The Effect of Nanobubble Water Containing Cordyceps Extract and Withaferin A on Free Fatty Acid-Induced Lipid Accumulation in HepG2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Reagents
2.2. Preparation of NBW
2.3. Determination of the Properties of NBW
2.4. Preparation of Culture Medium Containing NBW
2.5. Cell Viability Assay
2.6. Nile Red Assay for Determination of Intracellular Lipid
2.7. Data Analysis
3. Results
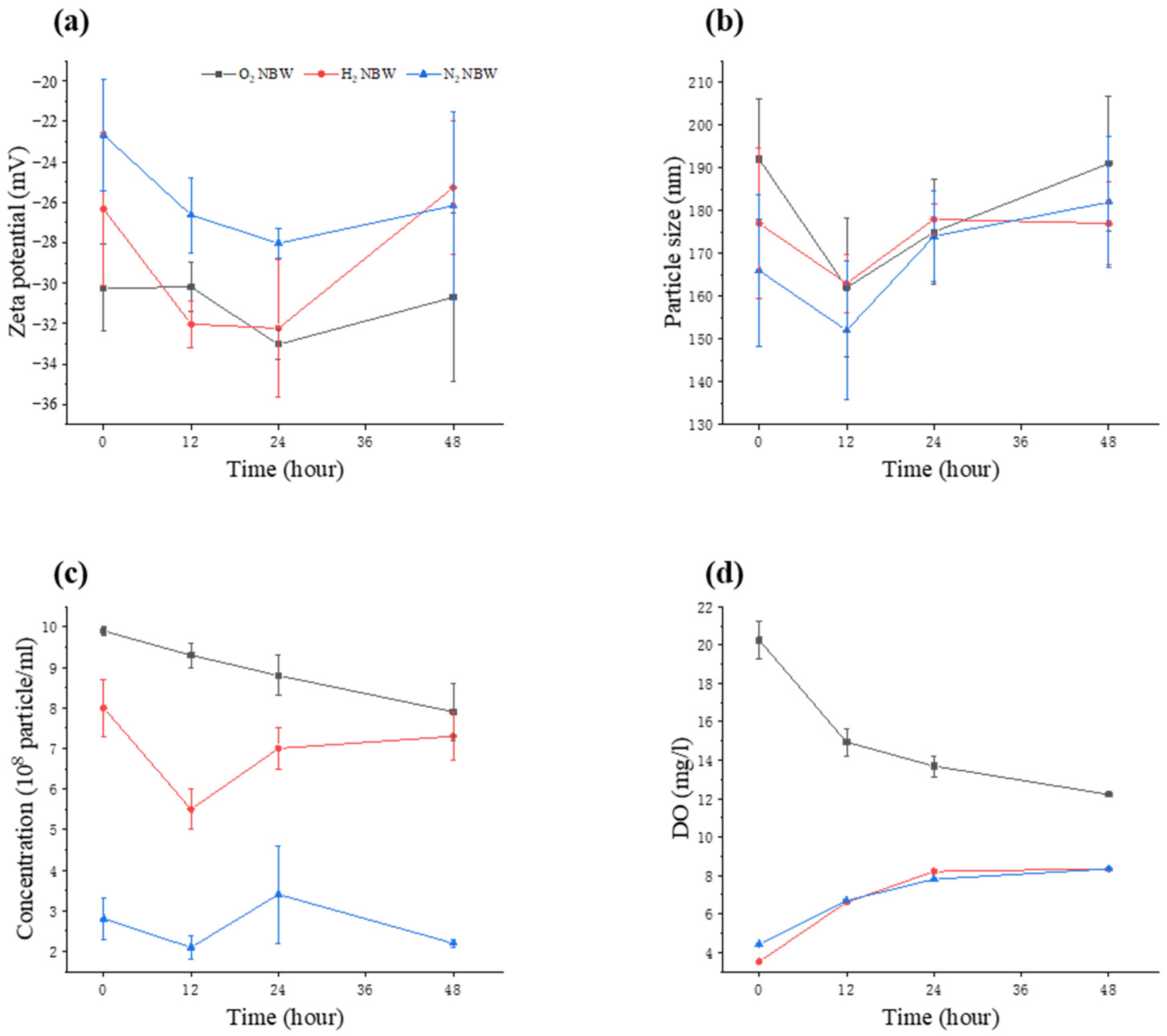
3.1. Physical Properties of NBW
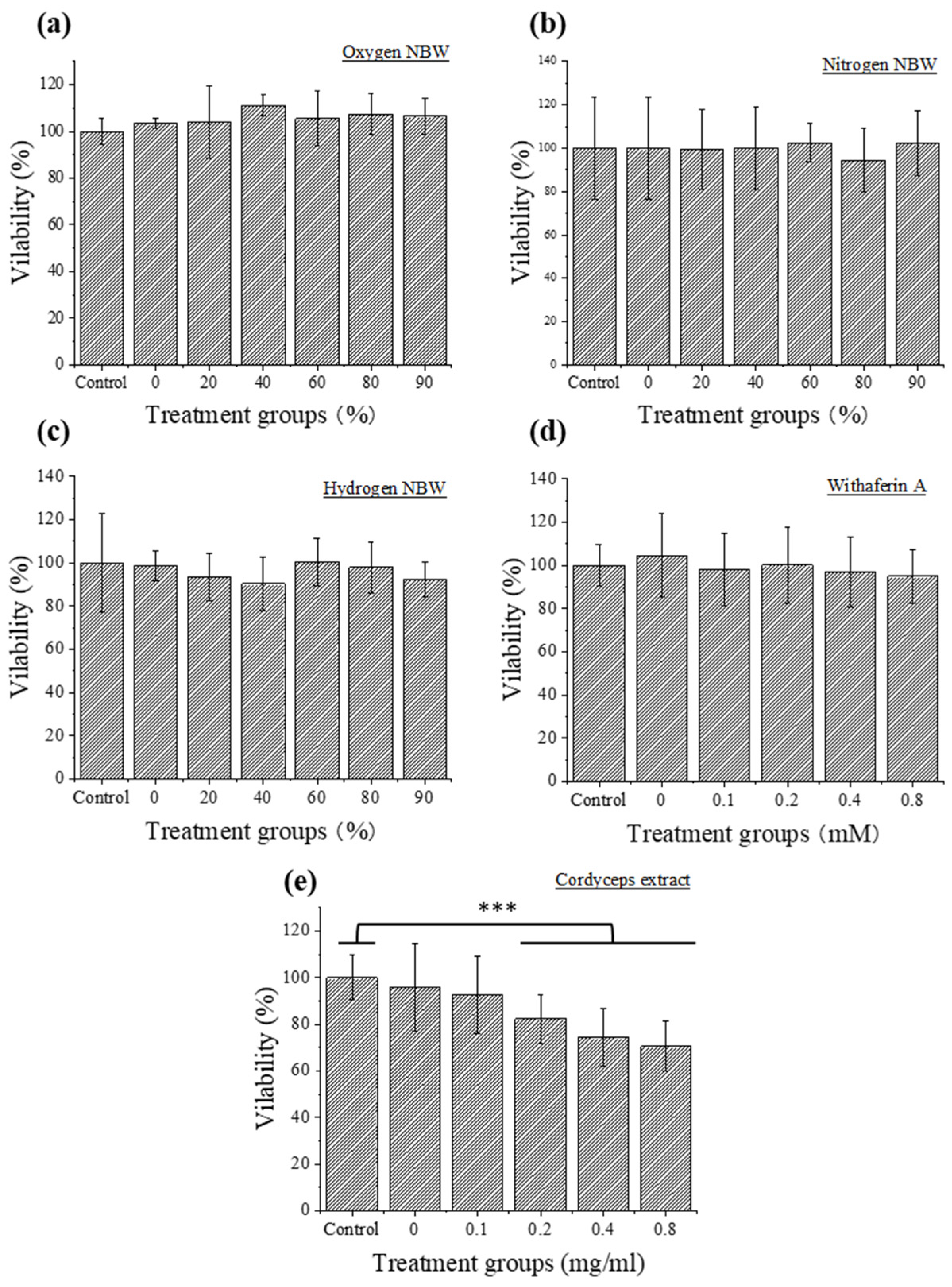
3.2. Cytotoxicity Evaluation
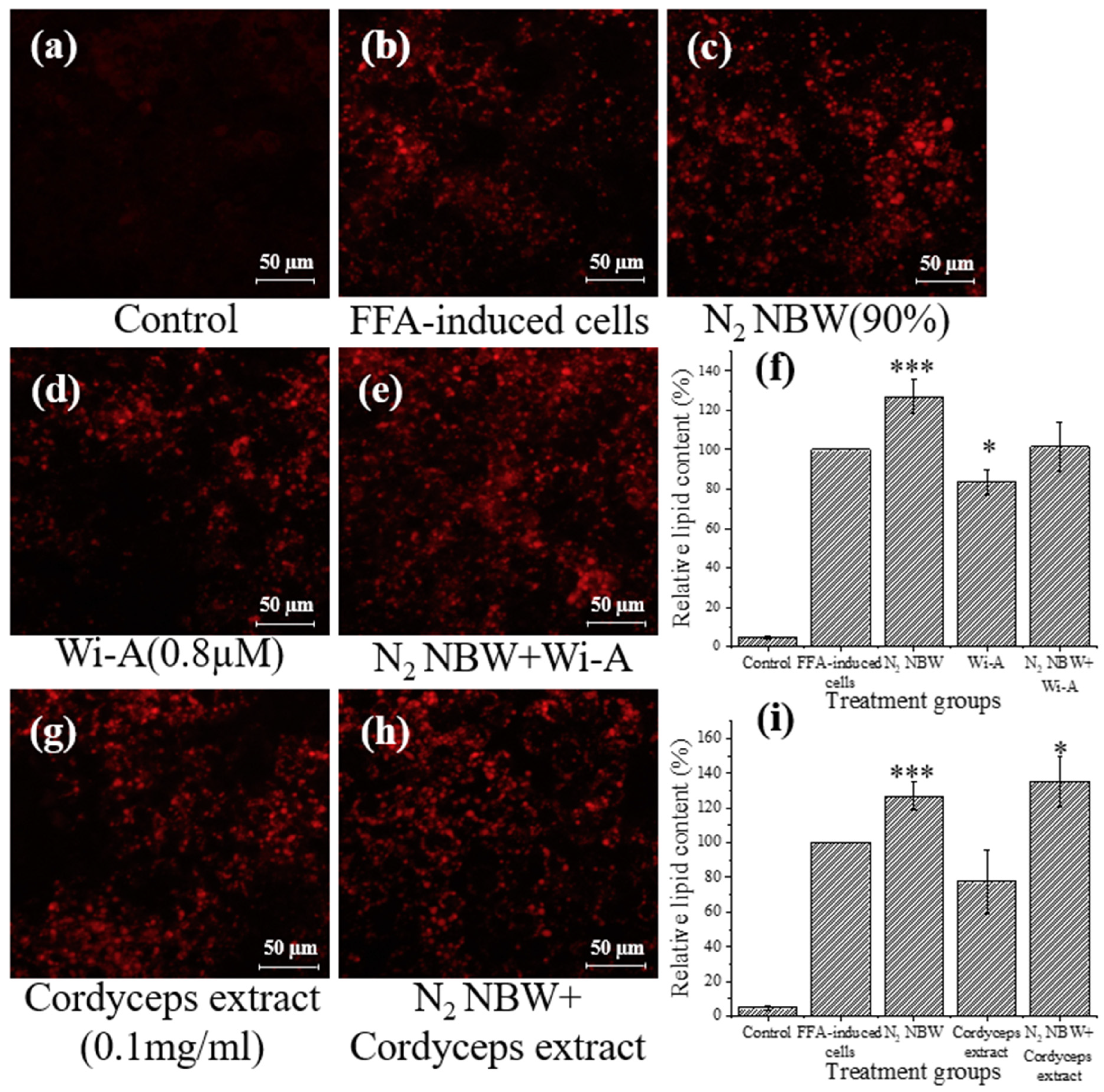
3.3. Synergistic Effect of Nitrogen NBW with Natural Compounds
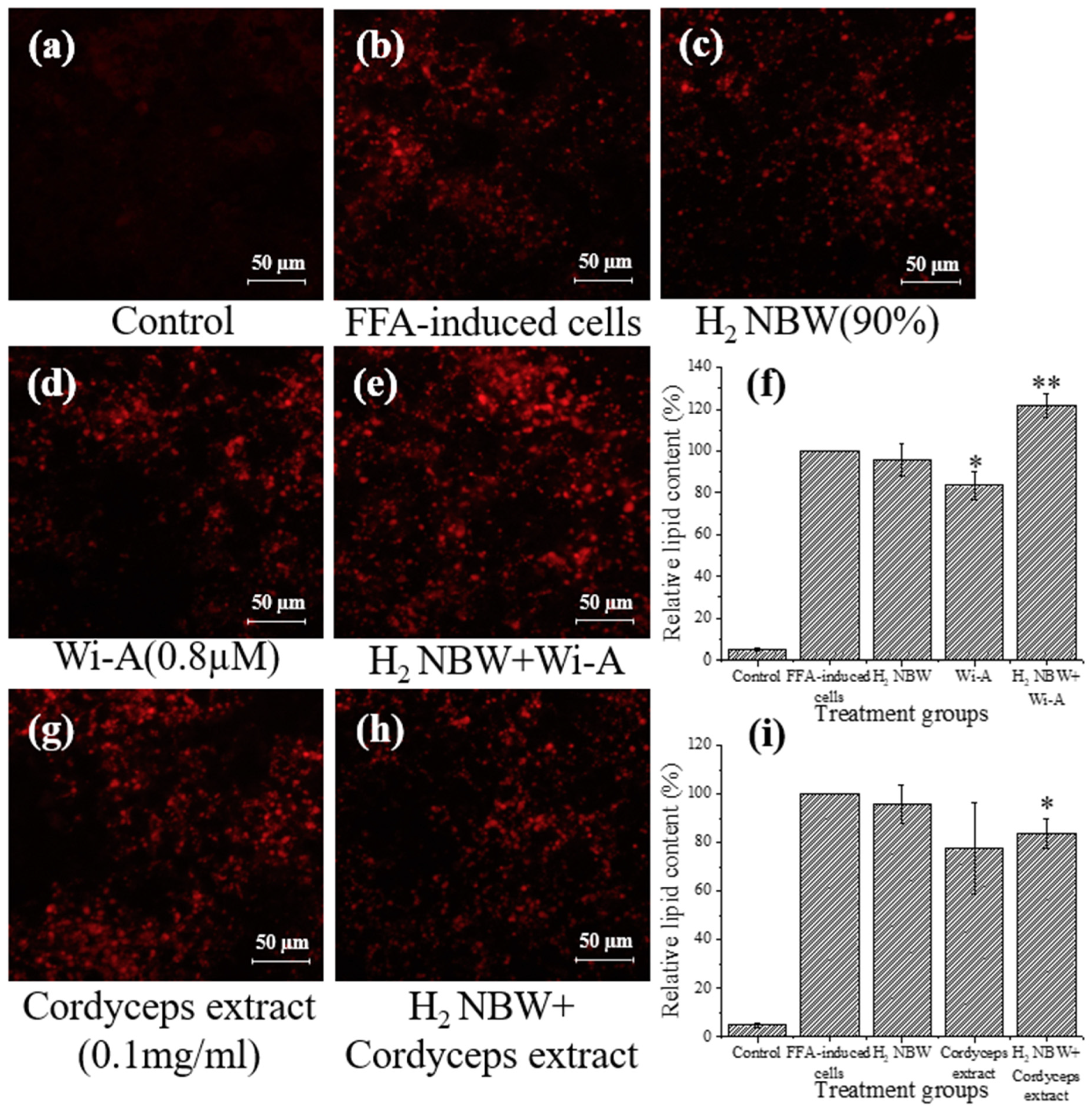
3.4. Synergistic Effect of Hydrogen NBW with Natural Compounds
3.5. Synergistic Effect of Oxygen NBW with Natural Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Promrat, K.; Lutchman, G.; Uwaifo, G.I.; Freedman, R.J.; Soza, A.; Heller, T.; Doo, E.; Ghany, M.; Premkumar, A.; Park, Y.; et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology 2004, 39, 188–196. [Google Scholar] [CrossRef]
- Ala, M. SGLT2 Inhibition for Cardiovascular Diseases, Chronic Kidney Disease, and NAFLD. Endocrinology 2021, 162, bqab157. [Google Scholar] [CrossRef]
- Petit, J.M.; Vergès, B. GLP-1 receptor agonists in NAFLD. Diabetes Metab. 2017, 43, 2S28–2S33. [Google Scholar] [CrossRef] [PubMed]
- Wharton, S.; Davies, M.; Dicker, D.; Lingvay, I.; Mosenzon, O.; Rubino, D.M.; Pedersen, S.D. Managing the gastrointestinal side effects of GLP-1 receptor agonists in obesity: Recommendations for clinical practice. Postgrad. Med. 2022, 134, 14–19. [Google Scholar] [PubMed]
- Wieland, A.; Frank, D.N.; Harnke, B.; Bambha, K. Systematic review: Microbial dysbiosis and nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2015, 42, 1051–1063. [Google Scholar]
- Loman, B.R.; Hernández-Saavedra, D.; An, R.; Rector, R.S. Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Nutr. Rev. 2018, 76, 822–839. [Google Scholar] [CrossRef]
- Li, T.; Wen, L.; Cheng, B. Cordycepin alleviates hepatic lipid accumulation by inducing protective autophagy via PKA/mTOR pathway. Biochem. Biophys. Res. Commun. 2019, 516, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Shiragannavar, V.D.; Sannappa Gowda, N.G.; Puttahanumantharayappa, L.D.; Karunakara, S.H.; Bhat, S.; Prasad, S.K.; Kumar, D.P.; Santhekadur, P.K. The ameliorating effect of withaferin A on high-fat diet-induced non-alcoholic fatty liver disease by acting as an LXR/FXR dual receptor activator. Front. Pharmacol. 2023, 14, 1135952. [Google Scholar] [CrossRef] [PubMed]
- Cheung, B.M.; Cheung, T.T.; Samaranayake, N.R. Safety of antiobesity drugs. Ther. Adv. Drug Saf. 2013, 4, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A.; Chilkoti, A. Nanomaterials for Drug Delivery. Science 2012, 337, 303–305. [Google Scholar] [CrossRef]
- Xiao, W.; Xu, G. Mass transfer of nanobubble aeration and its effect on biofilm growth: Microbial activity and structural properties. Sci. Total Environ. 2020, 703, 134976. [Google Scholar]
- Guo, Z.; Wang, X.; Wang, H.; Hu, B.; Lei, Z.; Kobayashi, M.; Adachi, Y.; Shimizu, K.; Zhang, Z. Effects of nanobubble water on the growth of Lactobacillus acidophilus 1028 and its lactic acid production. RSC Adv. 2019, 9, 30760–30767. [Google Scholar] [CrossRef]
- Asada, R.; Kageyama, K.; Tanaka, H.; Matsui, H.; Kimura, M.; Saitoh, Y.; Miwa, N. Antitumor effects of nano-bubble hydrogen-dissolved water are enhanced by coexistent platinum colloid and the combined hyperthermia with apoptosis-like cell death. Oncol. Rep. 2010, 24, 1463–1470. [Google Scholar]
- Mahjour, A.; Khazaei, M.; Nourmohammadi, E.; Khoshdel-Sarkarizi, H.; Ebrahimzadeh-Bideskan, A.; Rahimi, H.R.; Safipour Afshar, A. Evaluation of antitumor effect of oxygen nanobubble water on breast cancer-bearing BALB/c mice. J. Cell. Biochem. 2019, 120, 15546–15552. [Google Scholar] [CrossRef]
- Owen, J.; McEwan, C.; Nesbitt, H.; Bovornchutichai, P.; Averre, R.; Borden, M.; McHale, A.P.; Callan, J.F.; Stride, E. Reducing Tumour Hypoxia via Oral Administration of Oxygen Nanobubbles. PLoS ONE 2016, 11, e0168088. [Google Scholar] [CrossRef]
- Sofyana, N.T.; Refli, R.; Takahashi, M.; Sakamoto, K. Oxygen nanobubble water affects wound healing of fibroblast WI-38 cells. Biosci. Biotechnol. Biochem. 2023, 87, 620–626. [Google Scholar] [CrossRef]
- Xiao, L.; Sun, S.; Li, K.; Lei, Z.; Shimizu, K.; Zhang, Z.; Adachi, Y. Effects of nanobubble water supplementation on biomass accumulation during mycelium cultivation of Cordyceps militaris and the antioxidant activities of extracted polysaccharides. Bioresour. Technol. Rep. 2020, 12, 100600. [Google Scholar] [CrossRef]
- Bosca, F.; Bielecki, P.A.; Exner, A.A.; Barge, A. Porphyrin-Loaded Pluronic Nanobubbles: A New US-Activated Agent for Future Theranostic Applications. Bioconjugate Chem. 2018, 29, 234–240. [Google Scholar] [CrossRef] [PubMed]
- ISO 20480-1:2017; Fine Bubble Technology: General Principles for Usage and Measurement of Fine Bubbles. Part 1: Terminology. International Organization of Standardization: Geneva, Switzerland, 2017.
- Soyluoglu, M.; Kim, D.; Zaker, Y.; Karanfil, T. Removal mechanisms of geosmin and MIB by oxygen nanobubbles during water treatment. Chem. Eng. J. 2022, 443, 136535. [Google Scholar] [CrossRef]
- Ahmed, A.K.A.; Shi, X.; Hua, L.; Manzueta, L.; Qing, W.; Marhaba, T.; Zhang, W. Influences of Air, Oxygen, Nitrogen, and Carbon Dioxide Nanobubbles on Seed Germination and Plant Growth. J. Agric. Food Chem. 2018, 66, 5117–5124. [Google Scholar]
- Khaled Abdella Ahmed, A.; Sun, C.; Hua, L.; Zhang, Z.; Zhang, Y.; Marhaba, T.; Zhang, W.J.E.E.S. Colloidal properties of air, oxygen, and nitrogen nanobubbles in water: Effects of ionic strength, natural organic matters, and surfactants. Environ. Eng. Sci. 2018, 35, 720–727. [Google Scholar]
- Meegoda, J.N.; Aluthgun Hewage, S.; Batagoda, J.H. Stability of nanobubbles. Environ. Eng. Sci. 2018, 35, 1216–1227. [Google Scholar] [CrossRef]
- Hanaor, D.; Michelazzi, M.; Leonelli, C.; Sorrell, C.C. The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. J. Eur. Ceram. Soc. 2012, 32, 235–244. [Google Scholar] [CrossRef]
- Ushikubo, F.Y.; Furukawa, T.; Nakagawa, R.; Enari, M.; Makino, Y.; Kawagoe, Y.; Shiina, T.; Oshita, S. Evidence of the existence and the stability of nano-bubbles in water. Colloids Surf. A Physicochem. Eng. Asp. 2010, 361, 31–37. [Google Scholar] [CrossRef]
- Ahmed, A.K.A.; Sun, C.; Hua, L.; Zhang, Z.; Zhang, Y.; Zhang, W.; Marhaba, T. Generation of nanobubbles by ceramic membrane filters: The dependence of bubble size and zeta potential on surface coating, pore size and injected gas pressure. Chemosphere 2018, 203, 327–335. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, X.; Huang, Q. Protective effects of Cordyceps sinensis exopolysaccharide-selenium nanoparticles on H2O2-induced oxidative stress in HepG2 cells. Int. J. Biol. Macromol. 2022, 213, 339–351. [Google Scholar] [CrossRef]
- Sarkar, S.; Bhattacharya, S.; Alam, M.J.; Yadav, R.; Banerjee, S.K. Hypoxia aggravates non-alcoholic fatty liver disease in presence of high fat choline deficient diet: A pilot study. Life Sci. 2020, 260, 118404. [Google Scholar] [CrossRef]
- Gordon, G.B.; Barcza, M.A.; Bush, M.E.J.T.A.j.o.p. Lipid accumulation in hypoxic tissue culture cells. Am. J. Pathol. 1977, 88, 663. [Google Scholar]
- Aron-Wisnewsky, J.; Minville, C.; Tordjman, J.; Levy, P.; Bouillot, J.L.; Basdevant, A.; Bedossa, P.; Clement, K.; Pepin, J.L. Chronic intermittent hypoxia is a major trigger for non-alcoholic fatty liver disease in morbid obese. J. Hepatol. 2012, 56, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Mylonis, I.; Simos, G.; Paraskeva, E. Hypoxia-Inducible Factors and the Regulation of Lipid Metabolism. Cells 2019, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lei, Z.; Guo, Z.; Huang, W.; Wang, D.; Wang, X.; Zhang, Z.; Shimizu, K. Enhanced solubilization of solid organics and methane production by anaerobic digestion of swine manure under nano-bubble water addition. Bioresour. Technol. 2020, 299, 122512. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Sun, Y.; Zhang, W.; Zhang, Z.; Yuan, T. The Effect of Nanobubble Water Containing Cordyceps Extract and Withaferin A on Free Fatty Acid-Induced Lipid Accumulation in HepG2 Cells. Nanomaterials 2023, 13, 2265. https://doi.org/10.3390/nano13152265
Han H, Sun Y, Zhang W, Zhang Z, Yuan T. The Effect of Nanobubble Water Containing Cordyceps Extract and Withaferin A on Free Fatty Acid-Induced Lipid Accumulation in HepG2 Cells. Nanomaterials. 2023; 13(15):2265. https://doi.org/10.3390/nano13152265
Chicago/Turabian StyleHan, Hanlin, Yixin Sun, Weixu Zhang, Zhenya Zhang, and Tian Yuan. 2023. "The Effect of Nanobubble Water Containing Cordyceps Extract and Withaferin A on Free Fatty Acid-Induced Lipid Accumulation in HepG2 Cells" Nanomaterials 13, no. 15: 2265. https://doi.org/10.3390/nano13152265
APA StyleHan, H., Sun, Y., Zhang, W., Zhang, Z., & Yuan, T. (2023). The Effect of Nanobubble Water Containing Cordyceps Extract and Withaferin A on Free Fatty Acid-Induced Lipid Accumulation in HepG2 Cells. Nanomaterials, 13(15), 2265. https://doi.org/10.3390/nano13152265





