Optimizing the Performance of Low-Loaded Electrodes for CO2-to-CO Conversion Directly from Capture Medium: A Comprehensive Parameter Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Morphological Characterization of Ag GDEs
2.2. Electrochemical Tests and Product Analyses
2.3. Electrochemical Impedance Spectroscopy
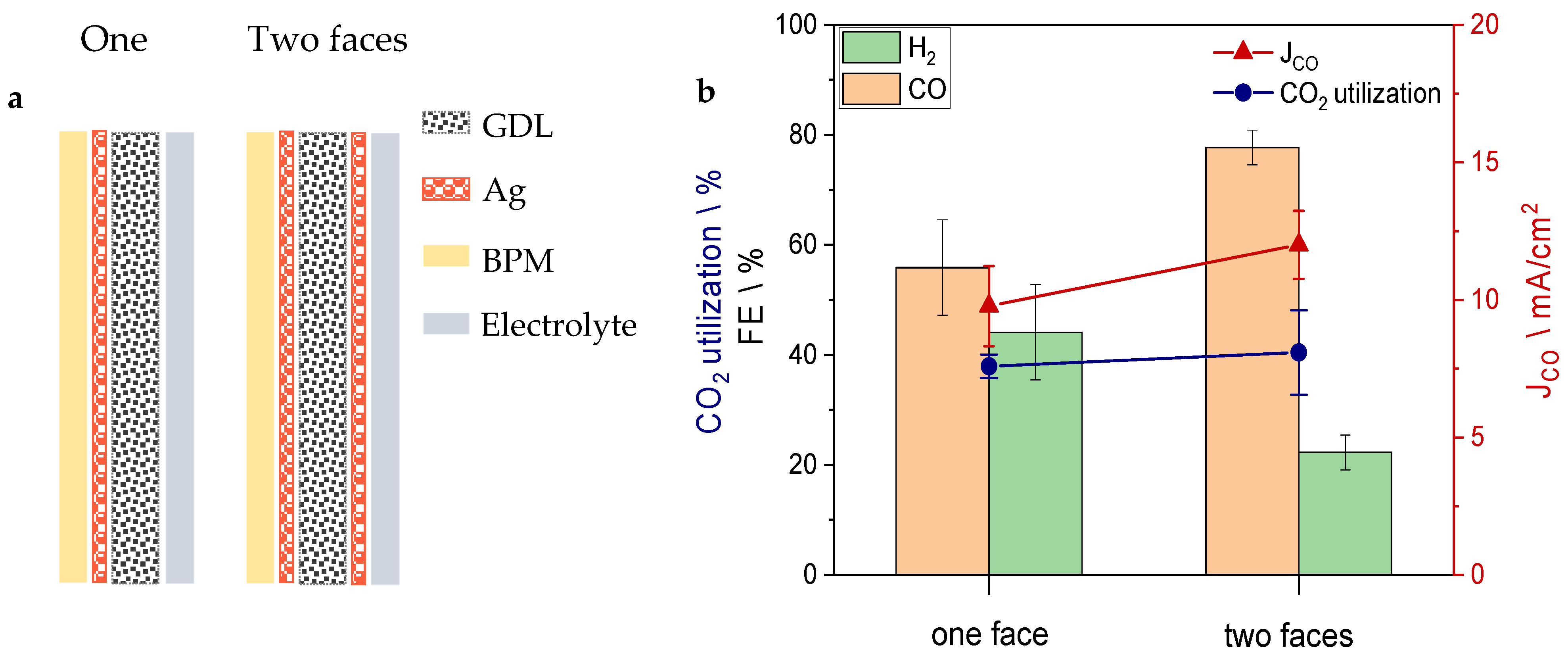
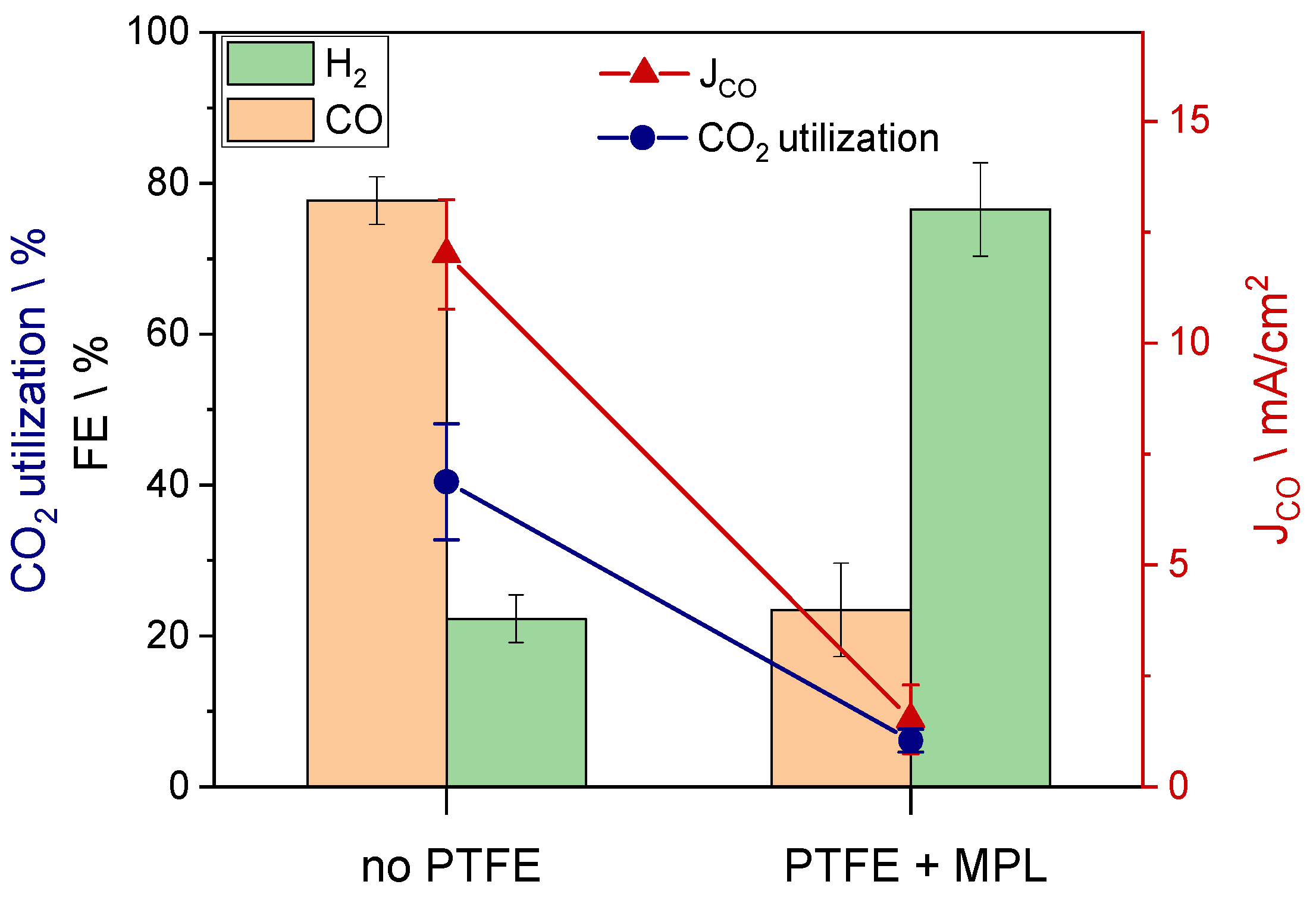
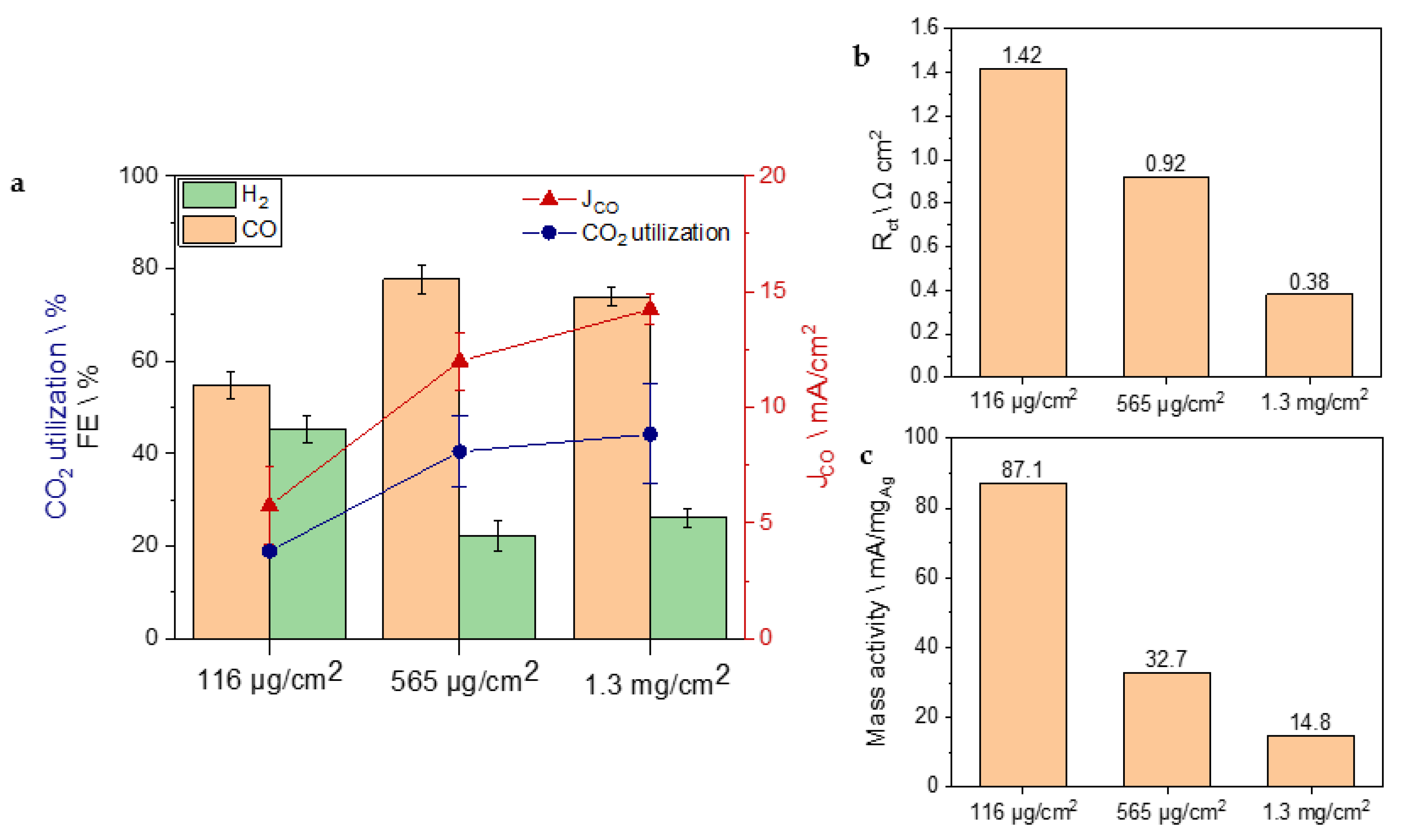
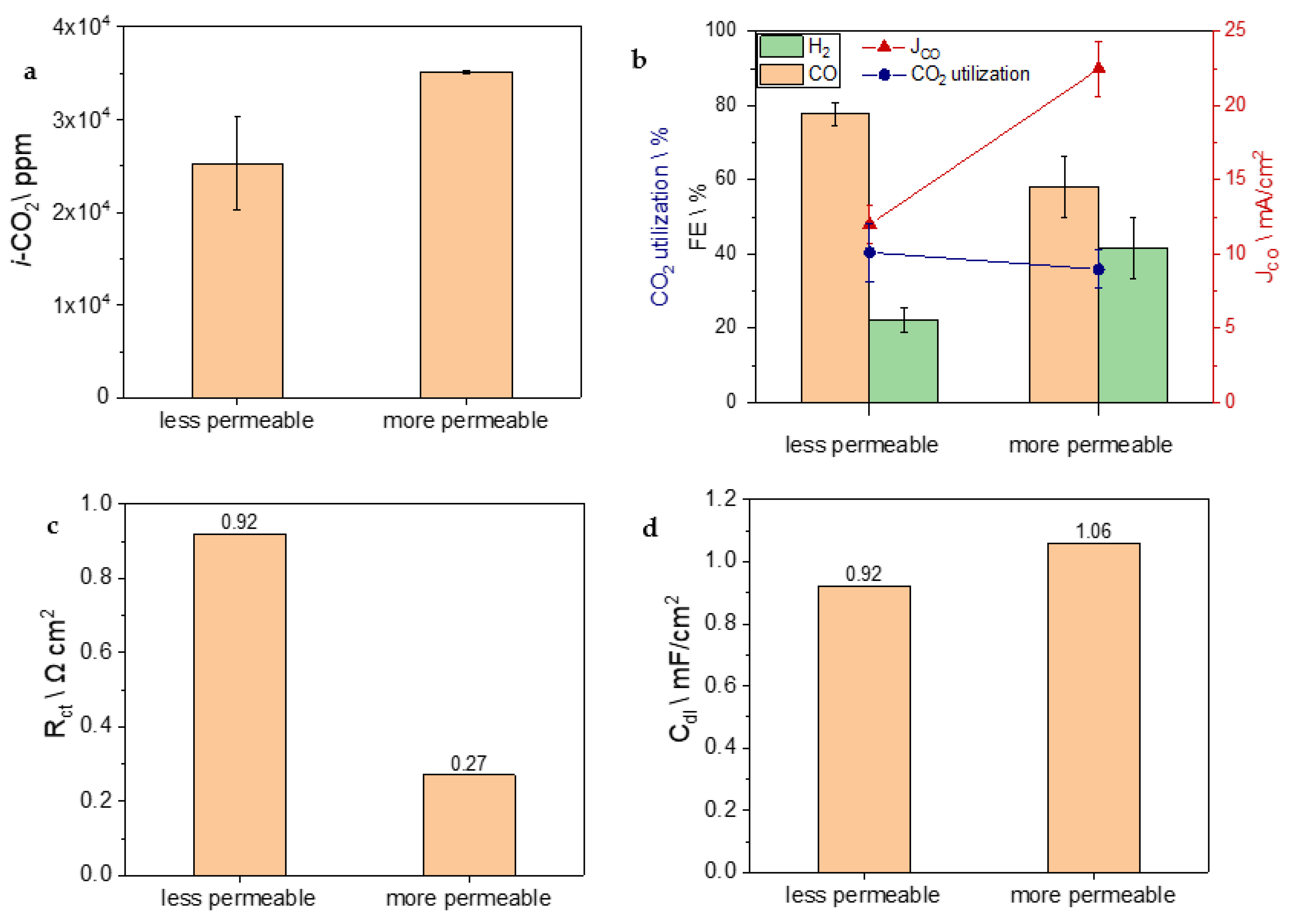
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pardal, T.; Messias, S.; Sousa, M.; Machado, A.S.R.; Rangel, C.M.; Nunes, D.; Pinto, J.V.; Martins, R.; da Ponte, M.N. Syngas production by electrochemical CO2 reduction in an ionic liquid based-electrolyte. J. CO2 Util. 2017, 18, 62–72. [Google Scholar] [CrossRef]
- Lees, E.W.; Mowbray, B.A.W.; Parlane, F.G.L.; Berlinguette, C.P. Gas diffusion electrodes and membranes for CO2 reduction electrolysers. Nat. Rev. Mater. 2021, 7, 55–64. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, B.; Li, L. Advance in Post-Combustion CO2 Capture with Alkaline Solution: A Brief Review. Energy Procedia 2012, 14, 1515–1522. [Google Scholar] [CrossRef]
- Iizuka, A.; Hashimoto, K.; Nagasawa, H.; Kumagai, K.; Yanagisawa, Y.; Yamasaki, A. Carbon dioxide recovery from carbonate solutions using bipolar membrane electrodialysis. Sep. Purif. Technol. 2012, 101, 49–59. [Google Scholar] [CrossRef]
- Mezza, A.; Pettigiani, A.; Monti, N.B.D.; Bocchini, S.; Farkhondehfal, M.A.; Zeng, J.; Chiodoni, A.; Pirri, C.F.; Sacco, A. An Electrochemical Platform for the Carbon Dioxide Capture and Conversion to Syngas. Energies 2021, 14, 7869. [Google Scholar] [CrossRef]
- Dinh, C.-T.; Li, Y.C.; Sargent, E.H. Boosting the Single-Pass Conversion for Renewable Chemical Electrosynthesis. Joule 2018, 3, 13–15. [Google Scholar] [CrossRef]
- Li, T.; Lees, E.W.; Goldman, M.; Salvatore, D.A.; Weekes, D.M.; Berlinguette, C.P. Electrolytic Conversion of Bicarbonate into CO in a Flow Cell. Joule 2019, 3, 1487–1497. [Google Scholar] [CrossRef]
- Li, Y.C.; Lee, G.; Yuan, T.; Wang, Y.; Nam, D.-H.; Wang, Z.; de Arquer, F.P.G.; Lum, Y.; Dinh, C.T.; Voznyy, O.; et al. CO2 Electroreduction from Carbonate Electrolyte. ACS Energy Lett. 2019, 4, 1427–1431. [Google Scholar] [CrossRef]
- Agliuzza, M.; Mezza, A.; Sacco, A. Solar-driven integrated carbon capture and utilization: Coupling CO2 electroreduction toward CO with capture or photovoltaic systems. Appl. Energy 2023, 334, 120649. [Google Scholar] [CrossRef]
- McDonald, M.B.; Ardo, S.; Lewis, N.S.; Freund, M.S. Use of Bipolar Membranes for Maintaining Steady-State pH Gradients in Membrane-Supported, Solar-Driven Water Splitting. ChemSusChem 2014, 7, 3021–3027. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Yan, Z.; Hitt, J.; Wycisk, R.; Pintauro, P.N.; Mallouk, T.E. Bipolar Membranes Inhibit Product Crossover in CO2 Electrolysis Cells. Adv. Sustain. Syst. 2018, 2, 1700187. [Google Scholar] [CrossRef]
- Welch, L.M.; Vijayaraghavan, M.; Greenwell, F.; Satherley, J.; Cowan, A.J. Electrochemical carbon dioxide reduction in ionic liquids at high pressure. Faraday Discuss. 2021, 230, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Hong, S.; Choi, M.; Lee, S.; Lee, J. Au on highly hydrophobic carbon substrate for improved selective CO production from CO2 in gas-phase electrolytic cell. Catal. Today 2019, 355, 340–346. [Google Scholar] [CrossRef]
- Larrea, C.; Torres, D.; Avilés-Moreno, J.R.; Ocón, P. Multi-parameter study of CO2 electrochemical reduction from concentrated bicarbonate feed. J. CO2 Util. 2022, 57, 101878. [Google Scholar] [CrossRef]
- Zhang, Z.; Lees, E.W.; Habibzadeh, F.; Salvatore, D.A.; Ren, S.; Simpson, G.L.; Wheeler, D.G.; Liu, A.; Berlinguette, C.P. Porous metal electrodes enable efficient electrolysis of carbon capture solutions. Energy Environ. Sci. 2022, 15, 705–713. [Google Scholar] [CrossRef]
- Kim, Y.; Lees, E.W.; Berlinguette, C.P. Permeability Matters When Reducing CO2 in an Electrochemical Flow Cell. ACS Energy Lett. 2022, 7, 2382–2387. [Google Scholar] [CrossRef]
- Lees, E.W.; Goldman, M.; Fink, A.G.; Dvorak, D.J.; Salvatore, D.A.; Zhang, Z.; Loo, N.W.X.; Berlinguette, C.P. Electrodes Designed for Converting Bicarbonate into CO. ACS Energy Lett. 2020, 5, 2165–2173. [Google Scholar] [CrossRef]
- Monti, N.B.D.; Fontana, M.; Sacco, A.; Chiodoni, A.; Lamberti, A.; Pirri, C.F.; Zeng, J. Facile Fabrication of Ag Electrodes for CO2-to-CO Conversion with Near-Unity Selectivity and High Mass Activity. ACS Appl. Energy Mater. 2022, 5, 14779–14788. [Google Scholar] [CrossRef]
- Sacco, A. Electrochemical impedance spectroscopy as a tool to investigate the electroreduction of carbon dioxide: A short review. J. CO2 Util. 2018, 27, 22–31. [Google Scholar] [CrossRef]
- Zhao, H.-Z.; Chang, Y.-Y.; Liu, C. Electrodes modified with iron porphyrin and carbon nanotubes: Application to CO2 reduction and mechanism of synergistic electrocatalysis. J. Solid State Electrochem. 2013, 17, 1657–1664. [Google Scholar] [CrossRef]
- Kleiminger, L.; Li, T.; Li, K.; Kelsall, G.H. Syngas (CO-H2) production using high temperature micro-tubular solid oxide electrolysers. Electrochim. Acta 2015, 179, 565–577. [Google Scholar] [CrossRef]
- Zeng, J.; Castellino, M.; Bejtka, K.; Sacco, A.; Di Martino, G.; Farkhondehfal, M.A.; Chiodoni, A.; Hernández, S.; Pirri, C.F. Facile synthesis of cubic cuprous oxide for electrochemical reduction of carbon dioxide. J. Mater. Sci. 2020, 56, 1255–1271. [Google Scholar] [CrossRef]
- Zeng, J.; Castellino, M.; Fontana, M.; Sacco, A.; Monti, N.B.D.; Chiodoni, A.; Pirri, C.F. Electrochemical Reduction of CO2 with Good Efficiency on a Nanostructured Cu-Al Catalyst. Front. Chem. 2022, 10, 931767. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Sánchez, O.; de Mot, B.; Bulut, M.; Pant, D.; Breugelmans, T. Engineering Aspects for the Design of a Bicarbonate Zero-Gap Flow Electrolyzer for the Conversion of CO2 to Formate. ACS Appl. Mater. Interfaces 2022, 14, 30760–30771. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Rino, T.; Bejtka, K.; Castellino, M.; Sacco, A.; Farkhondehfal, M.A.; Chiodoni, A.; Drago, F.; Pirri, P.C.F. Coupled Copper–Zinc Catalysts for Electrochemical Reduction of Carbon Dioxide. ChemSusChem 2020, 13, 4128–4139. [Google Scholar] [CrossRef]


| Ag Mass Loading (mg/cm2) | Deposition Technique | Feedstock [KHCO3 (M)] | FECO (%) | Cell Potential (V) | JCO (mA/cm2) | Partial Mass Activity (mA/mgAg) | Reference |
|---|---|---|---|---|---|---|---|
| 13 * | Spray coating | 3 | 80 | 3 | 20 | 2 * | [7] |
| 2 | PVD + spray coating | 3 | 25 | 3.5 | 25 | 13 | [8] |
| 2 | Spray coating | 2 | 58 | 3 | 14 | 7 | [14] |
| Foam ** | Free standing electrode ** | 3 | 60 | 3.7 | 60 | - | [15] |
| 3 | Electrodeposition | 3 | 70 | 3.5 | 70 | 23 | [16] |
| 2 | PVD + spray coating | 3 | 82 | 3.6 | 82 | 41 | [17] |
| 0.565 | PVD | 2 | 77 | 3 | 13 | 25 | This work |
| 0.565 | PVD | 2 | 58 | 3 | 22 | 40 | This work |
| 0.116 | PVD | 2 | 55 | 3 | 6 | 48 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezza, A.; Bartoli, M.; Chiodoni, A.; Zeng, J.; Pirri, C.F.; Sacco, A. Optimizing the Performance of Low-Loaded Electrodes for CO2-to-CO Conversion Directly from Capture Medium: A Comprehensive Parameter Analysis. Nanomaterials 2023, 13, 2314. https://doi.org/10.3390/nano13162314
Mezza A, Bartoli M, Chiodoni A, Zeng J, Pirri CF, Sacco A. Optimizing the Performance of Low-Loaded Electrodes for CO2-to-CO Conversion Directly from Capture Medium: A Comprehensive Parameter Analysis. Nanomaterials. 2023; 13(16):2314. https://doi.org/10.3390/nano13162314
Chicago/Turabian StyleMezza, Alessio, Mattia Bartoli, Angelica Chiodoni, Juqin Zeng, Candido F. Pirri, and Adriano Sacco. 2023. "Optimizing the Performance of Low-Loaded Electrodes for CO2-to-CO Conversion Directly from Capture Medium: A Comprehensive Parameter Analysis" Nanomaterials 13, no. 16: 2314. https://doi.org/10.3390/nano13162314
APA StyleMezza, A., Bartoli, M., Chiodoni, A., Zeng, J., Pirri, C. F., & Sacco, A. (2023). Optimizing the Performance of Low-Loaded Electrodes for CO2-to-CO Conversion Directly from Capture Medium: A Comprehensive Parameter Analysis. Nanomaterials, 13(16), 2314. https://doi.org/10.3390/nano13162314











