 29, 1904461. [Google Scholar] [CrossRef]
- Tang, Y.; Liang, M.; Chang, B.; Sun, H.; Zheng, K.; Pullerits, T.; Chi, Q. Lead-free double halide perovskite Cs3BiBr6 with well-defined crystal structure and high thermal stability for optoelectronics. J. Mater. Chem. C 2019, 7, 3369–3374. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Li, C.; Lu, Y.; Tong, X.-W.; Liang, F.-X.; Zhao, X.-Y.; Wu, D.; Xie, C.; Luo, L.-B. Sensitive deep ultraviolet photodetector and image sensor composed of inorganic lead-free CsCu2I5 perovskite with wide bandgap. J. Phys. Chem. Lett. 2019, 10, 5343–5350. [Google Scholar] [CrossRef]
- Pious, J.K.; Katre, A.; Muthu, C.; Chakraborty, S.; Krishna, S.; Vijayakumar, C. Zero-dimensional lead-free hybrid perovskite-like material with a quantum-well structure. Chem. Mater. 2019, 31, 1941–1945. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, K.; Zou, B. Bismuth halide perovskite-like materials: Current opportunities and challenges. ChemSusChem 2019, 12, 1612–1630. [Google Scholar] [CrossRef]
- Bi, C.; Zheng, X.; Chen, B.; Wei, H.; Huang, J. Spontaneous passivation of hybrid perovskite by sodium ions from glass substrates: Mysterious enhancement of device efficiency revealed. ACS Energy Lett. 2017, 2, 1400–1406. [Google Scholar] [CrossRef]
- Nazarenko, O.; Yakunin, S.; Morad, V.; Cherniukh, I.; Kovalenko, M.V. Single crystals of caesium formamidinium lead halide perovskites: Solution growth and gamma dosimetry. NPG Asia Mater. 2017, 9, e373. [Google Scholar] [CrossRef]
- Zhang, M.; Yun, J.S.; Ma, Q.; Zheng, J.; Lau, C.F.J.; Deng, X.; Kim, J.; Kim, D.; Seidel, J.; Green, M.A.; et al. High-efficiency rubidium-incorporated perovskite solar cells by gas quenching. ACS Energy Lett. 2017, 2, 438–444. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, M.; Li, J.; Dou, J.; Qiu, H.; Shao, J. Spray-coated CsPbBr3 quantum dot films for perovskite photodiodes. ACS Appl. Mater. Inter. 2018, 10, 26387–26395. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, J.; Wang, F.; Yin, Y.; Jin, Y.; Wang, J.; Peng, X.; Han, R.; Zhang, C.; Kong, J.; et al. Direct fabrication of CsPbxMn1−x(Br,Cl)3 thin film by a facile solution spraying approach. Nanomaterials 2021, 11, 3242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, C.; Chen, J.; Wang, F.; Kong, J.; Li, L.; Dong, H.; Sun, Y.; Peng, X.; Xu, J. Synthesis of perovskite nanocrystal films with a high luminous efficiency and an enhanced stability. Ceram. Int. 2021, 47, 33788–33797. [Google Scholar] [CrossRef]
- Lu, H.; Tian, W.; Cao, F.; Ma, Y.; Gu, B.; Li, L. A self-powered and stable all-perovskite photodetector–solar cell nanosystem. Adv. Funct. Mater. 2016, 26, 1296–1302. [Google Scholar] [CrossRef]
- Wang, M.; Tian, W.; Cao, F.; Wang, M.; Li, L. Flexible and self-powered lateral photodetector based on inorganic perovskite CsPbI3–CsPbBr3 heterojunction nanowire array. Adv. Funct. Mater. 2020, 30, 1909771. [Google Scholar] [CrossRef]
- Liu, T.; Tang, W.; Luong, S.; Fenwick, O. High charge carrier mobility in solution processed one-dimensional lead halide perovskite single crystals and their application as photodetectors. Nanoscale 2020, 12, 9688–9695. [Google Scholar] [CrossRef] [PubMed]
- Senanayak, S.P.; Yang, B.; Thomas, T.H.; Giesbrecht, N.; Huang, W.; Gann, E.; Nair, B.; Goedel, K.; Guha, S.; Moya, X.; et al. Understanding charge transport in lead iodide perovskite thin-film field-effect transistors. Sci. Adv. 2017, 3, 1601935. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Solar cells. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Z.; Wang, L.; Chen, Y.; Liang, W.; Wu, D.; Li, X.; Zhang, Y.; Shan, C.; Fang, X. Solution-processed one-dimensional CsCu2I3 nanowires for polarization-sensitive and flexible ultraviolet photodetectors. Mater. Horiz. 2020, 7, 1613–1622. [Google Scholar] [CrossRef]
- Xia, F.; Wang, H.; Xiao, D.; Dubey, M.; Ramasubramaniam, A. Two-dimensional material nanophotonics. Nat. Photonics 2014, 8, 899–907. [Google Scholar] [CrossRef]
- Britnell, L.; Ribeiro, R.M.; Eckmann, A.; Jalil, R.; Belle, B.D.; Mishchenko, A.; Kim, Y.J.; Gorbachev, R.V.; Georgiou, T.; Morozov, S.V.; et al. Strong light-matter interactions in heterostructures of atomically thin films. Science 2013, 340, 1311–1314. [Google Scholar] [CrossRef]
- Roy, K.; Padmanabhan, M.; Goswami, S.; Sai, T.P.; Ramalingam, G.; Raghavan, S.; Ghosh, A. Graphene-MoS2 hybrid structures for multifunctional photoresponsive memory devices. Nat. Nanotechnol. 2013, 8, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Weiss, N.O.; Duan, X.; Cheng, H.-C.; Huang, Y.; Duan, X. Van der Waals heterostructures and devices. Nat. Rev. Mater. 2016, 1, 16042. [Google Scholar] [CrossRef]
- Chen, X.; Lu, X.; Deng, B.; Sinai, O.; Shao, Y.; Li, C.; Yuan, S.; Tran, V.; Watanabe, K.; Taniguchi, T.; et al. Widely tunable black phosphorus mid-infrared photodetector. Nat. Commun. 2017, 8, 1672. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.H.; Stoumpos, C.C.; Farha, O.K.; Hupp, J.T.; Kanatzidis, M.G. 2D Homologous perovskites as light-absorbing materials for solar cell applications. J. Am. Chem. Soc. 2015, 137, 7843–7850. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Wang, P.; Fang, H.; Hu, W. Progress, challenges, and opportunities for 2D material based photodetectors. Adv. Funct. Mater. 2019, 29, 1803807. [Google Scholar] [CrossRef]
- Mao, L.; Stoumpos, C.C.; Kanatzidis, M.G. Two-dimensional hybrid halide perovskites: Principles and promises. J. Am. Chem. Soc. 2019, 141, 1171–1190. [Google Scholar] [CrossRef]
- Smith, I.C.; Hoke, E.T.; Solis-Ibarra, D.; McGehee, M.D.; Karunadasa, H.I. A layered hybrid perovskite solar-cell absorber with enhanced moisture stability. Angew Chem. Int. Ed. 2014, 53, 11232–11235. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Q.; Chmiel, F.P.; Sakai, N.; Herz, L.M.; Snaith, H.J. Efficient ambient-air-stable solar cells with 2D–3D heterostructured butylammonium-caesium-formamidinium lead halide perovskites. Nat. Energy 2017, 2, 17135. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Li, Z.; Liu, H.; Liu, X.; Chen, J.; Fang, X. High-performance two-dimensional perovskite Ca2Nb3O10 UV photodetectors. Nano Lett. 2021, 21, 382–388. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Lan, S.; Fang, C.; Shen, H.; Xiong, Q.; Li, D. Controllable growth of centimeter-sized 2D perovskite heterostructures for highly narrow dual-band photodetectors. ACS Nano 2019, 13, 5473–5484. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Ma, J.; Shen, H.; Li, L.; Duan, X.; Li, D. Self-trapped state enabled filterless narrowband photodetections in 2D layered perovskite single crystals. Nat. Commun. 2019, 10, 806. [Google Scholar] [CrossRef] [PubMed]
- Thirumal, K.; Chong, W.K.; Xie, W.; Ganguly, R.; Muduli, S.; Sherburne, M.P.; Asta, M.; Mhaisalkar, S.G.; Sum, T.C.; Soo, H.S.; et al. Morphology-independent stable white-light emission from self-assembled two-dimensional perovskites driven by strong exciton–phonon coupling to the organic framework. Chem. Mater. 2017, 29, 3947–3953. [Google Scholar] [CrossRef]
- Wu, X.; Trinh, M.T.; Niesner, D.; Zhu, H.; Norman, Z.; Owen, J.S.; Yaffe, O.; Kudisch, B.J.; Zhu, X.Y. Trap states in lead iodide perovskites. J. Am. Chem. Soc. 2015, 137, 2089–2096. [Google Scholar] [CrossRef]
- Xu, S.; Qin, Y.; Xu, C.; Wei, Y.; Yang, R.; Wang, Z.L. Self-powered nanowire devices. Nat. Nanotechnol. 2010, 5, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Wu, W. Nanotechnology-enabled energy harvesting for self-powered micro-/nanosystems. Angew. Chem. Int. Edit. 2012, 51, 11700–11721. [Google Scholar] [CrossRef] [PubMed]
- Shahiduzzaman, M.; Hossain, M.I.; Visal, S.; Kaneko, T.; Qarony, W.; Umezu, S.; Tomita, K.; Iwamori, S.; Knipp, D.; Tsang, Y.H.; et al. Spray pyrolyzed TiO2 embedded multi-layer front contact design for high-efficiency perovskite solar cells. Nano-Micro Lett. 2021, 13, 36. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Z.L.; Fukuda, K.; Someya, T. Flexible self-charging power sources. Nat. Rev. Mater. 2022, 7, 870–886. [Google Scholar] [CrossRef]
- Su, L.; Zhao, Z.X.; Li, H.Y.; Yuan, J.; Wang, Z.L.; Cao, G.Z.; Zhu, G. High-performance organolead halide perovskite-based self-powered triboelectric photodetector. ACS Nano 2015, 9, 11310–11316. [Google Scholar] [CrossRef]
- Zou, H.; Guo, L.; Xue, H.; Zhang, Y.; Shen, X.; Liu, X.; Wang, P.; He, X.; Dai, G.; Jiang, P.; et al. Quantifying and understanding the triboelectric series of inorganic non-metallic materials. Nat. Commun. 2020, 11, 2093. [Google Scholar] [CrossRef]
- Wu, W.; Wang, L.; Li, Y.; Zhang, F.; Lin, L.; Niu, S.; Chenet, D.; Zhang, X.; Hao, Y.; Heinz, T.F.; et al. Piezoelectricity of single-atomic-layer MoS2 for energy conversion and piezotronics. Nature 2014, 514, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Liu, J.; Cui, N.; Xu, Q.; Du, T.; Zhang, L.; Wang, Z.; Long, C.; Qin, Y. Enhancing the current density of a piezoelectric nanogenerator using a three-dimensional intercalation electrode. Nat. Commun. 2020, 11, 1030. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; You, J.-M.; Kim, N.-H.; Gu, Y.; Kumar, R.; Mohan, A.M.V.; Kurniawan, J.; Imani, S.; Nakagawa, T.; Parish, B.; et al. Soft, stretchable, high power density electronic skin-based biofuel cells for scavenging energy from human sweat. Energy Environ. Sci. 2017, 10, 1581–1589. [Google Scholar] [CrossRef]
- Yu, Y.; Nassar, J.; Xu, C.; Min, J.; Yang, Y.; Dai, A.; Doshi, R.; Huang, A.; Song, Y.; Gehlhar, R.; et al. Biofuel-powered soft electronic skin with multiplexed and wireless sensing for human-machine interfaces. Sci. Robot. 2020, 5, 7946. [Google Scholar] [CrossRef] [PubMed]
- Hinterleitner, B.; Knapp, I.; Poneder, M.; Shi, Y.; Müller, H.; Eguchi, G.; Eisenmenger-Sittner, C.; Stöger-Pollach, M.; Kkefuda, Y.; Kawamoto, N.; et al. Thermoelectric performance of a metastable thin-film heusler alloy. Nature 2019, 576, 85–90. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, Y.; Liu, L.; Jiang, Y.; Feng, J.; Zhu, W.; Zhang, J.; Dong, Z.; Deng, Y.; Luo, J.; et al. Semiconductor glass with superior flexibility and high room temperature thermoelectric performance. Sci. Adv. 2020, 6, 8423. [Google Scholar] [CrossRef]
- Shi, X.L.; Zou, J.; Chen, Z.G. Advanced thermoelectric design: From materials and structures to devices. Chem. Rev. 2020, 120, 7399–7515. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Fu, L.; Yu, T.; Zhou, S.; Zhang, L.; Yin, L. Surface passivation engineering strategy to fully-inorganic cubic CsPbI3 perovskites for high-performance solar cells. Nat. Commun. 2018, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Zhu, X.; Yang, Z.; Ke, W.; Feng, J.; Ren, X.; Zhao, K.; Liu, M.; Kanatzidis, M.G.; et al. Inch-sized high-quality perovskite single crystals by suppressing phase segregation for light-powered integrated circuits. Sci. Adv. 2021, 7, 8844. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Z.; Liang, W.; Wang, L.; Li, S.; Zhang, F.; Ma, Z.; Wang, Y.; Tian, Y.; Wu, D.; et al. Highly stable and spectrum-selective ultraviolet photodetectors based on lead-free copper-based perovskites. Mater.Horiz. 2020, 7, 530–540. [Google Scholar] [CrossRef]
- Liang, W.; Shi, Z.; Li, Y.; Ma, J.; Yin, S.; Chen, X.; Wu, D.; Tian, Y.; Tian, Y.; Zhang, Y.; et al. Strategy of all-inorganic Cs3Cu2I5/Si-core/shell nanowire heterojunction for stable and ultraviolet-enhanced broadband photodetectors with imaging capability. ACS Appl. Mater. Inter. 2020, 12, 37363–37374. [Google Scholar] [CrossRef]
- Gu, L.; Tavakoli, M.M.; Zhang, D.; Zhang, Q.; Waleed, A.; Xiao, Y.; Tsui, K.-H.; Lin, Y.; Liao, L.; Wang, J.; et al. 3D arrays of 1024-pixel image sensors based on lead halide perovskite nanowires. Adv. Mater. 2016, 28, 9713–9721. [Google Scholar] [CrossRef]
- Li, L.; Chen, H.; Fang, Z.; Meng, X.; Zuo, C.; Lv, M.; Tian, Y.; Fang, Y.; Xiao, Z.; Shan, C.; et al. An electrically modulated single-color/dual-color imaging photodetector. Adv. Mater. 2020, 32, 1907257. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Han, X.; Li, J.; Wang, X.; Zhang, Y.; Huo, Z.; Chen, Q.; Sun, X.; Xu, Z.; Tan, Y.; et al. Ultrathin and conformable lead halide perovskite photodetector arrays for potential application in retina-like vision sensing. Adv. Mater. 2021, 33, 2006006. [Google Scholar] [CrossRef]
- Li, J.; Pu, K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem. Soc. Rev. 2019, 48, 38–71. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Xu, W.; Yang, J.; Bai, S.; Teng, P.; Yang, Y.; Wang, J.; Zhao, N.; Zhang, W.; Huang, W.; et al. Bidirectional optical signal transmission between two identical devices using perovskite diodes. Nat. Electron. 2020, 3, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Yang, J.; Bai, S.; Xu, W.; Yan, Z.; Xu, Q.; Liu, J.; Zhang, W.; Gao, F. High Performance and stable all-Inorganic metal halide perovskite-based photodetectors for optical communication applications. Adv. Mater. 2018, 30, 1803422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, C.; Jiang, J.; Wang, B.; Shen, L. Sensitive and stable Tin–Lead hybrid perovskite photodetectors enabled by double-sided surface passivation for infrared upconversion detection. Small 2020, 16, 2001534. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Aviles, E.A.; Trejo-Valdez, M.; Torres, J.A.; Ramos-Torres, C.J.; Martínez-Gutiérrez, H.; Torres-Torres, C. Photo-induced structured waves by nanostructured topological insulator Bi2Te3. Opt. Laser Technol. 2021, 140, 107015. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Y.; Vogelbacher, F.; Zhan, Y.; Zhu, D.; Lan, Y.; Fang, W.; Zhang, Z.; Jiang, L.; Song, Y.; et al. Moiré perovskite photodetector toward high-sensitive digital polarization imaging. Adv. Energy Mater. 2021, 11, 2100742. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Ji, C.; Liu, X.; Li, Q.; Zhang, K.; Peng, Y.; Hong, M.; Luo, J. Rational design of high-quality 2D/3D perovskite heterostructure crystals for record-performance polarization-sensitive photodetection. Natl. Sci. Rev. 2021, 8, nwab044. [Google Scholar] [CrossRef]
- Huo, S.; Duan, P.; Jiao, T.; Peng, Q.; Liu, M. Self-assembled luminescent quantum dots to generate full-color and white circularly polarized light. Angew Chem. Int. Edit 2017, 56, 12174–12178. [Google Scholar] [CrossRef] [PubMed]
- Togan, E.; Chu, Y.; Trifonov, A.S.; Jiang, L.; Maze, J.; Childress, L.; Dutt, M.V.; Sørensen, A.S.; Hemmer, P.R.; Zibrov, A.S.; et al. Quantum entanglement between an optical photon and a solid-state spin qubit. Nature 2010, 466, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; da Costa, R.C.; Fuchter, M.J.; Campbell, A.J. Circularly polarized light detection by a chiral organic semiconductor transistor. Nat. Photonics 2013, 7, 634–638. [Google Scholar] [CrossRef]
- Wang, L.; Xue, Y.; Cui, M.; Huang, Y.; Xu, H.; Qin, C.; Yang, J.; Dai, H.; Yuan, M. A chiral reduced-dimension perovskite for an efficient flexible circularly polarized light photodetector. Angew Chem. Int. Edit 2020, 59, 6442–6450. [Google Scholar] [CrossRef]
- Yang, B.; Li, Y.-J.; Tang, Y.-X.; Mao, X.; Luo, C.; Wang, M.-S.; Deng, W.-Q.; Han, K.-L. Constructing sensitive and fast lead-Free Single-crystalline perovskite photodetectors. J. Phys. Chem. lett. 2018, 9, 3087–3092. [Google Scholar] [CrossRef]
- Tong, X.-W.; Zhang, Z.-X.; Wang, D.; Luo, L.-B.; Xie, C.; Wu, Y.-C. Inorganic CsBi3I10 perovskite/silicon heterojunctions for sensitive, self-driven and air-stable NIR photodetectors. J. Mater. Chem. C 2019, 7, 863–870. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, Y.; Li, W.; Zhao, C.; Mai, W. Reducing current fluctuation of Cs3Bi2Br9 perovskite photodetectors for diffuse reflection imaging with wide dynamic range. Sci. Bull. 2020, 65, 1371–1379. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Zuo, C.; Yang, W.; Fang, X. Supersaturation-controlled growth of monolithically integrated lead-free halide perovskite single-crystalline thin film for high-sensitivity photodetectors. Adv. Mater. 2021, 33, 2103010. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, Y.; Zhi, J.; Huang, R.; Li, W.; Huang, X.; Yan, G.; Ji, Z.; Mai, W. All-inorganic lead-free NiOx/Cs3Bi2Br9 perovskite heterojunction photodetectors for ultraviolet multispectral imaging. Nano Res. 2022, 15, 1094–1101. [Google Scholar] [CrossRef]
- Yan, G.; Ji, Z.; Li, Z.; Jiang, B.; Kuang, M.; Cai, X.; Yuan, Y.; Mai, W. All-inorganic Cs2AgBiBr6/CuSCN-based photodetectors for weak light imaging. Sci. China Mater. 2021, 64, 198–208. [Google Scholar] [CrossRef]
- Cortecchia, D.; Dewi, H.A.; Yin, J.; Bruno, A.; Chen, S.; Baikie, T.; Boix, P.P.; Grätzel, M.; Mhaisalkar, S.; Soci, C.; et al. Lead-free MA2CuClxBr4–x hybrid perovskites. Inorg. Chem. 2016, 55, 1044–1052. [Google Scholar] [CrossRef]
- Li, X.; Zhong, X.; Hu, Y.; Li, B.; Sheng, Y.; Zhang, Y.; Weng, C.; Feng, M.; Han, H.; Wang, J. Organic–inorganic copper(II)-based material: A low-toxic, highly stable light absorber for photovoltaic application. J. Phys. Chem. Lett. 2017, 8, 1804–1809. [Google Scholar] [CrossRef] [PubMed]
- Elseman, A.M.; Shalan, A.E.; Sajid, S.; Rashad, M.M.; Hassan, A.M.; Li, M. Copper-substituted lead perovskite materials constructed with different halides for working (CH3NH3)2CuX4-based perovskite solar cells from experimental and theoretical view. ACS Appl. Mater. Inter. 2018, 10, 11699–11707. [Google Scholar] [CrossRef] [PubMed]
- Peresh, E.Y.; Zubaka, O.V.; Sideĭ, V.I.; Barchii, I.E.; Kun, S.V.; Kun, A.V. Preparation, stability regions, and properties of M2TeI6 (M = Rb, Cs, Tl) crystals. Inorg. Mater. 2002, 38, 859–863. [Google Scholar] [CrossRef]
- Maughan, A.E.; Ganose, A.M.; Bordelon, M.M.; Miller, E.M.; Scanlon, D.O.; Neilson, J.R. Defect tolerance to intolerance in the vacancy-ordered double perovskite semiconductors Cs2SnI6 and Cs2TeI6. J. Am. Chem. Soc. 2016, 138, 8453–8464. [Google Scholar] [CrossRef]
- Xu, Y.; Jiao, B.; Song, T.-B.; Stoumpos, C.C.; He, Y.; Hadar, I.; Lin, W.; Jie, W.; Kanatzidis, M.G. Zero-dimensional Cs2TeI6 perovskite: Solution-processed thick films with high X-ray sensitivity. ACS Photonics 2019, 6, 196–203. [Google Scholar] [CrossRef]
- Deretzis, I.; Alberti, A.; Pellegrino, G.; Smecca, E.; Giannazzo, F.; Sakai, N.; Miyasaka, T.; Magna, A.L. Atomistic origins of CH3NH3PbI3 degradation to PbI2 in vacuum. Appl. Phys. Lett. 2015, 106, 131904. [Google Scholar] [CrossRef]
- Guerrero, A.; You, J.; Aranda, C.; Kang, Y.S.; Garcia-Belmonte, G.; Zhou, H.; Bisquert, J.; Yang, Y. Interfacial degradation of planar lead halide perovskite solar cells. ACS Nano 2016, 10, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tan, S.; Lund, P.D.; Zhou, H. Impact of H2O on organic–inorganic hybrid perovskite solar cells. Energ. Environ. Sci. 2017, 10, 2284–2311; 92. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Abdelhady, A.L.; Maculan, G.; Bakr, O.M. Retrograde solubility of formamidinium and methylammonium lead halide perovskites enabling rapid single crystal growth. Chem. Commun. 2015, 51, 17658–17661. [Google Scholar] [CrossRef]
- Niu, G.; Li, W.; Meng, F.; Wang, L.; Dong, H.; Qiu, Y. Study on the stability of CH3NH3PbI3 films and the effect of post-modification by aluminum oxide in all-solid-state hybrid solar cells. J. Mater. Chem. A 2014, 2, 705–710. [Google Scholar] [CrossRef]
- McMeekin, D.P.; Sadoughi, G.; Rehman, W.; Eperon, G.E.; Saliba, M.; Hörantner, M.T.; Haghighirad, A.; Sakai, N.; Korte, L.; Rech, B.; et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science 2016, 351, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, S.; Mosconi, E.; Fang, Y.; Xiao, X.; Wang, C.; Zhou, Y.; Yu, Z.; Zhao, J.; Gao, Y.; et al. Stabilizing halide perovskite surfaces for solar cell operation with wide-bandgap lead oxysalts. Science 2019, 365, 473–478. [Google Scholar] [CrossRef]
- Alsalloum, A.Y.; Turedi, B.; Zheng, X.; Mitra, S.; Zhumekenov, A.A.; Lee, K.J.; Maity, P.; Gereige, I.; Al-Saggaf, A.; Roqan, I.S.; et al. Low-Temperature crystallization enables 21.9% efficient single-crystal MAPbI3 inverted perovskite solar cells. ACS Energy Lett. 2020, 5, 657–662. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Yang, Z.; Feng, J.; Xu, Z.; Li, Q.; Hu, M.; Ye, H.; Zhang, X.; Liu, M.; et al. Low-temperature-gradient crystallization for multi-inch high-quality perovskite single crystals for record performance photodetectors. Mater. Today 2019, 22, 67–75. [Google Scholar] [CrossRef]
- Chen, L.; Tan, Y.-Y.; Chen, Z.-X.; Wang, T.; Hu, S.; Nan, Z.-A.; Xie, L.-Q.; Hui, Y.; Huang, J.-X.; Zhan, C.; et al. Toward long-term stability: Single-crystal alloys of cesium-containing mixed cation and mixed halide perovskite. J. Am. Chem. Soc. 2019, 141, 1665–1671. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Cacovich, S.; Stavrakas, C.; Philippe, B.; Richter, J.M.; Alsari, M.; Booker, E.P.; Hutter, E.M.; Pearson, A.J.; et al. Maximizing and stabilizing luminescence from halide perovskites with potassium passivation. Nature 2018, 555, 497–501. [Google Scholar] [CrossRef]
- Abdi-Jalebi, M.; Pazoki, M.; Philippe, B.; Dar, M.I.; Alsari, M.; Sadhanala, A.; Divitini, G.; Imani, R.; Lilliu, S.; Kullgren, J.; et al. Dedoping of lead halide perovskites incorporating monovalent cations. ACS Nano 2018, 12, 7301–7311. [Google Scholar] [CrossRef]
- Chen, B.; Rudd, P.N.; Yang, S.; Yuan, Y.; Huang, J. Imperfections and their passivation in halide perovskite solar cells. Chem. Soc. Rev. 2019, 48, 3842–3867. [Google Scholar] [CrossRef]
- Zhao, T.; Chueh, C.-C.; Chen, Q.; Rajagopal, A.; Jen, A.K.Y. Defect passivation of organic–inorganic hybrid perovskites by diammonium iodide toward high-performance photovoltaic devices. ACS Energy Lett. 2016, 1, 757–763. [Google Scholar] [CrossRef]
- Grancini, G.; Roldán-Carmona, C.; Zimmermann, I.; Mosconi, E.; Lee, X.; Martineau, D.; Narbey, S.; Oswald, F.; De Angelis, F.; Graetzel, M.; et al. One-Year stable perovskite solar cells by 2D/3D interface engineering. Nat. Commun. 2017, 8, 15684. [Google Scholar] [CrossRef]
- Jung, M.; Shin, T.J.; Seo, J.; Kim, G.; Seok, S.I. Structural features and their functions in surfactant-armoured methylammonium lead iodide perovskites for highly efficient and stable solar cells. Energy Environ. Sci. 2018, 11, 2188–2197. [Google Scholar] [CrossRef]
- Wu, D.; Xu, Y.; Zhou, H.; Feng, X.; Zhang, J.; Pan, X.; Gao, Z.; Wang, R.; Ma, G.; Tao, L.; et al. Ultrasensitive, flexible perovskite nanowire photodetectors with long-term stability exceeding 5000 h. InfoMat 2022, 4, e12320. [Google Scholar] [CrossRef]
- Fang, Y.; Huang, J. Resolving weak light of sub-picowatt per square centimeter by hybrid perovskite photodetectors enabled by noise reduction. Adv. Mater. 2015, 27, 2804–2810. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Li, G.; Huang, L.; Yang, J.; Qiu, X.; Sun, W. Rubidium iodide-doped spiro-OMeTAD as a hole-transporting material for efficient perovskite photodetectors. J. Phys. Chem. C 2022, 126, 9528–9540. [Google Scholar] [CrossRef]
- Jing, H.; Peng, R.; Ma, R.-M.; He, J.; Zhou, Y.; Yang, Z.; Li, C.-Y.; Liu, Y.; Guo, X.; Zhu, Y.; et al. Flexible ultrathin single-crystalline perovskite photodetector. Nano Lett. 2020, 20, 7144–7151. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Xu, H.; Li, X.; Guo, J.; Sathasivam, S.; Wang, M.; Ren, A.; Choy, K.L.; Parkin, I.P.; Guo, Z.; et al. Flexible and self-powered photodetector arrays based on all-inorganic CsPbBr3 quantum dots. Adv. Mater. 2020, 32, 2000004. [Google Scholar] [CrossRef]
- Island, J.O.; Blanter, S.I.; Buscema, M.; van der Zant, H.S.; Castellanos-Gomez, A. Gate Controlled Photocurrent generation mechanisms in high-gain In₂Se₃ phototransistors. Nano Lett. 2015, 15, 7853–7858. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Dong, D.; Yang, X.; Qiao, K.; Yang, D.; Deng, H.; Yuan, S.; Khan, J.; Lan, Y.; Song, H.; et al. Synergistic effect of hybrid PbS quantum dots/2D-WSe2 toward high performance and broadband phototransistors. Adv. Funct. Mater. 2017, 27, 1603605. [Google Scholar] [CrossRef]
- Yokota, T.; Zalar, P.; Kaltenbrunner, M.; Jinno, H.; Matsuhisa, N.; Kitanosako, H.; Tachibana, Y.; Yukita, W.; Koizumi, M.; Someya, T. Ultraflexible organic photonic skin. Sci. Adv. 2016, 2, e1501856. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Huang, R.; Shen, C.; Hu, J.; Wang, P.; Xu, Z.; Huang, Z.; Zhu, L. Hybrid halide perovskite-based near-infrared photodetectors and imaging arrays. Adv. Opt. Mater. 2022, 10, 2102656. [Google Scholar] [CrossRef]


| Performance Parameter | Perovskite Photoconductor | Perovskite Photodiode | Perovskite Phototransistor |
|---|---|---|---|
| Responsivity | high | low | high |
| Detectivity | low | high | high |
| EQE | high | low | high |
| Gain | high | low | high |
| Respond speed | slow | fast | slow |
| Dark current | high | low | high |
| Device Structure | Dimension | Illumination Wavelength (nm) | Responsivity (A W−1) | Detectiviy (Jones) | Rise/Fall Time (ms) | Illumination Intensity (mW cm−2) | Bias (V) | Year (Reference) |
|---|---|---|---|---|---|---|---|---|
| ITO/Cs3BiBr6/ITO | 0D | 400 nm | 0.025 | 0.8 × 109 | 50/60 | 4 | 6 | 2019 [23] |
| ITO/Cs3Cu2I5/ITO | 0D | 265 nm | 0.0649 | 6.9 × 1011 | 26.2/49.9 | / | 1 | 2019 [24] |
| Si/Au/ (DME)PbBr4/Au | 1D | 375 nm | 132.3 | / | 105/117 | 0.001 | 20 | 2017 [35] |
| Si/CsCu2I3/Au | 1D | 325 nm | 32.3 | 1.89 × 1012 | 0.00694/0.214 | / | 5 | 2020 [38] |
| quartz substrate/Ca2Nb3O10/Cr/Au | 2D | 280 nm | 14.94 | 8.7 × 1013 | 0.08/5.6 | / | 3 | 2021 [49] |
| ITO/(C4H9NH3)2PbI4/(C4H9NH3)2(CH3NH3)Pb2I7/Cr/Au | 2D | 540 nm | 0.69 | / | 150/170 | 45 | 3 | 2019 [50] |
| ITO/(BA)2(MA) Pb2I7/Au | 2D | 620 nm | / | 1 × 1011 | 125/74 | 0.02 | 5 | 2019 [51] |
| Device structure | Material Structure | EQE (%) | Responsivity (A W−1) | Detectiviy (Jones) | Rise/Fall Time (ms) | Spectrum Range (nm) | Bias (V) | Year (Reference) |
|---|---|---|---|---|---|---|---|---|
| ITO/Cs3BiBr6/ITO | Single crystal | 0.008 | 0.025@400 nm | 0.8 × 109@400 nm | 50/60 | 300–485 | 6 | 2019 [23] |
| ITO/MA3Sb2I9/ITO | Single-crystalline film | / | 40@460 nm | 1012@460 nm | 0.4/0.9 | 400–600 | 5 | 2018 [85] |
| Si/CsBi3I10/Au | Polycrystal-ine film | 75.2 | 0.492@808 nm | 1.38 × 1011@808 nm | 0.073/0.036 | 400–1200 | −1 | 2019 [86] |
| ITO/Cs3Cu2I5/ITO | Polycrystal-line film | 0.3 | 0.0649@265 nm | 6.9 × 1011@265nm | 26.2/49.9 | 200–405 | 1 | 2019 [24] |
| FTO//TiO2/Cs3BiBr9/Au | Polycrystal-line film | / | 0.006@405 nm | 3.39 × 1011@405 nm | 0.57/0.58 | 325–500 | / | 2020 [87] |
| Si/CsCu2I3/Au | Nanowire | / | 32.3@325 nm | 1.89 × 1012@325 nm | 0.00694/0.214 | 230–350 | 5 | 2020 [38] |
| Ag/Si/Cs3Bi2I9/Au | Single-crystalline film | / | / | 3.9 × 1011@450 nm | 0.0015/0.422 | 250–500 | 3 | 2021 [88] |
| FTO/NiOx/Cs3Bi2Br9/Ag | Polycrystal-ine film | / | 0.00433@450 nm | 1.3 × 1011@450 nm | 3.04/4.66 | 300–550 | 0 | 2022 [89] |
| FTO/Au/TiO2/Cs2AgBiBr6/CuSCN/Au | Polycrystal-ine film | / | 0.34 | 1.03 × 1013@405 nm | 28.75/32.95 | / | 0 | 2021 [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Sun, Y.; Chen, J.; Wang, F.; Han, R.; Zhang, C.; Kong, J.; Li, L.; Yang, J. A Review of Perovskite-Based Photodetectors and Their Applications. Nanomaterials 2022, 12, 4390. https://doi.org/10.3390/nano12244390
Wang H, Sun Y, Chen J, Wang F, Han R, Zhang C, Kong J, Li L, Yang J. A Review of Perovskite-Based Photodetectors and Their Applications. Nanomaterials. 2022; 12(24):4390. https://doi.org/10.3390/nano12244390
Chicago/Turabian StyleWang, Haiyan, Yu Sun, Jin Chen, Fengchao Wang, Ruiyi Han, Canyun Zhang, Jinfang Kong, Lan Li, and Jing Yang. 2022. "A Review of Perovskite-Based Photodetectors and Their Applications" Nanomaterials 12, no. 24: 4390. https://doi.org/10.3390/nano12244390
APA StyleWang, H., Sun, Y., Chen, J., Wang, F., Han, R., Zhang, C., Kong, J., Li, L., & Yang, J. (2022). A Review of Perovskite-Based Photodetectors and Their Applications. Nanomaterials, 12(24), 4390. https://doi.org/10.3390/nano12244390




