SCAPS-1D Modeling of Hydrogenated Lead-Free Cs2AgBiBr6 Double Perovskite Solar Cells with a Remarkable Efficiency of 26.3%
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Influence of the LFDP Layer on Solar Cell Efficiency
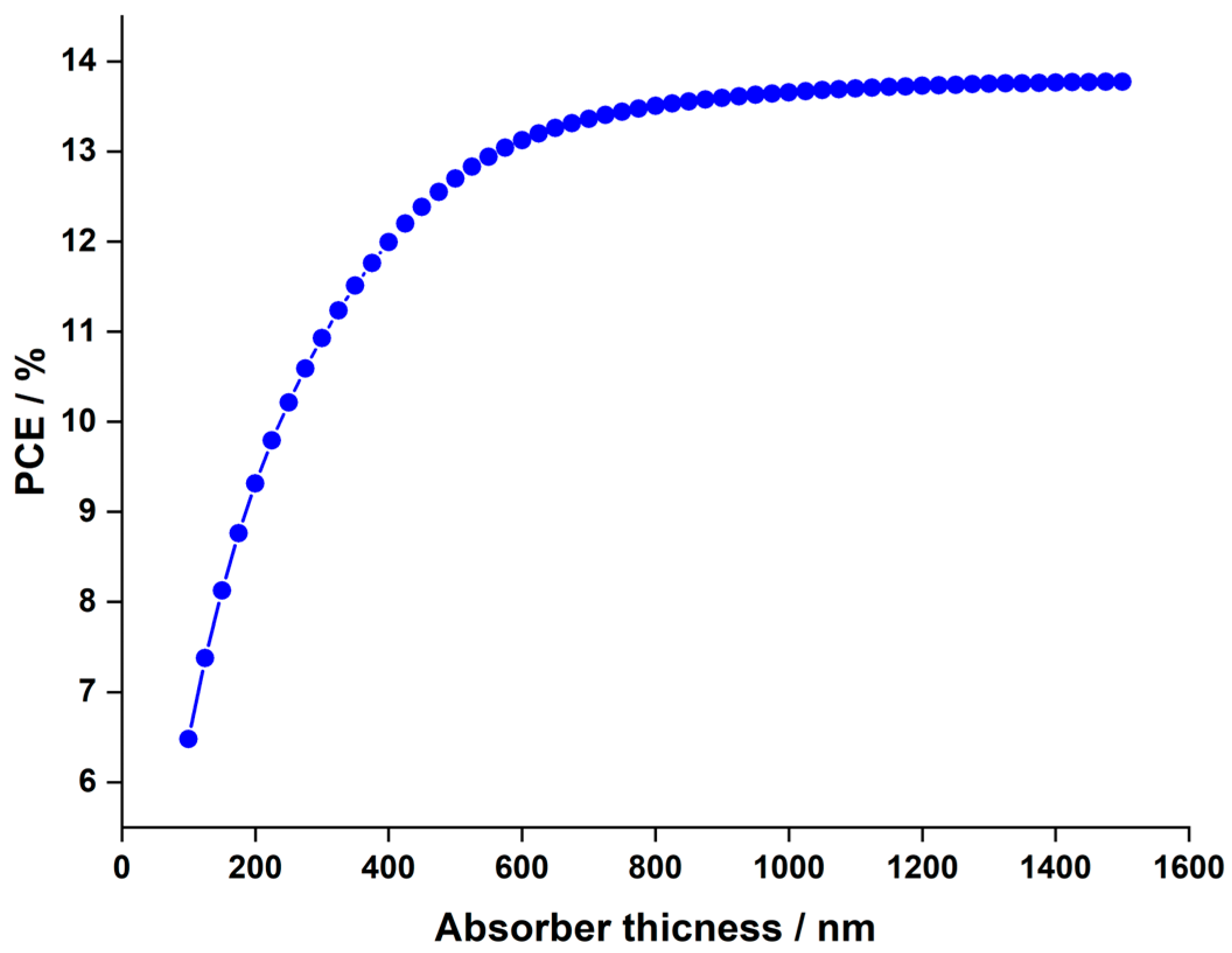
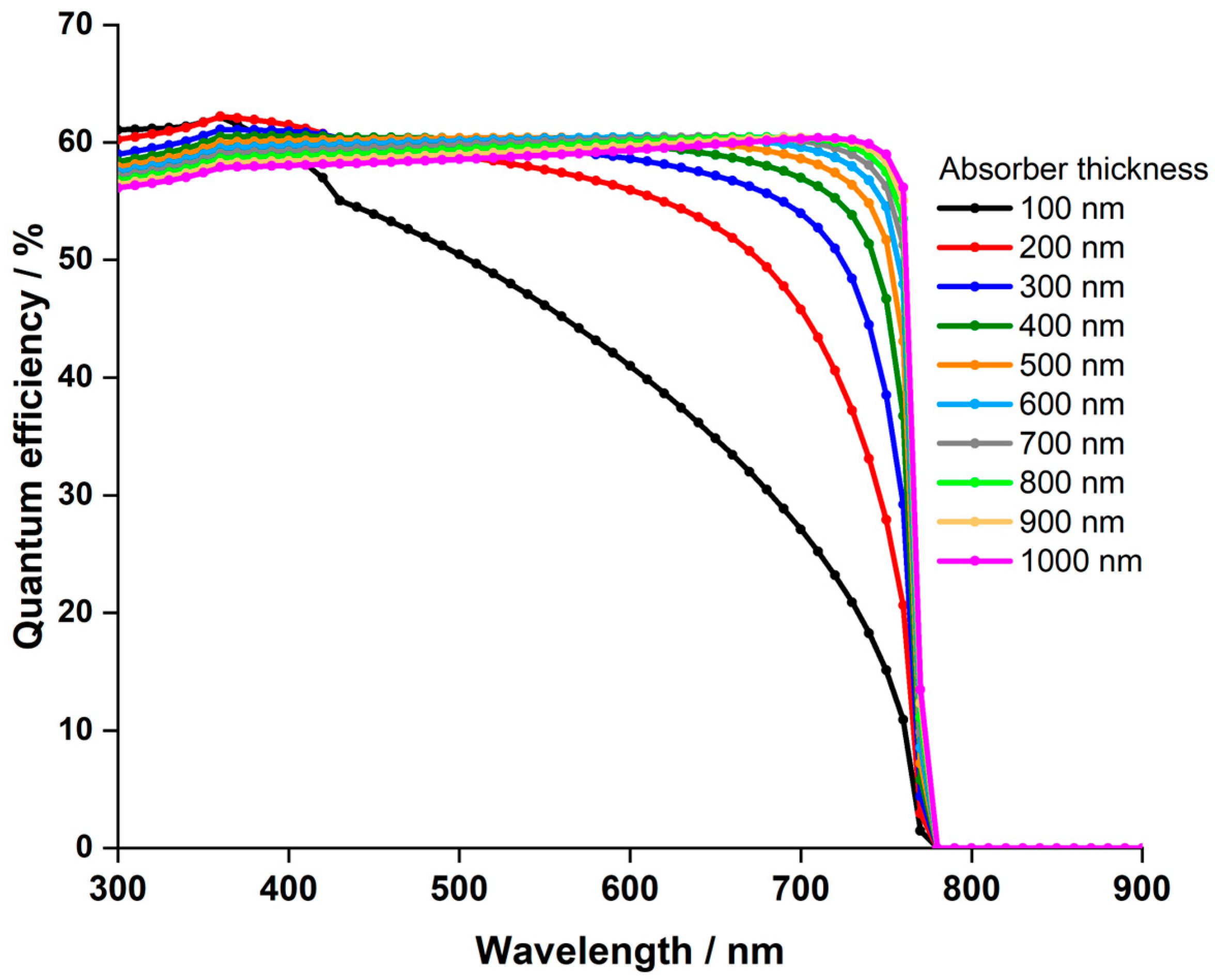
3.1.1. Impact of the LFDP Thickness
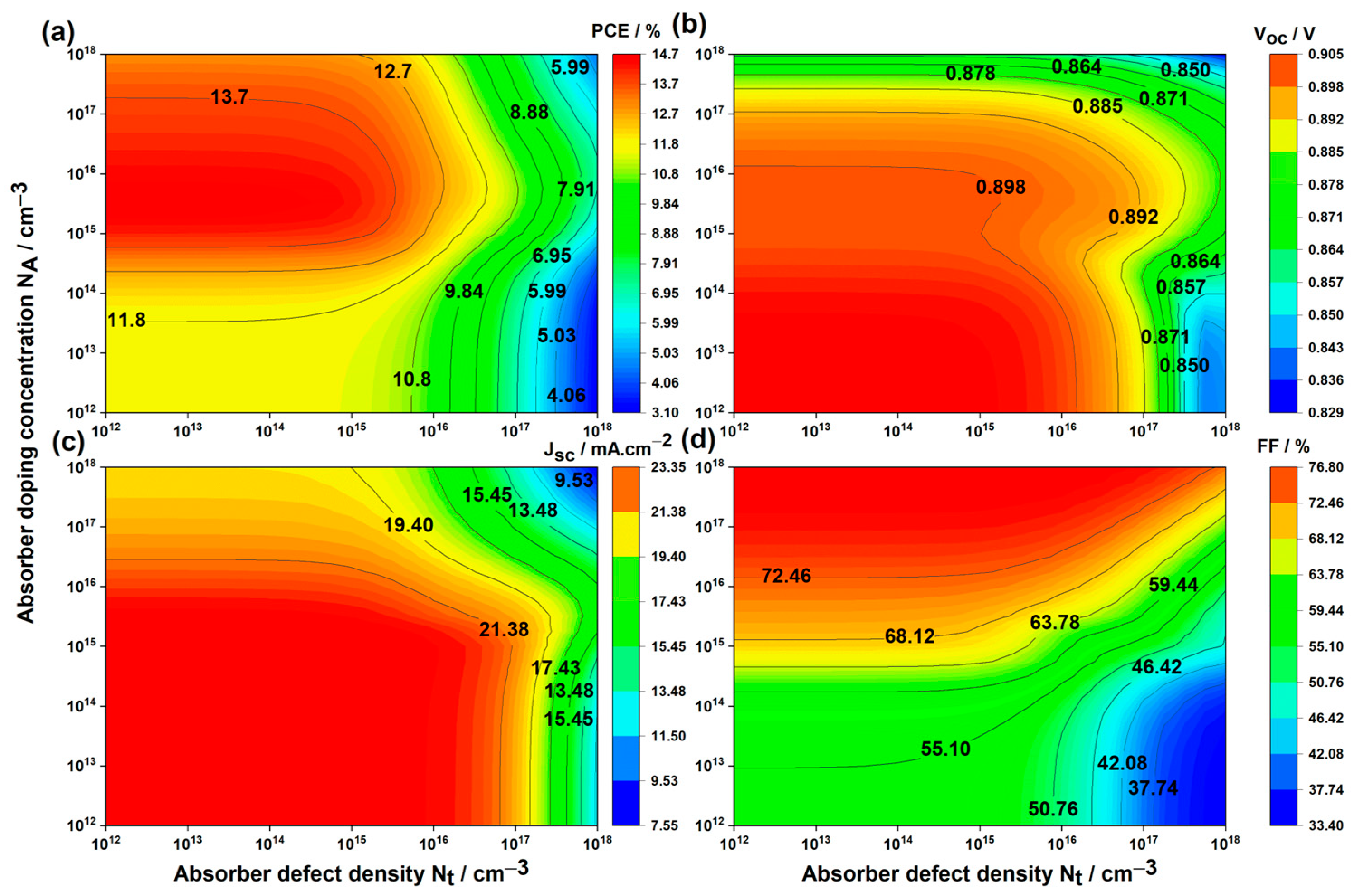
3.1.2. Impact of Doping Concentration and Defect Density in the Absorber Layer
3.2. Influence of the HTL on LFDP Solar Cell Efficiency
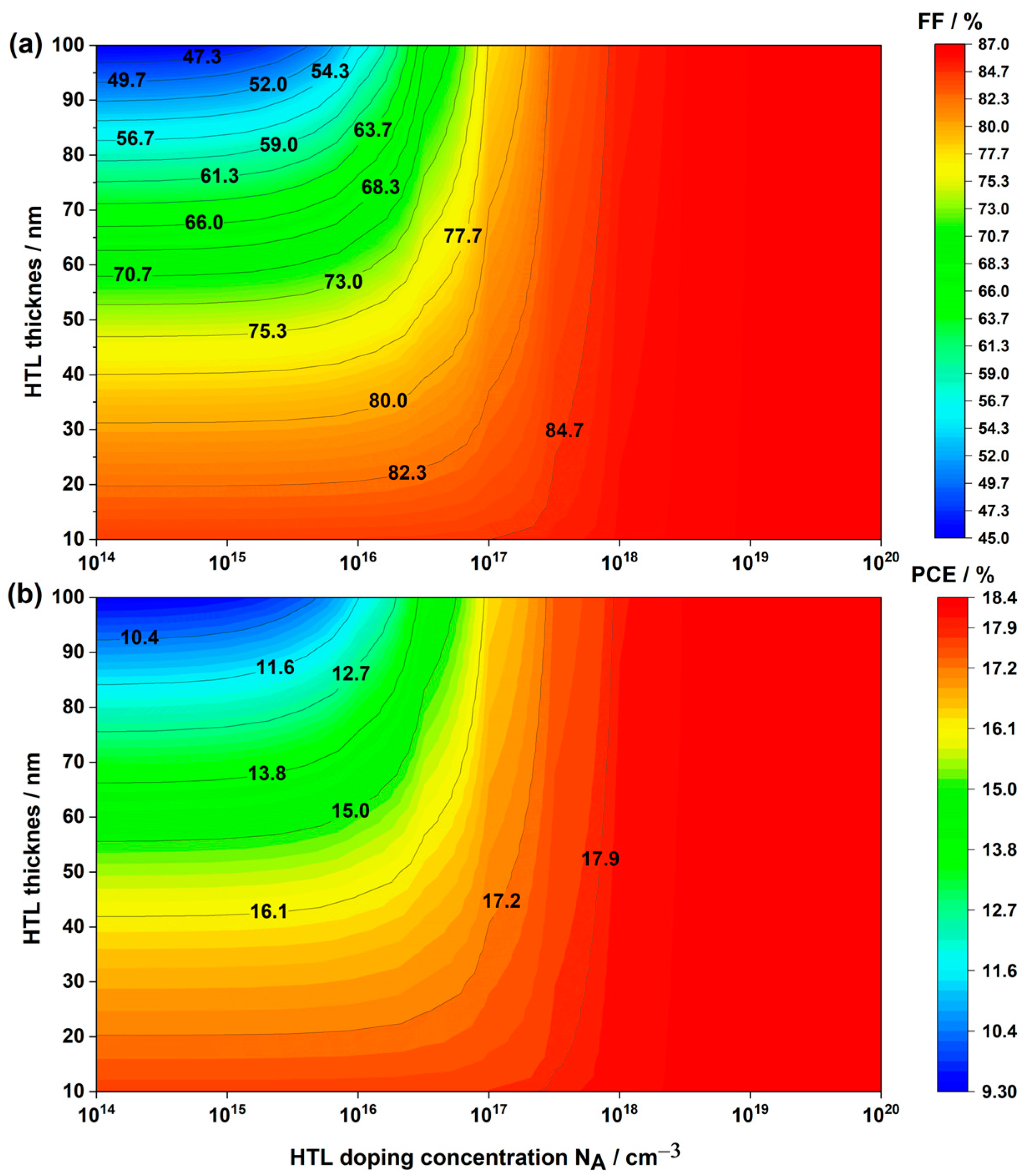
3.2.1. Impact of the HTL Thickness
3.2.2. Impact of the HTL Doping Concentration
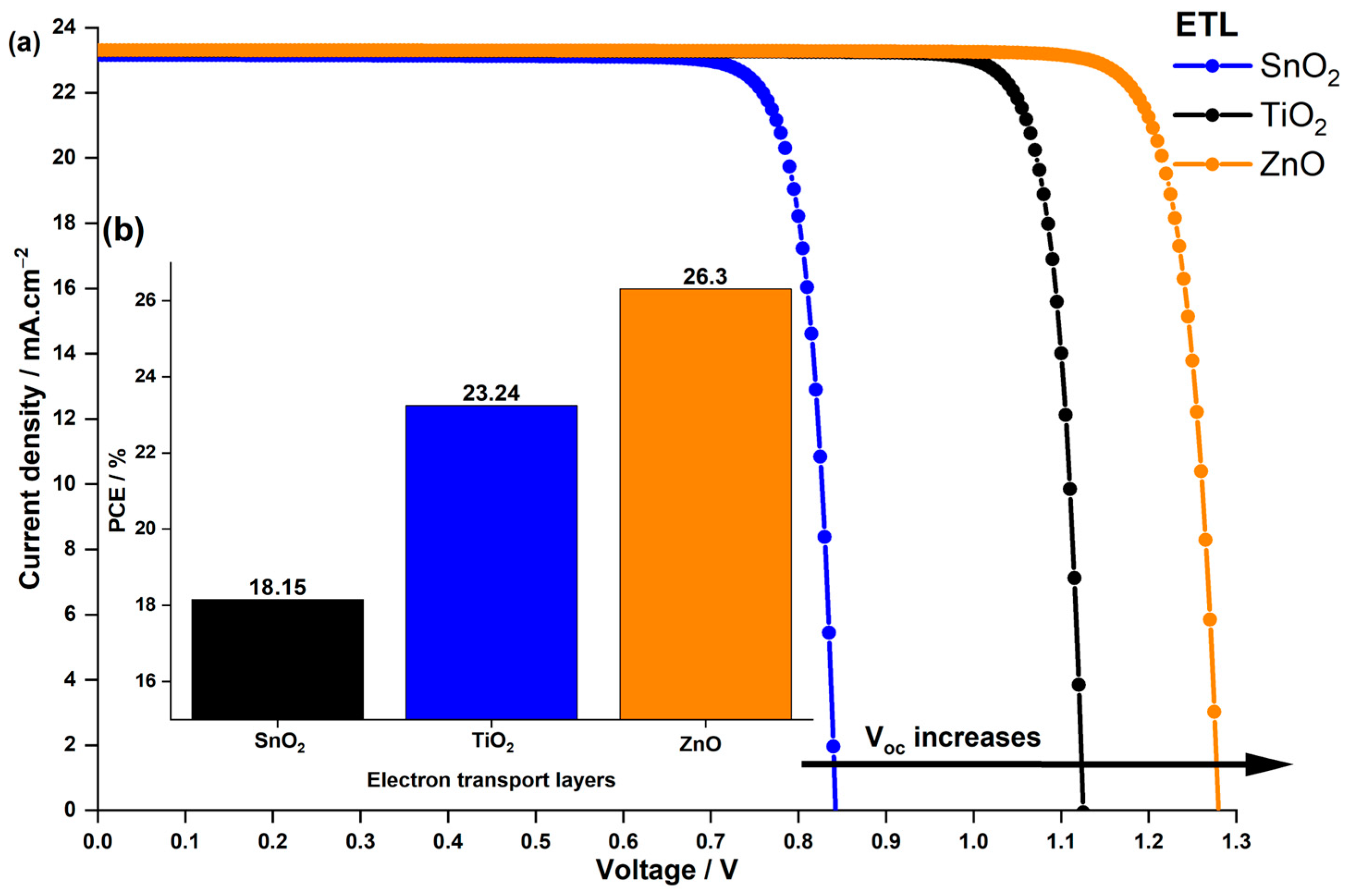
3.3. Influence of the ETL on LFDP Solar Cell Efficiency
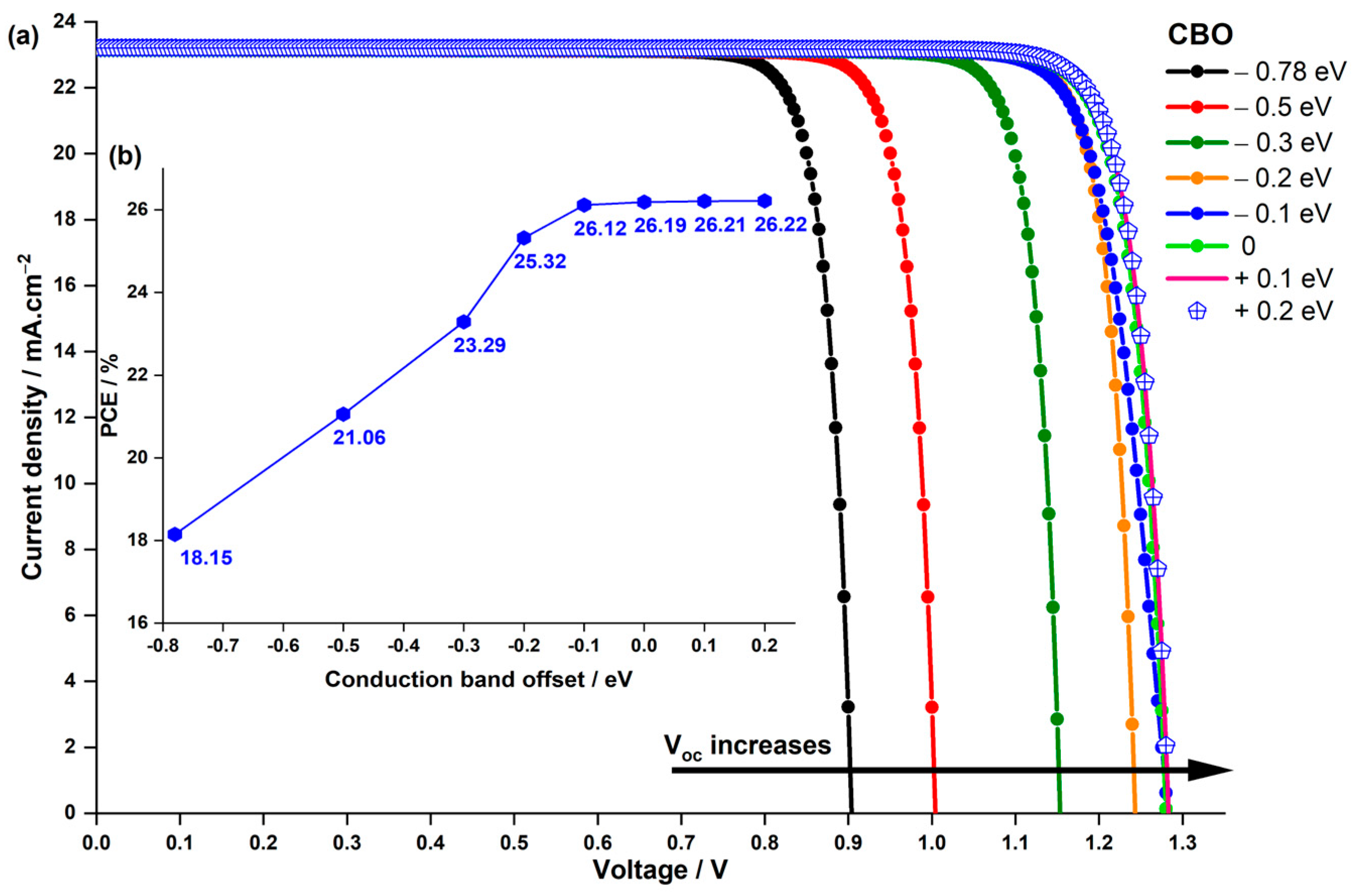
3.3.1. Impact of the CBO
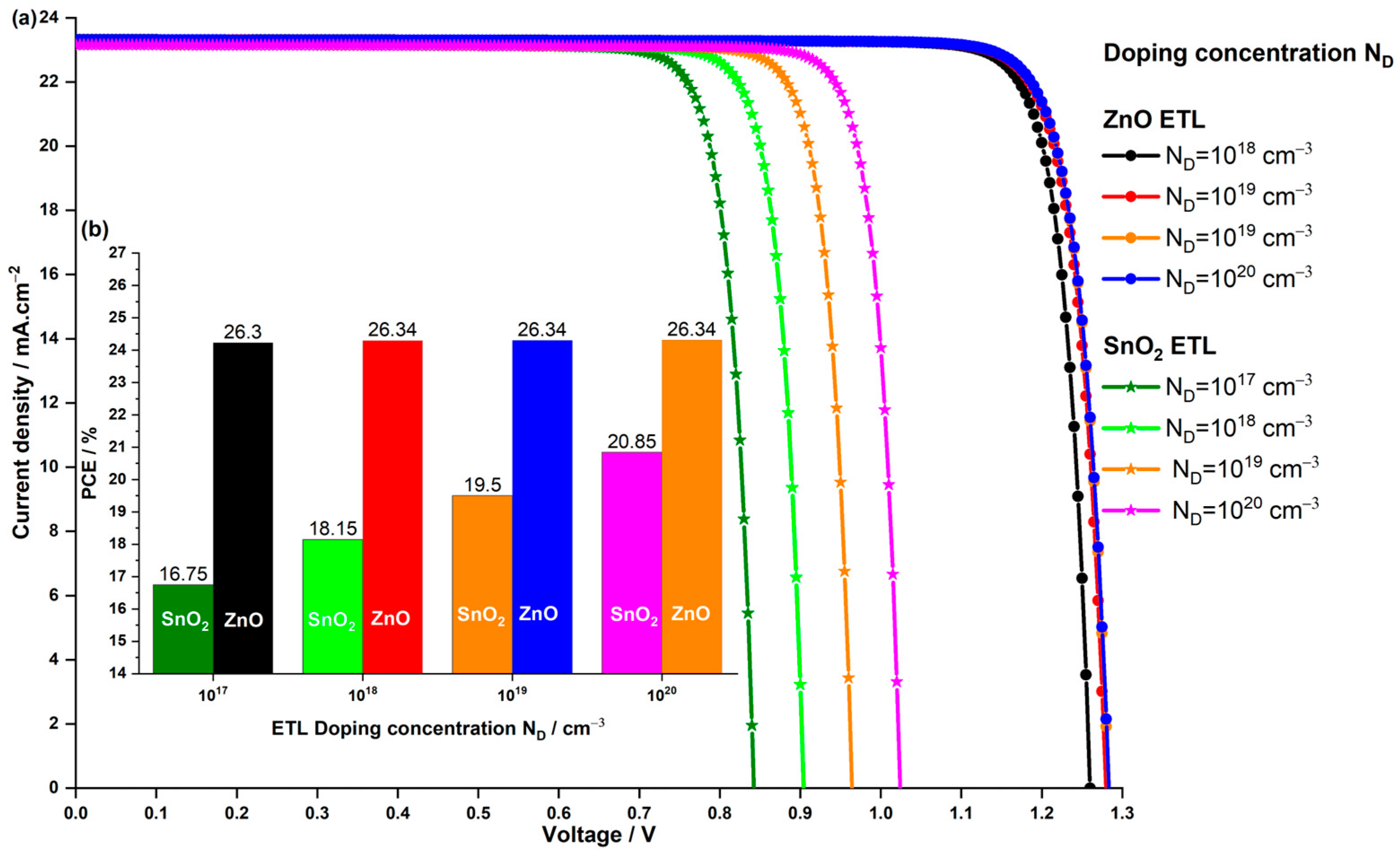
3.3.2. Impact of the ETL Doping Concentration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shih, Y.-C.; Lan, Y.-B.; Li, C.-S.; Hsieh, H.-C.; Wang, L.; Wu, C.-I.; Lin, K.-F. Amino-Acid-Induced Preferential Orientation of Perovskite Crystals for Enhancing Interfacial Charge Transfer and Photovoltaic Performance. Small 2017, 13, 1604305. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-H.; Shih, Y.-C.; Wang, L.; Lin, K.-F. Boosting the Ultra-Stable Unencapsulated Perovskite Solar Cells by Using Montmorillonite/CH3NH3PbI3 Nanocomposite as Photoactive Layer. Energy Environ. Sci. 2019, 12, 1265–1273. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide Management in Formamidinium-Lead-Halide–Based Perovskite Layers for Efficient Solar Cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Zeng, W.; Tang, H.; Wang, L.-X.; Liu, F.-S.; Tang, B.; Liu, Q.-J. Band Structures, Effective Masses and Exciton Binding Energies of Perovskite Polymorphs of CH3NH3PbI3. Solar Energy 2019, 190, 617–621. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, T.; Lou, Y.; Zhao, Y. All-Inorganic Lead-Free Perovskites for Optoelectronic Applications. Mater. Chem. Front. 2019, 3, 365–375. [Google Scholar] [CrossRef]
- Miyata, A.; Mitioglu, A.; Plochocka, P.; Portugall, O.; Wang, J.T.-W.; Stranks, S.D.; Snaith, H.J.; Nicholas, R.J. Direct Measurement of the Exciton Binding Energy and Effective Masses for Charge Carriers in Organic–Inorganic Tri-Halide Perovskites. Nature Phys. 2015, 11, 582–587. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, Y.; Shao, Y.; Yan, Y. Understanding the Physical Properties of Hybrid Perovskites for Photovoltaic Applications. Nat. Rev. Mater. 2017, 2, 17042. [Google Scholar] [CrossRef]
- Jeon, N.J.; Na, H.; Jung, E.H.; Yang, T.-Y.; Lee, Y.G.; Kim, G.; Shin, H.-W.; Il Seok, S.; Lee, J.; Seo, J. A Fluorene-Terminated Hole-Transporting Material for Highly Efficient and Stable Perovskite Solar Cells. Nat. Energy 2018, 3, 682–689. [Google Scholar] [CrossRef]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P.; et al. Efficient Perovskite Solar Cells via Improved Carrier Management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-Halide Anion Engineering for α-FAPbI3 Perovskite Solar Cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef]
- Macdonald, T.J.; Batmunkh, M.; Lin, C.-T.; Kim, J.; Tune, D.D.; Ambroz, F.; Li, X.; Xu, S.; Sol, C.; Papakonstantinou, I.; et al. Origin of Performance Enhancement in TiO2-Carbon Nanotube Composite Perovskite Solar Cells. Small Methods 2019, 3, 1900164. [Google Scholar] [CrossRef]
- Saliba, M.; Correa-Baena, J.-P.; Wolff, C.M.; Stolterfoht, M.; Phung, N.; Albrecht, S.; Neher, D.; Abate, A. How to Make over 20% Efficient Perovskite Solar Cells in Regular (n–i–p) and Inverted (p–i–n) Architectures. Chem. Mater. 2018, 30, 4193–4201. [Google Scholar] [CrossRef]
- Jodlowski, A.D.; Roldán-Carmona, C.; Grancini, G.; Salado, M.; Ralaiarisoa, M.; Ahmad, S.; Koch, N.; Camacho, L.; de Miguel, G.; Nazeeruddin, M.K. Large Guanidinium Cation Mixed with Methylammonium in Lead Iodide Perovskites for 19% Efficient Solar Cells. Nat. Energy 2017, 2, 972–979. [Google Scholar] [CrossRef]
- Ng, C.H.; Lim, H.N.; Hayase, S.; Zainal, Z.; Huang, N.M. Photovoltaic Performances of Mono- and Mixed-Halide Structures for Perovskite Solar Cell: A Review. Renew. Sustain. Energy Rev. 2018, 90, 248–274. [Google Scholar] [CrossRef]
- Kung, P.-K.; Li, M.-H.; Lin, P.-Y.; Jhang, J.-Y.; Pantaler, M.; Lupascu, D.C.; Grancini, G.; Chen, P. Lead-Free Double Perovskites for Perovskite Solar Cells. Solar RRL 2020, 4, 1900306. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-Range Balanced Electron- and Hole-Transport Lengths in Organic-Inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Jeong, J.; Kim, H.-B.; Kim, S.; Walker, B.; Kim, G.-H.; Kim, J.Y. Cesium-Doped Methylammonium Lead Iodide Perovskite Light Absorber for Hybrid Solar Cells. Nano Energy 2014, 7, 80–85. [Google Scholar] [CrossRef]
- Van Le, Q.; Jang, H.W.; Kim, S.Y. Recent Advances toward High-Efficiency Halide Perovskite Light-Emitting Diodes: Review and Perspective. Small Methods 2018, 2, 1700419. [Google Scholar] [CrossRef]
- Dou, L.; Yang, Y.; You, J.; Hong, Z.; Chang, W.-H.; Li, G.; Yang, Y. Solution-Processed Hybrid Perovskite Photodetectors with High Detectivity. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef]
- Xu, J.; Saklatvala, R.; Mittal, S.; Deshmukh, S.; Procopio, A. Recent Progress of Potentiating Immune Checkpoint Blockade with External Stimuli—An Industry Perspective. Adv. Sci. 2020, 7, 1903394. [Google Scholar] [CrossRef]
- Yang, Y.; You, J. Make Perovskite Solar Cells Stable. Nature 2017, 544, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Kopacic, I.; Friesenbichler, B.; Hoefler, S.F.; Kunert, B.; Plank, H.; Rath, T.; Trimmel, G. Enhanced Performance of Germanium Halide Perovskite Solar Cells through Compositional Engineering. ACS Appl. Energy Mater. 2018, 1, 343–347. [Google Scholar] [CrossRef]
- Shao, S.; Liu, J.; Portale, G.; Fang, H.-H.; Blake, G.R.; ten Brink, G.H.; Koster, L.J.A.; Loi, M.A. Highly Reproducible Sn-Based Hybrid Perovskite Solar Cells with 9% Efficiency. Adv. Energy Mater. 2018, 8, 1702019. [Google Scholar] [CrossRef]
- Yang, D.; Lv, J.; Zhao, X.; Xu, Q.; Fu, Y.; Zhan, Y.; Zunger, A.; Zhang, L. Functionality-Directed Screening of Pb-Free Hybrid Organic–Inorganic Perovskites with Desired Intrinsic Photovoltaic Functionalities. Chem. Mater. 2017, 29, 524–538. [Google Scholar] [CrossRef]
- Hao, F.; Stoumpos, C.C.; Cao, D.H.; Chang, R.P.H.; Kanatzidis, M.G. Lead-Free Solid-State Organic–Inorganic Halide Perovskite Solar Cells. Nat. Photon 2014, 8, 489–494. [Google Scholar] [CrossRef]
- Sabbah, H.; Baki, Z.A. Device Simulation of Highly Stable and 29% Efficient FA0.75MA0.25Sn0.95Ge0.05I3-Based Perovskite Solar Cell. Nanomaterials 2023, 13, 1537. [Google Scholar] [CrossRef]
- Nasti, G.; Abate, A. Tin Halide Perovskite (ASnX3) Solar Cells: A Comprehensive Guide toward the Highest Power Conversion Efficiency. Adv. Energy Mater. 2020, 10, 1902467. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting Tin and Lead Iodide Perovskites with Organic Cations: Phase Transitions, High Mobilities, and Near-Infrared Photoluminescent Properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Tong, J.; Gong, J.; Hu, M.; Yadavalli, S.K.; Dai, Z.; Zhang, F.; Xiao, C.; Hao, J.; Yang, M.; Anderson, M.A.; et al. High-Performance Methylammonium-Free Ideal-Band-Gap Perovskite Solar Cells. Matter 2021, 4, 1365–1376. [Google Scholar] [CrossRef]
- Rühle, S. Tabulated Values of the Shockley–Queisser Limit for Single Junction Solar Cells. Solar Energy 2016, 130, 139–147. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Gu, F.; Zhao, Z.; Li, H.; Jiang, H.; Bian, Z.; Liu, Z. Illumination Durability and High-Efficiency Sn-Based Perovskite Solar Cell under Coordinated Control of Phenylhydrazine and Halogen Ions. Matter 2021, 4, 709–721. [Google Scholar] [CrossRef]
- Arayro, J.; Mezher, R.; Sabbah, H. Comparative Simulation Study of the Performance of Conventional and Inverted Hybrid Tin-Based Perovskite Solar Cells. Coatings 2023, 13, 1258. [Google Scholar] [CrossRef]
- Byranvand, M.M.; Zuo, W.; Imani, R.; Pazoki, M.; Saliba, M. Tin-Based Halide Perovskite Materials: Properties and Applications. Chem. Sci. 2022, 13, 6766–6781. [Google Scholar] [CrossRef] [PubMed]
- Giustino, F.; Snaith, H.J. Toward Lead-Free Perovskite Solar Cells. ACS Energy Lett. 2016, 1, 1233–1240. [Google Scholar] [CrossRef]
- Wei, F.; Deng, Z.; Sun, S.; Zhang, F.; Evans, D.M.; Kieslich, G.; Tominaka, S.; Carpenter, M.A.; Zhang, J.; Bristowe, P.D.; et al. Synthesis and Properties of a Lead-Free Hybrid Double Perovskite: (CH3NH3)2AgBiBr6. Chem. Mater. 2017, 29, 1089–1094. [Google Scholar] [CrossRef]
- Creutz, S.E.; Crites, E.N.; De Siena, M.C.; Gamelin, D.R. Colloidal Nanocrystals of Lead-Free Double-Perovskite (Elpasolite) Semiconductors: Synthesis and Anion Exchange To Access New Materials. Nano Lett. 2018, 18, 1118–1123. [Google Scholar] [CrossRef]
- Slavney, A.H.; Hu, T.; Lindenberg, A.M.; Karunadasa, H.I. A Bismuth-Halide Double Perovskite with Long Carrier Recombination Lifetime for Photovoltaic Applications. J. Am. Chem. Soc. 2016, 138, 2138–2141. [Google Scholar] [CrossRef]
- McClure, E.T.; Ball, M.R.; Windl, W.; Woodward, P.M. Cs2AgBiX6 (X = Br, Cl): New Visible Light Absorbing, Lead-Free Halide Perovskite Semiconductors. Chem. Mater. 2016, 28, 1348–1354. [Google Scholar] [CrossRef]
- Savory, C.N.; Walsh, A.; Scanlon, D.O. Can Pb-Free Halide Double Perovskites Support High-Efficiency Solar Cells? ACS Energy Lett. 2016, 1, 949–955. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, P.; Bai, S.; Gu, J.; Li, F.; Yang, Z.; Liu, M. High-Quality Sequential-Vapor-Deposited Cs2AgBiBr6 Thin Films for Lead-Free Perovskite Solar Cells. Solar RRL 2018, 2, 1800217. [Google Scholar] [CrossRef]
- Wang, B.; Li, N.; Yang, L.; Dall’Agnese, C.; Jena, A.K.; Miyasaka, T.; Wang, X.-F. Organic Dye/Cs2AgBiBr6 Double Perovskite Heterojunction Solar Cells. J. Am. Chem. Soc. 2021, 143, 14877–14883. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Ran, C.; Xi, J.; Jiao, B.; Zhang, W.; Wu, M.; Hou, X.; Wu, Z. High-Quality Cs2AgBiBr6 Double Perovskite Film for Lead-Free Inverted Planar Heterojunction Solar Cells with 2.2% Efficiency. ChemPhysChem 2018, 19, 1696–1700. [Google Scholar] [CrossRef] [PubMed]
- Sirtl, M.T.; Hooijer, R.; Armer, M.; Ebadi, F.G.; Mohammadi, M.; Maheu, C.; Weis, A.; van Gorkom, B.T.; Häringer, S.; Janssen, R.A.J.; et al. 2D/3D Hybrid Cs2AgBiBr6 Double Perovskite Solar Cells: Improved Energy Level Alignment for Higher Contact-Selectivity and Large Open Circuit Voltage. Adv. Energy Mater. 2022, 12, 2103215. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Q.; Lu, Y.; Lu, F.; Mu, X.; Wei, S.-H.; Sui, M. Hydrogenated Cs2AgBiBr6 for Significantly Improved Efficiency of Lead-Free Inorganic Double Perovskite Solar Cell. Nat. Commun. 2022, 13, 3397. [Google Scholar] [CrossRef] [PubMed]
- Minemoto, T.; Murata, M. Theoretical Analysis on Effect of Band Offsets in Perovskite Solar Cells. Sol. Energy Mater. Sol. Cells 2015, 133, 8–14. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, J.; Wei, J.; Li, Y.; Lv, M.; Yang, S.; Zhang, B.; Yao, J.; Dai, S. Numerical Simulation: Toward the Design of High-Efficiency Planar Perovskite Solar Cells. Appl. Phys. Lett. 2014, 104, 253508. [Google Scholar] [CrossRef]
- Sabbah, H. Numerical Simulation of 30% Efficient Lead-Free Perovskite CsSnGeI3-Based Solar Cells. Materials 2022, 15, 3229. [Google Scholar] [CrossRef]
- Liu, Y.; Heinzel, D.; Rockett, A. A New Solar Cell Simulator: WxAMPS. In Proceedings of the 2011 37th IEEE Photovoltaic Specialists Conference, Seattle, WA, USA, 19–24 June 2011; pp. 002753–002756. [Google Scholar]
- Burgelman, M.; Nollet, P.; Degrave, S. Modelling Polycrystalline Semiconductor Solar Cells. Thin Solid. Films 2000, 361–362, 527–532. [Google Scholar] [CrossRef]
- Islam, T.; Jani, R.; Amin, S.M.A.; Shorowordi, K.M.; Nishat, S.S.; Kabir, A.; Taufique, M.F.N.; Chowdhury, S.; Banerjee, S.; Ahmed, S. Simulation Studies to Quantify the Impacts of Point Defects: An Investigation of Cs2AgBiBr6 Perovskite Solar Devices Utilizing ZnO and Cu2O as the Charge Transport Layers. Comput. Mater. Sci. 2020, 184, 109865. [Google Scholar] [CrossRef]
- Mohandes, A.; Moradi, M.; Nadgaran, H. Numerical Simulation of Inorganic Cs2AgBiBr6 as a Lead-Free Perovskite Using Device Simulation SCAPS-1D. Opt. Quant. Electron. 2021, 53, 319. [Google Scholar] [CrossRef]
- Liu, G.; Zhong, Y.; Mao, H.; Yang, J.; Dai, R.; Hu, X.; Xing, Z.; Sheng, W.; Tan, L.; Chen, Y. Highly Efficient and Stable ZnO-Based MA-Free Perovskite Solar Cells via Overcoming Interfacial Mismatch and Deprotonation Reaction. Chem. Eng. J. 2022, 431, 134235. [Google Scholar] [CrossRef]
- Raj, A.; Kumar, M.; Kumar, A.; Laref, A.; Singh, K.; Sharma, S.; Anshul, A. Effect of Doping Engineering in TiO2 Electron Transport Layer on Photovoltaic Performance of Perovskite Solar Cells. Mater. Lett. 2022, 313, 131692. [Google Scholar] [CrossRef]
- Hosseini, S.R.; Bahramgour, M.; Yardani Sefidi, P.; Tabatabaei Mashayekh, A.; Moradi, A.; Delibas, N.; Hosseini, M.G.; Niaei, A. Investigating the Effect of Non-Ideal Conditions on the Performance of a Planar CH3NH3PbI3-Based Perovskite Solar Cell through SCAPS-1D Simulation. Heliyon 2022, 8, e11471. [Google Scholar] [CrossRef] [PubMed]
- Seyed-Talebi, S.M.; Mahmoudi, M.; Lee, C.-H. A Comprehensive Study of CsSnI3-Based Perovskite Solar Cells with Different Hole Transporting Layers and Back Contacts. Micromachines 2023, 14, 1562. [Google Scholar] [CrossRef] [PubMed]
- Baena, J.P.C.; Steier, L.; Tress, W.; Saliba, M.; Neutzner, S.; Matsui, T.; Giordano, F.; Jacobsson, T.J.; Kandada, A.R.S.; Zakeeruddin, S.M.; et al. Highly Efficient Planar Perovskite Solar Cells through Band Alignment Engineering. Energy Environ. Sci. 2015, 8, 2928–2934. [Google Scholar] [CrossRef]
- Vasheghani Farahani, S.K.; Veal, T.D.; Mudd, J.J.; Scanlon, D.O.; Watson, G.W.; Bierwagen, O.; White, M.E.; Speck, J.S.; McConville, C.F. Valence-Band Density of States and Surface Electron Accumulation in Epitaxial SnO2 Films. Phys. Rev. B 2014, 90, 155413. [Google Scholar] [CrossRef]
- Doroody, C.; Rahman, K.S.; Rosly, H.N.; Harif, M.N.; Haque, F.; Tiong, S.K.; Amin, N. Impact of High Resistivity Transparent (HRT) Layer in Cadmium Telluride Solar Cells from Numerical Simulation. J. Renew. Sustain. Energy 2020, 12, 023702. [Google Scholar] [CrossRef]
- Hoye, R.L.Z.; Ehrler, B.; Böhm, M.L.; Muñoz-Rojas, D.; Altamimi, R.M.; Alyamani, A.Y.; Vaynzof, Y.; Sadhanala, A.; Ercolano, G.; Greenham, N.C.; et al. Improved Open-Circuit Voltage in ZnO–PbSe Quantum Dot Solar Cells by Understanding and Reducing Losses Arising from the ZnO Conduction Band Tail. Adv. Energy Mater. 2014, 4, 1301544. [Google Scholar] [CrossRef]
- Du, H.-J.; Wang, W.-C.; Zhu, J.-Z. Device Simulation of Lead-Free CH3NH3SnI3 Perovskite Solar Cells with High Efficiency. Chin. Phys. B 2016, 25, 108802. [Google Scholar] [CrossRef]
- Stamate, M.D. On the Dielectric Properties of Dc Magnetron TiO2 Thin Films. Appl. Surf. Sci. 2003, 218, 318–323. [Google Scholar] [CrossRef]
- Dipta, S.S.; Uddin, A.; Conibeer, G. Enhanced Light Management and Optimization of Perovskite Solar Cells Incorporating Wavelength Dependent Reflectance Modeling. Heliyon 2022, 8, e11380. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.N.; Zafar, Q.; Papadakis, A. A Study of Electromagnetic Light Propagation in a Perovskite-Based Solar Cell via a Computational Modelling Approach. Bull. Mater. Sci. 2019, 42, 169. [Google Scholar] [CrossRef]
- Chander, N.; Khan, A.F.; Chandrasekhar, P.S.; Thouti, E.; Swami, S.K.; Dutta, V.; Komarala, V.K. Reduced Ultraviolet Light Induced Degradation and Enhanced Light Harvesting Using YVO4:Eu3+ down-Shifting Nano-Phosphor Layer in Organometal Halide Perovskite Solar Cells. Appl. Phys. Lett. 2014, 105, 033904. [Google Scholar] [CrossRef]
- Haider, S.Z.; Anwar, H.; Wang, M. A Comprehensive Device Modelling of Perovskite Solar Cell with Inorganic Copper Iodide as Hole Transport Material. Semicond. Sci. Technol. 2018, 33, 035001. [Google Scholar] [CrossRef]
- Karimi, E.; Ghorashi, S.M.B. Investigation of the Influence of Different Hole-Transporting Materials on the Performance of Perovskite Solar Cells. Optik 2017, 130, 650–658. [Google Scholar] [CrossRef]
- Sabbah, H.; Arayro, J.; Mezher, R. Numerical Simulation and Optimization of Highly Stable and Efficient Lead-Free Perovskite FA1−xCsxSnI3-Based Solar Cells Using SCAPS. Materials 2022, 15, 4761. [Google Scholar] [CrossRef]
- Arumugam, G.M.; Karunakaran, S.K.; Liu, C.; Zhang, C.; Guo, F.; Wu, S.; Mai, Y. Inorganic Hole Transport Layers in Inverted Perovskite Solar Cells: A Review. Nano Select 2021, 2, 1081–1116. [Google Scholar] [CrossRef]
- Li, S.; Cao, Y.-L.; Li, W.-H.; Bo, Z.-S. A Brief Review of Hole Transporting Materials Commonly Used in Perovskite Solar Cells. Rare Met. 2021, 40, 2712–2729. [Google Scholar] [CrossRef]





| Parameters | Spiro-OMeTAD (HTL) [44,54,55] | (Absorber) [44,51] | (ETL) [44,56,57] | (ETL) [58,59] | (ETL) [60,61] |
|---|---|---|---|---|---|
| Thickness () | |||||
| Bandgap (eV) | |||||
| Electron affinity (eV) | |||||
| Dielectric permittivity | |||||
| CB effective density of states | |||||
| VB effective density of states | |||||
| Electron mobility | |||||
| Hole mobility | |||||
| Donor concentration | |||||
| Acceptor concentration |
| Parameters | ETL | HTL | Absorber | HTL/Absorber | Absorber/ETL |
|---|---|---|---|---|---|
| Defect Type | Neutral | Neutral | Neutral | Neutral | Neutral |
| Capture cross-section for electrons | |||||
| Capture cross-section for hole | |||||
| Energetic distribution | Single | Single | Gaussian | Single | Single |
| Energy level with respect to (above ) (eV) | 0.6 | 0.650 | 0.6 | 0.6 | 0.6 |
| Characteristic energy (eV) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Total density |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabbah, H.; Abdel Baki, Z.; Mezher, R.; Arayro, J. SCAPS-1D Modeling of Hydrogenated Lead-Free Cs2AgBiBr6 Double Perovskite Solar Cells with a Remarkable Efficiency of 26.3%. Nanomaterials 2024, 14, 48. https://doi.org/10.3390/nano14010048
Sabbah H, Abdel Baki Z, Mezher R, Arayro J. SCAPS-1D Modeling of Hydrogenated Lead-Free Cs2AgBiBr6 Double Perovskite Solar Cells with a Remarkable Efficiency of 26.3%. Nanomaterials. 2024; 14(1):48. https://doi.org/10.3390/nano14010048
Chicago/Turabian StyleSabbah, Hussein, Zaher Abdel Baki, Rabih Mezher, and Jack Arayro. 2024. "SCAPS-1D Modeling of Hydrogenated Lead-Free Cs2AgBiBr6 Double Perovskite Solar Cells with a Remarkable Efficiency of 26.3%" Nanomaterials 14, no. 1: 48. https://doi.org/10.3390/nano14010048
APA StyleSabbah, H., Abdel Baki, Z., Mezher, R., & Arayro, J. (2024). SCAPS-1D Modeling of Hydrogenated Lead-Free Cs2AgBiBr6 Double Perovskite Solar Cells with a Remarkable Efficiency of 26.3%. Nanomaterials, 14(1), 48. https://doi.org/10.3390/nano14010048






