Antibacterial Cu or Zn-MOFs Based on the 1,3,5-Tris-(styryl)benzene Tricarboxylate
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Physicochemical Characterization
2.3. Antibacterial Activity
3. Results
3.1. Synthesis and Physicochemical Characterization
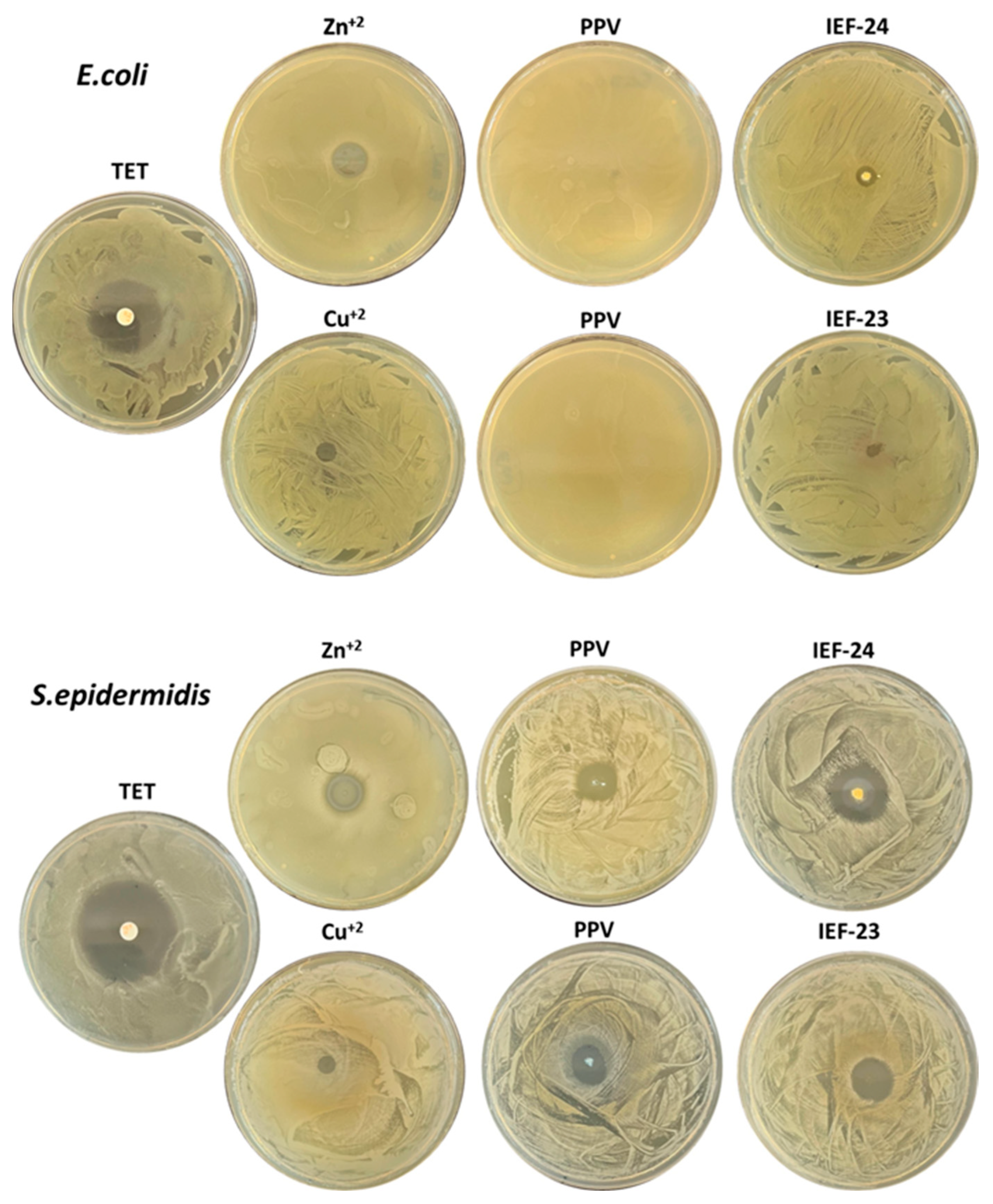
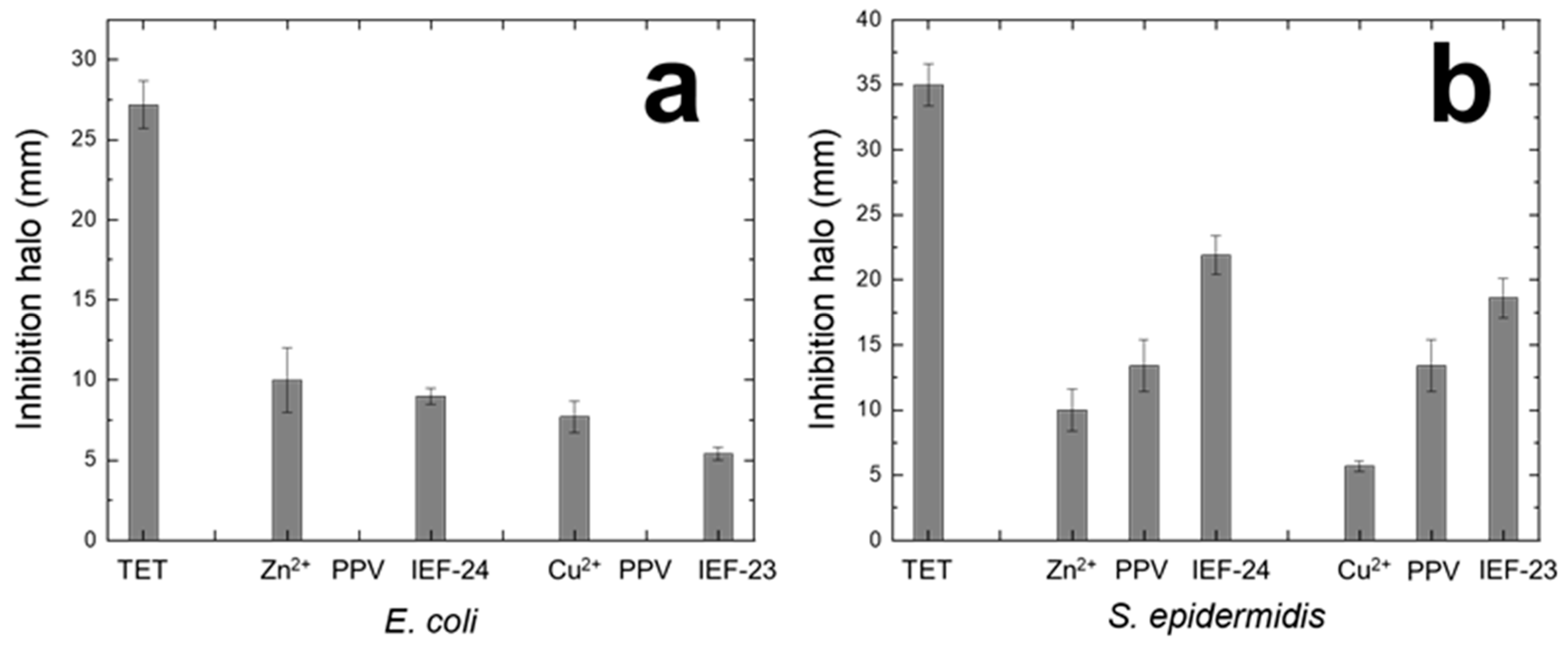
3.2. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, A.; Dubey, A.K. Various Biomaterials and Techniques for Improving Antibacterial Response. ACS Appl. Bio Mater. 2018, 1, 3–20. [Google Scholar] [CrossRef]
- Akram, F.; Imtiaz, M.; Haq, I. uL Emergent Crisis of Antibiotic Resistance: A Silent Pandemic Threat to 21st Century. Microb. Pathog. 2023, 174, 105923. [Google Scholar] [CrossRef]
- Arenas-vivo, A.; Amariei, G.; Aguado, S.; Rosal, R.; Horcajada, P. Acta Biomaterialia An Ag-Loaded Photoactive Nano-Metal Organic Framework as a Promising Biofilm Treatment. Acta Biomater. 2019, 97, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Alshahrani, M.Y.; Wahab, S.; Al-harbi, A.I.; Nisar, N.; Alraey, Y.; Alqahtani, A.; Ahmad, M.; Irfan, S.; Saeed, M. Journal of King Saud University—Science Zinc Oxide Nanoparticle: An Effective Antibacterial Agent against Pathogenic Bacterial Isolates. J. King Saud Univ.-Sci. 2022, 34, 102110. [Google Scholar] [CrossRef]
- Rojas, S.; García-García, A.; Hidalgo, T.; Rosales, M.; Ruiz-Camino, D.; Salcedo-Abraira, P.; Montes-Andrés, H.; Choquesillo-Lazarte, D.; Rosal, R.; Horcajada, P.; et al. Antibacterial Activity of Two Zn-MOFs Containing a Tricarboxylate Linker. Nanomaterials 2022, 12, 4139. [Google Scholar] [CrossRef] [PubMed]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc Status Is Associated with Inflammation, Oxidative Stress, Lipid, and Glucose Metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef]
- Maliki, M.; Ifijen, I.H.; Ikhuoria, E.U.; Jonathan, E.M.; Onaiwu, G.E.; Archibong, U.D.; Ighodaro, A. Copper Nanoparticles and Their Oxides: Optical, Anticancer and Antibacterial Properties. Int. Nano Lett. 2022, 12, 379–398. [Google Scholar] [CrossRef]
- Osredkar, J.; Sustar, N. Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. J. Clin. Toxicol. 2011, S3, 001. [Google Scholar] [CrossRef]
- Meyers, S.R.; Juhn, F.S.; Griset, A.P.; Luman, N.R.; Grinstaff, M.W. Anionic Amphiphilic Dendrimers as Antibacterial Agents. J. Am. Chem. Soc. 2008, 130, 14444–14445. [Google Scholar] [CrossRef]
- Staneva, D.; Manov, H.; Vasileva-Tonkova, E.; Kukeva, R.; Stoyanova, R.; Grabchev, I. Enhancing the Antibacterial Activity of PAMAM Dendrimer Modified with 1,8-Naphthalimides and Its Copper Complex via Light Illumination. Polym. Adv. Technol. 2022, 33, 3163–3172. [Google Scholar] [CrossRef]
- García-Martínez, J.C.; Díez-Barra, E.; Rodríguez-López, J. Conjugated Dendrimers with Poly(Phenylenevinylene) and Poly(Phenyleneethynylene) Scaffolds. Adv. Org. Synth. 2013, 5, 185–234. [Google Scholar] [CrossRef][Green Version]
- Ortiz-Gómez, I.; González-Alfaro, S.; Sánchez-Ruiz, A.; de Orbe-Payá, I.; Capitán-Vallvey, L.F.; Navarro, A.; Salinas-Castillo, A.; García-Martínez, J.C. Reversal of a Fluorescent Fluoride Chemosensor from Turn-Off to Turn-On Based on Aggregation Induced Emission Properties. ACS Sens. 2022, 7, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Villar, V.; Tolosa, J.; García-Martínez, J.C. AIE-Dots of Amphiphilic Oligostyrylbenzenes: Encapsulation and Release Monitored via FRET. J. Mol. Liq. 2022, 362, 119771. [Google Scholar] [CrossRef]
- Gómez-González, J.; Martínez-Castro, L.; Tolosa-Barrilero, J.; Alcalde-Ordóñez, A.; Learte-Aymamí, S.; Mascareñas, J.L.; García-Martínez, J.C.; Martínez-Costas, J.; Maréchal, J.D.; Vázquez López, M.; et al. Selective Recognition of A/T-Rich DNA 3-Way Junctions with a Three-Fold Symmetric Tripeptide. Chem. Commun. 2022, 58, 7769–7772. [Google Scholar] [CrossRef]
- Felix Sahayaraj, A.; Joy Prabu, H.; Maniraj, J.; Kannan, M.; Bharathi, M.; Diwahar, P.; Salamon, J. Metal–Organic Frameworks (MOFs): The Next Generation of Materials for Catalysis, Gas Storage, and Separation. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1757–1781. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Klet, R.C.; Howarth, A.J.; Hupp, J.T.; Farha, O.K. Metal—Organic Frameworks: An Emerging Class of Solid-State Materials. In Handbook of Solid State Chemistry; Wiley: Hoboken, NJ, USA, 2017; pp. 165–193. [Google Scholar]
- Wu, G.; Ma, J.; Li, S.; Li, J.; Wang, X.; Zhang, Z.; Chen, L. Functional Metal–Organic Frameworks as Adsorbents Used for Water Decontamination: Design Strategies and Applications. J. Mater. Chem. A 2023, 11, 6747–6771. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Fan, W.; Sun, D. Flexible Metal-Organic Frameworks for Gas Storage and Separation. Dalt. Trans. 2022, 51, 4608–4618. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Song, L.; Xu, W.; Guo, Z.; Yang, X.; Wu, X.; Chen, X. Au-HKUST—1 Composite Nanocapsules: Synthesis with a Coordination Replication Strategy and Catalysis on CO Oxidation. ACS Appl. Mater. Interfaces 2016, 8, 22745–22750. [Google Scholar] [CrossRef]
- Daniel, M.; Mathew, G.; Anpo, M.; Neppolian, B. MOF Based Electrochemical Sensors for the Detection of Physiologically Relevant Biomolecules: An Overview. Coord. Chem. Rev. 2022, 468, 214627. [Google Scholar] [CrossRef]
- Annapragada, R.; Vandavasi, K.R.; Kanuparthy, P.R. Metal-Organic Framework Membranes for Proton Exchange Membrane Fuel Cells: A Mini-Review. Inorganica Chim. Acta 2023, 546, 121304. [Google Scholar] [CrossRef]
- Moharramnejad, M.; Ehsani, A.; Shahi, M.; Gharanli, S.; Saremi, H.; Malekshah, R.E.; Basmenj, Z.S.; Salmani, S.; Mohammadi, M. MOF as Nanoscale Drug Delivery Devices: Synthesis and Recent Progress in Biomedical Applications. J. Drug Deliv. Sci. Technol. 2023, 81, 104285. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, F.; Wang, D. MOFs and MOF-Derived Materials for Antibacterial Application. J. Funct. Biomater. 2022, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Arenas-vivo, A.; Arias, V.C.; Amariei, G.; Rosal, R.; Izquierdo-barba, I.; Hidalgo, T.; Beltr, H.I.; Loera-serna, S.; Horcajada, P. Antiadherent AgBDC Metal—Organic Framework Coating for Escherichia Coli Biofilm Inhibition. Pharmaceutics 2023, 15, 301. [Google Scholar] [CrossRef] [PubMed]
- Tamames-Tabar, C.; Imbuluzqueta, E.; Guillou, N.; Serre, C.; Miller, S.R.; Elkaïm, E.; Horcajada, P.; Blanco-Prieto, M.J. A Zn Azelate MOF: Combining Antibacterial Effect. CrystEngComm 2015, 17, 456–462. [Google Scholar] [CrossRef]
- Kobayashi, D.; Hamakawa, A.; Yamaguchi, Y.; Takahashi, T.; Yanagita, M.; Arai, S.; Minoura, M. Broadened Bioactivity and Enhanced Durability of Two Structurally Distinct Metal-Organic Frameworks Containing Zn2+ ions and Thiabendazole. Dalt. Trans. 2021, 50, 7176–7180. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.H.; Kim, H.C.; Huh, S.; Kim, Y.; Lee, D.N. Antibacterial Activities of Cu-MOFs Containing Glutarates and Bipyridyl Ligands. Dalt. Trans. 2019, 48, 8084–8093. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, J.; Serrano de las Heras, G.; Carrión, B.; Segura, T.; Páez, P.L.; de Lera-Garrido, F.J.; Rodríguez-López, J.; García-Martínez, J.C. Structure-Activity Relationships for Poly(Phenylene)Vinylene Derivatives as Antibacterial Agents. ChemistrySelect 2018, 3, 7327–7332. [Google Scholar] [CrossRef]
- Boldog, I.; Xing, L.; Schulz, A.; Janiak, C. Influence of Sterically Non-Hindering Methyl Groups on Adsorption Properties of Two Classical Zinc and Copper MOF Types. Comptes Rendus Chim. 2012, 15, 866–877. [Google Scholar] [CrossRef]
- Hirscher, M.; Panella, B. Hydrogen Storage in Metal-Organic Frameworks. Scr. Mater. 2007, 56, 809–812. [Google Scholar] [CrossRef]
- Tran, N.T.T.; Tran, Q.H.; Truong, T. Removable Bidentate Directing Group Assisted-Recyclable Metal.Organic Frameworks-Catalyzed Direct Oxidative Amination of Sp2 C-H Bonds. J. Catal. 2014, 320, 9–15. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, X.; Liu, J.; Lin, J.; Zhang, X.; Kasemchainan, J.; Gao, H.; Wang, G.; Shu, X. Coordination Environment Dependent Stability of Cu-Based MOFs towards Selective Adsorption Desulfurization. Chem. Eng. J. 2023, 464, 142670. [Google Scholar] [CrossRef]
- Raza, H.; Yildiz, I.; Yasmeen, F.; Munawar, K.S.; Ashfaq, M.; Abbas, M.; Ahmed, M.; Younus, H.A.; Zhang, S.; Ahmad, N. Synthesis of a 2D Copper(II)-Carboxylate Framework Having Ultrafast Adsorption of Organic Dyes. J. Colloid Interface Sci. 2021, 602, 43–54. [Google Scholar] [CrossRef]
- Köberl, M.; Cokoja, M.; Herrmann, W.A.; Kühn, F.E. From Molecules to Materials: Molecular Paddle-Wheel Synthons of Macromolecules, Cage Compounds and Metal-Organic Frameworks. Dalt. Trans. 2011, 40, 6834–6859. [Google Scholar] [CrossRef]
- Kamal, S.; Khalid, M.; Khan, M.S.; Shahid, M.; Ahmad, M. A Zinc(II) MOF for Recognition of Nitroaromatic Explosive and Cr(III) Ion. J. Solid State Chem. 2022, 315, 123482. [Google Scholar] [CrossRef]
- Nakhaei, M.; Akhbari, K.; Kalati, M.; Phuruangrat, A. Antibacterial Activity of Three Zinc-Terephthalate MOFs and Its Relation to Their Structural Features. Inorganica Chim. Acta 2021, 522, 120353. [Google Scholar] [CrossRef]
- Vergara-Llanos, D.; Koning, T.; Pavicic, M.F.; Bello-Toledo, H.; Díaz-Gómez, A.; Jaramillo, A.; Melendrez-Castro, M.; Ehrenfeld, P.; Sánchez-Sanhueza, G. Antibacterial and Cytotoxic Evaluation of Copper and Zinc Oxide Nanoparticles as a Potential Disinfectant Material of Connections in Implant Provisional Abutments: An in-Vitro Study. Arch. Oral Biol. 2021, 122, 105031. [Google Scholar] [CrossRef]
- Xu, X.D.; Liang, Y.; Li, J.F.; Zhou, L.; Chen, L.Z.; Wang, F.M. A Stable Zinc(II)-Organic Framework as Rapid and Multi-Responsive Luminescent Sensor for Metal Ions in Water. J. Coord. Chem. 2020, 73, 867–876. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, X.; Cranston, E.D.; Zhu, S. Flexible and Porous Nanocellulose Aerogels with High Loadings of Metal–Organic-Framework Particles for Separations Applications. Adv. Mater. 2016, 28, 7652–7657. [Google Scholar] [CrossRef]
- Lee, S.; Bürgi, H.B.; Alshmimri, S.A.; Yaghi, O.M. Impact of Disordered Guest-Framework Interactions on the Crystallography of Metal-Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 8958–8964. [Google Scholar] [CrossRef]
- Zhu, N.; Lennox, M.J.; Düren, T.; Schmitt, W. Polymorphism of Metal-Organic Frameworks: Direct Comparison of Structures and Theoretical N2-Uptake of Topological Pto- and Tbo-Isomers. Chem. Commun. 2014, 50, 4207–4210. [Google Scholar] [CrossRef]
- Durini, S.; Ilić, N.; Ramazanova, K.; Grell, T.; Lönnecke, P.; Hey-Hawkins, E. Methanol Sensing by a Luminescent Zinc(II)-Based Metal−Organic Framework. Chempluschem 2019, 84, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Kim, T.K.; Kim, H.; Kim, D.; Jeong, S.; Moon, H.R.; Lah, M.S. Post-Synthetic Modifications of Framework Metal Ions in Isostructural Metal−Organic Frameworks: Core−Shell Heterostructures via Selective Transmetalations. Chem. Mater. 2012, 24, 3065–3073. [Google Scholar] [CrossRef]
- Zhang, S.; Lloveras, V.; Wu, Y.; Tolosa, J.; Garc, C. Fluorescent and Magnetic Radical Dendrimers as Potential Bimodal Imaging Probes. Pharmaceutics 2023, 15, 1776. [Google Scholar] [CrossRef] [PubMed]
- Vejzovic, D.; Piller, P.; Cordfunke, R.A.; Drijfhout, J.W.; Eisenberg, T.; Lohner, K.; Malanovic, N. Where Electrostatics Matter: Bacterial Surface Neutralization and Membrane Disruption by Antimicrobial Peptides SAAP-148 and OP-145. Biomolecules 2022, 12, 1252. [Google Scholar] [CrossRef] [PubMed]
- Raja, F.N.S.; Worthington, T.; Martin, R.A. The Antimicrobial Efficacy of Copper, Cobalt, Zinc and Silver Nanoparticles: Alone and in Combination. Biomed. Mater. 2023, 18, 045003. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lelouche, S.N.K.; Albentosa-González, L.; Clemente-Casares, P.; Biglione, C.; Rodríguez-Diéguez, A.; Tolosa Barrilero, J.; García-Martínez, J.C.; Horcajada, P. Antibacterial Cu or Zn-MOFs Based on the 1,3,5-Tris-(styryl)benzene Tricarboxylate. Nanomaterials 2023, 13, 2294. https://doi.org/10.3390/nano13162294
Lelouche SNK, Albentosa-González L, Clemente-Casares P, Biglione C, Rodríguez-Diéguez A, Tolosa Barrilero J, García-Martínez JC, Horcajada P. Antibacterial Cu or Zn-MOFs Based on the 1,3,5-Tris-(styryl)benzene Tricarboxylate. Nanomaterials. 2023; 13(16):2294. https://doi.org/10.3390/nano13162294
Chicago/Turabian StyleLelouche, Sorraya Najma Kinza, Laura Albentosa-González, Pilar Clemente-Casares, Catalina Biglione, Antonio Rodríguez-Diéguez, Juan Tolosa Barrilero, Joaquín Calixto García-Martínez, and Patricia Horcajada. 2023. "Antibacterial Cu or Zn-MOFs Based on the 1,3,5-Tris-(styryl)benzene Tricarboxylate" Nanomaterials 13, no. 16: 2294. https://doi.org/10.3390/nano13162294
APA StyleLelouche, S. N. K., Albentosa-González, L., Clemente-Casares, P., Biglione, C., Rodríguez-Diéguez, A., Tolosa Barrilero, J., García-Martínez, J. C., & Horcajada, P. (2023). Antibacterial Cu or Zn-MOFs Based on the 1,3,5-Tris-(styryl)benzene Tricarboxylate. Nanomaterials, 13(16), 2294. https://doi.org/10.3390/nano13162294






