A Sensitive Micro Conductometric Ethanol Sensor Based on an Alcohol Dehydrogenase-Gold Nanoparticle Chitosan Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
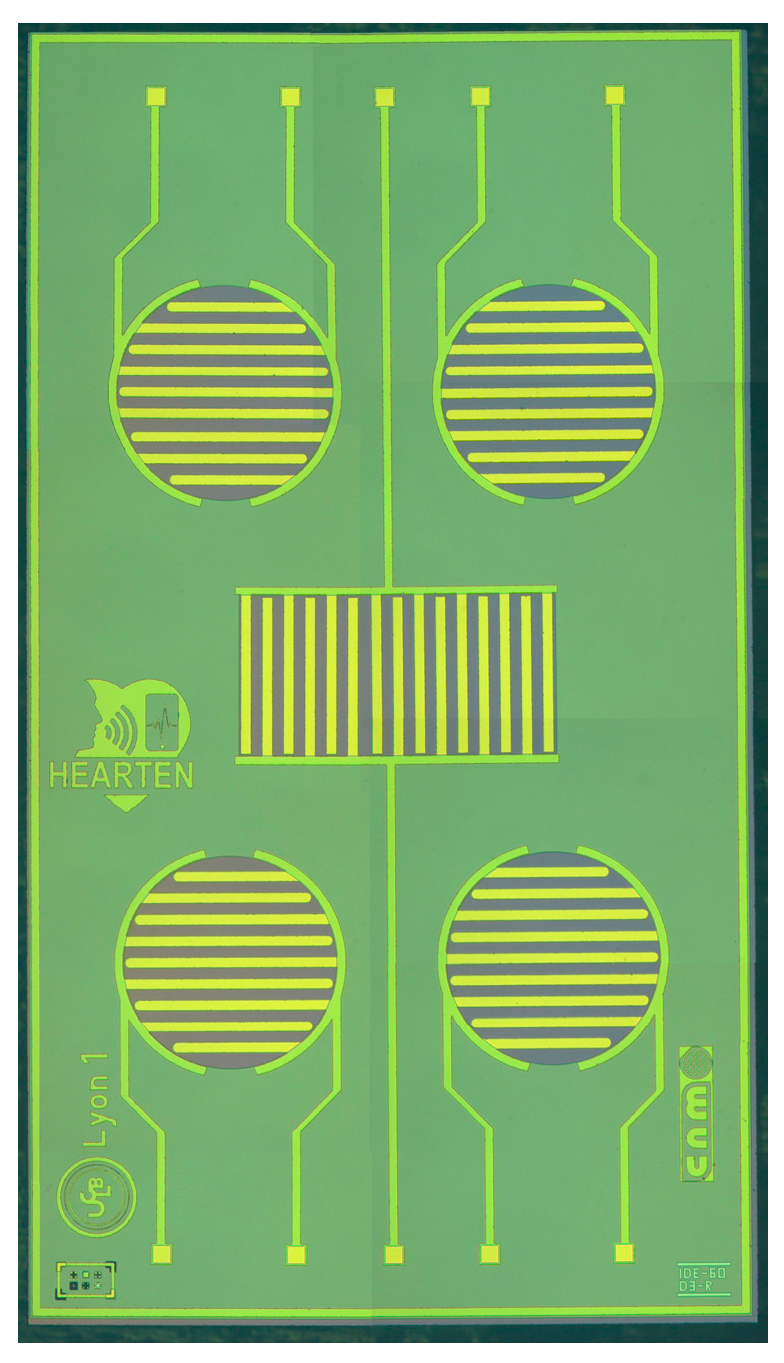
2.2. Microconductometric Chip
2.3. Synthesis of the Gold Nanoparticles (GNPs)
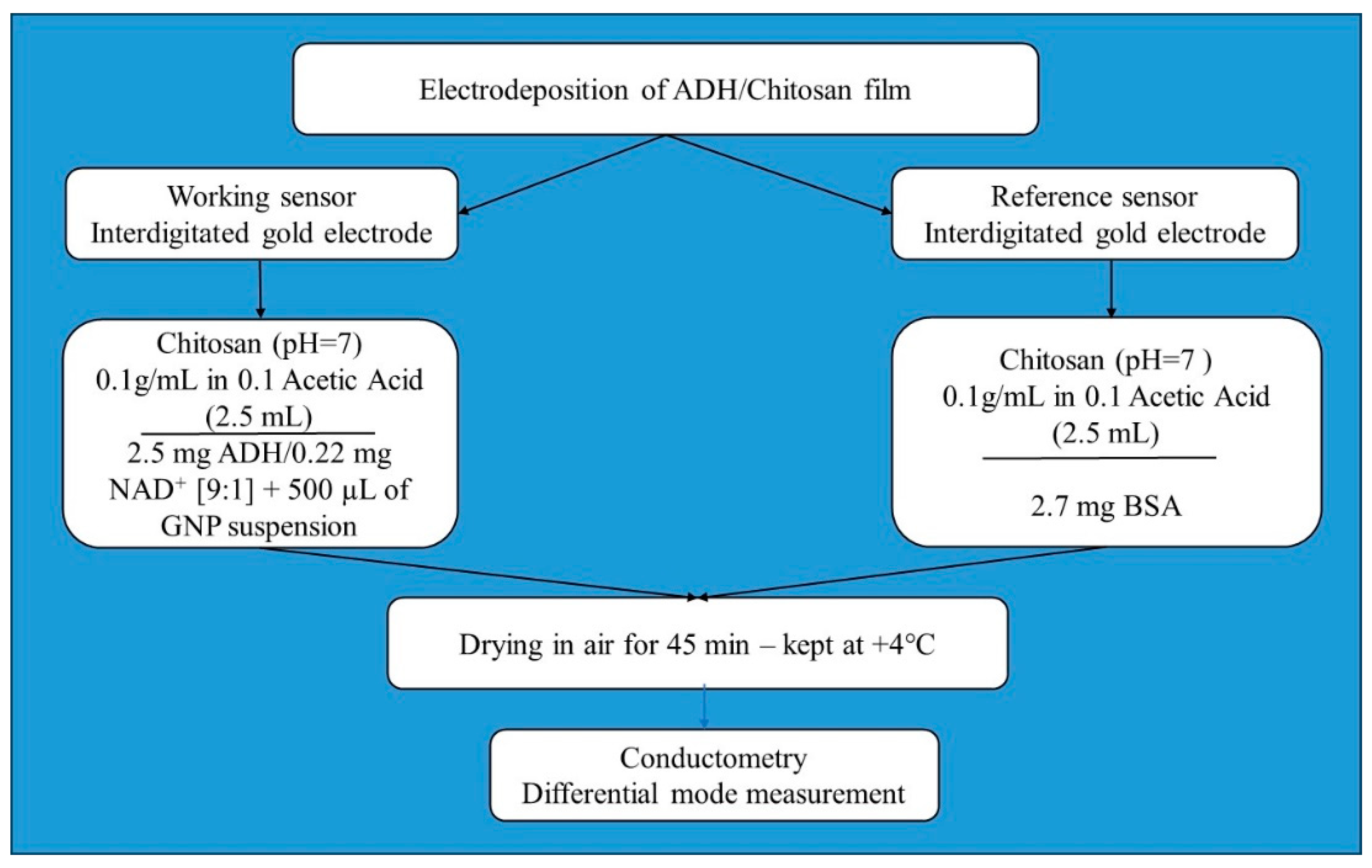
2.4. Fabrication of the Micro Conductometric Ethanol Sensor
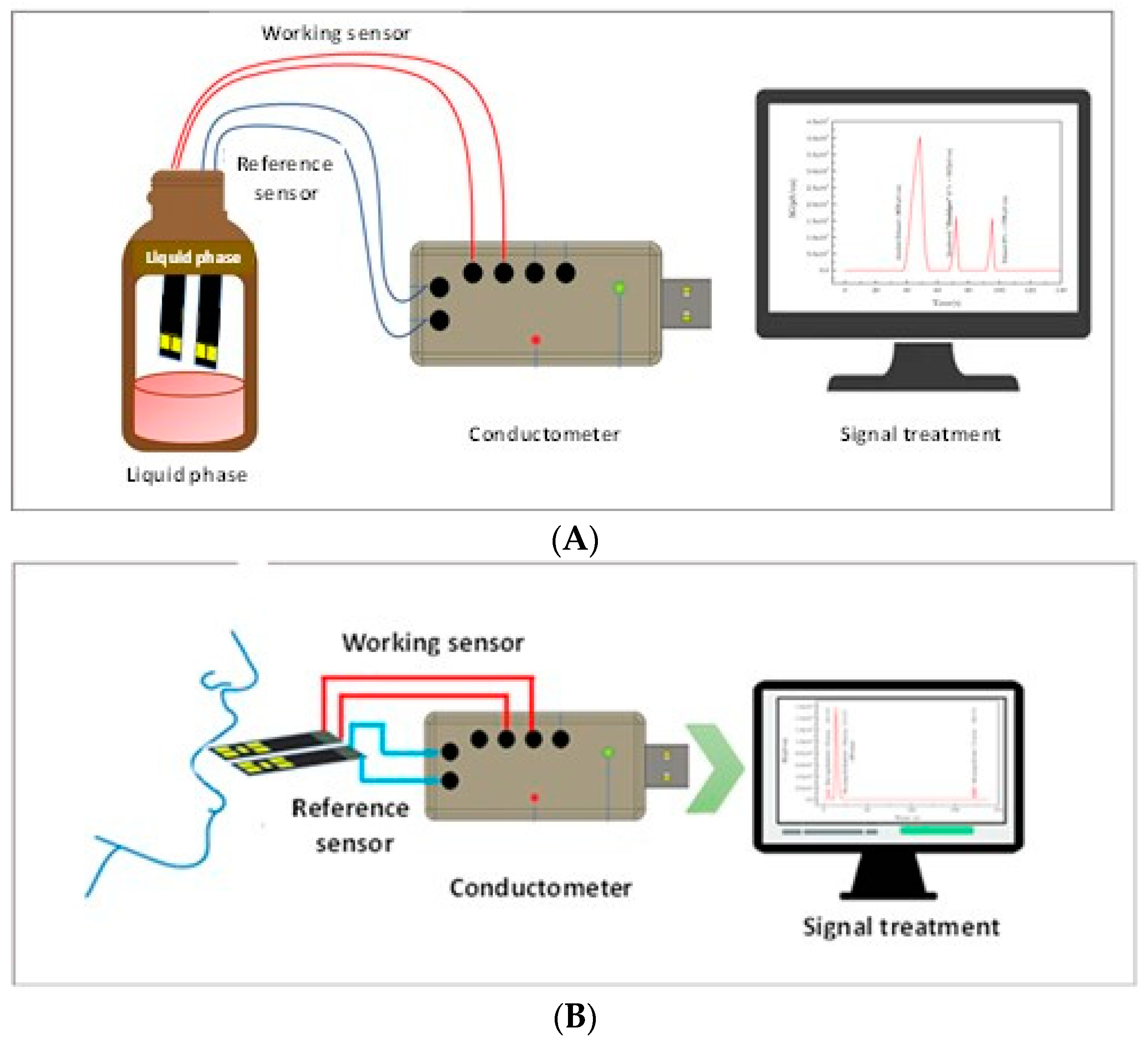
2.5. Micro Conductometric Measurements
2.6. Characterisation Techniques
3. Results and Discussion
3.1. Preparation of the Ethanol Microsensor
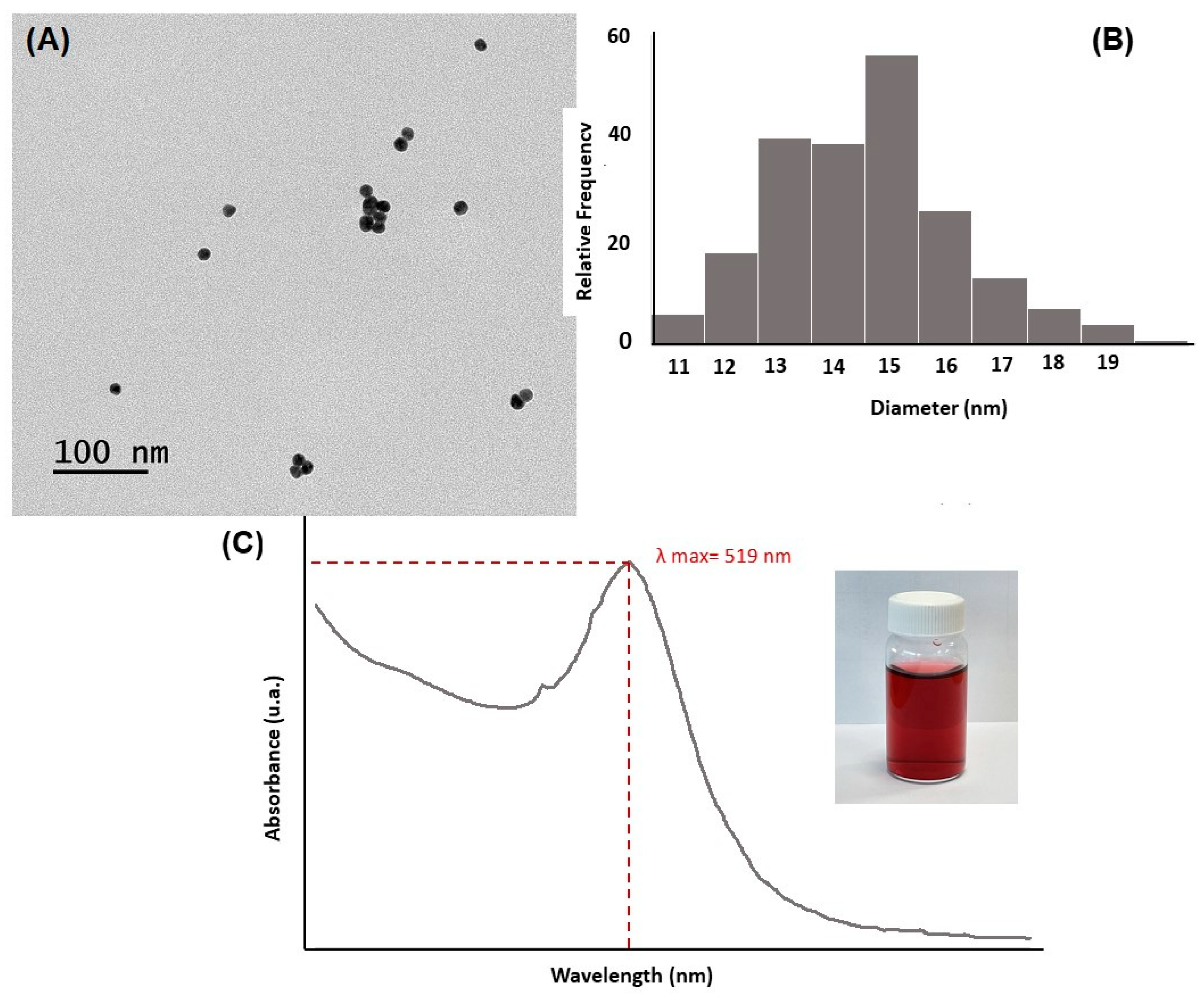
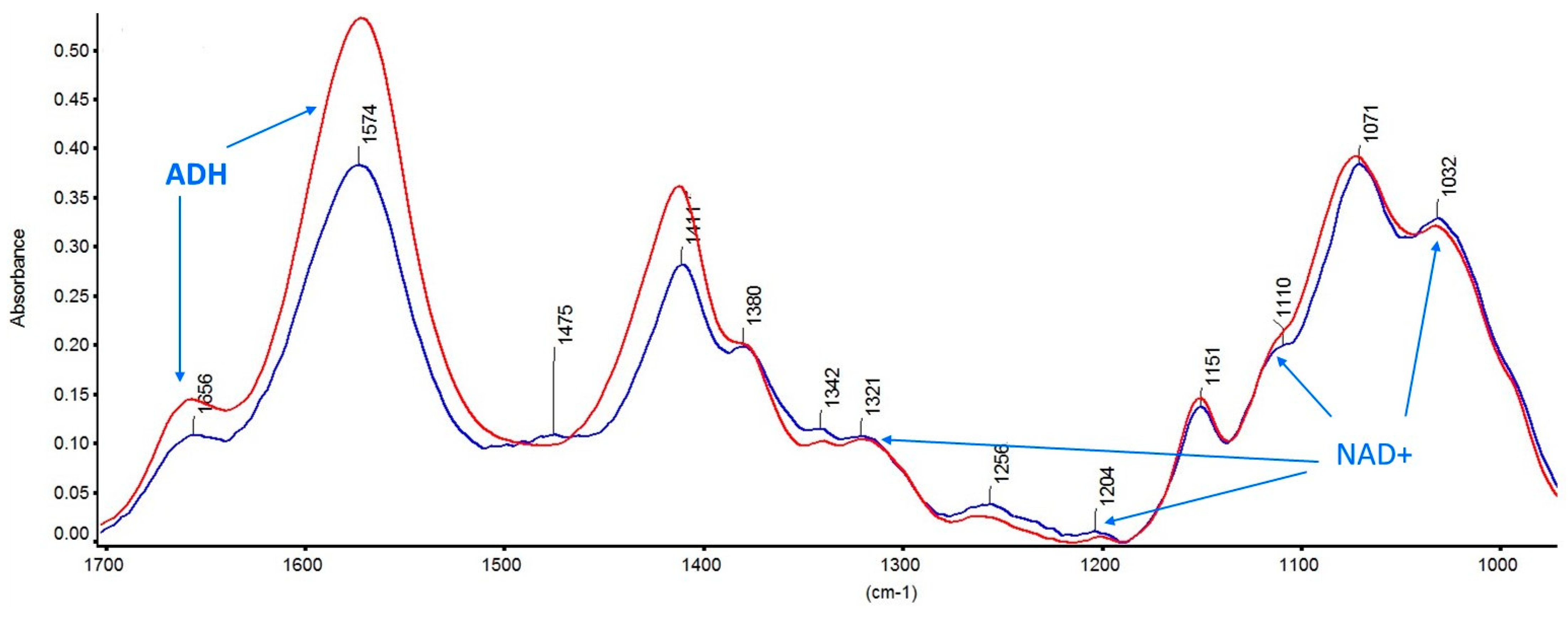
3.1.1. Characterization of the GNPs
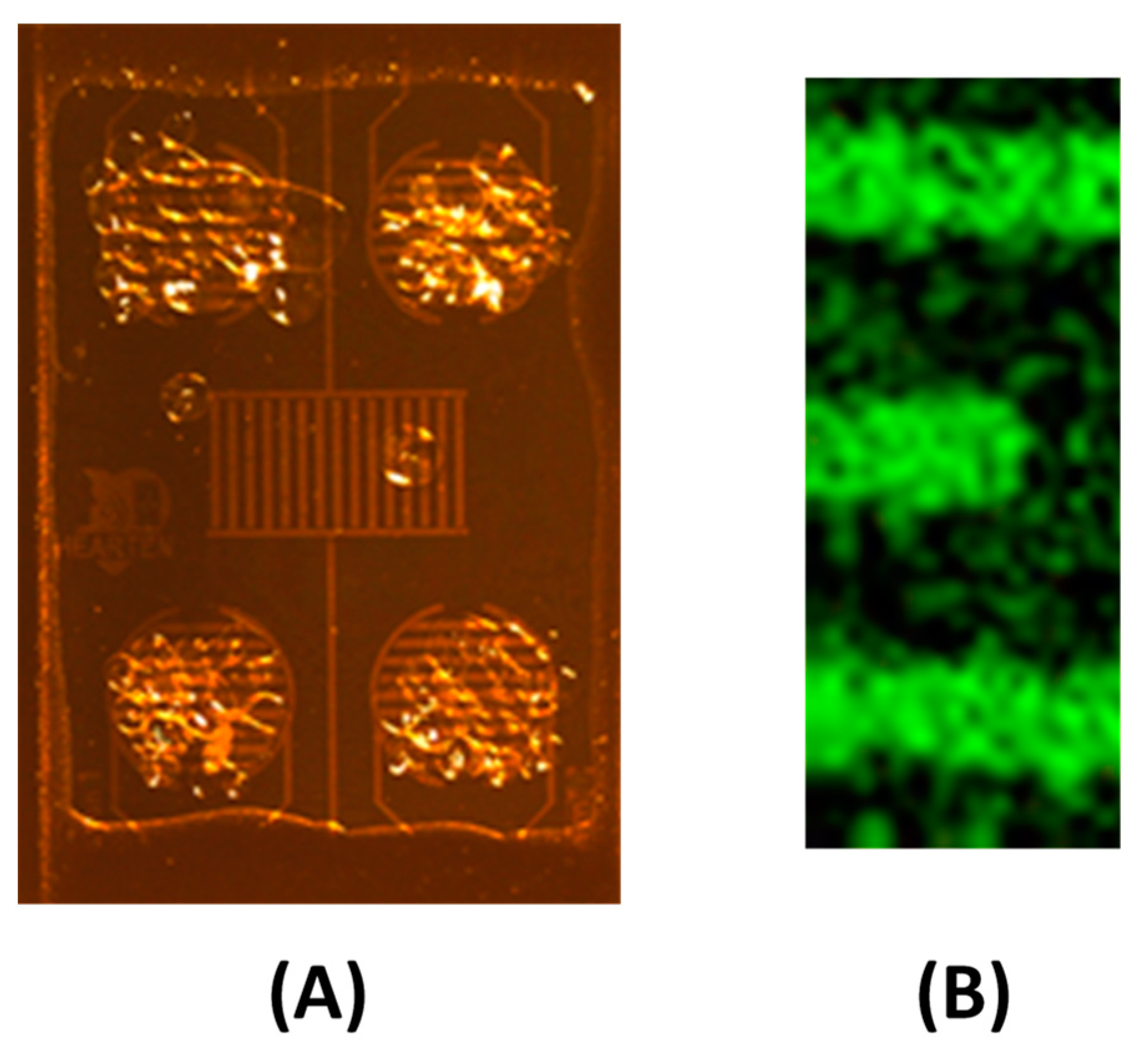
3.1.2. Electrodeposition of the Chitosan Composite Film
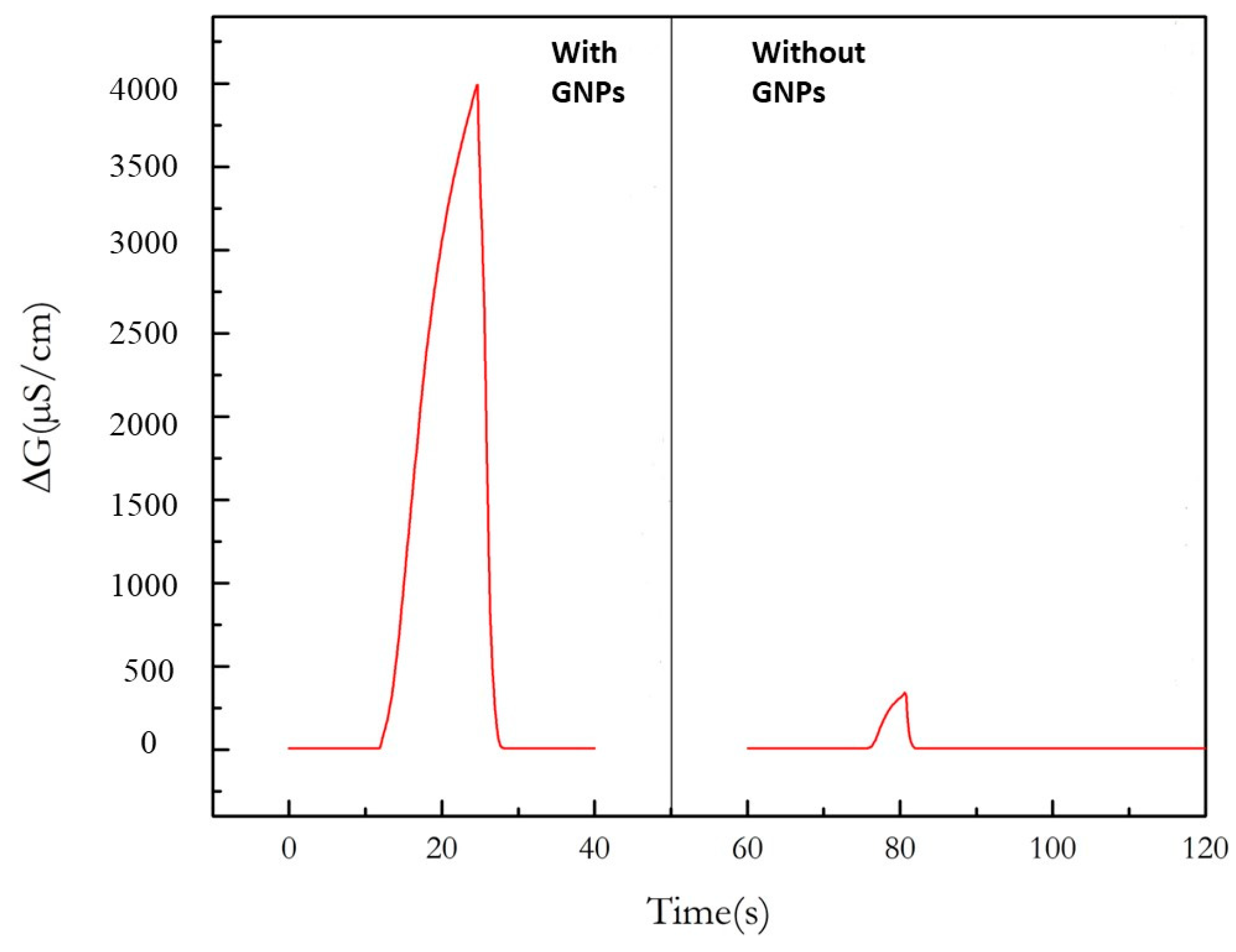
3.2. Effect of the Presence of GNP in the Chitosan Composite Film on the Conductometric Measurements of Ethanol Vapor
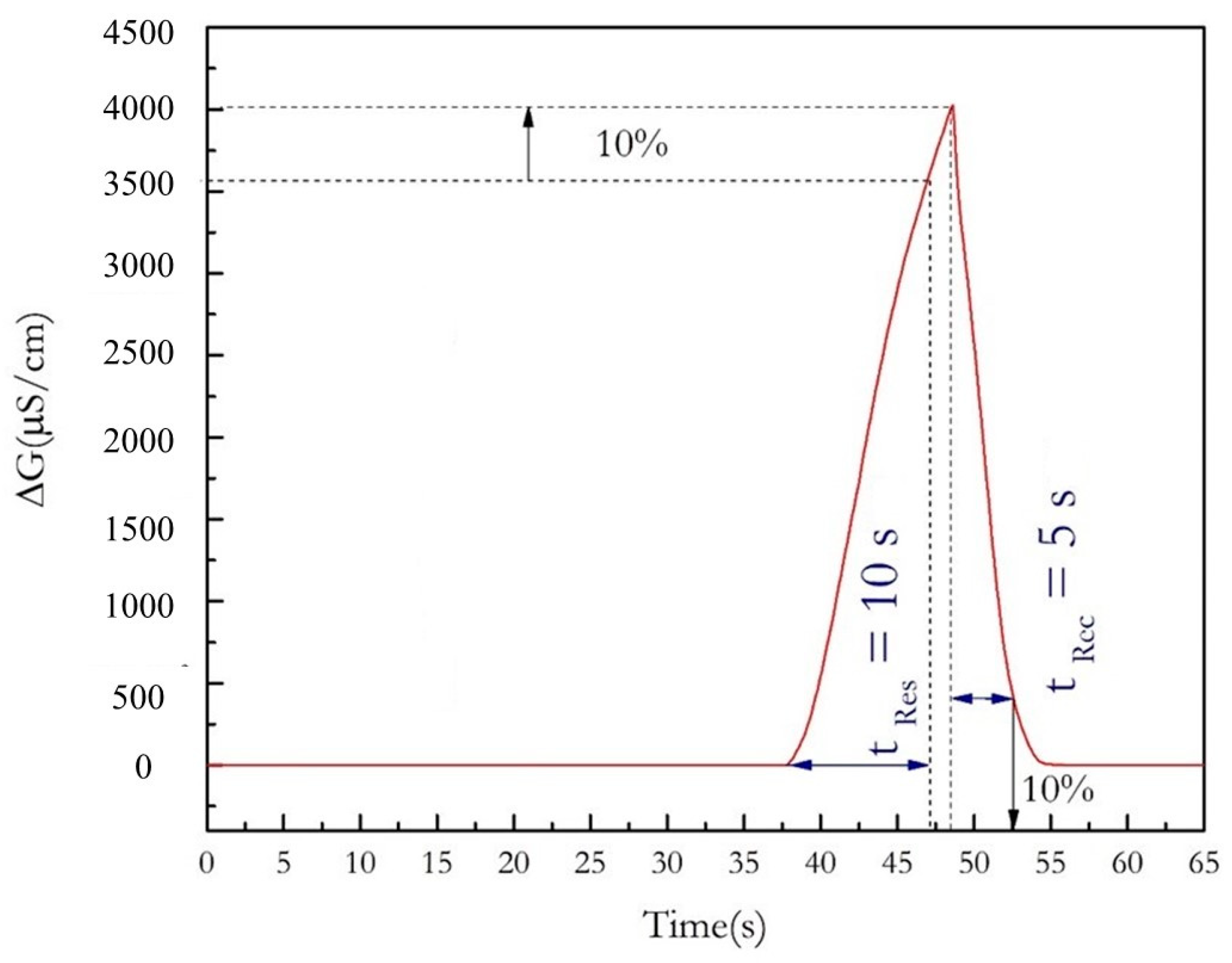
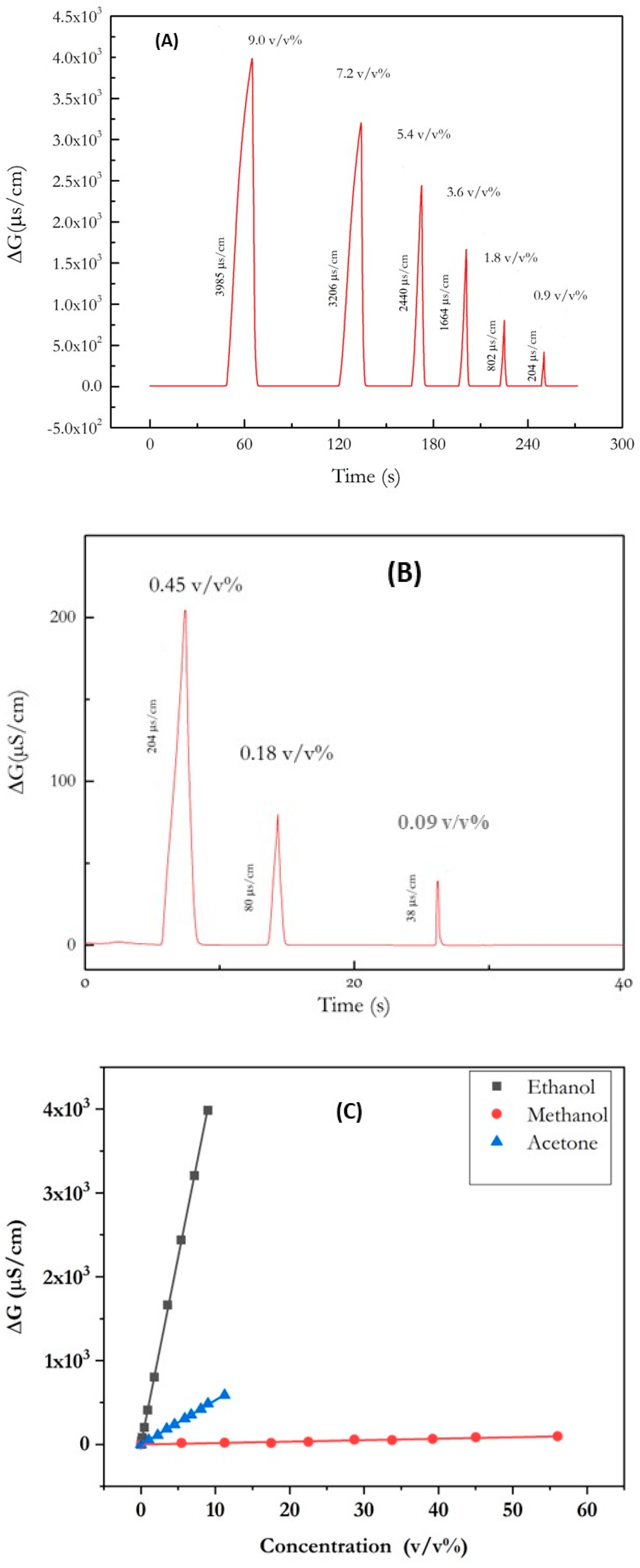
3.3. Analytical Performance of the Ethanol Sensor
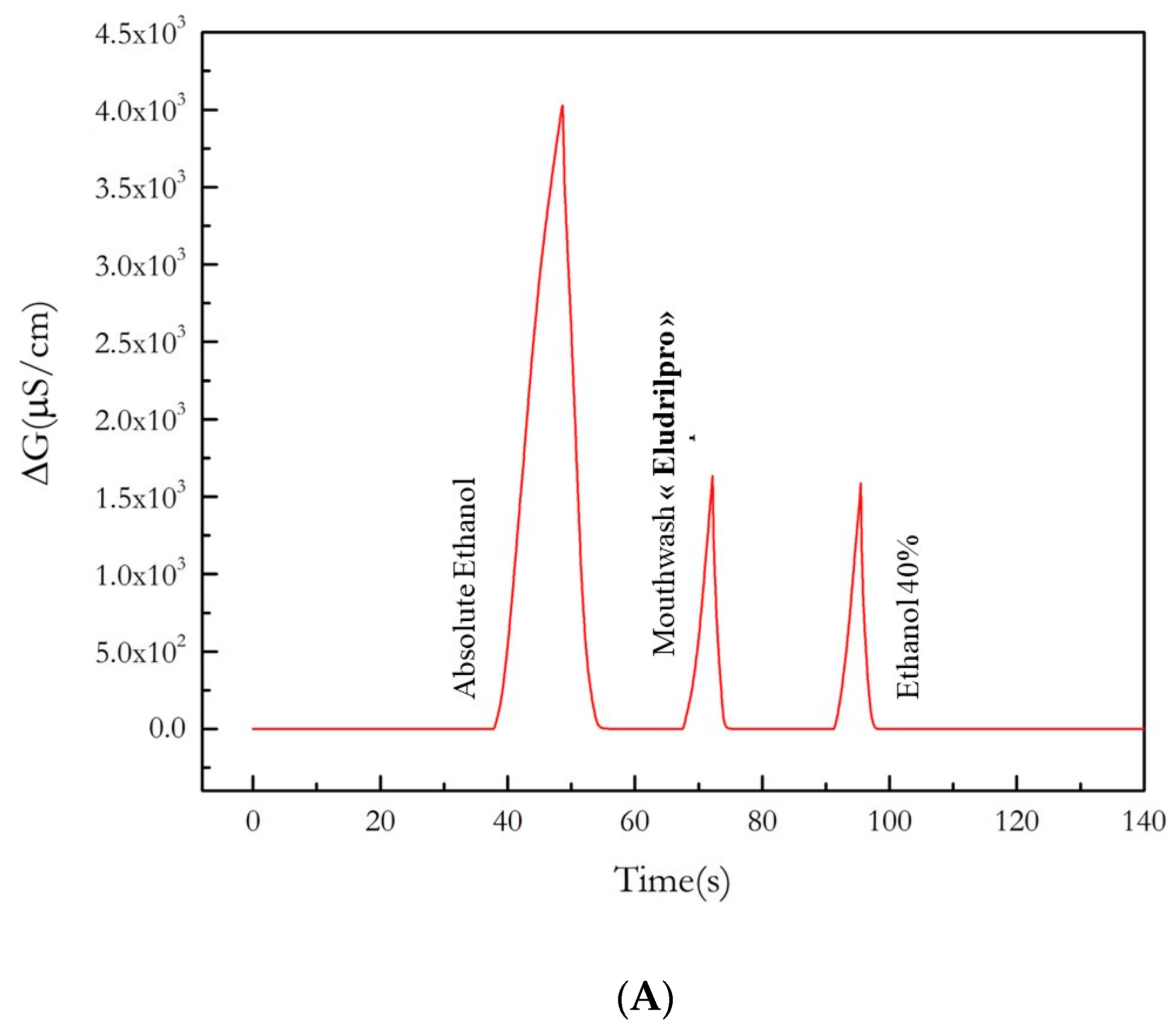
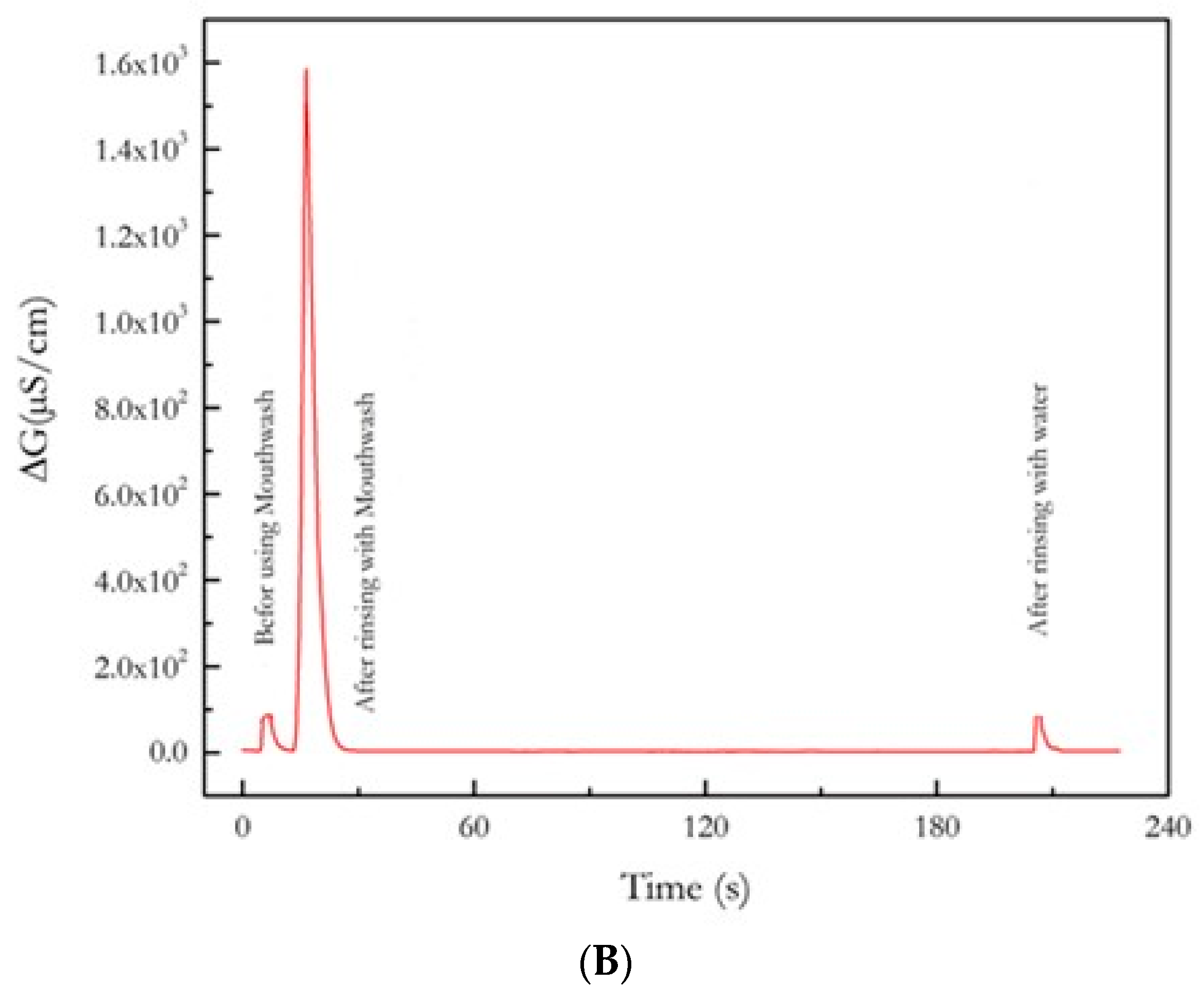
3.4. Effect of Mouthwash on the Content of Ethanol in the Exhaled Air
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sakthithasan, K.; Allanic, C.; Sicot, J.; Jouannique, V. Interactions entre bains de bouche et mesures d’alcool dans l’air expiré. Arch. Maladies Prof. l’Environ. 2020, 81, 718. [Google Scholar] [CrossRef]
- Staerz, A.; Weimar, U.; Barsan, N. Current state of knowledge on the metal oxide-based gas sensing mechanism. Sens. Actuator. B Chem. 2022, 358, 131531. [Google Scholar] [CrossRef]
- Santra, S.; De Luca, A.; Bhaumik, S.; Ali, Z.; Udrea, F.; Gardner, J.W.; Ray, S.K.; Guha, P.K. Dip pen nanolithography-deposited zinc oxide nanorods on a CMOS MEMS platform for ethanol sensing. RSC Adv. 2015, 5, 47609. [Google Scholar] [CrossRef][Green Version]
- Xia, Y.; Pan, A.; Gardner, D.W.; Zhao, S.; Davey, A.K.; Li, Z.; Zhao, L.; Carraro, C.; Maboudian, R. Well-connected ZnO nanoparticle network fabricated by in-situ annealing of ZIF-8 for enhanced sensitivity in gas sensing application. Sens. Actuator B Chem. 2021, 344, 130180. [Google Scholar] [CrossRef]
- Santra, S.; Sinha, A.K.; De Luca, A.; Ali, S.Z.; Udrea, F.; Guha, P.K.; Ray, S.K.; Gardner, J.W. Mask-less deposition of Au–SnO2 nanocomposites on CMOS MEMS platform for ethanol detection. Nanotechnology 2016, 27, 125502. [Google Scholar] [CrossRef] [PubMed]
- Sivalingam, Y.; Martinelli, E.; Catini, A.; Magna, G.; Giuseppe Pomarico, G.; Basoli, F.; Paolesse, R.; Di Natale, C. Gas-Sensitive Photoconductivity of Porphyrin-Functionalized ZnO Nanorods. J. Phys. Chem. C 2012, 116, 9151–9157. [Google Scholar] [CrossRef]
- Iwaki, T.; Covington, J.A.; Udrea, F.; Gardner, J.W. Identification and quantification of different vapours using a single polymer chemoresistor and the novel dual transient temperature modulation technique. Sens. Actuator B Chem. 2009, 141, 370–380. [Google Scholar] [CrossRef]
- Lipatov, A.; Varezhnikov, A.; Wilson, P.; Sysoev, V.; Kolmakov, A.; Sinitskii, A. Highly selective gas sensor arrays based on thermally reduced graphene oxide. Nanoscale 2013, 5, 5426. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Faheem Ahmed, F.; Rashad, M.; Saber, O.; Kumar, S.; Aljaafari, A.; Ashoaibi, A.; Mahmoud, A.Z.; Ezzeldien, M. Low-Temperature Ethanol Sensor via Defective Multiwalled Carbon Nanotubes. Materials 2022, 15, 4439. [Google Scholar] [CrossRef]
- Park, J.K.; Yee, H.J.; Lee, K.S.; Lee, W.Y.; Shin, M.C.; Kim, T.H.; Kim, S.R. Determination of breath alcohol using a differential-type amperometric biosensor based on alcohol dehydrogenase. Anal. Chim. Acta 1999, 390, 83–91. [Google Scholar] [CrossRef]
- Hämmerle, M.; Hilgert, K.; Horn, M.A.; Moos, R. Analysis of volatile alcohols in apple juices by an electrochemical biosensor measuring in the headspace above the liquid. Sens. Actuator B Chem. 2011, 158, 313–318. [Google Scholar] [CrossRef]
- Schlangen, C.; Hämmerle, M.; Moos, R. Amperometric enzyme electrodes for the determination of volatile alcohols in the headspace above fruit and vegetable juices. Microchim. Acta 2012, 179, 115–121. [Google Scholar] [CrossRef]
- Perinotto, A.C.; Caseli, L.; Hayasaka, C.O.; Riul, A., Jr.; Oliveira, O.N., Jr.; Zucolotto, V. Dendrimer-assisted immobilization of alcohol dehydrogenase in nanostructured films for biosensing: Ethanol detection using electrical capacitance measurements. Thin Solid Film. 2008, 516, 9002–9005. [Google Scholar] [CrossRef]
- Madaci, A.; Raffin, G.; Hangouet, M.; Pages, C.; Jose, C.; Martin, M.; Ferkous, H.; Bouzid, A.; Bausells, J.; Alcacer, A.; et al. A microconductometric ethanol sensor prepared through encapsulation of alcohol dehydrogenase in chitosan: Application to the determination of alcoholic content in headspace above beverages. J. Mater. Sci. Mater. Electron. 2021, 32, 17752–17763. [Google Scholar] [CrossRef]
- Arakawa, T.; Sato, T.; Iitani, K.; Toma, K.; Mitsubayashi, K. Fluorometric biosniffer camera “sniff-cam” for direct imaging of gaseous ethanol in breath and transdermal vapor. Anal. Chem. 2017, 89, 4495–4501. [Google Scholar] [CrossRef] [PubMed]
- Iitani, K.; Hayakawa, Y.; Toma, K.; Arakawa, T.; Mitsubayashi, K. Switchable sniff-cam (gas-imaging system) based on redox reactions of alcohol dehydrogenase for ethanol and acetaldehyde in exhaled breath. Talanta 2019, 197, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Aota, T.; Iitani, K.; Toma, K.; Iwasaki, Y.; Mitsubayashi, K. Skin ethanol gas measurement system with a biochemical gas sensor and gas concentrator toward monitoring of blood volatile compounds. Talanta 2020, 219, 121187. [Google Scholar] [CrossRef]
- Iitani, K.; Nakaya, M.; Tomono, T.; Toma, K.; Arakawa, T.; Tsuchido, Y.; Mitsubayashi, K.; Takeda, N. Enzyme-embedded electrospun fiber sensor of hydrophilic polymer for fluorometric ethanol gas imaging in vapor phase. Biosens. Bioelectron. 2022, 213, 114453. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-pour, A.; Verma, M.; Sarampalli, R.Y. Fabrication of nanobiocatalyst using encapsulated laccase onto chitosan-nanobiochar composite. Int. J. Biol. Macromol. 2019, 124, 530–536. [Google Scholar] [CrossRef]
- Zouaoui, F.; Bourouina-Bacha, S.; Bourouina, M.; Jaffrezic-Renault, N.; Zine, N.; Errachid, A. Electrochemical sensors based on molecularly imprinted chitosan: A review. Trends Anal. Chem. 2020, 130, 115982. [Google Scholar] [CrossRef]
- Nouira, W.; Maaref, A.; Vocanson, F.; Siadat, M.; Saulnier, J.; Lagarde, F.; Jaffrezic-Renault, N. Enhancement of enzymatic IDE biosensor response using gold nanoparticles. Example of the detection of urea. Electroanalysis 2012, 24, 1088–1094. [Google Scholar] [CrossRef]
- Temple-Boyer, P.; BenYahia, A.; Sant, W.; Pourciel-Gouzy, M.L.; Launay JMartinez, A. Modelling of urea-EnFETs for haemodialysis applications. Sens. Actuators B Chem. 2008, 131, 525. [Google Scholar] [CrossRef]
- Sheppard, N.F., Jr.; Mears, D.J.; Guiseppi-Elie, A. Model of an immobilized enzyme conductometric urea biosensor. Biosens. Bioelectron. 1996, 11, 967. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, B.; Duan, Z.; Yuan, Z.; Jiang, Y.; Tai, H. Batch fabrication of H2S sensors based on evaporated Pd/WO3 film with ppb-level detection limit. Mater. Chem. Phys. 2023, 302, 127768. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s Law Constants for Inorganic and Organic Species of Potential Importance in Environmental Chemistry, Version 3, 8 April 1999. Available online: http:/www.mpch-mainz.mpg.de∼sander/res/henry.html (accessed on 3 July 2023).
- Snider, J.R.; Dawson, G.A. Tropospheric light alcohols, carbonyls, and acetonitrile: Concentrations in the southwestern United States and Henry’s Law data. J. Geophys. Res Atmos. 1985, 90, 3797–3805. [Google Scholar] [CrossRef]
- Zheng, X.F.; Lian, Q.; Yang, H.; Wang, X. Surface Molecularly imprinted polymer of chitosan grafted poly(methyl methacrylate) for 5-fluorouracil and controlled release. Sci. Rep. 2016, 6, 21409. [Google Scholar] [CrossRef]
- Li, L.; Xie, Y.; Wang, Y.; Yang, X.; Chen, R.F.; Shen, R.F. Study on nicotinamide adenine dinucleotide adsorbed at nanoboehmite/water and nano-corundum/ware interfaces. Colloid Surf. B 2013, 102, 398–404. [Google Scholar] [CrossRef]
- Soldatkin, O.O.; Soldatkina, O.V.; Piliponskiy, I.I.; Rieznichenko, L.S.; Gruzina, T.G.; Dybkova, S.M.; Dzyadevych, S.V.; Soldatkin, A.P. Application of gold nanoparticles for improvement of analytical characteristics of conductometric enzyme biosensors. Appl. Nanosci. 2022, 12, 995–1003. [Google Scholar] [CrossRef]

| Volumetric Percentage in the Liquid Phase | Volumetric Percentage of Ethanol in the Gaseous Phase v/v% | Volumetric Percentage of Methanol in the Gaseous Phase v/v% | Volumetric Percentage of Acetone in the Gaseous Phase v/v% |
|---|---|---|---|
| 0% | 0 | 0 | 0 |
| 20% | 1.79 | 2.25 | 11.26 |
| 40% | 3.58 | 4.5 | 22.52 |
| 60% | 5.37 | 6.75 | 33.78 |
| 80% | 7.16 | 9 | 45.04 |
| 100% | 8.95 | 11.25 | 56.03 |
| Type of Sensor | Response Time (TRes) | Detection Limit | Ref. |
|---|---|---|---|
| ADH/amperometric | 5 s | 20 ppm | [10] |
| AO/amperometric | 69 s | 0.5 ppm | [11] |
| ADH/fluorometric | 20 s | 0.5 ppm | [15] |
| ADH/fluorometric | 20 s | 0.1 ppm | [16] |
| ADH/conductometric | 21s | 1200 ppm | [14] |
| ADH/conductometric | 10 s | 106 ppm | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madaci, A.; Suwannin, P.; Raffin, G.; Hangouet, M.; Martin, M.; Ferkous, H.; Bouzid, A.; Bausells, J.; Elaissari, A.; Errachid, A.; et al. A Sensitive Micro Conductometric Ethanol Sensor Based on an Alcohol Dehydrogenase-Gold Nanoparticle Chitosan Composite. Nanomaterials 2023, 13, 2316. https://doi.org/10.3390/nano13162316
Madaci A, Suwannin P, Raffin G, Hangouet M, Martin M, Ferkous H, Bouzid A, Bausells J, Elaissari A, Errachid A, et al. A Sensitive Micro Conductometric Ethanol Sensor Based on an Alcohol Dehydrogenase-Gold Nanoparticle Chitosan Composite. Nanomaterials. 2023; 13(16):2316. https://doi.org/10.3390/nano13162316
Chicago/Turabian StyleMadaci, Anis, Patcharapan Suwannin, Guy Raffin, Marie Hangouet, Marie Martin, Hana Ferkous, Abderrazak Bouzid, Joan Bausells, Abdelhamid Elaissari, Abdelhamid Errachid, and et al. 2023. "A Sensitive Micro Conductometric Ethanol Sensor Based on an Alcohol Dehydrogenase-Gold Nanoparticle Chitosan Composite" Nanomaterials 13, no. 16: 2316. https://doi.org/10.3390/nano13162316
APA StyleMadaci, A., Suwannin, P., Raffin, G., Hangouet, M., Martin, M., Ferkous, H., Bouzid, A., Bausells, J., Elaissari, A., Errachid, A., & Jaffrezic-Renault, N. (2023). A Sensitive Micro Conductometric Ethanol Sensor Based on an Alcohol Dehydrogenase-Gold Nanoparticle Chitosan Composite. Nanomaterials, 13(16), 2316. https://doi.org/10.3390/nano13162316












