N-Octadecane Encapsulated by Assembled BN/GO Aerogels for Highly Improved Thermal Conductivity and Energy Storage Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of BN
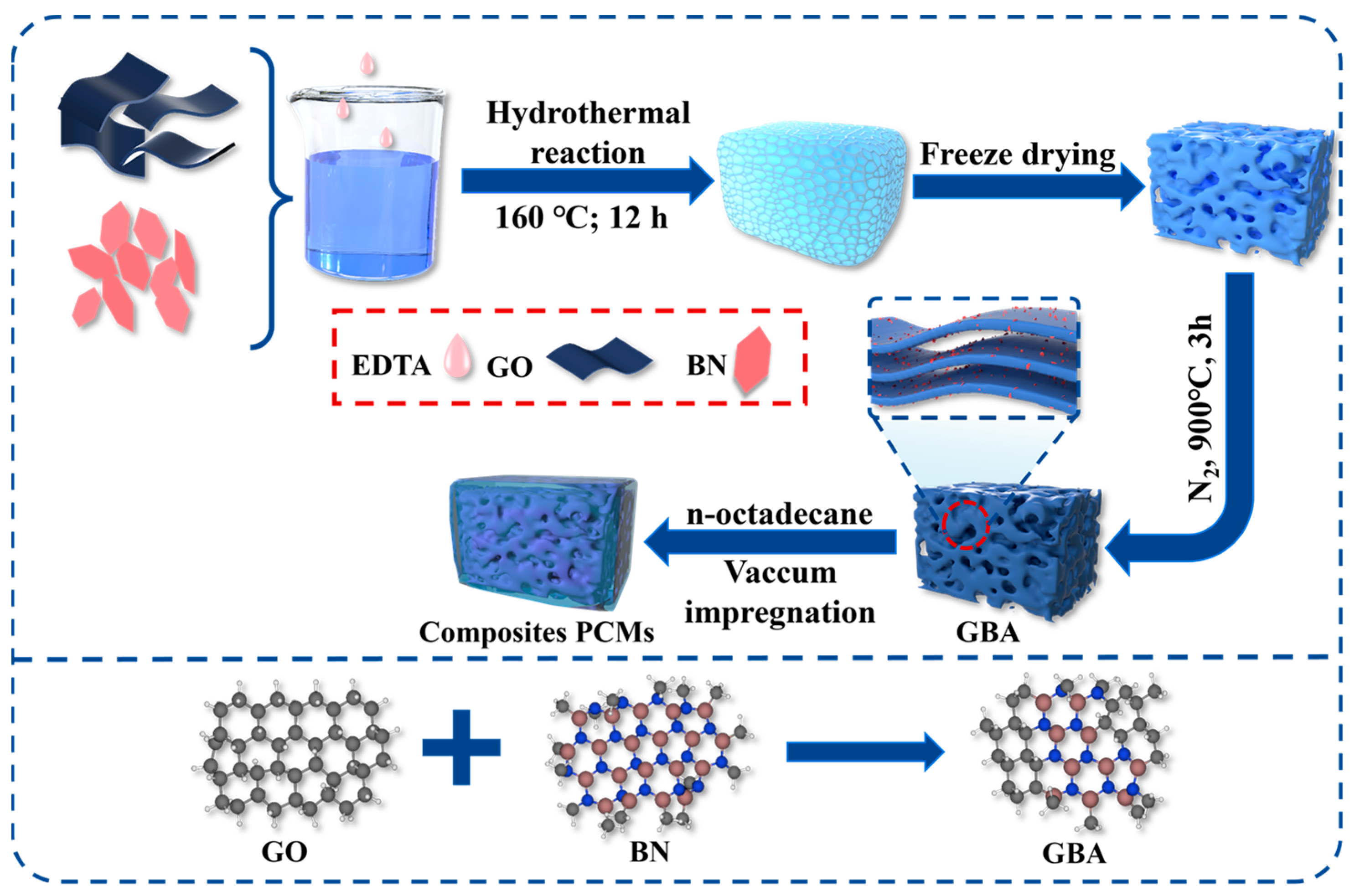
2.3. Preparation of the GBA Aerogels
2.4. Preparation of the OD/GBA Composite PCMs
2.5. Characterization and Tests
3. Results and Discussion
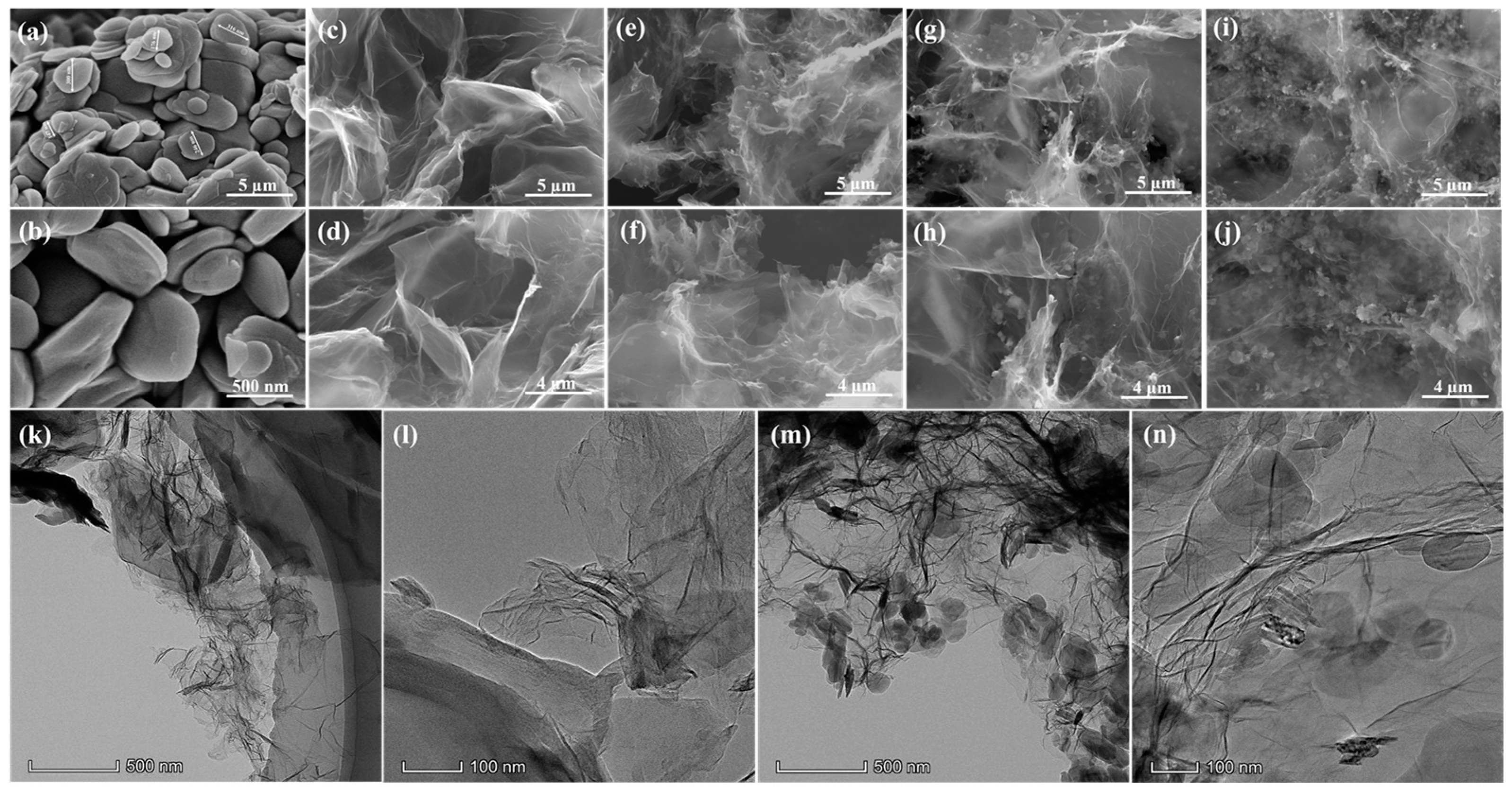
3.1. Micro-Morphologies of GBA
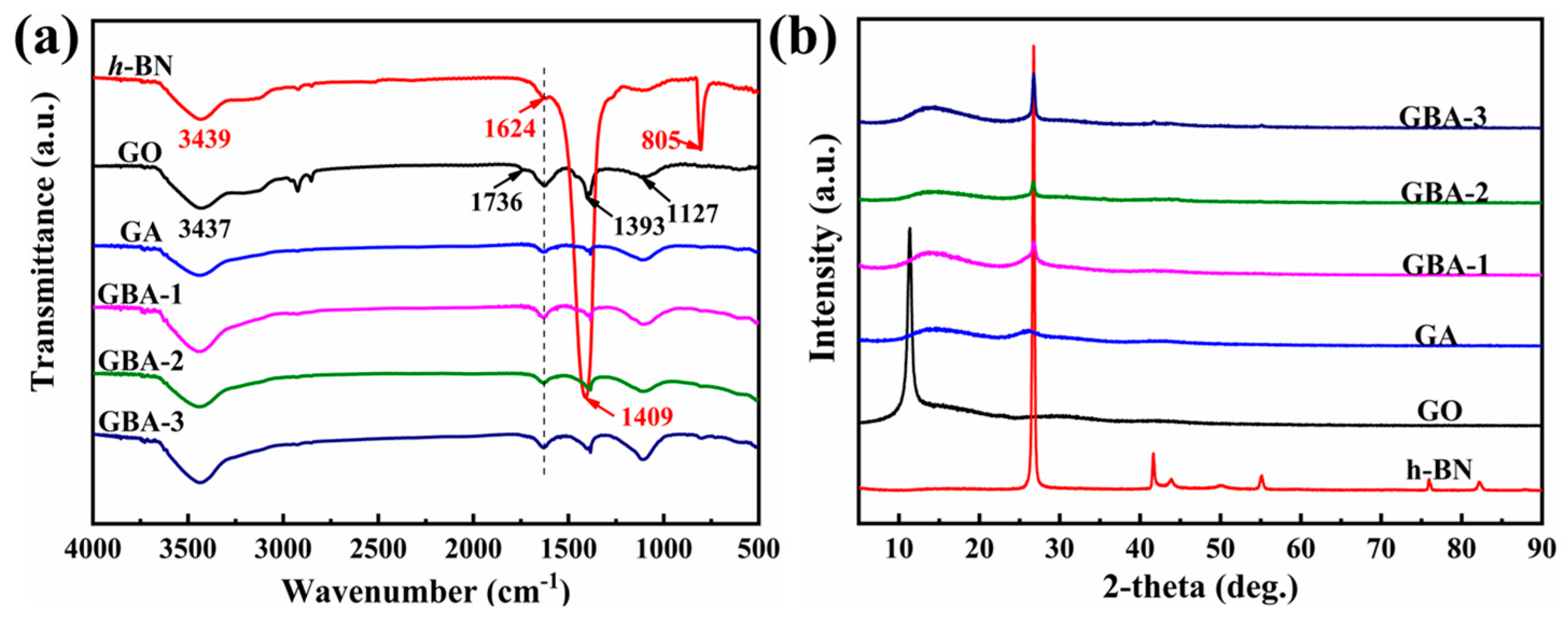
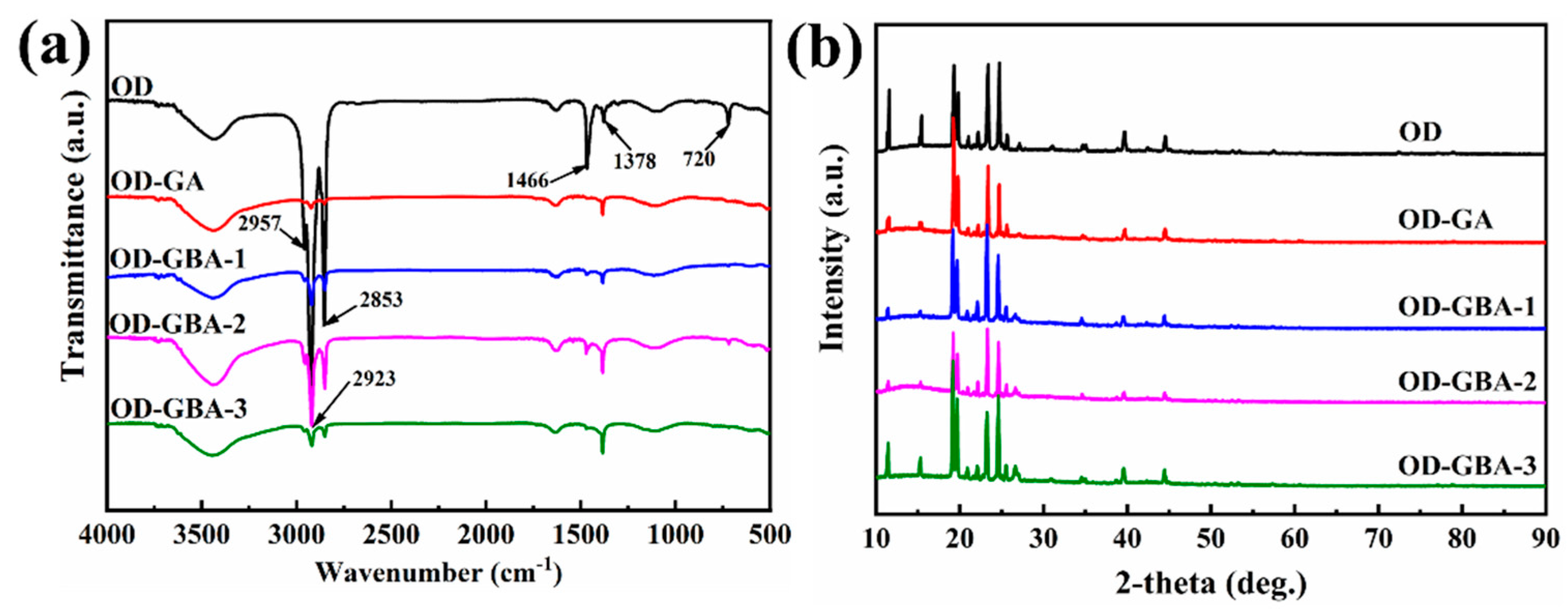
3.2. Chemical Compositions of the GBA
3.3. Adsorption Properties of GBA
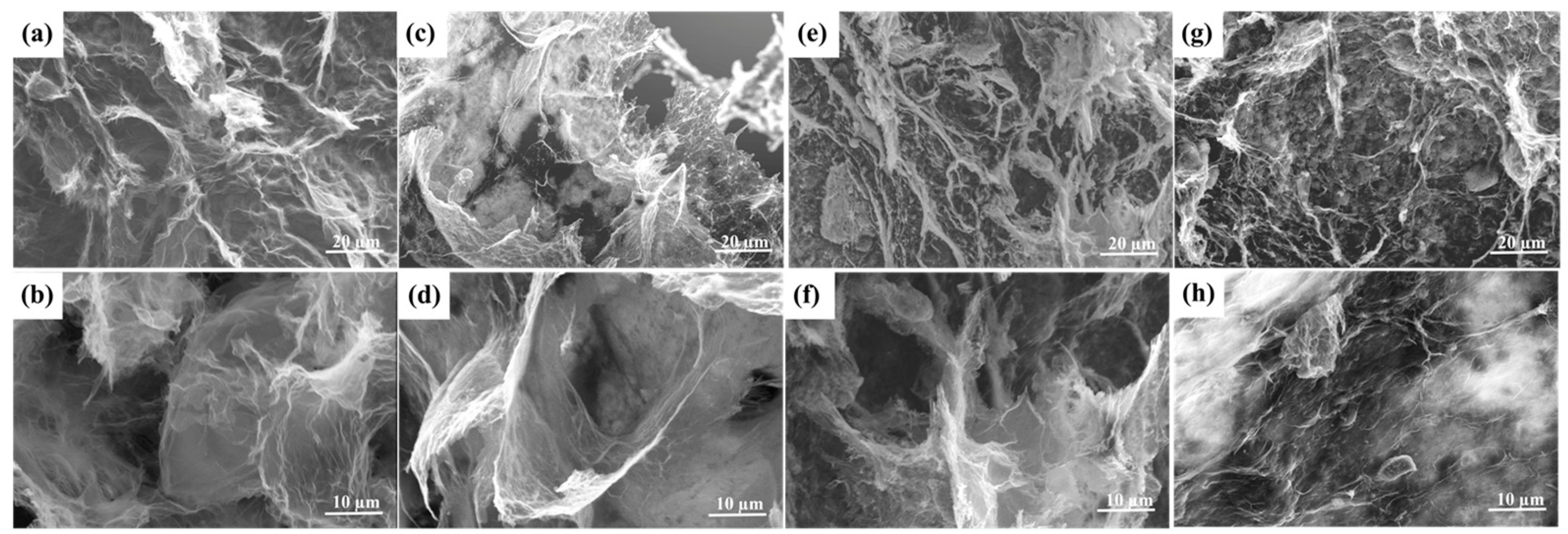
3.4. Micro-Morphologies of the OD/GBA
3.5. Microstructure Analysis of the OD/GBA Composite PCMs
3.6. Thermal Storage Properties of the OD/GBA Composite PCMs
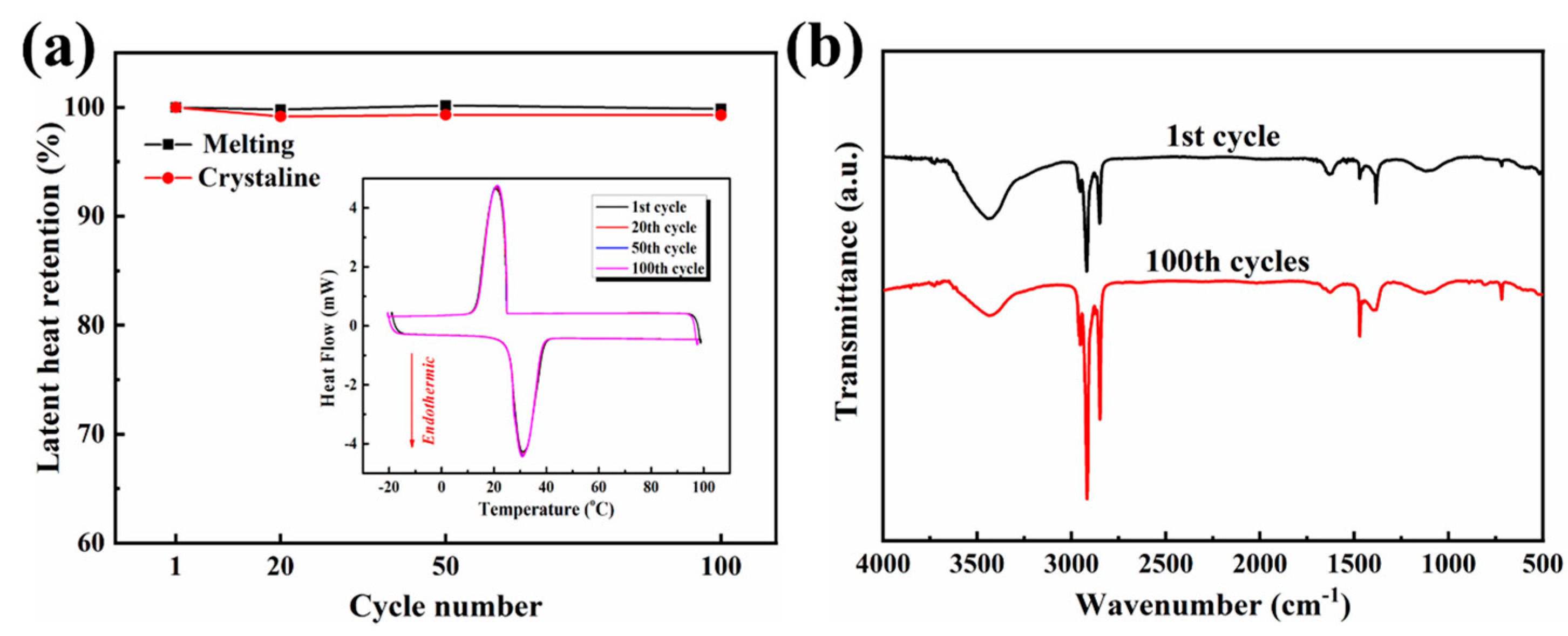
3.7. Thermal Cycling Stability of the OD/GBA Composite PCMs
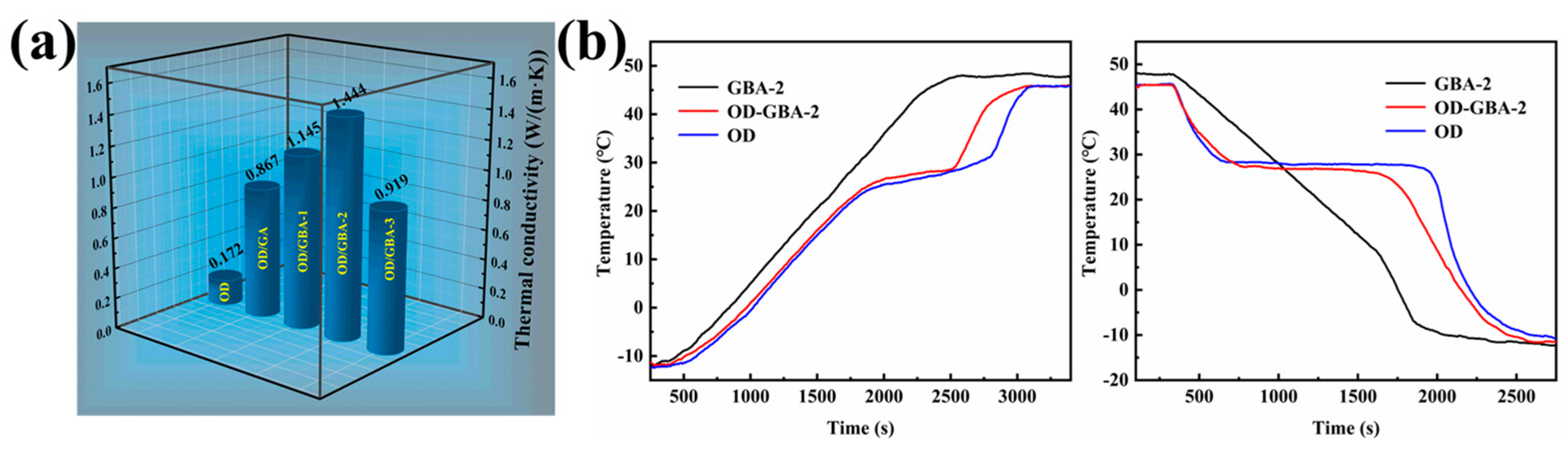
3.8. Thermal Conductivity of the OD/GBA Composite PCMs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Geng, X.; Huang, R.; Zhang, X.; Li, W. Research on Long-Chain Alkanol Etherified Melamine-Formaldehyde Resin MicroPCMs for Energy Storage. Energy 2021, 214, 119029. [Google Scholar] [CrossRef]
- Nie, B.; Palacios, A.; Zou, B.; Liu, J.; Zhang, T.; Li, Y. Review on Phase Change Materials for Cold Thermal Energy Storage Applications. Renew. Sustain. Energy Rev. 2020, 134, 110340. [Google Scholar] [CrossRef]
- Niu, Z.; Yuan, W. Highly Efficient Thermo- and Sunlight-Driven Energy Storage for Thermo-Electric Energy Harvesting Using Sustainable Nanocellulose-Derived Carbon Aerogels Embedded Phase Change Materials. ACS Sustain. Chem. Eng. 2019, 7, 17523–17534. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bhanja, D.; Nath, S. Numerical Investigation of Heat Transfer and Melting Process of Phase Change Material in Trapezoidal Cavities with Different Shapes and Different Heated Tube Positions. J. Energy Storage 2023, 72, 108285. [Google Scholar] [CrossRef]
- Fan, X.; Guan, Y.; Li, Y.; Yu, H.-Y.; Marek, J.; Wang, D.; Militky, J.; Zou, Z.-Y.; Yao, J. Shape-Stabilized Cellulose Nanocrystal-Based Phase-Change Materials for Energy Storage. ACS Appl. Nano Mater. 2020, 3, 1741–1748. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, W.; Zhang, H.; He, F.; Yan, H.; He, R.; Zhang, K.; Fan, J. Graphene Oxide Pickering Phase Change Material Emulsions with High Thermal Conductivity and Photo-Thermal Performance for Thermal Energy Management. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 42–49. [Google Scholar] [CrossRef]
- Jing, R.; Zhang, H.; Huang, C.; Su, F.; Wu, B.; Sun, Z.; Xu, F.; Sun, L.; Xia, Y.; Peng, H.; et al. Construction of Double Cross-Linking PEG/h-BN@GO Polymeric Energy-Storage Composites with High Structural Stability and Excellent Thermal Performances. Colloids Surf. A Physicochem. Eng. Asp. 2022, 638, 128193. [Google Scholar] [CrossRef]
- Mert, M.S.; Mert, H.H.; Arıcı, M. Development and Properties of N-Octadecane/Kaolinite Composites as Form-Stabilized Phase Change Materials for Energy Storage. J. Clean. Prod. 2023, 410, 137304. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of Solid–Liquid Phase Change Materials and Their Encapsulation Technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Su, J.; Zhang, X.; Han, N.; Wang, X. Microstructure and Thermal Reliability of Microcapsules Containing Phase Change Material with Self-Assembled Graphene/Organic Nano-Hybrid Shells. Nanomaterials 2018, 8, 364. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Tang, B.; Lu, R.; Zhang, S. Form-Stable Phase Change Materials with High Phase Change Enthalpy from the Composite of Paraffin and Cross-Linking Phase Change Structure. Appl. Energy 2016, 184, 241–246. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, X.; Kong, X. Preparation and Characterization of a Novel Composite Phase Change Material with Double Phase Change Points Based on Nanocapsules. Renew. Energy 2020, 147, 374–383. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Sulaiman, F.A.; Ibrahim, N.I.; Zahir, M.H.; Al-Ahmed, A.; Saidur, R.; Yılbaş, B.S.; Sahin, A.Z. A Review on Current Status and Challenges of Inorganic Phase Change Materials for Thermal Energy Storage Systems. Renew. Sustain. Energy Rev. 2017, 70, 1072–1089. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Chen, P.; Rojas, R.; Hajian, A.; Berglund, L. Cellulose Nanofibers Enable Paraffin Encapsulation and the Formation of Stable Thermal Regulation Nanocomposites. Nano Energy 2017, 34, 541–548. [Google Scholar] [CrossRef]
- Wang, C.; Liang, W.; Yang, Y.; Liu, F.; Sun, H.; Zhu, Z.; Li, A. Biomass Carbon Aerogels Based Shape-Stable Phase Change Composites with High Light-to-Thermal Efficiency for Energy Storage. Renew. Energy 2020, 153, 182–192. [Google Scholar] [CrossRef]
- Cao, Y.; Weng, M.; Mahmoud, M.H.H.; Elnaggar, A.Y.; Zhang, L.; El Azab, I.H.; Chen, Y.; Huang, M.; Huang, J.; Sheng, X. Flame-Retardant and Leakage-Proof Phase Change Composites Based on MXene/Polyimide Aerogels toward Solar Thermal Energy Harvesting. Adv. Compos. Hybrid Mater. 2022, 5, 1253–1267. [Google Scholar] [CrossRef]
- Lei, C.; Wu, K.; Wu, L.; Liu, W.; Du, R.; Chen, F.; Fu, Q. Phase Change Material with Anisotropically High Thermal Conductivity and Excellent Shape Stability Due to Its Robust Cellulose/BNNSs Skeleton. J. Mater. Chem. A 2019, 7, 19364–19373. [Google Scholar] [CrossRef]
- Qiu, J.; Fan, X.; Shi, Y.; Zhang, S.; Jin, X.; Wang, W.; Tang, B. PEG/3D Graphene Oxide Network Form-Stable Phase Change Materials with Ultrahigh Filler Content. J. Mater. Chem. A 2019, 7, 21371–21377. [Google Scholar] [CrossRef]
- Yang, J.; Tang, L.-S.; Bao, R.-Y.; Bai, L.; Liu, Z.-Y.; Xie, B.-H.; Yang, M.-B.; Yang, W. Hybrid Network Structure of Boron Nitride and Graphene Oxide in Shape-Stabilized Composite Phase Change Materials with Enhanced Thermal Conductivity and Light-to-Electric Energy Conversion Capability. Sol. Energy Mater. Sol. Cells 2018, 174, 56–64. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Liang, W.; Liu, F.; Wang, S.; Sun, H.; Zhu, Z.; Li, A. Enhanced Light-to-Thermal Conversion Performance of All-Carbon Aerogels Based Form-Stable Phase Change Material Composites. J. Colloid Interface Sci. 2022, 605, 60–70. [Google Scholar] [CrossRef]
- Chen, X.; Huang, X.; Shi, T.-Y.; Wang, J.-X.; Yuan, X.-R.; Huang, H.; Wang, J.; He, R.; Yu, X.-F. Synthesis and Properties of Shape-Stabilized Phase Change Materials Based on Poly(Triallyl Isocyanurate-Silicone)/n-Octadecane Composites. ACS Omega 2022, 7, 14952–14960. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zeng, L.; Chang, G.; Zhou, Y.; Yan, K.; Xie, L.; Xue, B.; Zheng, Q. Composite Phase Change Materials Embedded into Cellulose/Polyacrylamide/Graphene Nanosheets/Silver Nanowire Hybrid Aerogels Simultaneously with Effective Thermal Management and Anisotropic Electromagnetic Interference Shielding. Adv. Compos. Hybrid Mater. 2023, 6, 31. [Google Scholar] [CrossRef]
- Zehri, A.; Samani, M.K.; Latorre, M.G.; Nylander, A.; Nilsson, T.; Fu, Y.; Wang, N.; Ye, L.; Liu, J. High Porosity and Light Weight Graphene Foam Heat Sink and Phase Change Material Container for Thermal Management. Nanotechnology 2020, 31, 424003. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, S.; Fu, H.; Qu, J.; Zhang, Z. Yin-Yang Heat Flow Rate Converter: Based on Guar Gum Composite Phase Change Materials with Controllable Thermal Conductivity. Chem. Eng. J. 2023, 470, 144069. [Google Scholar]
- Sun, Z.; Zhang, H.; Zhang, Q.; Jing, R.; Wu, B.; Xu, F.; Sun, L.; Xia, Y.; Rosei, F.; Peng, H.; et al. Shape-Stabilized Phase Change Composites Enabled by Lightweight and Bio-Inspired Interconnecting Carbon Aerogels for Efficient Energy Storage and Photo-Thermal Conversion. J. Mater. Chem. A 2022, 10, 13556–13569. [Google Scholar] [CrossRef]
- Zambotti, A.; Caldesi, E.; Pellizzari, M.; Valentini, F.; Pegoretti, A.; Dorigato, A.; Speranza, G.; Chen, K.; Bortolotti, M.; Sorarù, G.D.; et al. Polymer-Derived Silicon Nitride Aerogels as Shape Stabilizers for Low and High-Temperature Thermal Energy Storage. J. Eur. Ceram. Soc. 2021, 41, 5484–5494. [Google Scholar] [CrossRef]
- Peydayesh, M.; Suter, M.K.; Bolisetty, S.; Boulos, S.; Handschin, S.; Nyström, L.; Mezzenga, R. Amyloid Fibrils Aerogel for Sustainable Removal of Organic Contaminants from Water. Adv. Mater. 2020, 32, 1907932. [Google Scholar] [CrossRef]
- Lv, L.; Wang, Y.; Ai, H.; Chen, T.; Zhang, X.; Song, S. 3D Graphene/Silver Nanowire Aerogel Encapsulated Phase Change Material with Significantly Enhanced Thermal Conductivity and Excellent Solar-Thermal Energy Conversion Capacity. J. Mater. Chem. A 2022, 10, 7773–7784. [Google Scholar] [CrossRef]
- Zhang, J.; You, J.; Wei, Q.; Han, J.-I.; Liu, Z. Hollow Porous CoO@Reduced Graphene Oxide Self-Supporting Flexible Membrane for High Performance Lithium-Ion Storage. Nanomaterials 2023, 13, 1986. [Google Scholar] [CrossRef]
- Macroscopic Zn-Doped α-Fe2O3/Graphene Aerogel Mediated Persulfate Activation for Heterogeneous Catalytic Degradation of Sulfamonomethoxine Wastewater. J. Ind. Eng. Chem. 2022, 108, 254–262. [CrossRef]
- Yu, C.; Song, Y.S. Modification of Graphene Aerogel Embedded Form-Stable Phase Change Materials for High Energy Harvesting Efficiency. Macromol. Res. 2022, 30, 198–204. [Google Scholar] [CrossRef]
- Yang, P.; Wu, B.; Tong, X.; Zeng, M.; Wang, Q.; Cheng, Z. Insight into Heat Transfer Process of Graphene Aerogel Composite Phase Change Material. Energy 2023, 279, 128051. [Google Scholar] [CrossRef]
- Min, P.; Liu, J.; Li, X.; An, F.; Liu, P.; Shen, Y.; Koratkar, N.; Yu, Z.-Z. Thermally Conductive Phase Change Composites Featuring Anisotropic Graphene Aerogels for Real-Time and Fast-Charging Solar-Thermal Energy Conversion. Adv. Funct. Mater. 2018, 28, 1805365. [Google Scholar] [CrossRef]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Metselaar, H.S.C.; Silakhori, M. Shape-Stabilized Phase Change Materials with High Thermal Conductivity Based on Paraffin/Graphene Oxide Composite. Energy Convers. Manag. 2013, 67, 275–282. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, K.; Min, X.; Xiao, J.; Xu, Z.; Huang, Z.; Liu, Y.; Wu, X.; Fang, M. Graphene Aerogel Stabilized Phase Change Material for Thermal Energy Storage. Case Stud. Therm. Eng. 2022, 40, 102497. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, C.; Wang, J.; Wang, Y.; Yu, F. Carbon Hybrid Aerogel-Based Phase Change Material with Reinforced Energy Storage and Electro-Thermal Conversion Performance for Battery Thermal Management. J. Energy Storage 2022, 52, 104905. [Google Scholar] [CrossRef]
- Wang, T.; Lin, Y.; Li, P.; Jiang, P.; Zhang, C.; Xu, H.; Xie, H.; Huang, X. Unidirectional Thermal Conduction in Electrically Insulating Phase Change Composites for Superior Power Output of Thermoelectric Generators. Compos. Sci. Technol. 2022, 225, 109500. [Google Scholar] [CrossRef]
- Li, Q.; Xue, Z.; Zhao, J.; Ao, C.; Jia, X.; Xia, T.; Wang, Q.; Deng, X.; Zhang, W.; Lu, C. Mass Production of High Thermal Conductive Boron Nitride/Nanofibrillated Cellulose Composite Membranes. Chem. Eng. J. 2020, 383, 123101. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Li, Q.; Chen, C.; Chen, Z.; Song, P.; Hao, J.; Li, Y.; Fakhrhoseini, S.; Naebe, M.; et al. Lightweight, Superelastic Yet Thermoconductive Boron Nitride Nanocomposite Aerogel for Thermal Energy Regulation. ACS Nano 2019, 13, 7860–7870. [Google Scholar] [CrossRef]
- Du, Z.; Zeng, X.; Zhu, M.; Kanta, A.; Liu, Q.; Li, J.; Kong, L.B. Alkyl Ethoxylate Assisted Liquid Phase Exfoliation of BN Nanosheet and Its Application as Interphase for Oxide/Oxide Composites. Ceram. Int. 2018, 44, 21461–21469. [Google Scholar] [CrossRef]
- Guo, Y.; Lyu, Z.; Yang, X.; Lu, Y.; Ruan, K.; Wu, Y.; Kong, J.; Gu, J. Enhanced Thermal Conductivities and Decreased Thermal Resistances of Functionalized Boron Nitride/Polyimide Composites. Compos. B Eng. 2019, 164, 732–739. [Google Scholar] [CrossRef]
- Jiang, F.; Cui, S.; Song, N.; Shi, L.; Ding, P. Hydrogen Bond-Regulated Boron Nitride Network Structures for Improved Thermal Conductive Property of Polyamide-Imide Composites. ACS Appl. Mater. Interfaces 2018, 10, 16812–16821. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on Thermal Energy Storage with Phase Change Materials and Applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]

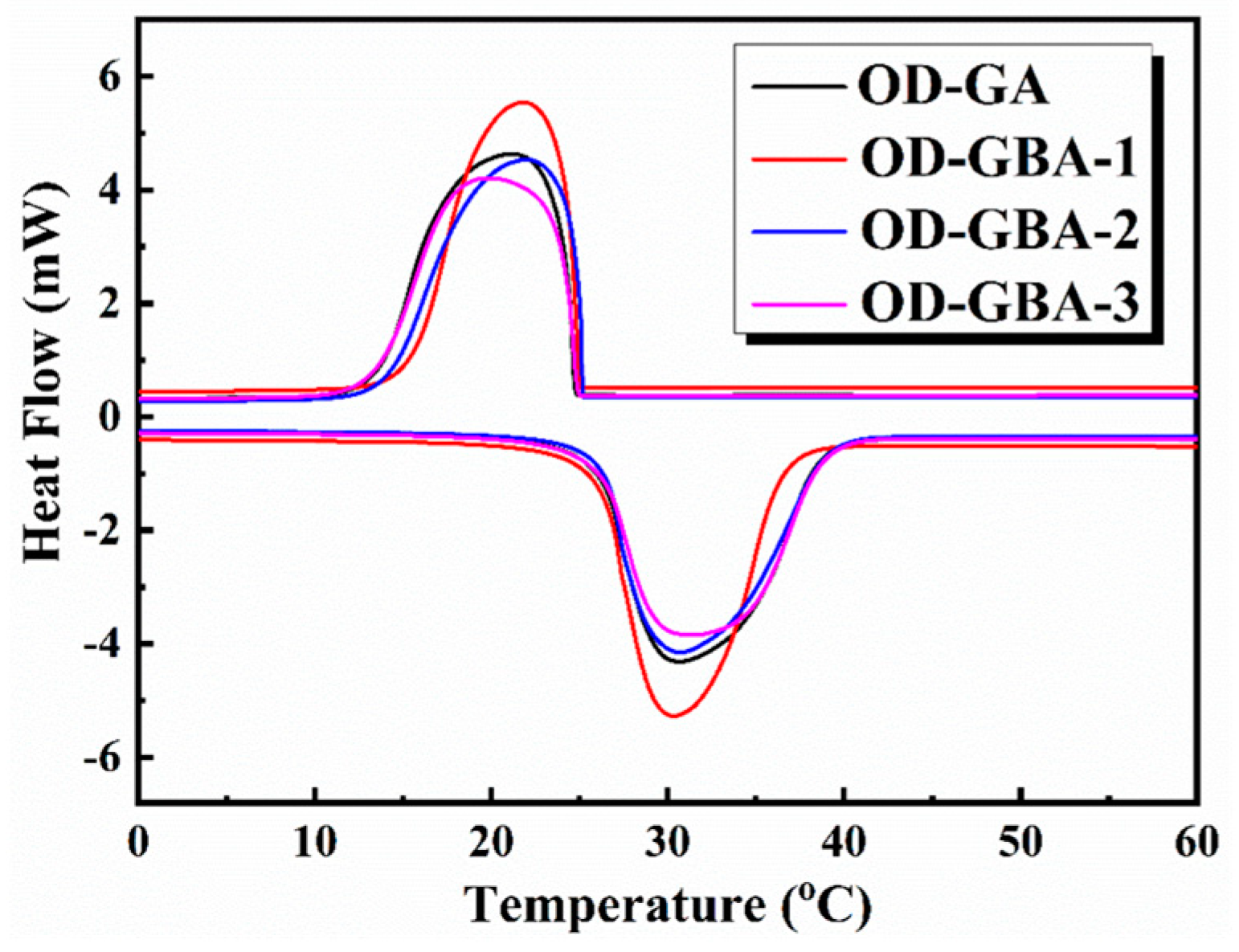
| Sample | Crystallization Process | Melting Process | ||
|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | |
| OD | 29.9 | 236.4 | 23.9 | 235.4 |
| OD-GA | 30.6 | 218.8 | 21.3 | 215.6 |
| OD-GBA-1 | 30.4 | 214.4 | 21.8 | 212.2 |
| OD-GBA-2 | 30.7 | 208.3 | 22.0 | 207.1 |
| OD-GBA-3 | 31.4 | 203.6 | 19.8 | 201.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, S.; Ji, R.; Zhang, H.; Huang, C.; Xu, F.; Sun, L.; Xia, Y.; Lin, X.; Ma, L.; Peng, H.; et al. N-Octadecane Encapsulated by Assembled BN/GO Aerogels for Highly Improved Thermal Conductivity and Energy Storage Capacity. Nanomaterials 2023, 13, 2317. https://doi.org/10.3390/nano13162317
Hui S, Ji R, Zhang H, Huang C, Xu F, Sun L, Xia Y, Lin X, Ma L, Peng H, et al. N-Octadecane Encapsulated by Assembled BN/GO Aerogels for Highly Improved Thermal Conductivity and Energy Storage Capacity. Nanomaterials. 2023; 13(16):2317. https://doi.org/10.3390/nano13162317
Chicago/Turabian StyleHui, Siyue, Rong Ji, Huanzhi Zhang, Chaowei Huang, Fen Xu, Lixian Sun, Yongpeng Xia, Xiangcheng Lin, Lei Ma, Hongliang Peng, and et al. 2023. "N-Octadecane Encapsulated by Assembled BN/GO Aerogels for Highly Improved Thermal Conductivity and Energy Storage Capacity" Nanomaterials 13, no. 16: 2317. https://doi.org/10.3390/nano13162317
APA StyleHui, S., Ji, R., Zhang, H., Huang, C., Xu, F., Sun, L., Xia, Y., Lin, X., Ma, L., Peng, H., Li, B., Wang, Y., Yan, E., & Huang, P. (2023). N-Octadecane Encapsulated by Assembled BN/GO Aerogels for Highly Improved Thermal Conductivity and Energy Storage Capacity. Nanomaterials, 13(16), 2317. https://doi.org/10.3390/nano13162317









