The Influence of Reduced Graphene Oxide on the Texture and Chemistry of N,S-Doped Porous Carbon. Implications for Electrocatalytic and Energy Storage Applications
Abstract
:1. Introduction
2. Materials and Methods
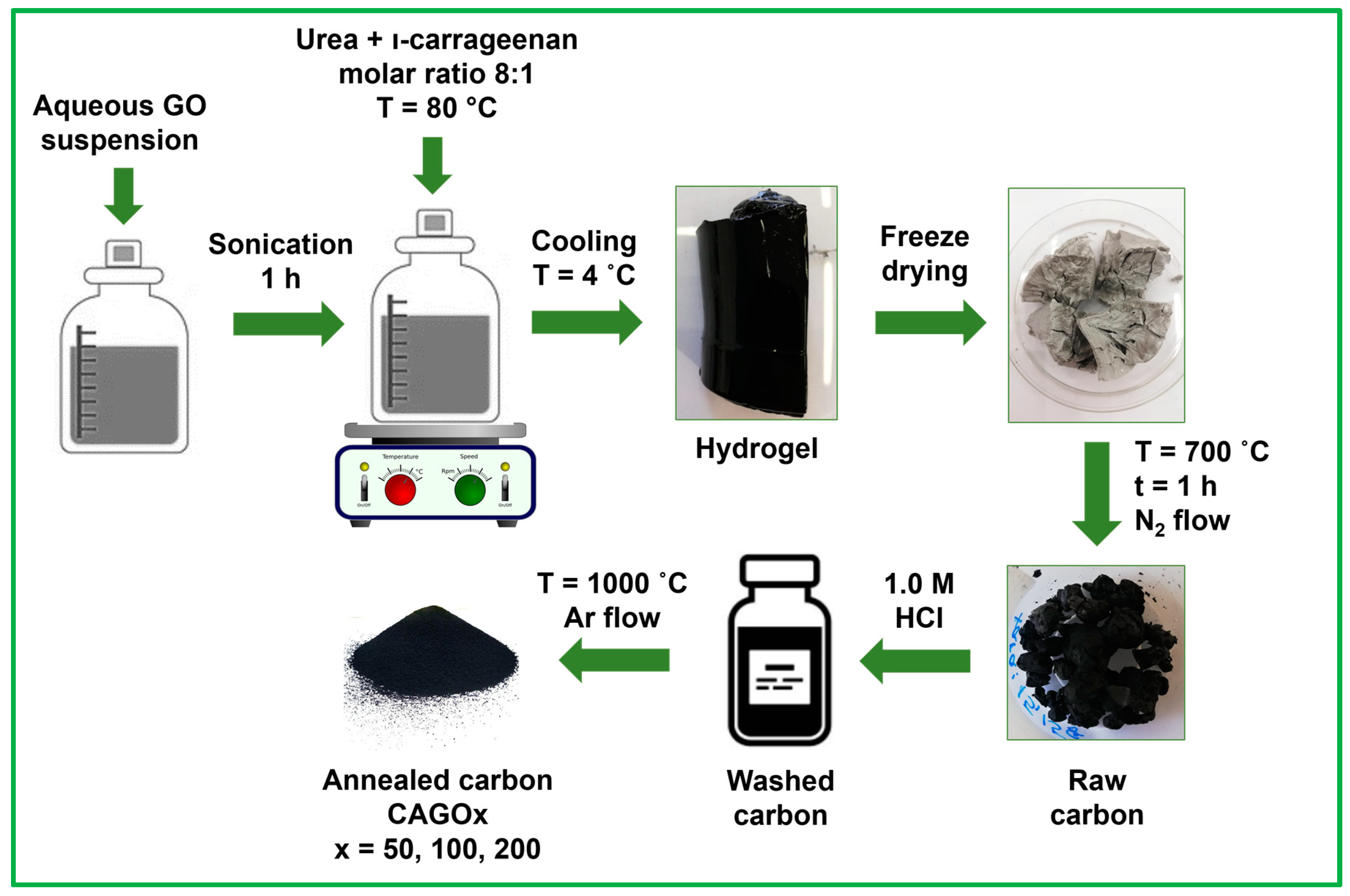
2.1. Synthesis
2.2. Characterization Methods
2.3. Electrochemical Performance Tests
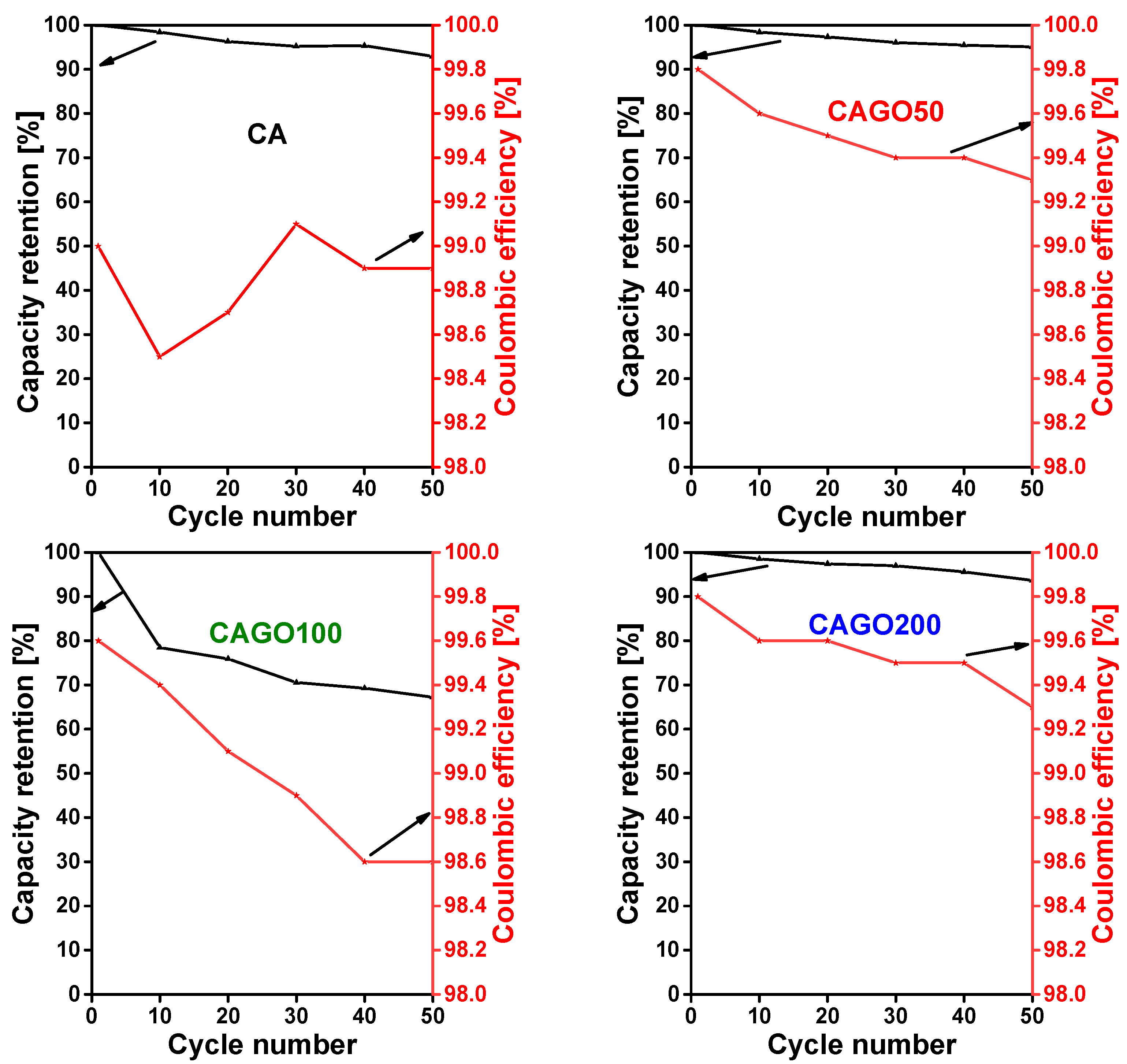
3. Results and Discussion
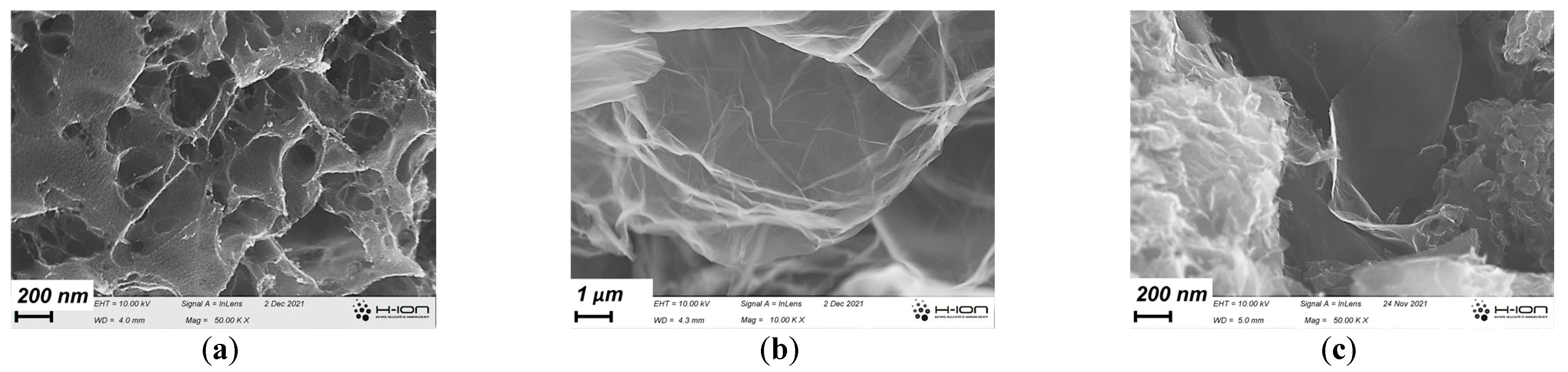
3.1. Development of the Morphology during the Annealing
3.2. Surface Chemistry of the Samples
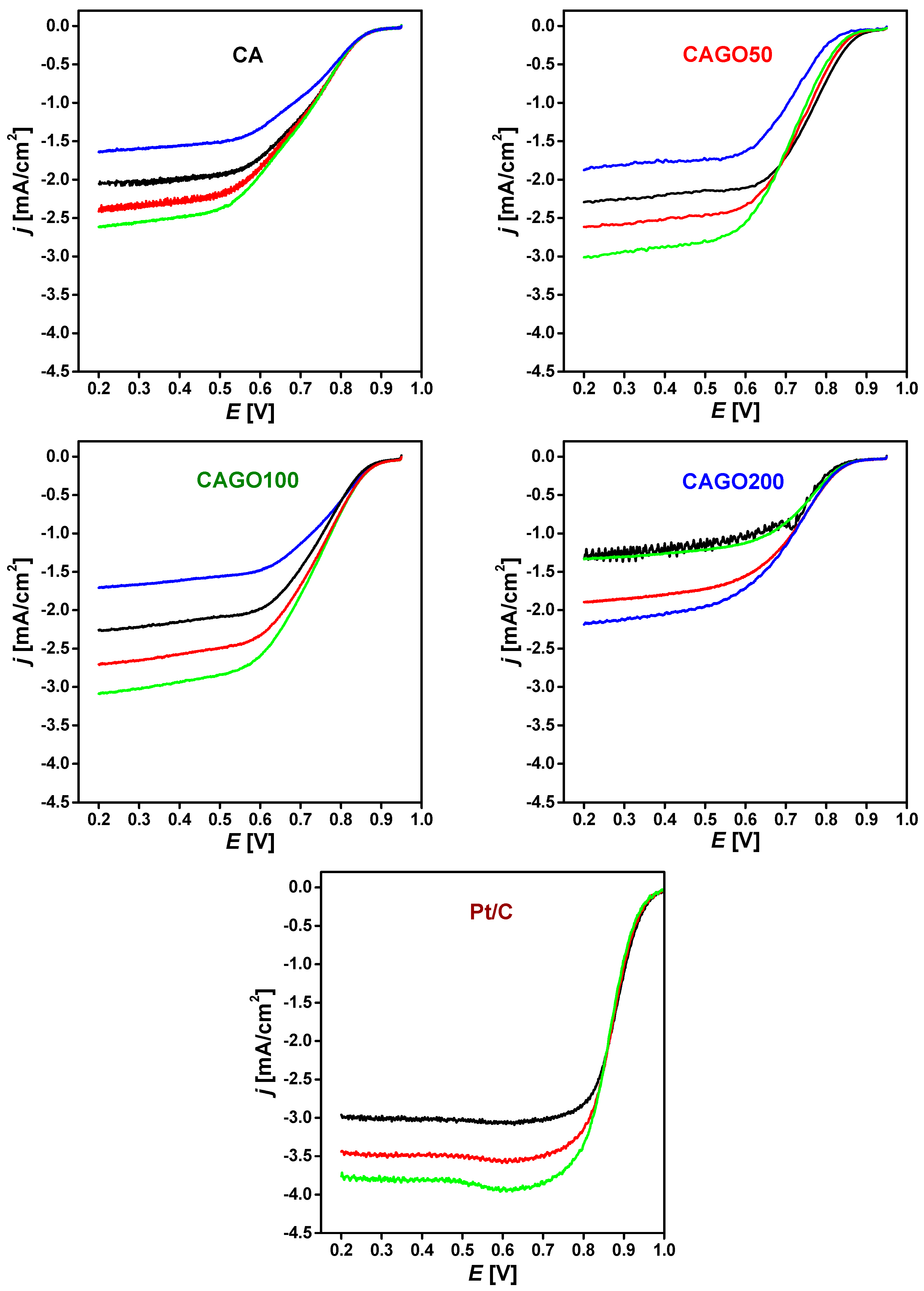
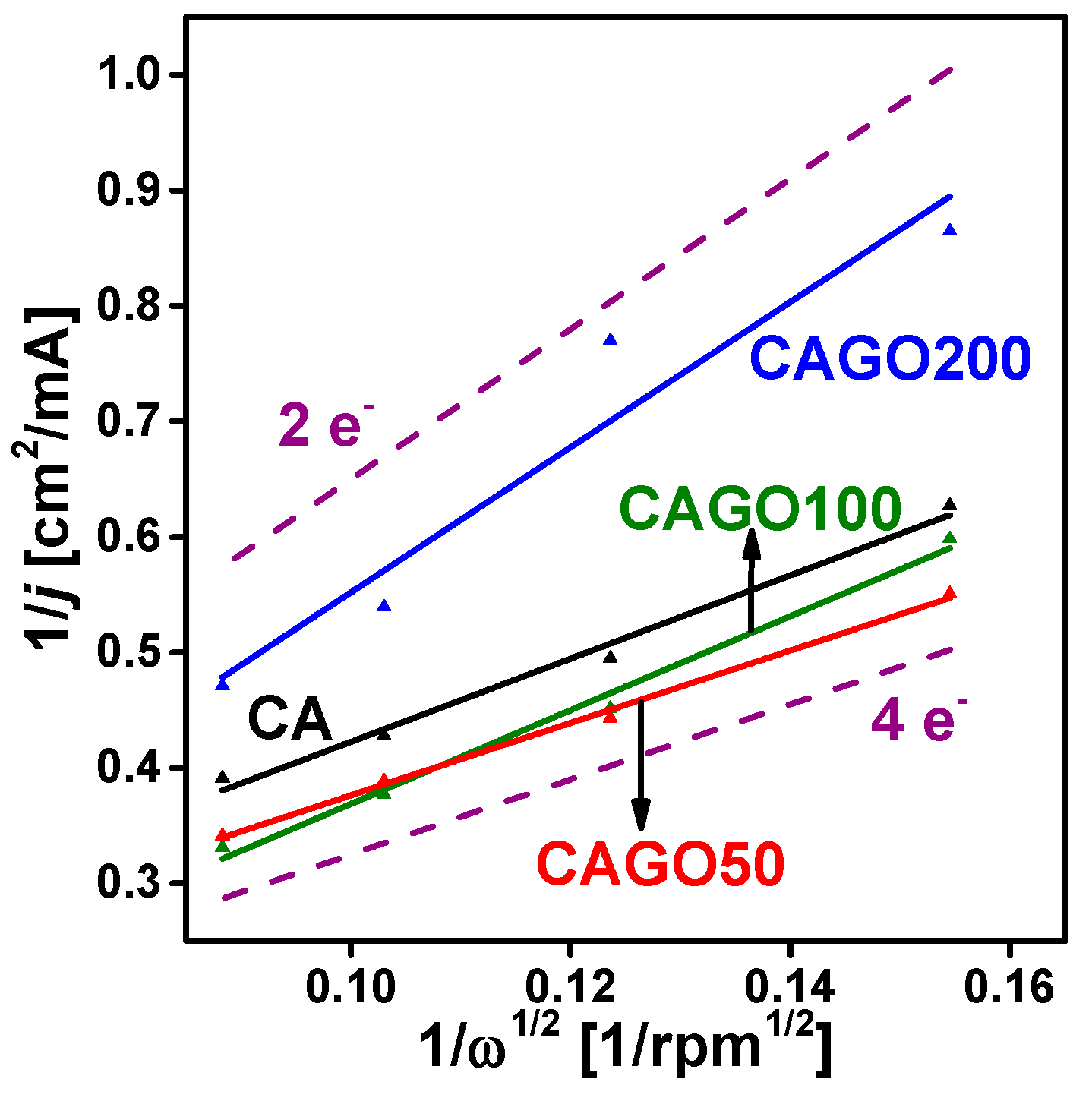
3.3. Effect of GO on ORR Performance
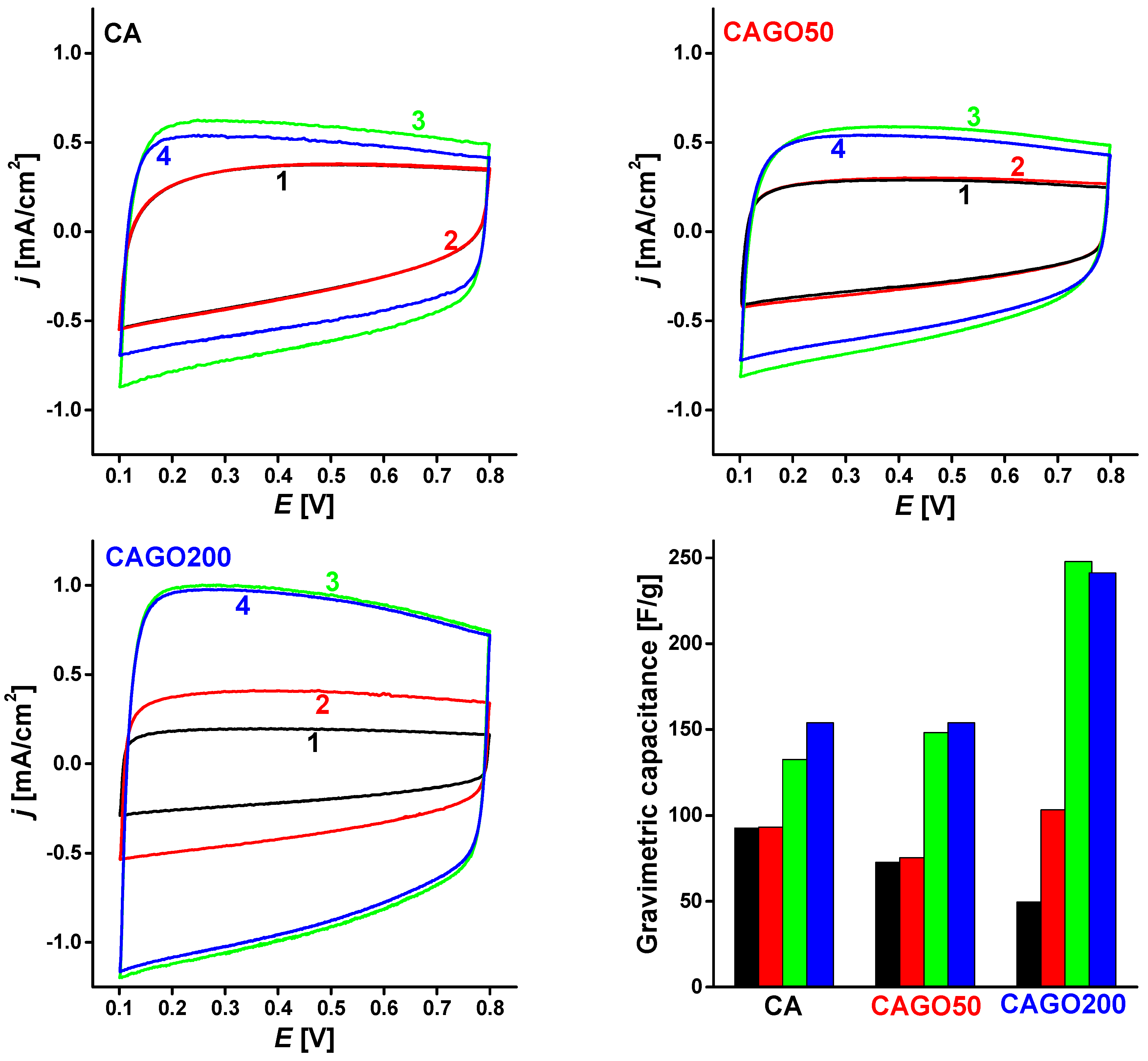
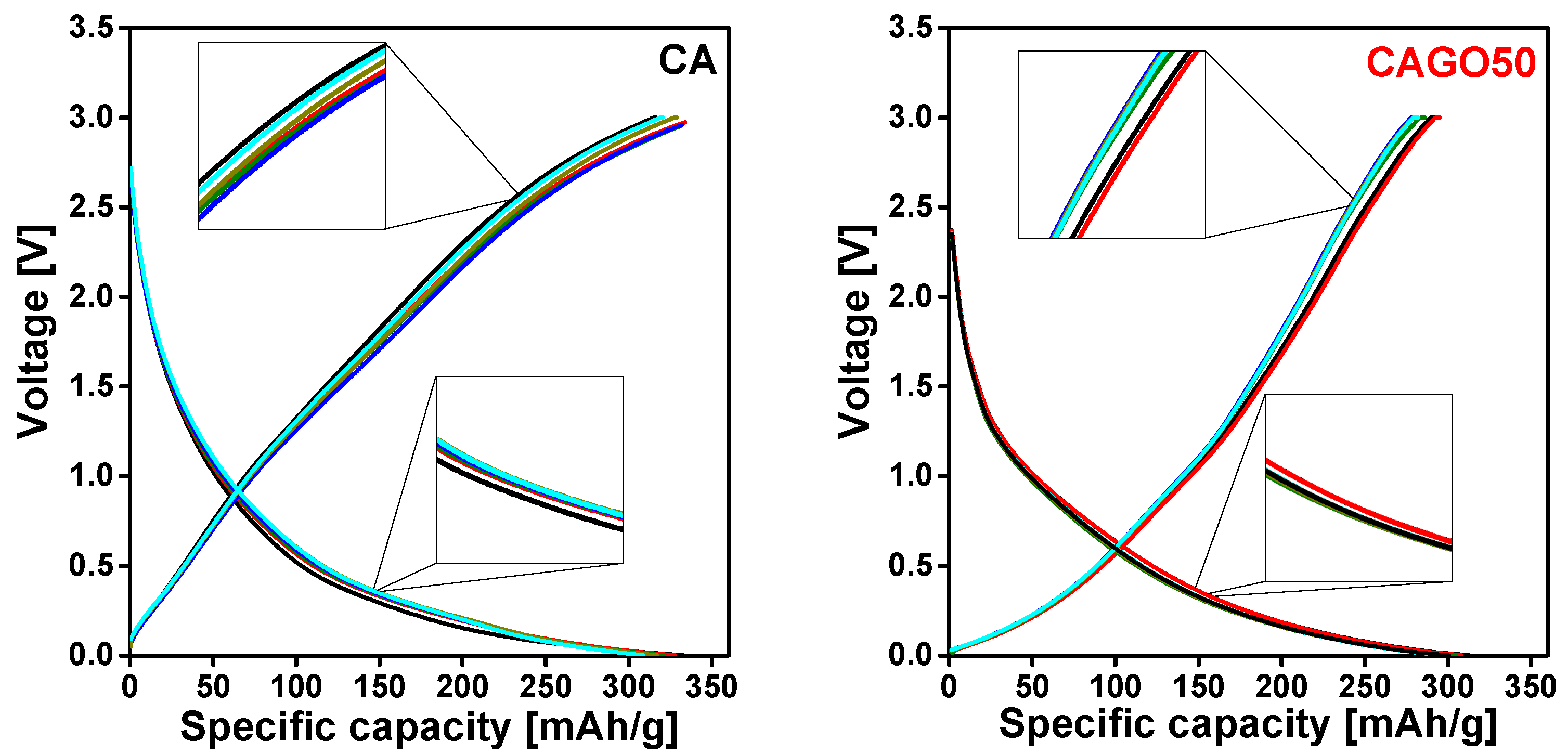
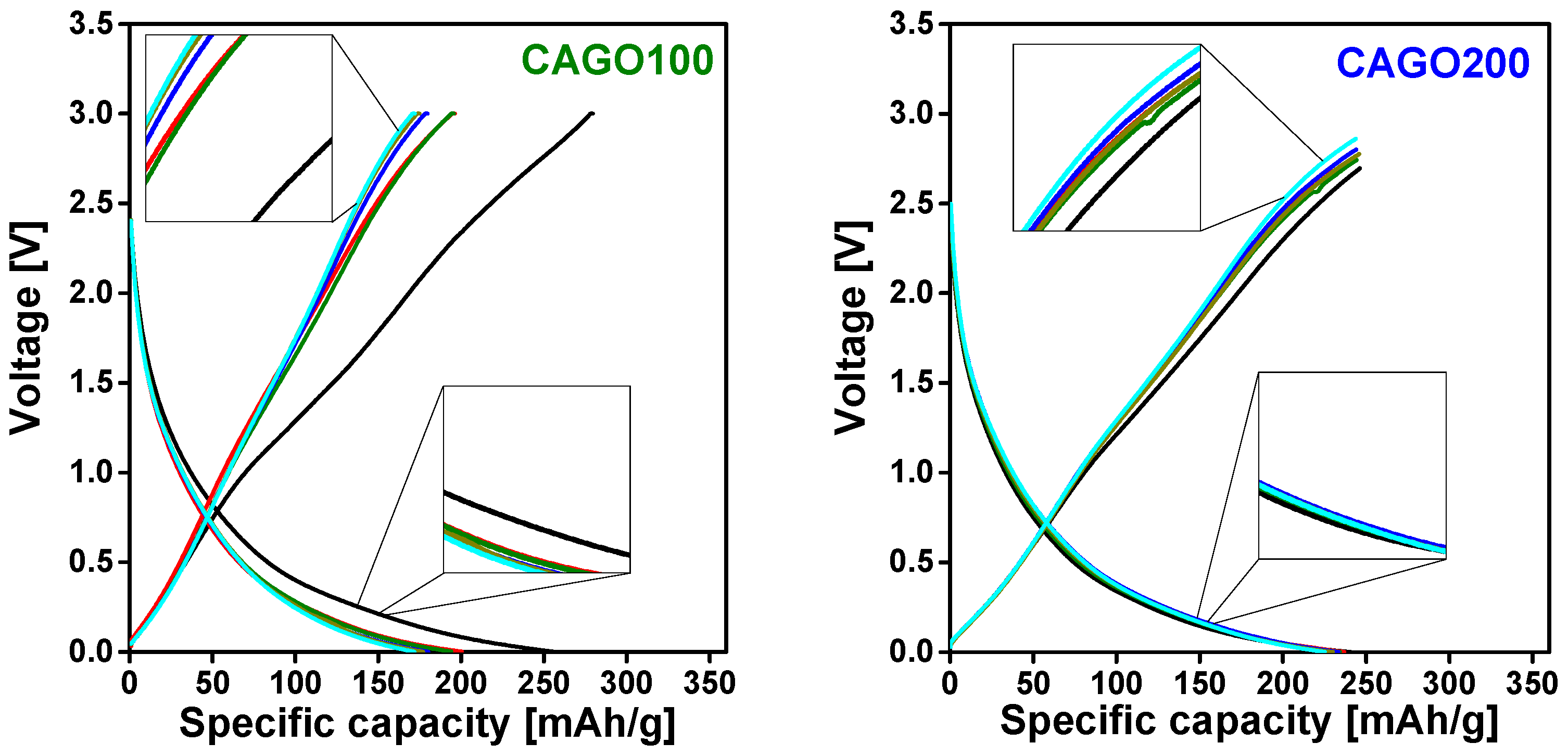
3.4. Effect of the GO on Li-Ion Battery Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, P.; Wang, L.-K.; Wang, G.; Gao, M.-R.; Ge, J.; Yuan, W.-J.; Shen, Y.-H.; Xie, A.-J.; Yu, S.-H. Nitrogen-doped nanoporous carbon nanosheets derived from plant biomass: An efficient catalyst for oxygen reduction reaction. Energy Environ. Sci. 2014, 7, 4095–4103. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Quan, X.; Chen, S.; Yu, H.; Zhao, H. Nitrogen-doped carbon with a high degree of graphitization derived from biomass as high-performance electrocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 2017, 396, 986–993. [Google Scholar] [CrossRef]
- Zhao, Q.; Ma, Q.; Pan, F.; Wang, Z.; Yang, B.; Zhang, J.; Zhang, J. Facile synthesis of nitrogen-doped carbon nanosheets as metal-free catalyst with excellent oxygen reduction performance in alkaline and acidic media. J. Solid State Electrochem. 2016, 20, 1469–1479. [Google Scholar] [CrossRef]
- Cheng, P.; Li, T.; Yu, H.; Zhi, L.; Liu, Z.-H.; Lei, Z. Biomass-Derived Carbon Fiber Aerogel as a Binder-Free Electrode for High-Rate Supercapacitors. J. Phys. Chem. C 2016, 120, 2079–2086. [Google Scholar] [CrossRef]
- You, Z.; Zhao, L.; Zhao, K.; Liao, H.; Wen, S.; Xiao, Y.; Cheng, B.; Lei, S. Highly tunable three-dimensional porous carbon produced from tea seed meal crop by-products for high performance supercapacitors. Appl. Surf. Sci. 2023, 607, 155080. [Google Scholar] [CrossRef]
- Wang, L.; Schnepp, Z.; Titirici, M.M. Rice husk-derived carbon anodes for lithium ion batteries. J. Mater. Chem. A 2013, 1, 5269–5273. [Google Scholar] [CrossRef]
- Li, D.; Guo, H.; Jiang, S.; Zeng, G.; Zhou, W.; Li, Z. Microstructures and electrochemical performances of TiO2-coated Mg–Zr co-doped NCM as a cathode material for lithium-ion batteries with high power and long circular life. New J. Chem. 2021, 45, 19446–19455. [Google Scholar] [CrossRef]
- Liu, T.; Li, X. Biomass-derived nanostructured porous carbons for sodium ion batteries: A review. Mater. Technol. 2018, 34, 232–245. [Google Scholar] [CrossRef]
- Li, H.; Cheng, Z.; Zhang, Q.; Natan, A.; Yang, Y.; Cao, D.; Zhu, H. Bacterial-Derived, Compressible, and Hierarchical Porous Carbon for High-Performance Potassium-Ion Batteries. Nano Lett. 2018, 18, 7407–7413. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zeng, G.; Jin, H.; Jiang, S.; Huang, M.; Zhang, C.; Chen, H. Bio-Template Synthesis of V2O3@Carbonized Dictyophora Composites for Advanced Aqueous Zinc-Ion Batteries. Molecules 2023, 28, 2147. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Q.; Tang, Y.; Zhang, L.; Li, Y. Zinc–air batteries: Are they ready for prime time? Chem. Sci. 2019, 10, 8924–8929. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Xu, Y.; Zhang, X.; Li, C.; Liu, Y.; Xiang, K.; Chen, H. (NH4)2Co2V10O28·16H2O/(NH4)2V10O25·8H2O heterostructure as cathode for high-performance aqueous Zn-ion batteries. J. Alloys Compd. 2022, 903, 163824. [Google Scholar] [CrossRef]
- Deng, W.-N.; Li, Y.-H.; Xu, D.-F.; Zhou, W.; Xiang, K.-X.; Chen, H. Three-dimensional hierarchically porous nitrogen-doped carbon from water hyacinth as selenium host for high-performance lithium–selenium batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- Wen, X.; Luo, J.; Xiang, K.; Zhou, W.; Zhang, C.; Chen, H. High-performance monoclinic WO3 nanospheres with the novel NH4+ diffusion behaviors for aqueous ammonium-ion batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar] [CrossRef]
- Sebestyén, Z.; Jakab, E.; Domán, A.; Bokrossy, P.; Bertóti, I.; Madarász, J.; László, K. Thermal degradation of crab shell biomass, a nitrogen-containing carbon precursor. J. Therm. Anal. Calorim. 2020, 142, 301–308. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- Li, D.; Jia, Y.; Chang, G.; Chen, J.; Liu, H.; Wang, J.; Hu, Y.; Xia, Y.; Yang, D.; Yao, X. A Defect-Driven Metal-free Electrocatalyst for Oxygen Reduction in Acidic Electrolyte. Chem 2018, 4, 2345–2356. [Google Scholar] [CrossRef]
- Du, C.; Li, P.; Zhuang, Z.; Fang, Z.; He, S.; Feng, L.; Chen, W. Highly porous nanostructures: Rational fabrication and promising application in energy electrocatalysis. Coord. Chem. Rev. 2022, 466, 214604. [Google Scholar] [CrossRef]
- Hou, J.; Cao, C.; Idrees, F.; Ma, X. Hierarchical Porous Nitrogen-Doped Carbon Nanosheets Derived from Silk for Ultrahigh-Capacity Battery Anodes and Supercapacitors. ACS Nano 2015, 9, 2556–2564. [Google Scholar] [CrossRef]
- Wang, D.-W.; Li, F.; Liu, M.; Lu, G.Q.; Cheng, H.-M. 3D Aperiodic Hierarchical Porous Graphitic Carbon Material for High-Rate Electrochemical Capacitive Energy Storage. Angew. Chem. Int. Ed. 2007, 47, 373–376. [Google Scholar] [CrossRef]
- Xiao, J.; Li, H.; Zhang, H.; He, S.; Zhang, Q.; Liu, K.; Jiang, S.; Duan, G.; Zhang, K. Nanocellulose and its derived composite electrodes toward supercapacitors: Fabrication, properties, and challenges. J. Bioresour. Bioprod. 2022, 7, 245–269. [Google Scholar] [CrossRef]
- Gabe, A.; Ruiz-Rosas, R.; González-Gaitán, C.; Morallón, E.; Cazorla-Amorós, D. Modeling of oxygen reduction reaction in porous carbon materials in alkaline medium. Effect of microporosity. J. Power Sources 2018, 412, 451–464. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Ge, B.; Pu, L.; Liu, Z. Influence of Micropore and Mesoporous in Activated Carbon Air-cathode Catalysts on Oxygen Reduction Reaction in Microbial Fuel Cells. Electrochimica Acta 2016, 214, 110–118. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, C.; Aoki, Y.; Habazaki, H. Starch-Derived Hierarchical Porous Carbon with Controlled Porosity for High Performance Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 7292–7303. [Google Scholar] [CrossRef]
- Bandosz, T.J. Revealing the impact of small pores on oxygen reduction on carbon electrocatalysts: A journey through recent findings. Carbon 2021, 188, 289–304. [Google Scholar] [CrossRef]
- Huang, M.; Yoo, S.J.; Lee, J.-S.; Yoon, T.-H. Electrochemical properties of an activated carbon xerogel monolith from resorcinol–formaldehyde for supercapacitor electrode applications. RSC Adv. 2021, 11, 33192–33201. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Bai, Y.; Liu, M.; Li, Y.; Yang, H.; Wang, X.; Wu, C. Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials. Energy Environ. Sci. 2021, 14, 2036–2089. [Google Scholar] [CrossRef]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon Materials for Chemical Capacitive Energy Storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Y.; Zhong, Y.; Shen, T.; Wang, X.; Xia, X.; Tu, J. Natural biomass-derived carbons for electrochemical energy storage. Mater. Res. Bull. 2017, 88, 234–241. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Z. The rational design of biomass-derived carbon materials towards next-generation energy storage: A review. Renew. Sustain. Energy Rev. 2020, 134, 110308. [Google Scholar] [CrossRef]
- Dahn, J.R.; Zheng, T.; Liu, Y.; Xue, J.S. Mechanisms for Lithium Insertion in Carbonaceous Materials. Science 1995, 270, 590–593. [Google Scholar] [CrossRef]
- Sun, S.; Yan, Q.; Wu, M.; Zhao, X. Carbon aerogel based materials for secondary batteries. Sustain. Mater. Technol. 2021, 30, e00342. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Sun, Y.; Lu, Y.; Chen, S.; Wang, B.; Zhang, H.; Xia, Y.; Yang, D. Turning gelidium amansii residue into nitrogen-doped carbon nanofiber aerogel for enhanced multiple energy storage. Carbon 2018, 137, 31–40. [Google Scholar] [CrossRef]
- Xiong, Q.; Ji, Z. Controllable growth of MoS2/C flower-like microspheres with enhanced electrochemical performance for lithium ion batteries. J. Alloys Compd. 2016, 673, 215–219. [Google Scholar] [CrossRef]
- Enterría, M.; Figueiredo, J.L. Nanostructured mesoporous carbons: Tuning texture and surface chemistry. Carbon 2016, 108, 79–102. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; He, F.; Chen, Y.; Zhu, J.; Wang, D.; Mu, S.; Yang, H.Y. Defect and Doping Co-Engineered Non-Metal Nanocarbon ORR Electrocatalyst. Nano-Micro Lett. 2021, 13, 65. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Li, R.; Chen, Y.; Wu, Z.; Zhong, B.; Guo, X. Heteroatom-doped Ginkgo Folium porous carbon modified separator for high-capacity and long-cycle lithium-sulfur batteries. Appl. Surf. Sci. 2022, 602, 154342. [Google Scholar] [CrossRef]
- Yan, B.; Feng, L.; Zheng, J.; Zhang, Q.; Dong, Y.; Ding, Y.; Yang, W.; Han, J.; Jiang, S.; He, S. Nitrogen-doped carbon layer on cellulose derived free-standing carbon paper for high-rate supercapacitors. Appl. Surf. Sci. 2023, 608, 155144. [Google Scholar] [CrossRef]
- Duan, Z.; Henkelman, G. Identification of Active Sites of Pure and Nitrogen-Doped Carbon Materials for Oxygen Reduction Reaction Using Constant-Potential Calculations. J. Phys. Chem. C 2020, 124, 12016–12023. [Google Scholar] [CrossRef]
- Nagy, B.; Villar-Rodil, S.; Tascón, J.M.; Bakos, I.; László, K. Nitrogen doped mesoporous carbon aerogels and implications for electrocatalytic oxygen reduction reactions. Microporous Mesoporous Mater. 2016, 230, 135–144. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Yuan, W.; Li, C.; Song, J.; Xie, A.; Shen, Y. Synergistic effect of Nitrogen-doped hierarchical porous carbon/graphene with enhanced catalytic performance for oxygen reduction reaction. Appl. Surf. Sci. 2017, 393, 144–150. [Google Scholar] [CrossRef]
- Lee, K.; Shabnam, L.; Faisal, S.N.; Hoang, V.C.; Gomes, V.G. Aerogel from fruit biowaste produces ultracapacitors with high energy density and stability. J. Energy Storage 2019, 27, 101152. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Badhulika, S. Effect of self-doped heteroatoms on the performance of biomass-derived carbon for supercapacitor applications. J. Power Sources 2020, 480, 228830. [Google Scholar] [CrossRef]
- He, D.; Zhao, W.; Li, P.; Liu, Z.; Wu, H.; Liu, L.; Han, K.; Liu, L.; Wan, Q.; Butt, F.K.; et al. Bifunctional biomass-derived 3D nitrogen-doped porous carbon for oxygen reduction reaction and solid-state supercapacitor. Appl. Surf. Sci. 2018, 465, 303–312. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, D.; Zhou, H.; Wu, J.; Xu, G.; Huang, Z.; Liu, M.; Yang, J.; Chen, D. Boosting gravimetric and volumetric energy density of supercapacitors by 3D pomegranate-like porous carbon structure design. Appl. Surf. Sci. 2020, 534, 147613. [Google Scholar] [CrossRef]
- Ito, S.; Murata, T.; Hasegawa, M.; Bito, Y.; Toyoguchi, Y. Study on CxN and CxS with disordered carbon structure as the anode materials for secondary lithium batteries. J. Power Sources 1997, 68, 245–248. [Google Scholar] [CrossRef]
- Seredych, M.; László, K.; Rodríguez-Castellón, E.; Bandosz, T.J. S-doped carbon aerogels/GO composites as oxygen reduction catalysts. J. Energy Chem. 2016, 25, 236–245. [Google Scholar] [CrossRef]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and Nitrogen Dual-Doped Mesoporous Graphene Electrocatalyst for Oxygen Reduction with Synergistically Enhanced Performance. Angew. Chem. Int. Ed. 2012, 51, 11496–11500. [Google Scholar] [CrossRef]
- Jiang, M.; Yu, X.; Yang, H.; Chen, S. Optimization Strategies of Preparation of Biomass-Derived Carbon Electrocatalyst for Boosting Oxygen Reduction Reaction: A Minireview. Catalysts 2020, 10, 1472. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Chen, Y.; Liu, X.; Li, X.; Gao, S. A treasure map for nonmetallic catalysts: Optimal nitrogen and fluorine distribution of biomass-derived carbon materials for high-performance oxygen reduction catalysts. J. Mater. Chem. A 2021, 9, 18251–18259. [Google Scholar] [CrossRef]
- Jiao, S.; Yao, Y.; Zhang, J.; Zhang, L.; Li, C.; Zhang, H.; Zhao, X.; Chen, H.; Jiang, J. Nano-flower-like porous carbon derived from soybean straw for efficient N-S co-doped supercapacitors by coupling in-situ heteroatom doping with green activation method. Appl. Surf. Sci. 2023, 615, 156365. [Google Scholar] [CrossRef]
- Jin, H.; Wang, X.; Gu, Z.; Polin, J. Carbon materials from high ash biochar for supercapacitor and improvement of capacitance with HNO3 surface oxidation. J. Power Sources 2013, 236, 285–292. [Google Scholar] [CrossRef]
- Chen, F.; Yang, J.; Bai, T.; Long, B.; Zhou, X. Biomass waste-derived honeycomb-like nitrogen and oxygen dual-doped porous carbon for high performance lithium-sulfur batteries. Electrochimica Acta 2016, 192, 99–109. [Google Scholar] [CrossRef]
- Qiu, Y.; Huo, J.; Jia, F.; Shanks, B.H.; Li, W. N- and S-doped mesoporous carbon as metal-free cathode catalysts for direct biorenewable alcohol fuel cells. J. Mater. Chem. A 2015, 4, 83–95. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, S.-J. Recent advances in preparations and applications of carbon aerogels: A review. Carbon 2020, 163, 1–18. [Google Scholar] [CrossRef]
- Martín-Jimeno, F.; Suárez-García, F.; Paredes, J.; Martínez-Alonso, A.; Tascón, J. Activated carbon xerogels with a cellular morphology derived from hydrothermally carbonized glucose-graphene oxide hybrids and their performance towards CO2 and dye adsorption. Carbon 2015, 81, 137–147. [Google Scholar] [CrossRef]
- Enterría, M.; Martín-Jimeno, F.; Suárez-García, F.; Paredes, J.; Pereira, M.; Martins, J.; Martínez-Alonso, A.; Tascón, J.; Figueiredo, J. Effect of nanostructure on the supercapacitor performance of activated carbon xerogels obtained from hydrothermally carbonized glucose-graphene oxide hybrids. Carbon 2016, 105, 474–483. [Google Scholar] [CrossRef]
- Bakierska, M.; Lis, M.; Pacek, J.; Świętosławski, M.; Gajewska, M.; Tąta, A.; Proniewicz, E.; Molenda, M. Bio-derived carbon nanostructures for high-performance lithium-ion batteries. Carbon 2019, 145, 426–432. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Jaleh, B.; Nasrollahzadeh, M.; Eslamipanah, M.; Nasri, A.; Shabanlou, E.; Manwar, N.R.; Zboril, R.; Fornasiero, P.; Gawande, M.B. The Role of Carbon-Based Materials for Fuel Cells Performance. Carbon 2022, 198, 301–352. [Google Scholar] [CrossRef]
- Song, H.; Na, R.; Hong, C.; Zhang, G.; Li, X.; Kang, Y.; Zhang, Q.; Xie, H. In situ measurement and mechanism analysis of the lithium storage behavior of graphene electrodes. Carbon 2021, 188, 146–154. [Google Scholar] [CrossRef]
- Li, L.; Zhang, D.; Deng, J.; Gou, Y.; Fang, J.; Cui, H.; Zhao, Y.; Cao, M. Carbon-based materials for fast charging lithium-ion batteries. Carbon 2021, 183, 721–734. [Google Scholar] [CrossRef]
- Wu, S.; Wu, H.; Zou, M.; Shi, X.; Yuan, Y.; Bai, W.; Cao, A. Short-range ordered graphitized-carbon nanotubes with large cavity as high-performance lithium-ion battery anodes. Carbon 2019, 158, 642–650. [Google Scholar] [CrossRef]
- Gómez-Urbano, J.L.; Moreno-Fernández, G.; Arnaiz, M.; Ajuria, J.; Rojo, T.; Carriazo, D. Graphene-coffee waste derived carbon composites as electrodes for optimized lithium ion capacitors. Carbon 2020, 162, 273–282. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, F.; Zhang, L.; Lu, Y.; Zhang, Y.; Yang, X.; Ma, Y.; Huang, Y. High energy density Li-ion capacitor assembled with all graphene-based electrodes. Carbon 2015, 92, 106–118. [Google Scholar] [CrossRef]
- Lim, M.B.; Hu, M.; Manandhar, S.; Sakshaug, A.; Strong, A.; Riley, L.; Pauzauskie, P.J. Ultrafast sol–gel synthesis of graphene aerogel materials. Carbon 2015, 95, 616–624. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, J.; Huang, L. A review of three-dimensional graphene-based materials: Synthesis and applications to energy conversion/storage and environment. Carbon 2018, 143, 610–640. [Google Scholar] [CrossRef]
- Rao, C.E.N.E.R.; Sood, A.E.K.; Subrahmanyam, K.E.S.; Govindaraj, A. Graphene: The New Two-Dimensional Nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, L.; Zhu, Y.; Zhang, C.; Jiang, S.; Hou, H. In Situ Fabrication of High Dielectric Constant Composite Films with Good Mechanical and Thermal Properties by Controlled Reduction. Molecules 2023, 28, 2535. [Google Scholar] [CrossRef] [PubMed]
- Canal-Rodríguez, M.; Arenillas, A.; Rey-Raap, N.; Ramos-Fernández, G.; Martín-Gullón, I.; Menéndez, J.A. Graphene-doped carbon xerogel combining high electrical conductivity and surface area for optimized aqueous supercapacitors. Carbon 2017, 118, 291–298. [Google Scholar] [CrossRef]
- Ramos-Fernández, G.; Canal-Rodríguez, M.; Arenillas, A.; Menéndez, J.A.; Rodríguez-Pastor, I.; Martin-Gullon, I. Determinant influence of the electrical conductivity versus surface area on the performance of graphene oxide-doped carbon xerogel supercapacitors. Carbon 2018, 126, 456–463. [Google Scholar] [CrossRef]
- Sato, K.; Noguchi, M.; Demachi, A.; Oki, N.; Endo, M. A Mechanism of Lithium Storage in Disordered Carbons. Science 1994, 264, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.S.; Dahn, J.R. Dramatic Effect of Oxidation on Lithium Insertion in Carbons Made from Epoxy Resins. J. Electrochem. Soc. 1995, 142, 3668–3677. [Google Scholar] [CrossRef]
- Wang, L.; Schütz, C.; Salazar-Alvarez, G.; Titirici, M.-M. Carbon aerogels from bacterial nanocellulose as anodes for lithium ion batteries. RSC Adv. 2014, 4, 17549–17554. [Google Scholar] [CrossRef]
- Pan, D.; Wang, S.; Zhao, B.; Wu, M.; Zhang, H.; Wang, Y.; Jiao, Z. Li Storage Properties of Disordered Graphene Nanosheets. Chem. Mater. 2009, 21, 3136–3142. [Google Scholar] [CrossRef]
- Yazami, R.; Deschamps, M. High reversible capacity carbon-lithium negative electrode in polymer electrolyte. J. Power Sources 1995, 54, 411–415. [Google Scholar] [CrossRef]
- Li, J.-C.; Hou, P.-X.; Cheng, M.; Liu, C.; Cheng, H.-M.; Shao, M. Carbon nanotube encapsulated in nitrogen and phosphorus co-doped carbon as a bifunctional electrocatalyst for oxygen reduction and evolution reactions. Carbon 2018, 139, 156–163. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Huang, J.; Zhou, Y.; Xu, K.; Zhao, N.; Cheng, X. Soft template-assisted method for synthesis of nitrogen and sulfur co-doped three-dimensional reduced graphene oxide as an efficient metal free catalyst for oxygen reduction reaction. Carbon 2017, 122, 237–246. [Google Scholar] [CrossRef]
- Nagy, B.; Bakos, I.; Bertóti, I.; Domán, A.; Menyhárd, A.; Mohai, M.; László, K. Synergism of nitrogen and reduced graphene in the electrocatalytic behavior of resorcinol—Formaldehyde based carbon aerogels. Carbon 2018, 139, 872–879. [Google Scholar] [CrossRef]
- Andrade, S.K.S.; Bakos, I.; Dobos, G.; Farkas, A.; Kiss, G.; Klébert, S.; Madarász, J.; László, K. Biomass Related Highly Porous Metal Free Carbon for Gas Storage and Electrocatalytic Applications. Materials 2021, 14, 3488. [Google Scholar] [CrossRef]
- Berke, B.; Sós, L.; Bérczes, V.; Domján, A.; Porcar, L.; Czakkel, O.; László, K. Graphene derivatives in responsive hydrogels: Effect of concentration and surface chemistry. Eur. Polym. J. 2017, 93, 717–725. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. The Equation of the characteristic curve of activated charcoal. Dokl. Akad. Nauk. SSSR 1947, 55, 327–329. [Google Scholar]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surf. A Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Bertóti, I.; Mohai, M.; László, K. Surface modification of graphene and graphite by nitrogen plasma: Determination of chemical state alterations and assignments by quantitative X-ray photoelectron spectroscopy. Carbon 2015, 84, 185–196. [Google Scholar] [CrossRef]
- Mohai, M. XPS MultiQuant: Multimodel XPS quantification software. Surf. Interface Anal. 2004, 36, 828–832. [Google Scholar] [CrossRef]
- Evans, S.; Pritchard, R.G.; Thomas, J.M. Relative differential subshell photoionisation cross-sections (MgKα) from lithium to uranium. J. Electron Spectrosc. Relat. Phenom. 1978, 14, 341–358. [Google Scholar] [CrossRef]
- Reilman, R.F.; Msezane, A.; Manson, S.T. Relative intensities in photoelectron spectroscopy of atoms and molecules. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 389–394. [Google Scholar] [CrossRef]
- Nagy, B.; Domán, A.; Menyhárd, A.; László, K. Influence of Graphene Oxide Incorporation on Resorcinol-Formaldehyde Polymer and Carbon Aerogels. Period. Polytech. Chem. Eng. 2018, 62, 441–449. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Wang, Y.; Alsmeyer, D.C.; McCreery, R.L. Raman spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater. 1990, 2, 557–563. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martínez-Alonso, A.; Tascón, J. Raman microprobe studies on carbon materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The Importance of Interbands on the Interpretation of the Raman Spectrum of Graphene Oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Couzi, M.; Bruneel, J.-L.; Talaga, D.; Bokobza, L. A multi wavelength Raman scattering study of defective graphitic carbon materials: The first order Raman spectra revisited. Carbon 2016, 107, 388–394. [Google Scholar] [CrossRef]
- McDonald-Wharry, J.; Manley-Harris, M.; Pickering, K. Carbonisation of biomass-derived chars and the thermal reduction of a graphene oxide sample studied using Raman spectroscopy. Carbon 2013, 59, 383–405. [Google Scholar] [CrossRef]
- Rhim, Y.-R.; Zhang, D.; Fairbrother, D.H.; Wepasnick, K.A.; Livi, K.J.; Bodnar, R.J.; Nagle, D.C. Changes in electrical and microstructural properties of microcrystalline cellulose as function of carbonization temperature. Carbon 2010, 48, 1012–1024. [Google Scholar] [CrossRef]
- Gavrilov, N.; Pašti, I.A.; Mitrić, M.; Travas-Sejdić, J.; Ćirić-Marjanović, G.; Mentus, S.V. Electrocatalysis of oxygen reduction reaction on polyaniline-derived nitrogen-doped carbon nanoparticle surfaces in alkaline media. J. Power Sources 2012, 220, 306–316. [Google Scholar] [CrossRef]
- Yin, D.; Lu, N.; Li, Z.; Yang, J. A computational infrared spectroscopic study of graphene oxide. J. Chem. Phys. 2013, 139, 084704. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Qiu, Y.; Zhang, L.; Zhang, X. Comparison of the characteristic properties of reduced graphene oxides synthesized from natural graphites with different graphitization degrees. RSC Adv. 2017, 7, 52337–52344. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, G.; Dong, Q.; Qian, B.; Zhang, M.; Li, C.; Qiu, J. An ionic liquid template approach to graphene–carbon xerogel composites for supercapacitors with enhanced performance. J. Mater. Chem. A 2014, 2, 14329–14333. [Google Scholar] [CrossRef]
- Lu, H.; Yang, C.; Chen, J.; Li, J.; Jin, H.; Wang, J.; Wang, S. Tailoring Hierarchically Porous Nitrogen-, Sulfur-Codoped Carbon for High-Performance Supercapacitors and Oxygen Reduction. Small 2020, 16, e1906584. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Wang, D.; Yang, P.; Xu, H.; Xiao, L.; Zhang, G.-X.; Lu, X.; Duan, Z.; Meng, F.; Zhang, J.; et al. Graphite N–C–P dominated three-dimensional nitrogen and phosphorus co-doped holey graphene foams as high-efficiency electrocatalysts for Zn–air batteries. Nanoscale 2019, 11, 17010–17017. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, S.; Xia, M.; Rehman, S.; Mu, S.; Kou, Z.; Zhang, Z.; Chen, Z.; Gao, F.; Hou, Y. N-P-O co-doped high performance 3D graphene prepared through red phosphorous-assisted “cutting-thin” technique: A universal synthesis and multifunctional applications. Nano Energy 2016, 28, 346–355. [Google Scholar] [CrossRef]
- Tong, J.; Ma, W.; Wang, W.; Ma, J.; Li, W.; Bo, L.; Fan, H. Nitrogen/phosphorus dual-doped hierarchically porous graphitic biocarbon with greatly improved performance on oxygen reduction reaction in alkaline media. J. Electroanal. Chem. 2018, 809, 163–170. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, L.; Shi, G.; Liu, J.; Chen, J.; Dai, L. N,P-Codoped Carbon Networks as Efficient Metal-free Bifunctional Catalysts for Oxygen Reduction and Hydrogen Evolution Reactions. Angew. Chem. 2015, 128, 2270–2274. [Google Scholar] [CrossRef]
- Zhu, Y.N.; Cao, C.Y.; Jiang, W.J.; Yang, S.L.; Hu, J.S.; Song, W.G.; Wan, L.J. Nitrogen, phosphorus and sulfur co-doped ultrathin carbon nanosheets as a metal-free catalyst for selective oxidation of aromatic alkanes and the oxygen reduction reaction. J. Mater. Chem. A 2016, 4, 18470–18477. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, X.; Jin, C.; Tian, J.; Yang, R. Ternary doping of phosphorus, nitrogen, and sulfur into porous carbon for enhancing electrocatalytic oxygen reduction. Carbon 2015, 92, 327–338. [Google Scholar] [CrossRef]
- Zheng, X.; Cao, X.; Wu, J.; Tian, J.; Jin, C.; Yang, R. Yolk-shell N/P/B ternary-doped biocarbon derived from yeast cells for enhanced oxygen reduction reaction. Carbon 2016, 107, 907–916. [Google Scholar] [CrossRef]
- Chandran, P.; Ghosh, A.; Ramaprabhu, S. High-performance Platinum-free oxygen reduction reaction and hydrogen oxidation reaction catalyst in polymer electrolyte membrane fuel cell. Sci. Rep. 2018, 8, 3591. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Dawidziuk, M.B.; Carrasco-Marín, F.; Morallón, E. Electrochemical performance of carbon gels with variable surface chemistry and physics. Carbon 2012, 50, 3324–3332. [Google Scholar] [CrossRef]
- Lobato, B.; Suárez, L.; Guardia, L.; Centeno, T.A. Capacitance and surface of carbons in supercapacitors. Carbon 2017, 122, 434–445. [Google Scholar] [CrossRef]
- Lu, Y.; Ye, Z.; Zhao, Y.; Li, Q.; He, M.; Bai, C.; Wang, X.; Han, Y.; Wan, X.; Zhang, S.; et al. Graphene supported double-layer carbon encapsulated silicon for high-performance lithium-ion battery anode materials. Carbon 2023, 201, 962–971. [Google Scholar] [CrossRef]
- Yokokura, T.J.; Rodriguez, J.R.; Pol, V.G. Waste Biomass-Derived Carbon Anode for Enhanced Lithium Storage. ACS Omega 2020, 5, 19715–19720. [Google Scholar] [CrossRef]
- Wang, J.; Nie, P.; Ding, B.; Dong, S.; Hao, X.; Dou, H.; Zhang, X. Biomass derived carbon for energy storage devices. J. Mater. Chem. A 2017, 5, 2411–2428. [Google Scholar] [CrossRef]
- Alex, A.S.; Lekshmi, M.S.A.; Sekkar, V.; John, B.; Gouri, C.; Ilangovan, S.A. Microporous carbon aerogel prepared through ambient pressure drying route as anode material for lithium ion cells. Polym. Adv. Technol. 2017, 28, 1945–1950. [Google Scholar] [CrossRef]
- Taleghani, S.T.; Marcos, B.; Zaghib, K.; Lantagne, G. A Study on the Effect of Porosity and Particles Size Distribution on Li-Ion Battery Performance. J. Electrochem. Soc. 2017, 164, E3179–E3189. [Google Scholar] [CrossRef]
- Duraisamy, E.; Prasath, A.; Devi, V.S.; Ansari, M.N.M.; Elumalai, P. Sustainably-derived hierarchical porous carbon from spent honeycomb for high-performance lithium-ion battery and ultracapacitors. Energy Storage 2020, 2, e136. [Google Scholar] [CrossRef]
- Kim, K.; Adams, R.A.; Kim, P.J.; Arora, A.; Martinez, E.; Youngblood, J.P.; Pol, V.G. Li-ion storage in an amorphous, solid, spheroidal carbon anode produced by dry-autoclaving of coffee oil. Carbon 2018, 133, 62–68. [Google Scholar] [CrossRef]
- Stephan, A.M.; Kumar, P.; Ramesh, R.; Thomas, S.; Jeong, S.K.; Nahm, K.S. Pyrolitic carbon from biomass precursors as anode materials for lithium batteries. Mater. Sci. Eng. A 2006, 430, 132–137. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Yang, D.; Narayan, R.; Raju, K.; Kumar, N.A.; Zhao, X. Biomass derived carbon nanoparticle as anodes for high performance sodium and lithium ion batteries. Nano Energy 2016, 26, 346–352. [Google Scholar] [CrossRef]
- Xing, W.; Xue, J.S.; Dahn, J.R. Optimizing Pyrolysis of Sugar Carbons for Use as Anode Materials in Lithium-Ion Batteries. J. Electrochem. Soc. 1996, 143, 3046–3052. [Google Scholar] [CrossRef]
- Wang, W.; Sun, Y.; Liu, B.; Wang, S.; Cao, M. Porous carbon nanofiber webs derived from bacterial cellulose as an anode for high performance lithium ion batteries. Carbon 2015, 91, 56–65. [Google Scholar] [CrossRef]
- Paudics, A.; Farah, S.; Bertóti, I.; Farkas, A.; László, K.; Mohai, M.; Sáfrán, G.; Szilágyi, A.; Kubinyi, M. Fluorescence probing of binding sites on graphene oxide nanosheets with Oxazine 1 dye. Appl. Surf. Sci. 2021, 541, 148451. [Google Scholar] [CrossRef]
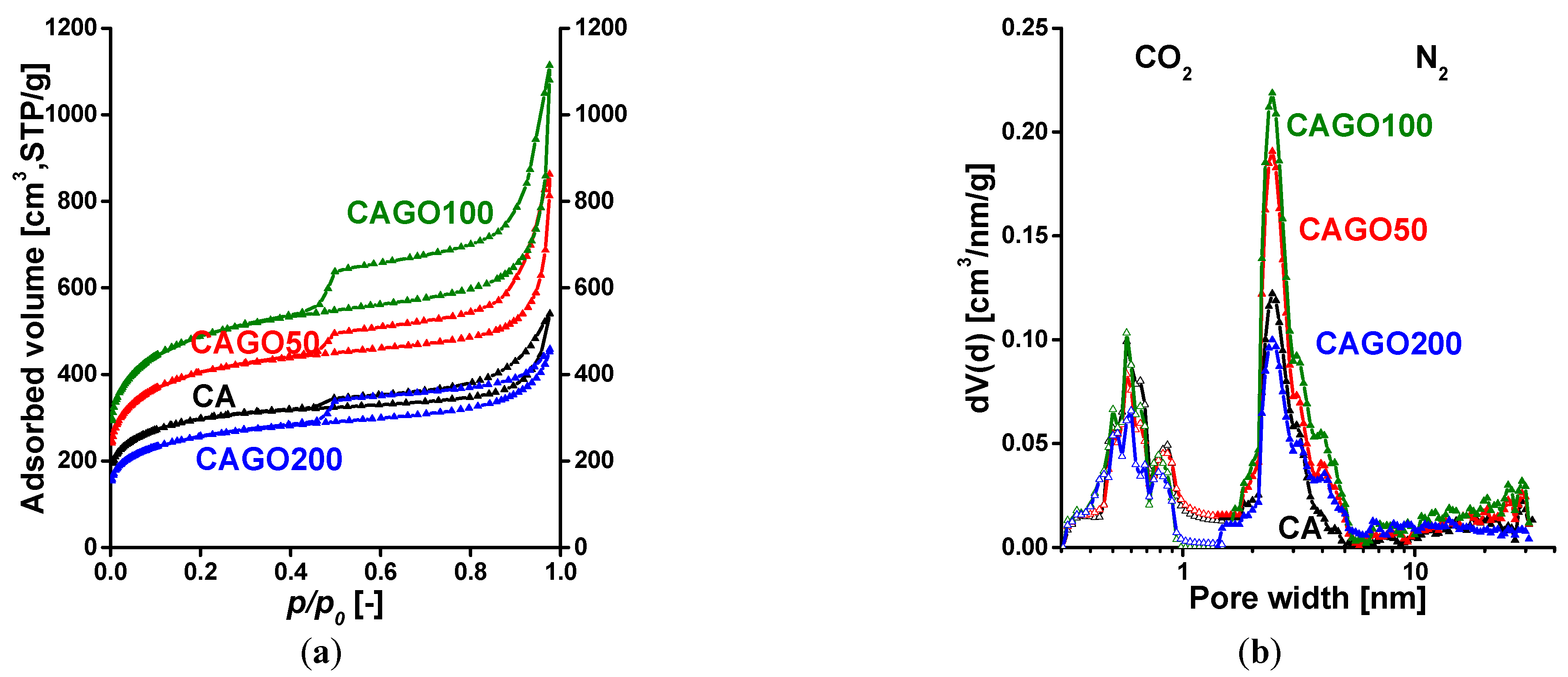

| Method | Parameter | Units | CA | CAGO50 | CAGO100 | CAGO200 |
|---|---|---|---|---|---|---|
| From N2 | SBET | [m2/g] | 1070 | 1479 | 1779 | 933 |
| V0.98 | [cm3/g] | 0.83 | 1.33 | 1.72 | 0.71 | |
| Vmicro,DR | [cm3/g] | 0.42 | 0.54 | 0.64 | 0.34 | |
| [%] | 51 | 40 | 37 | 48 | ||
| Vmicro,DFT | [cm3/g] | 0.31 | 0.40 | 0.48 | 0.25 | |
| [%] | 37 | 30 | 28 | 35 | ||
| From CO2 | Vumicro,DR | [cm3/g] | 0.073 | 0.062 | 0.059 | 0.041 |
| Vumicro,DFT | [cm3/g] | 0.042 | 0.039 | 0.030 | 0.025 |
| Sample | C | O | N | S | O/C | N/C | S/C | O + N + S | S/N |
|---|---|---|---|---|---|---|---|---|---|
| C | |||||||||
| CA | 90.6 | 3.3 | 5.1 | 1.0 | 0.036 | 0.056 | 0.011 | 0.104 | 0.196 |
| CAGO50 | 92.0 | 3.1 | 3.7 | 1.3 | 0.034 | 0.039 | 0.014 | 0.087 | 0.361 |
| CAGO100 | 90.7 | 4.1 | 4.1 | 1.2 | 0.045 | 0.045 | 0.013 | 0.104 | 0.293 |
| CAGO200 | 90.4 | 3.7 | 4.4 | 1.4 | 0.041 | 0.049 | 0.015 | 0.105 | 0.318 |
| GO film | 67.4 | 32.1 | - | 0.5 | 0.476 | - | 0.007 | 0.484 | - |
| C1s | O1s | |||||
|---|---|---|---|---|---|---|
| C1 | C2 | C3 | O1 | O2 | O3 | |
| Chemical state | sp2 C=C | C–O C–N C–S | C=O O–C–O N–C–O | S–O | C–O–C C–OH C=O | OC–O–CO (H2O) |
| Binding energy [eV] | 284.3–284.4 | 285.7–285.8 | 287.5–287.9 | 530.2–530.6 | 532.1–532.5 | 533.9–534.3 |
| CA | 74.0 | 10.9 | 5.4 | 1.5 | 1.7 | - |
| CAGO50 | 78.8 | 7.4 | 5.5 | 1.9 | 1.3 | - |
| CAGO100 | 74.7 | 11.0 | 4.8 | 1.8 | 1.7 | 0.7 |
| CAGO200 | 75.9 | 9.4 | 4.8 | 1.8 | 1.6 | 0.5 |
| N1s | S2p | ||||
|---|---|---|---|---|---|
| N1 | N2 | N3 | S1 | S2 | |
| Chemical state | C–N | OO–C–N | C–N+ | C–S | C–SO3 |
| Binding energy [eV] | 397.8–398.0 | 400.4–400.5 | 402.4–402.7 | 164.9–165.0 | 168.3–168.6 |
| CA | 2.3 | 2.3 | 0.8 | 0.9 | 0.2 |
| CAGO50 | 1.6 | 1.7 | 0.6 | 1.2 | - |
| CAGO100 | 1.9 | 1.9 | 0.4 | 1.0 | 0.2 |
| CAGO200 | 2.0 | 2.0 | 0.7 | 1.1 | 0.3 |
| Sample | BET Surface Area [m2/g] | Onset Potential [mV] | E1/2 [mV] | Number of e− Transferred | Ref. |
|---|---|---|---|---|---|
| SWCNT@N,P doped carbon | 616 | 920 | 850 | 3.91 | [79] |
| N,S co-doped 3D rGO | 392 | 895 | 732 | 3.87 | [80] |
| N,S porous carbon materials | 732 | 940 | 840 | - | [106] |
| N,P-holey graphene foams | 758 | 983 | 865 | 3.70 | [107] |
| 3D-high performance graphene | 1406 | 928 | 836 | 3.83 | [108] |
| N,P porous graphitic biocarbon | 845 | −14 (vs. Ag/AgCl) | −115 (vs. Ag/AgCl) | 3.9 | [109] |
| N,P co-doped carbon | 375 | 950 | 820 | 3.7 | [110] |
| N,P,S co-doped carbon nanosheets | 1198 | 938 | 800 | 3.8–4.0 | [111] |
| P,N,S-porous carbon | 711 | 905 | 780 | 3.68–3.96 | [112] |
| N,P,B biocarbon | 1155 | 904 | 790 | 3.78–3.90 | [113] |
| Pt/C (20 wt.% Pt on Vulcan XC-72) | - | 960 | 869 | 3.96 | [113] |
| CA | 1070 | 855 | 700 | 3.5 | Our work |
| CAGO50 | 1479 | 850 | 760 | 4.0 | |
| CAGO100 | 1779 | 845 | 730 | 3.1 | |
| CAGO200 | 933 | 825 | 700 | 2.0 | |
| Pt/C | 957 | 886 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samaniego Andrade, S.K.; Lakshmi, S.S.; Bakos, I.; Klébert, S.; Kun, R.; Mohai, M.; Nagy, B.; László, K. The Influence of Reduced Graphene Oxide on the Texture and Chemistry of N,S-Doped Porous Carbon. Implications for Electrocatalytic and Energy Storage Applications. Nanomaterials 2023, 13, 2364. https://doi.org/10.3390/nano13162364
Samaniego Andrade SK, Lakshmi SS, Bakos I, Klébert S, Kun R, Mohai M, Nagy B, László K. The Influence of Reduced Graphene Oxide on the Texture and Chemistry of N,S-Doped Porous Carbon. Implications for Electrocatalytic and Energy Storage Applications. Nanomaterials. 2023; 13(16):2364. https://doi.org/10.3390/nano13162364
Chicago/Turabian StyleSamaniego Andrade, Samantha K., Shiva Shankar Lakshmi, István Bakos, Szilvia Klébert, Robert Kun, Miklós Mohai, Balázs Nagy, and Krisztina László. 2023. "The Influence of Reduced Graphene Oxide on the Texture and Chemistry of N,S-Doped Porous Carbon. Implications for Electrocatalytic and Energy Storage Applications" Nanomaterials 13, no. 16: 2364. https://doi.org/10.3390/nano13162364
APA StyleSamaniego Andrade, S. K., Lakshmi, S. S., Bakos, I., Klébert, S., Kun, R., Mohai, M., Nagy, B., & László, K. (2023). The Influence of Reduced Graphene Oxide on the Texture and Chemistry of N,S-Doped Porous Carbon. Implications for Electrocatalytic and Energy Storage Applications. Nanomaterials, 13(16), 2364. https://doi.org/10.3390/nano13162364








