Recent Progress in Multifunctional Graphene-Based Nanocomposites for Photocatalysis and Electrocatalysis Application
Abstract
1. Introduction
2. Photocatalytic Water Splitting for Hydrogen Production and Electrocatalysis
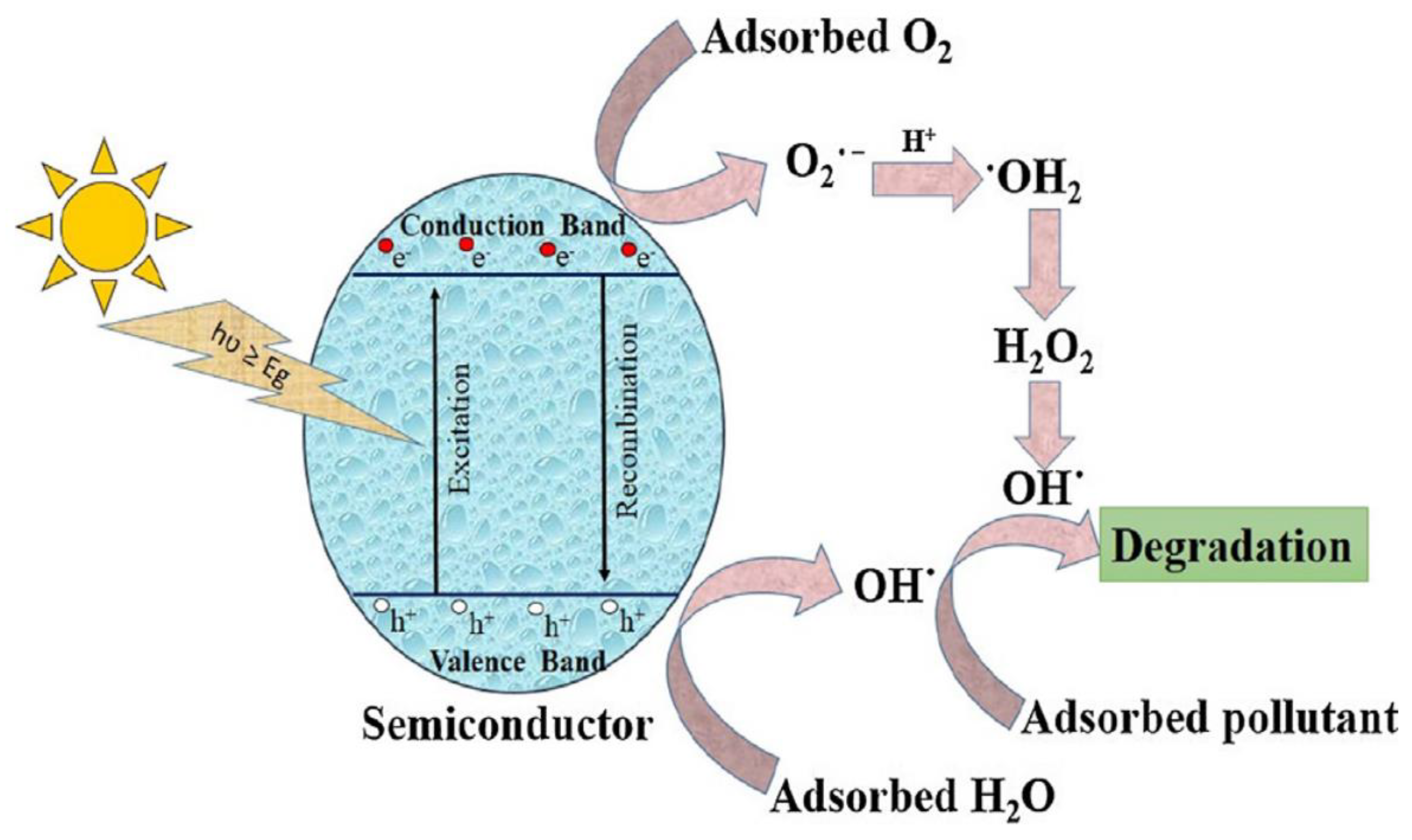
2.1. Mechanism
2.2. Graphene Optimization Method for the Photocatalytic Effect
2.2.1. Graphene Matrix Composites and Graphene-Doped-Metal Matrix Composite
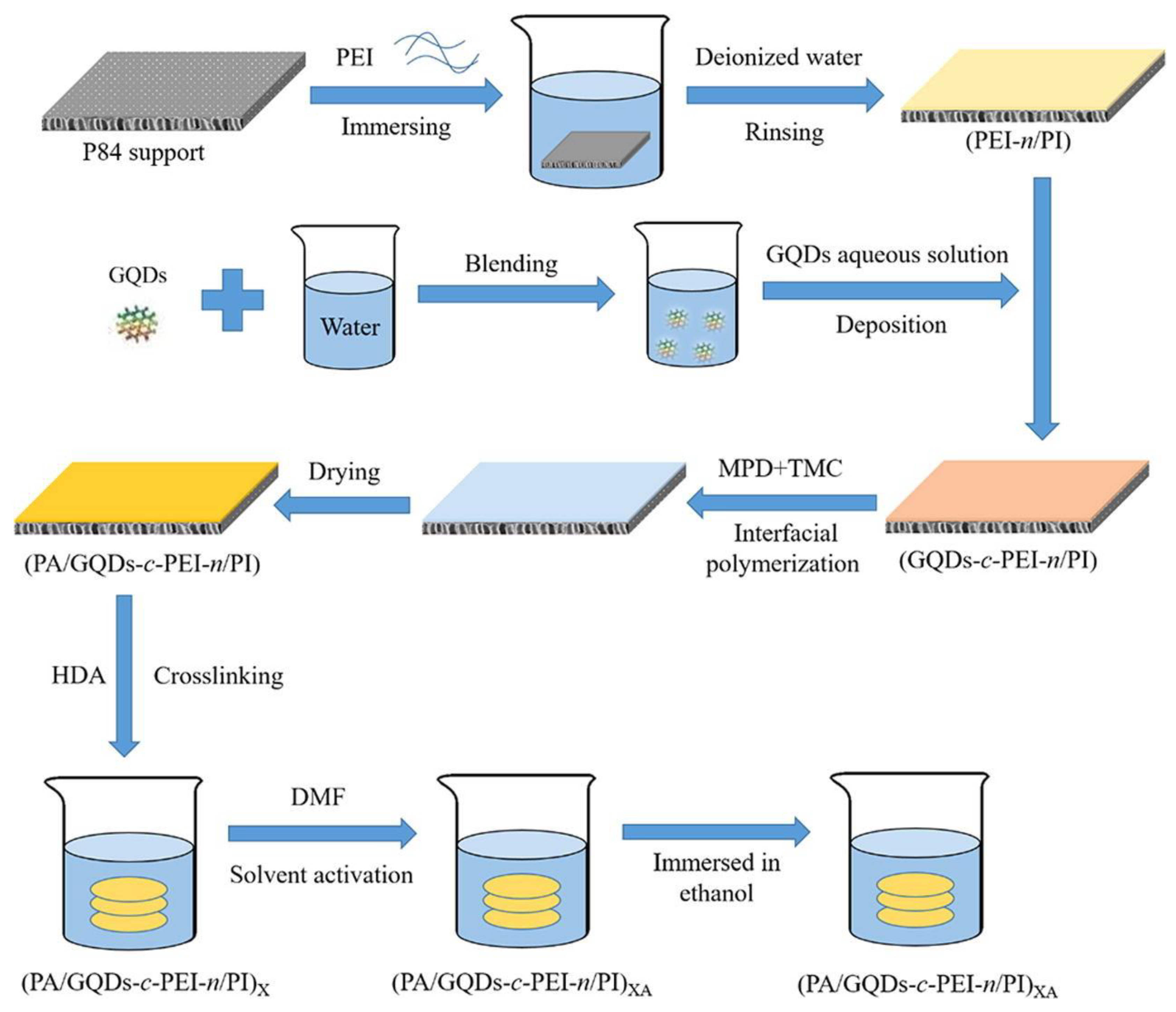
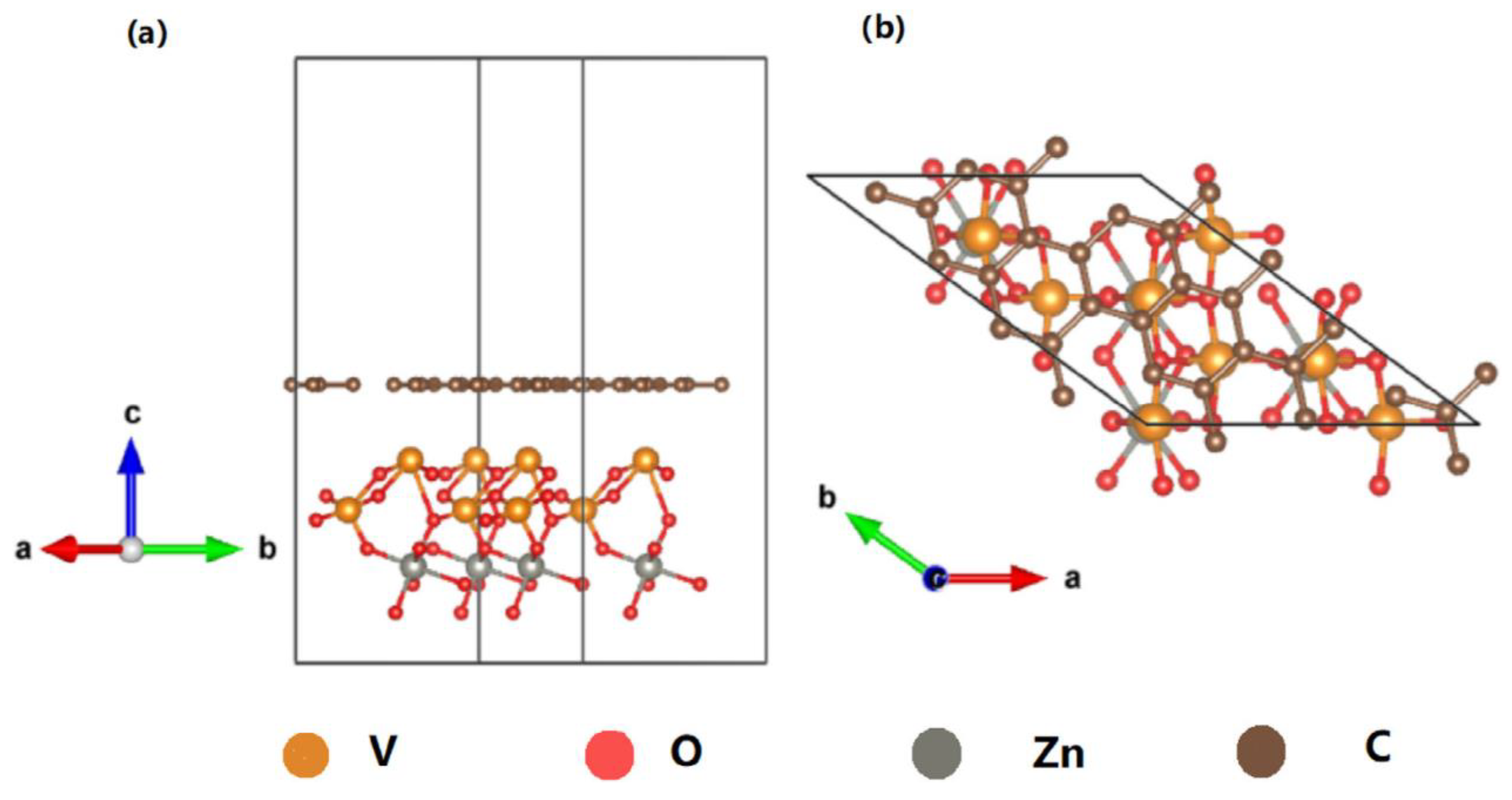
2.2.2. Structure Design
2.3. Graphene Electrocatalysis
3. Pollutant Degradation
3.1. Removal of Typical Pollutants
3.1.1. Antibiotics
3.1.2. Dye
3.1.3. Oil and Organic Solvents
3.2. Adsorption of Heavy Metals in Sewage
3.3. Degradation of Gaseous Pollutants
4. Photocatalytic Reduction of CO2
4.1. Mechanism
4.2. Main Optimization Strategies of Graphene
4.2.1. Metal Doping, Non-Metal Doping and Graphene Heterostructure
4.2.2. Compound Materials
4.2.3. Bandgap Engineering
5. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Gu, P.; Ma, R.; Luo, C.; Wen, T.; Zhao, G.; Cheng, W.; Wang, X. Recent developments in fabrication and structure regulation of visible-light-driven g-C3N4-based photocatalysts towards water purification: A critical review. Catal. Today 2019, 335, 65–77. [Google Scholar] [CrossRef]
- Freund, H.J.; Roberts, M.W. Surface chemistry of carbon dioxide. Surf. Sci. Rep. 1996, 25, 225–273. [Google Scholar] [CrossRef]
- Sakakura, T.; Choi, J.C.; Yasuda, H. Transformation of carbon dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef]
- Feng, J.; Huang, H.; Yan, S.; Luo, W.; Yu, T.; Li, Z.; Zou, Z. Non-oxide semiconductors for artificial photosynthesis: Progress on photoelectrochemical water splitting and carbon dioxide reduction. Nano Today 2020, 30, 100830. [Google Scholar] [CrossRef]
- Ahmad, A.; Ali, M.; Al-Sehemi, A.G.; Al-Ghamdi, A.A.; Park, J.-W.; Algarni, H.; Anwer, H. Carbon-integrated semiconductor photocatalysts for removal of volatile organic compounds in indoor environments. Chem. Eng. J. 2023, 452, 139436. [Google Scholar] [CrossRef]
- Chi, K.; Wu, Z.; Tian, X.; Wang, Z.; Xiao, F.; Xiao, J.; Wang, S. Boosting hydrogen evolution via integrated construction and synergistic cooperation of confined graphene/CoSe2 active interfaces and 3D graphene nanomesh arrays. Appl. Catal. B Environ. 2023, 324, 122256. [Google Scholar] [CrossRef]
- Lin, S.; Lu, Y.; Xu, J.; Feng, S.; Li, J. High performance graphene/semiconductor van der Waals heterostructure optoelectronic devices. Nano Energy 2017, 40, 122–148. [Google Scholar] [CrossRef]
- Lee, S.J.; Theerthagiri, J.; Nithyadharseni, P.; Arunachalam, P.; Balaji, D.; Kumar, A.M.; Madhavan, J.; Mittal, V.; Choi, M.Y. Heteroatom-doped graphene-based materials for sustainable energy applications: A review. Renew. Sustain. Energy Rev. 2021, 143, 110849. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, C.; Wang, H.; Chen, M.; Zeng, G.; Liu, Z.; Liu, Y.; Wang, W.; Wu, T.; Shao, B.; et al. Recent advance of graphene/semiconductor composite nanocatalysts: Synthesis, mechanism, applications and perspectives. Chem. Eng. J. 2021, 414, 128795. [Google Scholar] [CrossRef]
- Cheng, C.; Liang, Q.; Yan, M.; Liu, Z.; He, Q.; Wu, T.; Luo, S.; Pan, Y.; Zhao, C.; Liu, Y. Advances in preparation, Y., mechanism and applications of graphene quantum dots/semiconductor composite photocatalysts: A review. J. Hazard. Mater. 2022, 424 Pt D, 127721. [Google Scholar] [CrossRef]
- Yan, W.; Xiao, F.; Li, X.; He, W.; Yao, Y.; Wan, D.; Liu, Y.; Feng, F.; Zhang, Q.; Lu, C.; et al. Nickel and oxygen-containing functional groups co-decorated graphene-like shells as catalytic sites with excellent selective hydrogenation activity and robust stability. Chem. Eng. J. 2023, 452, 139361. [Google Scholar] [CrossRef]
- Wang, D.; Dong, S.; Hu, H.; He, Z.; Dong, F.; Tang, J.; Lu, X.; Wang, L.; Song, S.; Ma, J. Catalytic ozonation of atrazine with stable boron-doped graphene nanoparticles derived from waste polyvinyl alcohol film: Performance and mechanism. Chem. Eng. J. 2023, 455, 140316. [Google Scholar] [CrossRef]
- Kumwimba, M.N.; Zhu, B.; Wang, T.; Dzakpasu, M.; Li, X. Nutrient dynamics and retention in a vegetated drainage ditch receiving nutrient-rich sewage at low temperatures. Sci. Total Environ. 2020, 741, 140268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, B.; Wang, X.; Zhao, G.; Hu, B.; Lu, Z.; Wen, T.; Chen, J.; Wang, X. Recent developments of two-dimensional graphene-based composites in visible-light photocatalysis for eliminating persistent organic pollutants from wastewater. Chem. Eng. J. 2020, 390, 124642. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Senthil, R.; Senthilkumar, B.; Polu, A.R.; Madhavan, J.; Ashokkumar, M. Recent advances in MoS 2 nanostructured materials for energy and environmental applications—A review. J. Solid State Chem. 2017, 252, 43–71. [Google Scholar] [CrossRef]
- Jayaraman, T.; Murthy, A.P.; Elakkiya, V.; Chandrasekaran, S.; Nithyadharseni, P.; Khan, Z.; Senthil, R.A.; Shanker, R.; Raghavender, M.; Kuppusami, P.; et al. Recent development on carbon based heterostructures for their applications in energy and environment: A review. J. Ind. Eng. Chem. 2018, 64, 16–59. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Salla, S.; A Senthil, R.; Nithyadharseni, P.; Madankumar, A.; Arunachalam, P.; Maiyalagan, T.; Kim, H.-S. A review on ZnO nanostructured materials: Energy, environmental and biological applications. Nanotechnology 2019, 30, 392001. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Madhavan, J.; Lee, S.J.; Choi, M.Y.; Ashokkumar, M.; Pollet, B.G. Sonoelectrochemistry for energy and environmental applications. Ultrason. Sonochem. 2020, 63, 104960. [Google Scholar] [CrossRef]
- Bora, A.P.; Gupta, D.P.; Durbha, K.S. Sewage sludge to bio-fuel: A review on the sustainable approach of transforming sewage waste to alternative fuel. Fuel 2020, 259, 116262. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, F.; Zhang, R.; Zhao, L.; Qi, J. Recent progress on biodiesel production from municipal sewage sludge. Renew. Sustain. Energy Rev. 2021, 135, 110260. [Google Scholar] [CrossRef]
- Świerczek, L.; Cieślik, B.M.; Konieczka, P. Challenges and opportunities related to the use of sewage sludge ash in cement-based building materials—A review. J. Clean. Prod. 2021, 287, 125054. [Google Scholar] [CrossRef]
- Zhong, L.; Ding, J.; Wu, T.; Zhao, Y.-L.; Pang, J.W.; Jiang, J.-P.; Jiang, J.-Q.; Li, Y.; Ren, N.-Q.; Yang, S.-S. Bibliometric overview of research progress, challenges, and prospects of rural domestic sewage: Treatment techniques, resource recovery, and ecological risk. J. Water Process Eng. 2023, 51, 103389. [Google Scholar] [CrossRef]
- Lu, F.; Astruc, D. Nanomaterials for removal of toxic elements from water. Coord. Chem. Rev. 2018, 356, 147–164. [Google Scholar] [CrossRef]
- Al-Omari, A.; Muhammetoglu, A.; Karadirek, E.; Jiries, A.; Batarseh, M.; Topkaya, B.; Soyupak, S. A Review on Formation and Decay Kinetics of Trihalomethanes in Water of Different Qualities. Clean-Soil Air Water 2014, 42, 1687–1700. [Google Scholar] [CrossRef]
- Duranceau, S.J.; Smith, C.T. Trihalomethane Formation Downstream of Spray Aerators Treating Disinfected Groundwater. J. Am. Water Work. Assoc. 2016, 108, E99–E108. [Google Scholar] [CrossRef]
- Kinani, S.; Roumiguières, A.; Bouchonnet, S. A Critical Review on Chemical Speciation of Chlorine-Produced Oxidants (CPOs) in Seawater. Part 1: Chlorine Chemistry in Seawater and Its Consequences in Terms of Biocidal Effectiveness and Environmental Impact. Crit. Rev. Anal. Chem. 2022. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Farrok, O.; Haque, M. Environmental impact of renewable energy source based electrical power plants: Solar, wind, hydroelectric, biomass, geothermal, tidal, ocean, and osmotic. Renew. Sustain. Energy Rev. 2022, 161, 112279. [Google Scholar] [CrossRef]
- Daaboul, J.; Moriarty, P.; Honnery, D. Net green energy potential of solar photovoltaic and wind energy generation systems. J. Clean. Prod. 2023, 415, 137806. [Google Scholar] [CrossRef]
- Brillas, E. Solar photoelectro-Fenton: A very effective and cost-efficient electrochemical advanced oxidation process for the removal of organic pollutants from synthetic and real wastewaters. Chemosphere 2023, 327, 138532. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Hammad, M.; Angel, S.; Al-Kamal, A.K.; Asghar, A.; Amin, A.S.; Kräenbring, M.-A.; Wiedemann, H.T.A.; Vinayakumar, V.; Ali, M.Y.; Fortugno, P.; et al. Synthesis of novel LaCoO3/graphene catalysts as highly efficient peroxymonosulfate activator for the degradation of organic pollutants. Chem. Eng. J. 2023, 454, 139900. [Google Scholar] [CrossRef]
- Liu, G.-Y.; Li, K.-K.; Jia, J.; Zhang, Y.-T. Coal-based graphene as a promoter of TiO2 catalytic activity for the photocatalytic degradation of organic dyes. Carbon 2023, 203, 899. [Google Scholar] [CrossRef]
- Min, D.H.; Han, X.; Li, N.; Jung, M.G.; Lee, S.J.; Park, H.W.; Lee, J.Y.; Park, H.S. Catalytic active interfacial B–C bonds of boron nanosheet/reduced graphene oxide heterostructures for efficient oxygen reduction reaction. Compos. Part B Eng. 2023, 252, 110496. [Google Scholar] [CrossRef]
- Lu, K.-Q.; Yuan, L.; Xin, X.; Xu, Y.-J. Hybridization of graphene oxide with commercial graphene for constructing 3D metal-free aerogel with enhanced photocatalysis. Appl. Catal. B Environ. 2018, 226, 16–22. [Google Scholar] [CrossRef]
- Bilal, M.; Rashid, E.U.; Zdarta, J.; Jesionowski, T. Graphene-based nanoarchitectures as ideal supporting materials to develop multifunctional nanobiocatalytic systems for strengthening the biotechnology industry. Chem. Eng. J. 2023, 452, 139509. [Google Scholar] [CrossRef]
- Yu, W.; Liu, L.; Yang, Y.; Li, N.; Chen, Y.; Yin, X.; Niu, J.; Wang, J.; Ding, S. N, O-diatomic dopants activate catalytic activity of 3D self-standing graphene carbon aerogel for long-cycle and high-efficiency Li-CO2 batteries. Chem. Eng. J. 2023, 465, 142787. [Google Scholar] [CrossRef]
- Esfandiari, M.; Lalbakhsh, A.; Shehni, P.N.; Jarchi, S.; Ghaffari-Miab, M.; Mahtaj, H.N.; Reisenfeld, S.; Alibakhshikenari, M.; Koziel, S.; Szczepanski, S. Recent and emerging applications of Graphene-based metamaterials in electromagnetics. Mater. Des. 2022, 221, 110920. [Google Scholar] [CrossRef]
- Esfandiyari, M.; Lalbakhsh, A.; Jarchi, S.; Ghaffari-Miab, M.; Mahtaj, H.N.; Simorangkir, R.B.V.B. Tunable terahertz filter/antenna-sensor using graphene-based metamaterials. Mater. Des. 2022, 220, 110855. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, A.; Ding, L.; Liu, Y.; Lu, H. A three-dimensional absorber hybrid with polar oxygen functional groups of MWNTs/graphene with enhanced microwave absorbing properties. Compos. Part B Eng. 2017, 108, 386–392. [Google Scholar] [CrossRef]
- Lin, K.T.; Lin, H.; Yang, T.; Jia, B. Structured graphene metamaterial selective absorbers for high efficiency and omnidirectional solar thermal energy conversion. Nat. Commun. 2020, 11, 1389. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-L.; Li, E.-P.; Wei, X.-C.; Yi, D. A Novel Tunable Absorber Based on Vertical Graphene Strips. IEEE Microw. Wirel. Compon. Lett. 2016, 26, 10–12. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, J.; Li, L. A Dual-Band Tunable Metamaterial Near-Unity Absorber Composed of Periodic Cross and Disk Graphene Arrays. IEEE Photonics J. 2018, 10, 1–12. [Google Scholar] [CrossRef]
- Borah, D.; Bhattacharyya, N.S. Design and Development of Expanded Graphite-Based Non-metallic and Flexible Metamaterial Absorber for X-band Applications. J. Electron. Mater. 2017, 46, 226–232. [Google Scholar] [CrossRef]
- Chen, X.; Jia, X.; Wu, Z.; Tang, Z.; Zeng, Y.; Wang, X.; Fu, X.; Zou, Y. A Graphite-Based Metamaterial Microwave Absorber. IEEE Antennas Wirel. Propag. Lett. 2019, 18, 1016–1020. [Google Scholar] [CrossRef]
- Guo, T.; Sun, Y.; Evans, J.; Wang, N.; Fu, Y.; He, S. Thermal management with a highly emissive and thermally conductive graphite absorber. Aip Adv. 2019, 9, 025224. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.-H.; Li, L.; Fan, Y.-X.; Tao, Z.-Y. Vanadium dioxide-assisted broadband tunable terahertz metamaterial absorber. Sci. Rep. 2019, 9, 5751. [Google Scholar] [CrossRef]
- Singhal, S. Wide angle insensitive and polarization independent graphite based superwideband absorber. Opt. Quantum Electron. 2022, 54, 671. [Google Scholar] [CrossRef]
- Norouzi, M.; Jarchi, S.; Ghaffari-Miab, M.; Esfandiari, M.; Lalbakhsh, A.; Koziel, S.; Reisenfeld, S.; Moloudian, G. 3D metamaterial ultra-wideband absorber for curved surface. Sci. Rep. 2023, 13, 1043. [Google Scholar] [CrossRef]
- Borah, D.; Bhattacharyya, N.S. Design, fabrication and characterization of flexible and ultrathin microwave metamaterial absorber. In Proceedings of the International Conference on Innovations in Electronics, Signal Processing and Communication (IESC), Shillong, India, 6–7 April 2017; pp. 190–193. [Google Scholar]
- Rani, N.; Saha, S. Graphite based metal-free and polarization-insensitive multiband THz absorber with wide incident angle. Optik 2022, 266, 169601. [Google Scholar] [CrossRef]
- Soni, A.K.; Giri, P.; Varshney, G. Metal-free super-wideband THz absorber for electromagnetic shielding. Phys. Scr. 2021, 96, 125866. [Google Scholar] [CrossRef]
- Soni, A.K.; Varshney, G. Multiband Generation and Absorption Enhancement in a Graphite-Based Metal-Free Absorber. Plasmonics 2021, 16, 241–252. [Google Scholar] [CrossRef]
- Varshney, G. Wideband THz Absorber: By Merging the Resonance of Dielectric Cavity and Graphite Disk Resonator. IEEE Sens. J. 2021, 21, 1635–1643. [Google Scholar] [CrossRef]
- Varshney, G.; Rani, N.; Pandey, V.S.; Yaduvanshi, R.S.; Singh, D. Graphite/graphene disk stack-based metal-free wideband terahertz absorber. J. Opt. Soc. Am. B-Opt. Phys. 2021, 38, 530–538. [Google Scholar] [CrossRef]
- Korkmaz, S.; Kariper, I.A. Graphene and graphene oxide based aerogels: Synthesis, characteristics and supercapacitor applications. J. Energy Storage 2020, 27, 101038. [Google Scholar] [CrossRef]
- Liu, X.; Liang, B.; Long, J. Research progress on graphene aerogel in catalysis. N. Chem. Mater. 2021, 49, 60–63. [Google Scholar]
- Shaikh, J.S.; Shaikh, N.S.; Mishra, Y.K.; Pawar, S.S.; Parveen, N.; Shewale, P.M.; Sabale, S.; Kanjanaboos, P.; Praserthdam, S.; Lokhande, C.D. The implementation of graphene-based aerogel in the field of supercapacitor. Nanotechnology 2021, 32, 362001. [Google Scholar] [CrossRef]
- Zhong, K.; Zhang, C.; Zhong, Y.; Cui, S.; Shen, X. Research progress in the preparation and adsorption capability of graphene aerogel composite materials. Ind. Water Treat. 2019, 39, 1–6. [Google Scholar]
- Wu, F.; Xie, A.; Sun, M.; Wang, Y.; Wang, M. Reduced graphene oxide (RGO) modified spongelike polypyrrole (PPy) aerogel for excellent electromagnetic absorption. J. Mater. Chem. A 2015, 3, 14358–14369. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Liu, Y.-J.; Sui, G.-X. Lightweight spongy bone-like graphene@SiC aerogel composites for high-performance microwave absorption. Chem. Eng. J. 2018, 337, 522–531. [Google Scholar] [CrossRef]
- Gorgolis, G.; Galiotis, C. Graphene aerogels: A review. 2D Mater. 2017, 4, 032001. [Google Scholar] [CrossRef]
- Jing, J.; Qian, X.; Si, Y.; Liu, G.; Shi, C. Recent Advances in the Synthesis and Application of Three-Dimensional Graphene-Based Aerogels. Molecules 2022, 27, 924. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Pei, C.; Zhu, Z.; An, J.; Qin, X.; Bao, X. Progress in graphene aerogels. Mod. Chem. Ind. 2013, 33, 20–23. [Google Scholar]
- Wang, Z.; Liu, L.; Zhang, Y.; Huang, Y.; Liu, J.; Zhang, X.; Liu, X.; Teng, H.; Zhang, X.; Zhang, J.; et al. A Review of Graphene-Based Materials/Polymer Composite Aerogels. Polymers 2023, 15, 1888. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, R.; Gu, J.; Liu, H.; Liu, C.; Luo, C.; Kong, J.; Shao, Q.; Wang, N.; Guo, Z.; et al. Ultralight, highly compressible and fire-retardant graphene aerogel with self-adjustable electromagnetic wave absorption. Carbon 2018, 139, 1126–1135. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Meng, H.; Xie, S.; Zhang, B.; Li, L.; Ma, H.; Zhang, J.; Yu, M. Compressible and fire-resistant graphene aerogel as a highly efficient and recyclable absorbent for organic liquids. J. Mater. Chem. A 2014, 2, 2934–2941. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Sohn, E.-H.; Park, S.; Park, H.S. Highly-efficient and recyclable oil absorbing performance of functionalized graphene aerogel. Chem. Eng. J. 2015, 269, 229–235. [Google Scholar] [CrossRef]
- Yao, X.; Zhao, Y. Three-Dimensional Porous Graphene Networks and Hybrids for Lithium-Ion Batteries and Supercapacitors. Chem 2017, 2, 171–200. [Google Scholar] [CrossRef]
- Cao, L.; Wang, C.; Huang, Y. Structure optimization of graphene aerogel-based composites and applications in batteries and supercapacitors. Chem. Eng. J. 2023, 454, 140094. [Google Scholar] [CrossRef]
- Xie, W.; Yao, F.; Gu, H.; Du, A.; Lei, Q.; Naik, N.; Guo, Z. Magnetoresistive and piezoresistive polyaniline nanoarrays in-situ polymerized surrounding magnetic graphene aerogel. Adv. Compos. Hybrid Mater. 2022, 5, 1003–1016. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Leng, Y.; Tian, J.; Sang, Y.; Boughton, R.I.; Wong, C.P.; Liu, H.; Yang, B. Graphene-based nitrogen self-doped hierarchical porous carbon aerogels derived from chitosan for high performance supercapacitors. Nano Energy 2015, 15, 9–23. [Google Scholar] [CrossRef]
- Tayebi, M.; Tayyebi, A.; Masoumi, Z.; Lee, B.-K. Photocorrosion suppression and photoelectrochemical (PEC) enhancement of ZnO via hybridization with graphene nanosheets. Appl. Surf. Sci. 2020, 502, 144189. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, J.; Mao, J.; Guo, Q.; Chen, Z.; Lai, Y. Metal-organic frameworks and their derivatives with graphene composites: Preparation and applications in electrocatalysis and photocatalysis. J. Mater. Chem. A 2020, 8, 2934–2961. [Google Scholar] [CrossRef]
- Li, Y.-H.; Tang, Z.-R.; Xu, Y.-J. Multifunctional graphene-based composite photocatalysts oriented by multifaced roles of graphene in photocatalysis. Chin. J. Catal. 2022, 43, 708–730. [Google Scholar] [CrossRef]
- Subramanyam, P.; Vinodkumar, T.; Nepak, D.; Deepa, M.; Subrahmanyam, C. Mo-doped BiVO4@reduced graphene oxide composite as an efficient photoanode for photoelectrochemical water splitting. Catal. Today 2019, 325, 73–80. [Google Scholar] [CrossRef]
- Ranjan, R.; Kumar, M.; Sinha, A.S.K. Development and characterization of rGO supported CdS MoS2 photoelectrochemical catalyst for splitting water by visible light. Int. J. Hydrogen Energy 2019, 44, 16176–16189. [Google Scholar] [CrossRef]
- Qiu, H.J.; Du, P.; Hu, K.; Gao, J.; Li, H.; Liu, P.; Ina, T.; Ohara, K.; Ito, Y.; Chen, M. Metal and Nonmetal Codoped 3D Nanoporous Graphene for Efficient Bifunctional Electrocatalysis and Rechargeable Zn-Air Batteries. Adv. Mater. 2019, 31, e1900843. [Google Scholar] [CrossRef]
- Liu, J.; Kong, X.; Zheng, L.; Guo, X.; Liu, X.; Shui, J. Rare Earth Single-Atom Catalysts for Nitrogen and Carbon Dioxide Reduction. ACS Nano 2020, 14, 1093–1101. [Google Scholar] [CrossRef]
- Bie, C.; Yu, H.; Cheng, B.; Ho, W.; Fan, J.; Yu, J. Design, Fabrication, and Mechanism of Nitrogen-Doped Graphene-Based Photocatalyst. Adv. Mater. 2021, 33, e2003521. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; von Colbe, J.B.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage—Past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Li, Y.; Kimura, S. Economic competitiveness and environmental implications of hydrogen energy and fuel cell electric vehicles in ASEAN countries: The current and future scenarios. Energy Policy 2021, 148, 111980. [Google Scholar] [CrossRef]
- Thomas, J.M.; Edwards, P.P.; Dobson, P.J.; Owen, G.P. Decarbonising energy: The developing international activity in hydrogen technologies and fuel cells. J. Energy Chem. 2020, 51, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Ajanovic, A.; Sayer, M.; Haas, R. The economics and the environmental benignity of different colors of hydrogen. Int. J. Hydrogen Energy 2022, 47, 24136–24154. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy; economy; storage: Review; recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Tarhan, C.; Çil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- She, H.; Yue, P.; Ma, X.; Huang, J.; Wang, L.; Wang, Q. Fabrication of BiVO4 photoanode cocatalyzed with NiCo-layered double hydroxide for enhanced photoactivity of water oxidation. Appl. Catal. B Environ. 2020, 263, 118280. [Google Scholar] [CrossRef]
- Pan, J.; Wang, P.; Wang, P.; Yu, Q.; Wang, J.; Song, C.; Zheng, Y.; Li, C. The photocatalytic overall water splitting hydrogen production of g-C3N4/CdS hollow core–shell heterojunction via the HER/OER matching of Pt/MnOx. Chem. Eng. J. 2021, 405, 126622. [Google Scholar] [CrossRef]
- Guayaquil-Sosa, J.F.; Serrano-Rosales, B.; Valadés-Pelayo, P.J.; de Lasa, H. Photocatalytic hydrogen production using mesoporous TiO2 doped with Pt. Appl. Catal. B Environ. 2017, 211, 337–348. [Google Scholar] [CrossRef]
- Mei, F.; Li, Z.; Dai, K.; Zhang, J.; Liang, C. Step-scheme porous g-C3N4/Zn0.2Cd0.8S-DETA composites for efficient and stable photocatalytic H2 production. Chin. J. Catal. 2020, 41, 41–49. [Google Scholar] [CrossRef]
- An, X.; Yu, J.C.; Wang, Y.; Hu, Y.; Yu, X.; Zhang, G. WO3 nanorods/graphene nanocomposites for high-efficiency visible-light-driven photocatalysis and NO2 gas sensing. J. Mater. Chem. 2012, 22, 8525–8531. [Google Scholar] [CrossRef]
- Marlinda, A.R.; Yusoff, N.; Sagadevan, S.; Johan, M.R. Recent developments in reduced graphene oxide nanocomposites for photoelectrochemical water-splitting applications. Int. J. Hydrogen Energy 2020, 45, 11976–11994. [Google Scholar] [CrossRef]
- Desai, M.A.; Vyas, A.N.; Saratale, G.D.; Sartale, S.D. Zinc oxide superstructures: Recent synthesis approaches and application for hydrogen production via photoelectrochemical water splitting. Int. J. Hydrogen Energy 2019, 44, 2091–2127. [Google Scholar] [CrossRef]
- Kumaravel, V.; Imam, M.; Badreldin, A.; Chava, R.; Do, J.; Kang, M.; Abdel-Wahab, A. Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO(2) Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, e1901997. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Singh, A.; Rahimi, F.A.; Sarkar, P.; Nath, S.; Pati, S.K.; Maji, T.K. Charge-transfer regulated visible light driven photocatalytic H(2) production and CO(2) reduction in tetrathiafulvalene based coordination polymer gel. Nat. Commun. 2021, 12, 7313. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. Fabrication of highly efficient and stable indirect Z-scheme assembly of AgBr/TiO2 via graphene as a solid-state electron mediator for visible light induced enhanced photocatalytic H2 production. Appl. Surf. Sci. 2019, 463, 445–455. [Google Scholar] [CrossRef]
- Li, S.H.; Qi, M.Y.; Tang, Z.R.; Xu, Y.J. Nanostructured metal phosphides: From controllable synthesis to sustainable catalysis. Chem. Soc. Rev. 2021, 50, 7539–7586. [Google Scholar] [CrossRef]
- Song, Y.-D.; Wang, L.; Wu, L.-M. Theoretical study of the CO, NO, and N2 adsorptions on Li-decorated graphene and boron-doped graphene. Can. J. Chem. 2018, 96, 30–39. [Google Scholar] [CrossRef]
- Lotfi, E.; Neek-Amal, M. Temperature distribution in graphene doped with nitrogen and graphene with grain boundary. J. Mol. Graph. Model. 2017, 74, 100–104. [Google Scholar] [CrossRef]
- Sturala, J.; Luxa, J.; Plutnar, J.; Janoušek, Z.; Sofer, Z.; Pumera, M. Selenium covalently modified graphene: Towards gas sensing. 2D Mater. 2019, 6, 034006. [Google Scholar] [CrossRef]
- Yoo, M.S.; Lee, H.C.; Lee, S.B.; Cho, K. Cu-Phosphorus Eutectic Solid Solution for Growth of Multilayer Graphene with Widely Tunable Doping. Adv. Funct. Mater. 2020, 31, 2006499. [Google Scholar] [CrossRef]
- Chahal, S.; Nair, A.K.; Ray, S.J.; Yi, J.; Vinu, A.; Kumar, P. Microwave flash synthesis of phosphorus and sulphur ultradoped graphene. Chem. Eng. J. 2022, 450, 138447. [Google Scholar] [CrossRef]
- Liu, A.; Li, W.; Jin, H.; Yu, X.; Bu, Y.; He, Y.; Huang, H.; Wang, S.; Wang, J. The enhanced electrocatalytic activity of graphene co-doped with chlorine and fluorine atoms. Electrochim. Acta 2015, 177, 36–42. [Google Scholar] [CrossRef]
- Tongay, S.; Hwang, J.; Tanner, D.B.; Pal, H.K.; Maslov, D.; Hebard, A.F. Supermetallic conductivity in bromine-intercalated graphite. Phys. Rev. B 2010, 81, 115428. [Google Scholar] [CrossRef]
- Albero, J.; Peng, Y.; García, H. Photocatalytic CO2 Reduction to C2+ Products. ACS Catal. 2020, 10, 5734–5749. [Google Scholar] [CrossRef]
- Guo, Q.; Ma, Z.; Zhou, C.; Ren, Z.; Yang, X. Single Molecule Photocatalysis on TiO(2) Surfaces. Chem. Rev. 2019, 119, 11020–11041. [Google Scholar] [CrossRef]
- He, F.; Meng, A.; Cheng, B.; Ho, W.; Yu, J. Enhanced photocatalytic H2-production activity of WO3/TiO2 step-scheme heterojunction by graphene modification. Chin. J. Catal. 2020, 41, 9–20. [Google Scholar] [CrossRef]
- Kiranakumar, H.V.; Thejas, R.; Naveen, C.S.; Khan, M.I.; Prasanna, G.D.; Reddy, S.; Oreijah, M.; Guedri, K.; Bafakeeh, O.T.; Jameel, M. A review on electrical and gas-sensing properties of reduced graphene oxide-metal oxide nanocomposites. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Padmanabhan, N.T.; Thomas, N.; Louis, J.; Mathew, D.T.; Ganguly, P.; John, H.; Pillai, S.C. Graphene coupled TiO2 photocatalysts for environmental applications: A review. Chemosphere 2021, 271, 129506. [Google Scholar] [CrossRef]
- Purabgola, A.; Mayilswamy, N.; Kandasubramanian, B. Graphene-based TiO2 composites for photocatalysis & environmental remediation: Synthesis and progress. Environ. Sci. Pollut. Res. 2022, 29, 32305–32325. [Google Scholar]
- Qian, R.; Zong, H.; Schneider, J.; Zhou, G.; Zhao, T.; Li, Y.; Yang, J.; Bahnemann, D.W.; Pan, J.H. Charge carrier trapping, recombination and transfer during TiO2 photocatalysis: An overview. Catal. Today 2019, 335, 78–90. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Shen, X.; Duoerkun, G.; Zhu, B.; Zhang, L.; Li, M.; Chen, Z. Fabrication of g-C3N4/BiOBr heterojunctions on carbon fibers as weaveable photocatalyst for degrading tetracycline hydrochloride under visible light. Chem. Eng. J. 2020, 386, 124010. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Lee, T.; Bui, H.T.; Yoo, J.; Ra, M.; Han, S.H.; Kim, W.; Kwon, W. Formation of TiO(2)@Carbon Core/Shell Nanocomposites from a Single Molecular Layer of Aromatic Compounds for Photocatalytic Hydrogen Peroxide Generation. ACS Appl. Mater. Interfaces 2019, 11, 41196–41203. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Cho, Y.; Go, E.M.; Sharma, P.; Cho, H.; Lee, B.; Lee, S.M.; Park, S.O.; Ko, M.; Kwak, S.K.; et al. Unassisted photocatalytic H2O2 production under visible light by fluorinated polymer-TiO2 heterojunction. Chem. Eng. J. 2021, 418, 129346. [Google Scholar] [CrossRef]
- Kamal, K.M.; Narayan, R.; Chandran, N.; Popović, S.; Nazrulla, M.A.; Kovač, J.; Vrtovec, N.; Bele, M.; Hodnik, N.; Kržmanc, M.M.; et al. Synergistic enhancement of photocatalytic CO2 reduction by plasmonic Au nanoparticles on TiO2 decorated N-graphene heterostructure catalyst for high selectivity methane production. Appl. Catal. B Environ. 2022, 307, 121181. [Google Scholar] [CrossRef]
- Baran, T.; Wojtyła, S.; Minguzzi, A.; Rondinini, S.; Vertova, A. Achieving efficient H2O2 production by a visible-light absorbing, highly stable photosensitized TiO2. Appl. Catal. B Environ. 2019, 244, 303–312. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yao, X.; Narita, A.; Mullen, K. Heteroatom-Doped Nanographenes with Structural Precision. Acc. Chem. Res. 2019, 52, 2491–2505. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Dong, H.; Chen, X.; Leng, L.; Wu, Z.; Peng, L. In situ synthesis of In2S3@MIL-125(Ti) core–shell microparticle for the removal of tetracycline from wastewater by integrated adsorption and visible-light-driven photocatalysis. Appl. Catal. B Environ. 2016, 186, 19–29. [Google Scholar] [CrossRef]
- Tayebi, M.; Kolaei, M.; Tayyebi, A.; Masoumi, Z.; Belbasi, Z.; Lee, B.-K. Reduced graphene oxide (RGO) on TiO2 for an improved photoelectrochemical (PEC) and photocatalytic activity. Sol. Energy 2019, 190, 185–194. [Google Scholar] [CrossRef]
- Shu, R.; Wan, Z.; Zhang, J.; Wu, Y.; Liu, Y.; Shi, J.; Zheng, M. Facile Design of Three-Dimensional Nitrogen-Doped Reduced Graphene Oxide/Multi-Walled Carbon Nanotube Composite Foams as Lightweight and Highly Efficient Microwave Absorbers. ACS Appl. Mater. Interfaces 2020, 12, 4689–4698. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Sun, J.; Li, S.; Huang, C.; Yao, W.; Chen, W.; Zeng, T.; Wu, Q.; Xu, Q. Single Nickel Atoms Anchored on Nitrogen-Doped Graphene as a Highly Active Cocatalyst for Photocatalytic H2 Evolution. ACS Catal. 2018, 8, 11863–11874. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Zheng, S.; Lv, S.; Li, H.; Si, Z.; Wu, X.; Ran, R.; Weng, D.; Kang, F. Ni single atoms anchored on nitrogen-doped graphene as H2-Evolution cocatalyst of SrTiO3(Al)/CoO for photocatalytic overall water splitting. Carbon 2021, 183, 763–773. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Duan, X.; Li, Y.; Fan, X.; Zhang, G.; Zhang, F.; Peng, W. Fine-Tuning Radical/Nonradical Pathways on Graphene by Porous Engineering and Doping Strategies. ACS Catal. 2021, 11, 4848–4861. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, B.; Wang, L.; Cheng, B.; Zhang, L.; Yu, J. In-situ grown N, S co-doped graphene on TiO2 fiber for artificial photosynthesis of H2O2 and mechanism study. Appl. Catal. B Environ. 2022, 317, 121788. [Google Scholar] [CrossRef]
- Kurra, N.; Jiang, Q.; Nayak, P.; Alshareef, H.N. Laser-derived graphene: A three-dimensional printed graphene electrode and its emerging applications. Nano Today 2019, 24, 81–102. [Google Scholar] [CrossRef]
- Yang, L.; Peng, Y.; Luo, X.; Dan, Y.; Ye, J.; Zhou, Y.; Zou, Z. Beyond C3N4 pi-conjugated metal-free polymeric semiconductors for photocatalytic chemical transformations. Chem. Soc. Rev. 2021, 50, 2147–2172. [Google Scholar] [CrossRef]
- Lu, C.; Meng, J.; Zhang, J.; Chen, X.; Du, M.; Chen, Y.; Hou, C.; Wang, J.; Ju, A.; Wang, X.; et al. Three-Dimensional Hierarchically Porous Graphene Fiber-Shaped Supercapacitors with High Specific Capacitance and Rate Capability. ACS Appl. Mater. Interfaces 2019, 11, 25205–25217. [Google Scholar] [CrossRef]
- Xiong, C.; Li, B.; Lin, X.; Liu, H.; Xu, Y.; Mao, J.; Duan, C.; Li, T.; Ni, Y. The recent progress on three-dimensional porous graphene-based hybrid structure for supercapacitor. Compos. Part B Eng. 2019, 165, 10–46. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, B.; Shan, C.; Zhang, W.; Dionysiou, D.D.; Pan, B. Roles of oxygen-containing functional groups of O-doped g-C3N4 in catalytic ozonation: Quantitative relationship and first-principles investigation. Appl. Catal. B Environ. 2021, 292, 120155. [Google Scholar] [CrossRef]
- Han, C.; Su, P.; Tan, B.; Ma, X.; Lv, H.; Huang, C.; Wang, P.; Tong, Z.; Li, G.; Huang, Y.; et al. Defective ultra-thin two-dimensional g-C3N4 photocatalyst for enhanced photocatalytic H2 evolution activity. J. Colloid Interface Sci. 2021, 581 Pt A, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Wang, Y.; Yang, W.; Yu, Y. Nitrogen-rich g-C3N4@AgPd Mott-Schottky heterojunction boosts photocatalytic hydrogen production from water and tandem reduction of NO3− and NO2−. J. Colloid Interface Sci. 2021, 581 Pt B, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, M.; Peng, T.; Zhang, C.; Li, T.; Hussain, I.; Wang, J.; Tan, B. Porous hypercrosslinked polymer-TiO2-graphene composite photocatalysts for visible-light-driven CO2 conversion. Nat. Commun. 2019, 10, 676. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, H.; Ahn, C.; Park, J.; Jeon, S. Strategies to improve the photocatalytic activity of TiO2: 3D nanostructuring and heterostructuring with graphitic carbon nanomaterials. Nanoscale 2019, 11, 7025–7040. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, X.; Xu, Y.; Du, Y.; Yan, C. Ultrathin 2D Rare-Earth Nanomaterials: Compositions, Syntheses, and Applications. Adv. Mater. 2020, 32, e1806461. [Google Scholar] [CrossRef]
- Zhang, L.; Long, R.; Zhang, Y.; Duan, D.; Xiong, Y.; Zhang, Y.; Bi, Y. Direct Observation of Dynamic Bond Evolution in Single-Atom Pt/C3N4 Catalysts. Angew. Chem. Int. Ed. Engl. 2020, 59, 6224–6229. [Google Scholar] [CrossRef]
- Jiang, J.; Yu, J.; Cao, S. Au/PtO nanoparticle-modified g-C3N4 for plasmon-enhanced photocatalytic hydrogen evolution under visible light. J. Colloid Interface Sci. 2016, 461, 56–63. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Li, J.; Zhao, X. Facile synthesis of metal free perylene imide-carbon nitride membranes for efficient photocatalytic degradation of organic pollutants in the presence of peroxymonosulfate. Appl. Catal. B Environ. 2020, 278, 118981. [Google Scholar] [CrossRef]
- Xiao, J.; Xie, Y.; Rabeah, J.; Bruckner, A.; Cao, H. Visible-Light Photocatalytic Ozonation Using Graphitic C3N4 Catalysts: A Hydroxyl Radical Manufacturer for Wastewater Treatment. Acc. Chem. Res. 2020, 53, 1024–1033. [Google Scholar] [CrossRef]
- Wen, P.; Sun, Y.; Li, H.; Liang, Z.; Wu, H.; Zhang, J.; Zeng, H.; Geyer, S.M.; Jiang, L. A highly active three-dimensional Z-scheme ZnO/Au/g-C3N4 photocathode for efficient photoelectrochemical water splitting. Appl. Catal. B Environ. 2020, 263, 118180. [Google Scholar] [CrossRef]
- Li, W.; Chu, X.-S.; Wang, F.; Dang, Y.-Y.; Liu, X.-Y.; Wang, X.-C.; Wang, C.-Y. Enhanced cocatalyst-support interaction and promoted electron transfer of 3D porous g-C3N4/GO-M (Au, Pd, Pt) composite catalysts for hydrogen evolution. Appl. Catal. B Environ. 2021, 288, 120034. [Google Scholar] [CrossRef]
- Wang, J.W.; Qiao, L.Z.; Nie, H.D.; Huang, H.H.; Li, Y.; Yao, S.; Liu, M.; Zhang, Z.M.; Kang, Z.H.; Lu, T.B. Facile electron delivery from graphene template to ultrathin metal-organic layers for boosting CO2 photoreduction. Nat. Commun. 2021, 12, 813. [Google Scholar] [CrossRef]
- Yang, J.; Miao, H.; Jing, J.; Zhu, Y.; Choi, W. Photocatalytic activity enhancement of PDI supermolecular via π-π action and energy level adjusting with graphene quantum dots. Appl. Catal. B Environ. 2021, 281, 119547. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef]
- He, J.; Hartmann, G.; Lee, M.; Hwang, G.S.; Chen, Y.; Manthiram, A. Freestanding 1T MoS2/graphene heterostructures as a highly efficient electrocatalyst for lithium polysulfides in Li-S batteries. Energy Environ. Sci. 2019, 12, 344–350. [Google Scholar] [CrossRef]
- Huang, H.; Shi, H.; Das, P.; Qin, J.; Li, Y.; Wang, X.; Su, F.; Wen, P.; Li, S.; Lu, P.; et al. The Chemistry and Promising Applications of Graphene and Porous Graphene Materials. Adv. Funct. Mater. 2020, 30, 1909035. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Jiang, G.; Li, G.; Zhu, J.; Xiao, M.; Zhu, Y.; Gao, R.; Yu, A.; Feng, M.; et al. Graphene Quantum Dots-Based Advanced Electrode Materials: Design, Synthesis and Their Applications in Electrochemical Energy Storage and Electrocatalysis. Adv. Energy Mater. 2020, 10, 2001275. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Chen, Y.; Bao, J.; Zhou, X.; Xie, Z.; Wei, J.; Zhou, Z. The First Introduction of Graphene to Rechargeable Li-CO2 Batteries. Angew. Chem.-Int. Ed. 2015, 54, 6550–6553. [Google Scholar] [CrossRef]
- Ding, W.; Wei, Z.; Chen, S.; Qi, X.; Yang, T.; Hu, J.; Wang, D.; Wan, L.-J.; Alvi, S.F.; Li, L. Space-Confinement-Induced Synthesis of Pyridinic- and Pyrrolic-Nitrogen-Doped Graphene for the Catalysis of Oxygen Reduction. Angew. Chem.-Int. Ed. 2013, 52, 11755–11759. [Google Scholar] [CrossRef]
- Dou, S.; Tao, L.; Huo, J.; Wang, S.; Dai, L. Etched and doped Co9S8/graphene hybrid for oxygen electrocatalysis. Energy Environ. Sci. 2016, 9, 1320–1326. [Google Scholar] [CrossRef]
- Ma, Z.; Dou, S.; Shen, A.; Tao, L.; Dai, L.; Wang, S. Sulfur-Doped Graphene Derived from Cycled Lithium-Sulfur Batteries as a Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. Angew. Chem.-Int. Ed. 2015, 54, 1888–1892. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Voiry, D.; Ahn, S.J.; Kang, D.; Kim, A.Y.; Chhowalla, M.; Shin, H.S. Two-Dimensional Hybrid Nanosheets of Tungsten Disulfide and Reduced Graphene Oxide as Catalysts for Enhanced Hydrogen Evolution. Angew. Chem.-Int. Ed. 2013, 52, 13751–13754. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Zhu, J.; Yan, Q.; Dou, S.X.; Sun, W. Nanostructured Metal Chalcogenides for Energy Storage and Electrocatalysis. Adv. Funct. Mater. 2017, 27, 1702317. [Google Scholar] [CrossRef]
- Zeng, M.; Liu, Y.; Zhao, F.; Nie, K.; Han, N.; Wang, X.; Huang, W.; Song, X.; Zhong, J.; Li, Y. Metallic Cobalt Nanoparticles Encapsulated in Nitrogen-Enriched Graphene Shells: Its Bifunctional Electrocatalysis and Application in Zinc-Air Batteries. Adv. Funct. Mater. 2016, 26, 4397–4404. [Google Scholar] [CrossRef]
- Chia, X.; Pumera, M. Characteristics and performance of two-dimensional materials for electrocatalysis. Nat. Catal. 2018, 1, 909–921. [Google Scholar] [CrossRef]
- Huang, H.; Yan, M.; Yang, C.; He, H.; Jiang, Q.; Yang, L.; Lu, Z.; Sun, Z.; Xu, X.; Bando, Y.; et al. Graphene Nanoarchitectonics: Recent Advances in Graphene-Based Electrocatalysts for Hydrogen Evolution Reaction. Adv. Mater. 2019, 31, e1903415. [Google Scholar] [CrossRef]
- Jahan, M.; Liu, Z.; Loh, K.P. A Graphene Oxide and Copper-Centered Metal Organic Framework Composite as a Tri-Functional Catalyst for HER, OER, and ORR. Adv. Funct. Mater. 2013, 23, 5363–5372. [Google Scholar] [CrossRef]
- Prabhu, P.; Jose, V.; Lee, J.M. Heterostructured Catalysts for Electrocatalytic and Photocatalytic Carbon Dioxide Reduction. Adv. Funct. Mater. 2020, 30, 1910768. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Fan, C.; Meng, Q.; Tang, Y.; Qiu, X.; Fu, G.; Ma, T. Dual Single-Atomic Ni-N4 and Fe-N4 Sites Constructing Janus Hollow Graphene for Selective Oxygen Electrocatalysis. Adv. Mater. 2020, 32, 2003134. [Google Scholar] [CrossRef]
- Li, M.F.; Liu, Y.G.; Zeng, G.M.; Liu, N.; Liu, S.B. Graphene and graphene-based nanocomposites used for antibiotics removal in water treatment: A review. Chemosphere 2019, 226, 360–380. [Google Scholar] [CrossRef] [PubMed]
- Bano, Z.; Mazari, S.A.; Saeed, R.M.Y.; Majeed, M.A.; Xia, M.; Memon, A.Q.; Abro, R.; Wang, F. Water decontamination by 3D graphene based materials: A review. J. Water Process Eng. 2020, 36, 101404. [Google Scholar] [CrossRef]
- Wang, X.; Yin, R.; Zeng, L.; Zhu, M. A review of graphene-based nanomaterials for removal of antibiotics from aqueous environments. Environ. Pollut. 2019, 253, 100–110. [Google Scholar] [CrossRef]
- Vaizoğullar, A.I. ZnO/ZrO2 composites: Synthesis characterization and photocatalytic performance in the degradation of oxytetracycline antibiotic. Mater. Technol. 2019, 34, 433–443. [Google Scholar] [CrossRef]
- Cerro-Lopez, M.; Méndez-Rojas, M.A. Application of Nanomaterials for Treatment of Wastewater Containing Pharmaceuticals. In Ecopharmacovigilance; Springer: Cham, Switzerland, 2017; pp. 201–219. [Google Scholar]
- Ibrahim, F.A.; Al-Ghobashy, M.A.; El-Rahman, M.K.A.; Abo-Elmagd, I.F. Optimization and in line potentiometric monitoring of enhanced photocatalytic degradation kinetics of gemifloxacin using TiO2 nanoparticles/H2O2. Environ. Sci. Pollut. Res. Int. 2017, 24, 23880–23892. [Google Scholar] [CrossRef]
- Yang, Y.; Song, W.; Lin, H.; Wang, W.; Du, L.; Xing, W. Antibiotics and antibiotic resistance genes in global lakes: A review and meta-analysis. Environ. Int. 2018, 116, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, X.; Duan, X.; Fu, Y.; Dionysiou, D.D. Photochemical degradation of oxytetracycline: Influence of pH and role of carbonate radical. Chem. Eng. J. 2015, 276, 113–121. [Google Scholar] [CrossRef]
- Liu, L.; Wu, W.; Zhang, J.; Lv, P.; Xu, L.; Yan, Y. Progress of research on the toxicology of antibiotic pollution in aquatic organisms. Acta Ecol. Sin. 2018, 38, 36–41. [Google Scholar] [CrossRef]
- Guo, H.; Li, Z.; Lin, S.; Li, D.; Jiang, N.; Wang, H.; Han, J.; Li, J. Multi-catalysis induced by pulsed discharge plasma coupled with graphene-Fe3O4 nanocomposites for efficient removal of ofloxacin in water: Mechanism, degradation pathway and potential toxicity. Chemosphere 2021, 265, 129089. [Google Scholar] [CrossRef]
- Dong, S.; Zhao, Y.; Yang, J.; Liu, X.; Li, W.; Zhang, L.; Wu, Y.; Sun, J.; Feng, J.; Zhu, Y. Visible-light responsive PDI/rGO composite film for the photothermal catalytic degradation of antibiotic wastewater and interfacial water evaporation. Appl. Catal. B Environ. 2021, 291, 120127. [Google Scholar] [CrossRef]
- Juengchareonpoon, K.; Wanichpongpan, P.; Boonamnuayvitaya, V. Graphene oxide and carboxymethylcellulose film modified by citric acid for antibiotic removal. J. Environ. Chem. Eng. 2020, 9, 104637. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Yao, X.; Zhang, Y.; Li, Z.; Pan, S.; Han, J.; Xu, L.; Qiao, W.; Li, J.; et al. A comprehensive insight into plasma-catalytic removal of antibiotic oxytetracycline based on graphene-TiO2-Fe3O4 nanocomposites. Chem. Eng. J. 2021, 425, 130614. [Google Scholar] [CrossRef]
- Romao, J.; Barata, D.; Ribeiro, N.; Habibovic, P.; Fernandes, H.; Mul, G. High throughput screening of photocatalytic conversion of pharmaceutical contaminants in water. Environ. Pollut. 2017, 220 Pt B, 1199–1207. [Google Scholar] [CrossRef]
- Shaniba, C.; Akbar, M.; Ramseena, K.; Raveendran, P.; Narayanan, B.N.; Ramakrishnan, R.M. Sunlight-assisted oxidative degradation of cefixime antibiotic from aqueous medium using TiO2/nitrogen doped holey graphene nanocomposite as a high performance photocatalyst. J. Environ. Chem. Eng. 2020, 8, 102204. [Google Scholar] [CrossRef]
- Qiao, D.; Li, Z.; Duan, J.; He, X. Adsorption and photocatalytic degradation mechanism of magnetic graphene oxide/ZnO nanocomposites for tetracycline contaminants. Chem. Eng. J. 2020, 400, 125952. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, Y.; Yuan, X.; Zou, D.; Fang, J.; Jiang, L.; Zhang, J.; Yang, H.; Xiao, Z. MXene Ti3C2 derived Z–scheme photocatalyst of graphene layers anchored TiO2/g–C3N4 for visible light photocatalytic degradation of refractory organic pollutants. Chem. Eng. J. 2020, 394, 124921. [Google Scholar] [CrossRef]
- Guo, H.; Li, Z.; Xiang, L.; Jiang, N.; Zhang, Y.; Wang, H.; Li, J. Efficient removal of antibiotic thiamphenicol by pulsed discharge plasma coupled with complex catalysis using graphene-WO3-Fe3O4 nanocomposites. J. Hazard. Mater. 2021, 403, 123673. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y. Enhanced electron and mass transfer flow-through cell with C3N4-MoS2 supported on three-dimensional graphene photoanode for the removal of antibiotic and antibacterial potencies in ampicillin wastewater. Appl. Catal. B Environ. 2021, 282, 119574. [Google Scholar] [CrossRef]
- Zhu, L.; Ji, J.; Liu, J.; Mine, S.; Matsuoka, M.; Zhang, J.; Xing, M. Designing 3D-MoS2 Sponge as Excellent Cocatalysts in Advanced Oxidation Processes for Pollutant Control. Angew. Chem. Int. Ed. Engl. 2020, 59, 13968–13976. [Google Scholar] [CrossRef]
- Zhu, T.-T.; Su, Z.-X.; Lai, W.-X.; Zhang, Y.-B.; Liu, Y.-W. Insights into the fate and removal of antibiotics and antibiotic resistance genes using biological wastewater treatment technology. Sci. Total Environ. 2021, 776, 145906. [Google Scholar] [CrossRef]
- Ullah, S.; Hashmi, M.; Hussain, N.; Ullah, A.; Sarwar, M.N.; Saito, Y.; Kim, S.H.; Kim, I.S. Stabilized nanofibers of polyvinyl alcohol (PVA) crosslinked by unique method for efficient removal of heavy metal ions. J. Water Process Eng. 2020, 33, 101111. [Google Scholar] [CrossRef]
- Citulski, J.; Farahbakhsh, K.; Kent, F. Optimization of phosphorus removal in secondary effluent using immersed ultrafiltration membranes with in-line coagulant pretreatment—Implications for advanced water treatment and reuse applications. J. Environ. Eng. Sci. 2013, 8, 359–370. [Google Scholar] [CrossRef]
- Zhang, P.; Hou, D.; Li, X.; Pehkonen, S.; Varma, R.S.; Wang, X. Greener and size-specific synthesis of stable Fe-Cu oxides as earth-abundant adsorbents for malachite green. J. Mater. Chem. A Mater. 2018, 6, 9229–9236. [Google Scholar]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef]
- Verma, A.; Thakur, S.; Mamba, G.; Prateek; Gupta, R.K.; Thakur, P.; Thakur, V.K. Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Int. J. Biol. Macromol. 2020, 148, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Arabkhani, P.; Asfaram, A. Development of a novel three-dimensional magnetic polymer aerogel as an efficient adsorbent for malachite green removal. J. Hazard. Mater. 2020, 384, 121394. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, H.; Umar, M.; Ullah, A.; Razzaq, H.; Zia, K.M.; Liu, X. Polyvinylidene fluoride nanocomposite super hydrophilic membrane integrated with Polyaniline-Graphene oxide nano fillers for treatment of textile effluents. J. Hazard. Mater. 2021, 403, 123587. [Google Scholar] [CrossRef] [PubMed]
- Mittal, H.; Al Alili, A.; Morajkar, P.P.; Alhassan, S.M. GO crosslinked hydrogel nanocomposites of chitosan/carboxymethyl cellulose—A versatile adsorbent for the treatment of dyes contaminated wastewater. Int. J. Biol. Macromol. 2021, 167, 1248–1261. [Google Scholar] [CrossRef]
- Jiang, L.; Wen, Y.; Zhu, Z.; Liu, X.; Shao, W. A Double cross-linked strategy to construct graphene aerogels with highly efficient methylene blue adsorption performance. Chemosphere 2021, 265, 129169. [Google Scholar] [CrossRef]
- Pashaei-Fakhri, S.; Peighambardoust, S.J.; Foroutan, R.; Arsalani, N.; Ramavandi, B. Crystal violet dye sorption over acrylamide/graphene oxide bonded sodium alginate nanocomposite hydrogel. Chemosphere 2021, 270, 129419. [Google Scholar] [CrossRef]
- Cao, M.; Shen, Y.; Yan, Z.; Wei, Q.; Jiao, T.; Shen, Y.; Han, Y.; Wang, Y.; Wang, S.; Xia, Y.; et al. Extraction-like removal of organic dyes from polluted water by the graphene oxide/PNIPAM composite system. Chem. Eng. J. 2021, 405, 126647. [Google Scholar] [CrossRef]
- Hao, J.; Wang, Z.; Xiao, C.; Zhao, J.; Chen, L. In situ reduced graphene oxide-based polyurethane sponge hollow tube for continuous oil removal from water surface. Environ. Sci. Pollut. Res. Int. 2018, 25, 4837–4845. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Yang, B.; Barras, A.; Szunerits, S.; Boukherroub, R. Polyurethane sponge functionalized with superhydrophobic nanodiamond particles for efficient oil/water separation. Chem. Eng. J. 2017, 307, 319–325. [Google Scholar] [CrossRef]
- Ma, W.; Li, Y.; Zhang, M.; Gao, S.; Cui, J.; Huang, C.; Fu, G. Biomimetic Durable Multifunctional Self-Cleaning Nanofibrous Membrane with Outstanding Oil/Water Separation, Photodegradation of Organic Contaminants, and Antibacterial Performances. ACS Appl. Mater. Interfaces 2020, 12, 34999–35010. [Google Scholar] [CrossRef] [PubMed]
- Khalilifard, M.; Javadian, S. Magnetic superhydrophobic polyurethane sponge loaded with Fe3O4@oleic acid@graphene oxide as high performance adsorbent oil from water. Chem. Eng. J. 2021, 408, 127369. [Google Scholar] [CrossRef]
- Alammar, A.; Park, S.-H.; Williams, C.J.; Derby, B.; Szekely, G. Oil-in-water separation with graphene-based nanocomposite membranes for produced water treatment. J. Membr. Sci. 2020, 603, 118007. [Google Scholar] [CrossRef]
- Cao, J.; Chen, Y.; Zhang, J.; Wang, X.; Wang, J.; Shi, C.; Ning, Y.; Wang, X. Preparation and application of nanofluid flooding based on polyoxyethylated graphene oxide nanosheets for enhanced oil recovery. Chem. Eng. Sci. 2022, 247, 117023. [Google Scholar] [CrossRef]
- Abdalla, O.; Wahab, M.A.; Abdala, A. Mixed matrix membranes containing aspartic acid functionalized graphene oxide for enhanced oil-water emulsion separation. J. Environ. Chem. Eng. 2020, 8, 104269. [Google Scholar] [CrossRef]
- Liang, Y.; Li, C.; Li, S.; Su, B.; Hu, M.Z.; Gao, X.; Gao, C. Graphene quantum dots (GQDs)-polyethyleneimine as interlayer for the fabrication of high performance organic solvent nanofiltration (OSN) membranes. Chem. Eng. J. 2020, 380, 122462. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Sankaran, R.; Show, P.L.; Ooi, C.-W.; Ling, T.C.; Shu-Jen, C.; Chen, S.-Y.; Chang, Y.-K. Feasibility assessment of removal of heavy metals and soluble microbial products from aqueous solutions using eggshell wastes. Clean Technol. Environ. Policy 2020, 22, 773–786. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Show, P.L.; Lau, B.F.; Chang, J.S.; Ling, T.C. New Prospects for Modified Algae in Heavy Metal Adsorption. Trends Biotechnol. 2019, 37, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- O’connell, D.W.; Birkinshaw, C.; O’dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef]
- Kong, Q.; Shi, X.; Ma, W.; Zhang, F.; Yu, T.; Zhao, F.; Zhao, D.; Wei, C. Strategies to improve the adsorption properties of graphene-based adsorbent towards heavy metal ions and their compound pollutants: A review. J. Hazard. Mater. 2021, 415, 125690. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Ploychompoo, S.; Chen, J.; Zhou, T.; Luo, H. Simultaneous Cr(VI) reduction and bisphenol A degradation by a 3D Z-scheme Bi2S3-BiVO4 graphene aerogel under visible light. Chem. Eng. J. 2020, 384, 123256. [Google Scholar] [CrossRef]
- Chen, R.; Cheng, Y.; Wang, P.; Wang, Q.; Wan, S.; Huang, S.; Su, R.; Song, Y.; Wang, Y. Enhanced removal of Co(II) and Ni(II) from high-salinity aqueous solution using reductive self-assembly of three-dimensional magnetic fungal hyphal/graphene oxide nanofibers. Sci. Total Environ. 2021, 756, 143871. [Google Scholar] [CrossRef]
- Bao, S.; Yang, W.; Wang, Y.; Yu, Y.; Sun, Y. One-pot synthesis of magnetic graphene oxide composites as an efficient and recoverable adsorbent for Cd(II) and Pb(II) removal from aqueous solution. J. Hazard. Mater. 2020, 381, 120914. [Google Scholar] [CrossRef]
- Pakulski, D.; Gorczynski, A.; Marcinkowski, D.; Czepa, W.; Chudziak, T.; Witomska, S.; Nishina, Y.; Patroniak, V.; Ciesielski, A.; Samori, P. High-sorption terpyridine-graphene oxide hybrid for the efficient removal of heavy metal ions from wastewater. Nanoscale 2021, 13, 10490–10499. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Dvoranova, D.; Lajaunie, L.; Rozman, N.; Figueiredo, B.; Seabra, M.P.; Skapin, A.S.; Calvino, J.J.; Brezova, V.; Labrincha, J.A. Graphene-TiO2 hybrids for photocatalytic aided removal of VOCs and nitrogen oxides from outdoor environment. Chem. Eng. J. 2021, 405, 126651. [Google Scholar] [CrossRef]
- Shajari, S.; Kowsari, E.; Seifvand, N.; Ajdari, F.B.; Chinnappan, A.; Ramakrishna, S.; Saianand, G.; Najafi, M.D.; Haddadi-Asl, V.; Abdpour, S. Efficient Photocatalytic Degradation of Gaseous Benzene and Toluene over Novel Hybrid PIL@TiO2/m-GO Composites. Catalysts 2021, 11, 126. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Zhang, X.; Zhang, J.; Duan, Y.; Li, X.; Jiang, D.; Kozawa, T.; Naito, M. Development of graphene aerogels with high strength and ultrahigh adsorption capacity for gas purification. Mater. Des. 2021, 208, 109903. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, B.; Yu, J. Photokatalysatoren auf Graphenbasis für die Produktion von Solarbrennstoffen. Angew. Chem. 2015, 127, 11508–11524. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Ali, S.; Razzaq, A.; In, S.-I. Development of graphene based photocatalysts for CO2 reduction to C1 chemicals: A brief overview. Catal. Today 2019, 335, 39–54. [Google Scholar] [CrossRef]
- Thoai, D.B.; Hu, Y.Z.; Koch, S.W. Influence of the confinement potential on the electron-hole-pair states in semiconductor microcrystallites. Phys. Rev. B Condens. Matter 1990, 42, 11261–11266. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Iqbal, M.Z.; Iqbal, M.W.; Eom, J. Improving the electrical properties of graphene layers by chemical doping. Sci. Technol. Adv. Mater. 2014, 15, 055004. [Google Scholar] [CrossRef]
- Tian, P.; Zang, J.; Jia, S.; Zhang, Y.; Gao, H.; Zhou, S.; Wang, W.; Xu, H.; Wang, Y. Preparation of S/N co-doped graphene through a self-generated high gas pressure for high rate supercapacitor. Appl. Surf. Sci. 2018, 456, 781–788. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Gopalakrishnan, K.; Govindaraj, A. Synthesis, properties and applications of graphene doped with boron, nitrogen and other elements. Nano Today 2014, 9, 324–343. [Google Scholar] [CrossRef]
- He, Y.; Chen, Q.; Yang, S.; Lu, C.; Feng, M.; Jiang, Y.; Cao, G.; Zhang, J.; Liu, C. Micro-crack behavior of carbon fiber reinforced Fe3O4/graphene oxide modified epoxy composites for cryogenic application. Compos. Part A Appl. Sci. Manuf. 2018, 108, 12–22. [Google Scholar] [CrossRef]
- Singh, D.P.; Herrera, C.E.; Singh, B.; Singh, S.; Singh, R.K.; Kumar, R. Graphene oxide: An efficient material and recent approach for biotechnological and biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 86, 173–197. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Kumar, R.; Singh, D.P.; Savu, R.; Moshkalev, S.A. Progress in microwave-assisted synthesis of quantum dots (graphene/carbon/semiconducting) for bioapplications: A review. Mater. Today Chem. 2019, 12, 282–314. [Google Scholar] [CrossRef]
- Awasthi, K.; Kumar, R.; Tiwari, R.S.; Srivastava, O.N. Large scale synthesis of bundles of aligned carbon nanotubes using a natural precursor: Turpentine oil. J. Exp. Nanosci. 2010, 5, 498–508. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, B.; Bao, Q.; Liu, Y. Controllable synthesis of doped graphene and its applications. Small 2014, 10, 2975–2991. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Lacey, S.D.; Xu, L.; Xie, H.; Li, T.; Danner, V.A.; Hu, L. Reduced graphene oxide film with record-high conductivity and mobility. Mater. Today 2018, 21, 186–192. [Google Scholar] [CrossRef]
- Du, J.; Duan, J.; Yang, X.; Duan, Y.; Zhou, Q.; Tang, Q. p-Type Charge Transfer Doping of Graphene Oxide with (NiCo)1-yFeyOx for Air-Stable, All-Inorganic CsPbIBr2 Perovskite Solar Cells. Angew. Chem. Int. Ed. Engl. 2021, 60, 10608–10613. [Google Scholar] [CrossRef]
- Meng, F.; Li, J.; Cushing, S.K.; Zhi, M.; Wu, N. Solar hydrogen generation by nanoscale p-n junction of p-type molybdenum disulfide/n-type nitrogen-doped reduced graphene oxide. J. Am. Chem. Soc. 2013, 135, 10286–10289. [Google Scholar] [CrossRef]
- Nag, A.; Mitra, A.; Mukhopadhyay, S.C. Graphene and its sensor-based applications: A review. Sens. Actuators A Phys. 2018, 270, 177–194. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Chen, X.; Chen, B. Macroscopic and spectroscopic investigations of the adsorption of nitroaromatic compounds on graphene oxide, reduced graphene oxide, and graphene nanosheets. Environ. Sci. Technol. 2015, 49, 6181–6189. [Google Scholar] [CrossRef]
- Bi, W.; Li, X.; You, R.; Chen, M.; Yuan, R.; Huang, W.; Wu, X.; Chu, W.; Wu, C.; Xie, Y. Surface Immobilization of Transition Metal Ions on Nitrogen-Doped Graphene Realizing High-Efficient and Selective CO2 Reduction. Adv. Mater. 2018, 30, e1706617. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Jhankal, D.; Shakya, P.; Sharma, A.K.; Banerjee, M.K.; Sachdev, K. Ultraslim and highly flexible supercapacitor based on chemical vapor deposited nitrogen-doped bernal graphene for wearable electronics. Carbon 2023, 208, 227–237. [Google Scholar] [CrossRef]
- Sinha, S.; Barman, P.B.; Hazra, S.K. Probing the electronic properties of chemically synthesised doped and undoped graphene derivative. Mater. Sci. Eng. B 2023, 287, 116145. [Google Scholar] [CrossRef]
- Backes, C.; Abdelkader, A.M.; Alonso, C.; Andrieux-Ledier, A.; Arenal, R.; Azpeitia, J.; Balakrishnan, N.; Banszerus, L.; Barjon, J.; Bartali, R.; et al. Production and processing of graphene and related materials. 2D Mater. 2020, 7, 022001. [Google Scholar] [CrossRef]
- Khudair, S.A.M.; Jappor, H.R. Adsorption of Gas Molecules on Graphene Doped with Mono and Dual Boron as Highly Sensitive Sensors and Catalysts. J. Nanostruct. 2020, 10, 217–229. [Google Scholar]
- Chen, X.; Chen, S.; Wang, J. Screening of catalytic oxygen reduction reaction activity of metal-doped graphene by density functional theory. Appl. Surf. Sci. 2016, 379, 291–295. [Google Scholar] [CrossRef]
- Dai, X.; Shen, T.; Feng, Y.; Yang, B.; Liu, H. DFT investigations on photoelectric properties of graphene modified by metal atoms. Ferroelectrics 2020, 568, 143–154. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, W.; Zhao, K.; Guan, F.; Wang, Y. Investigations on the electronic structure and optical properties of (Ga, N, Ga-N) doped graphene by first-principle calculations. Int. J. Mod. Phys. B 2021, 35, 2150067. [Google Scholar] [CrossRef]
- Kumar, P.; Mungse, H.P.; Khatri, O.P.; Jain, S.L. Nitrogen-doped graphene-supported copper complex: A novel photocatalyst for CO2 reduction under visible light irradiation. RSC Adv. 2015, 5, 54929–54935. [Google Scholar] [CrossRef]
- Tang, Z.; He, W.; Wang, Y.; Wei, Y.; Yu, X.; Xiong, J.; Wang, X.; Zhang, X.; Zhao, Z.; Liu, J. Ternary heterojunction in rGO-coated Ag/Cu2O catalysts for boosting selective photocatalytic CO2 reduction into CH4. Appl. Catal. B Environ. 2022, 311, 121371. [Google Scholar] [CrossRef]
- Yang, A.; Luo, J.; Xie, Z. First-principles study of Graphene/ZnV2O6(001) heterostructure photocatalyst. J. Mater. Res. Technol. 2021, 15, 1479–1486. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.; Tong, J.; Liu, Y.; Xu, S.; Cao, Y.; Cao, S. Photocatalytic CO2 conversion to methanol by Cu2O/graphene/TNA heterostructure catalyst in a visible-light-driven dual-chamber reactor. Nano Energy 2016, 27, 320–329. [Google Scholar] [CrossRef]
- Sun, R.; Yin, H.; Zhang, Z.; Wang, Y.; Liang, T.; Zhang, S.; Jing, L. Graphene-Modulated PDI/g-C3N4 All-Organic S-Scheme Heterojunction Photocatalysts for Efficient CO2 Reduction under Full-Spectrum Irradiation. J. Phys. Chem. C 2021, 125, 23830–23839. [Google Scholar] [CrossRef]
- Radovic, L.R.; Salgado-Casanova, A.J.A.; Mora-Vilches, C.V. On the active sites for the oxygen reduction reaction catalyzed by graphene-based materials. Carbon 2020, 156, 389–398. [Google Scholar] [CrossRef]
- Ren, S.; Cui, M.; Liu, C.; Wang, L. A comprehensive review on ultrathin, multi-functionalized, and smart graphene and graphene-based composite protective coatings. Corros. Sci. 2023, 212, 110939. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, X.; Li, J.; Xiao, L.; Gao, H.; Zhao, F.; Ma, H. Progress on the application of graphene-based composites toward energetic materials: A review. Def. Technol. 2023. [Google Scholar] [CrossRef]
- Choi, M.G.; Park, S.; Lee, H.; Kim, S. Correlating surface structures and nanoscale friction of CVD Multi-Layered graphene. Appl. Surf. Sci. 2022, 584, 152572. [Google Scholar] [CrossRef]
- Muschi, M.; Devautour-Vinot, S.; Aureau, D.; Heymans, N.; Sene, S.; Emmerich, R.; Ploumistos, A.; Geneste, A.; Steunou, N.; Patriarche, G.; et al. Metal–organic framework/graphene oxide composites for CO2 capture by microwave swing adsorption. J. Mater. Chem. A 2021, 9, 13135–13142. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, Y.H. 3D Graphene Materials from the Reduction of CO2. Acc. Mater. Res. 2021, 2, 48–58. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Phuriragpitikhon, J.; Choma, J.; Jaroniec, M. Mechanochemical synthesis of three-component graphene oxide/ordered mesoporous carbon/metal-organic framework composites. J. Colloid Interface Sci. 2020, 577, 163–172. [Google Scholar] [CrossRef]
- Chebrolu, N.R.; Chittari, B.L.; Jung, J. Flat bands in twisted double bilayer graphene. Phys. Rev. B 2019, 99, 235417. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Xu, S.; Huo, Y.; Jiang, S.; Yang, C.; Li, Z.; Zhao, X.; Zhang, S.; Man, B. Experimental and theoretical investigation for a hierarchical SERS activated platform with 3D dense hot spots. Sens. Actuators B Chem. 2018, 263, 408–416. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, S.; Zhou, P.; Sun, Q.; Wang, P.; Wan, L.; Li, J.; Chen, L.; Wang, X.; Ding, S.; et al. Evolution of the band-gap and optical properties of graphene oxide with controllable reduction level. Carbon 2013, 62, 157–164. [Google Scholar] [CrossRef]
- Hunt, A.; Kurmaev, E.Z.; Moewes, A. Band gap engineering of graphene oxide by chemical modification. Carbon 2014, 75, 366–371. [Google Scholar] [CrossRef]
- Hunt, A.; Dikin, D.A.; Kurmaev, E.Z.; Lee, Y.H.; Luan, N.V.; Chang, G.S.; Moewes, A. Modulation of the band gap of graphene oxide: The role of AA-stacking. Carbon 2014, 66, 539–546. [Google Scholar] [CrossRef]
- Sugawara, K.; Suzuki, K.; Sato, M.; Sato, T.; Takahashi, T. Enhancement of band gap and evolution of in-gap states in hydrogen-adsorbed monolayer graphene on SiC(0001). Carbon 2017, 124, 584–587. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Wang, B.; Wang, G.; Wan, J. Significant band gap induced by uniaxial strain in graphene/blue phosphorene bilayer. Carbon 2018, 130, 120–126. [Google Scholar] [CrossRef]
- Méndez-Romero, U.A.; Pérez-García, S.A.; Xu, X.; Wang, E.; Licea-Jiménez, L. Functionalized reduced graphene oxide with tunable band gap and good solubility in organic solvents. Carbon 2019, 146, 491–502. [Google Scholar] [CrossRef]
- Pradhan, J.; Srivastava, S.K.; Palanivelu, M.; Kothalamuthu, S.; Balakrishnan, S.; Sarkar, S.; Mathew, S.; Venkatesan, T. Band gap opening and surface morphology of monolayer graphene induced by single ion impacts of argon monomer and dimer ions. Carbon 2021, 184, 322–330. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, W.; Yang, T.; Zhang, Z. Magic angles and flat Chern bands in alternating-twist multilayer graphene system. J. Mater. Sci. Technol. 2022, 111, 28–34. [Google Scholar] [CrossRef]
- Dompreh, K.A.; Sekyi-Arthur, D.; Mensah, S.Y.; Adu, K.W.; Edziah, R.; Amekpewu, M. Effect of band-gap tuning on absorption of phonons and acoustoelectric current in graphene nanoribbon. Phys. E Low-Dimens. Syst. Nanostructures 2023, 147, 115516. [Google Scholar] [CrossRef]
- Nandee, R.; Chowdhury, M.A.; Shahid, A.; Hossain, N.; Rana, M. Band gap formation of 2D materialin graphene: Future prospect and challenges. Results Eng. 2022, 15, 100474. [Google Scholar] [CrossRef]
- Chen, D.-M.; Shenai, P.M.; Zhao, Y. Tight binding description on the band gap opening of pyrene-dispersed graphene. Phys. Chem. Chem. Phys. 2011, 13, 1515–1520. [Google Scholar] [CrossRef]
- Gong, E.; Shahzad, A.; Hiragond, C.B.; Kim, H.S.; Powar, N.S.; Kim, D.; Kim, H.; In, S.-I. Solar fuels: Research and development strategies to accelerate photocatalytic CO2 conversion into hydrocarbon fuels. Energy Environ. Sci. 2022, 15, 880–937. [Google Scholar] [CrossRef]
- Wu, J.-T.; Shi, X.-H.; Wei, Y.-J. Tunable band structures of polycrystalline graphene by external and mismatch strains. Acta Mech. Sin. 2012, 28, 1539–1544. [Google Scholar] [CrossRef]
- Yuan, W.; Zhou, Y.; Li, Y.; Li, C.; Peng, H.; Zhang, J.; Liu, Z.; Dai, L.; Shi, G. The edge-and basal-plane-specific electrochemistry of a single-layer graphene sheet. Sci. Rep. 2013, 3, 2248. [Google Scholar] [CrossRef] [PubMed]















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Zhou, S.; Feng, X.; Wang, N.; Ola, O.; Zhu, Y. Recent Progress in Multifunctional Graphene-Based Nanocomposites for Photocatalysis and Electrocatalysis Application. Nanomaterials 2023, 13, 2028. https://doi.org/10.3390/nano13132028
Yang Z, Zhou S, Feng X, Wang N, Ola O, Zhu Y. Recent Progress in Multifunctional Graphene-Based Nanocomposites for Photocatalysis and Electrocatalysis Application. Nanomaterials. 2023; 13(13):2028. https://doi.org/10.3390/nano13132028
Chicago/Turabian StyleYang, Zanhe, Siqi Zhou, Xiangyu Feng, Nannan Wang, Oluwafunmilola Ola, and Yanqiu Zhu. 2023. "Recent Progress in Multifunctional Graphene-Based Nanocomposites for Photocatalysis and Electrocatalysis Application" Nanomaterials 13, no. 13: 2028. https://doi.org/10.3390/nano13132028
APA StyleYang, Z., Zhou, S., Feng, X., Wang, N., Ola, O., & Zhu, Y. (2023). Recent Progress in Multifunctional Graphene-Based Nanocomposites for Photocatalysis and Electrocatalysis Application. Nanomaterials, 13(13), 2028. https://doi.org/10.3390/nano13132028





