In Situ Ultra-Small- and Small-Angle X-ray Scattering Study of ZnO Nanoparticle Formation and Growth through Chemical Bath Deposition in the Presence of Polyvinylpyrrolidone
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnO Nanoparticles
2.2. Characterization
2.2.1. In-House Small-Angle X-ray Scattering
2.2.2. Ultra-Small- and Small-Angle Synchrotron X-ray Scattering
2.2.3. Transmission Electron Microscopy
3. Results and Discussion
3.1. PVP Structure in the Precursor Solution
 ) shows a Guinier region at low q with almost constant scattering intensity. As q increases, a power law decay is observed until a minimum is reached at q ≈ 1 Å−1, followed by the intermolecular C-C chain interaction peak [66] at q ≈ 1.5 Å−1. Similar behavior was found when 12.5 mM ZnAc2 (
) shows a Guinier region at low q with almost constant scattering intensity. As q increases, a power law decay is observed until a minimum is reached at q ≈ 1 Å−1, followed by the intermolecular C-C chain interaction peak [66] at q ≈ 1.5 Å−1. Similar behavior was found when 12.5 mM ZnAc2 ( ) or 26.3 mM TEAOH (
) or 26.3 mM TEAOH ( ) were added while keeping the polymer amount constant at ϕPVP = 7.1 × 10−2. However, for the final composition of the precursor solution (
) were added while keeping the polymer amount constant at ϕPVP = 7.1 × 10−2. However, for the final composition of the precursor solution ( ), instead of a Guinier region for q < 0.1 Å−1, a power law decay with I(q) ≈ q−1.4 can be seen. Thus, the presence of both ZnAc2 and TEAOH results in a significantly different structure of the polymer.
), instead of a Guinier region for q < 0.1 Å−1, a power law decay with I(q) ≈ q−1.4 can be seen. Thus, the presence of both ZnAc2 and TEAOH results in a significantly different structure of the polymer.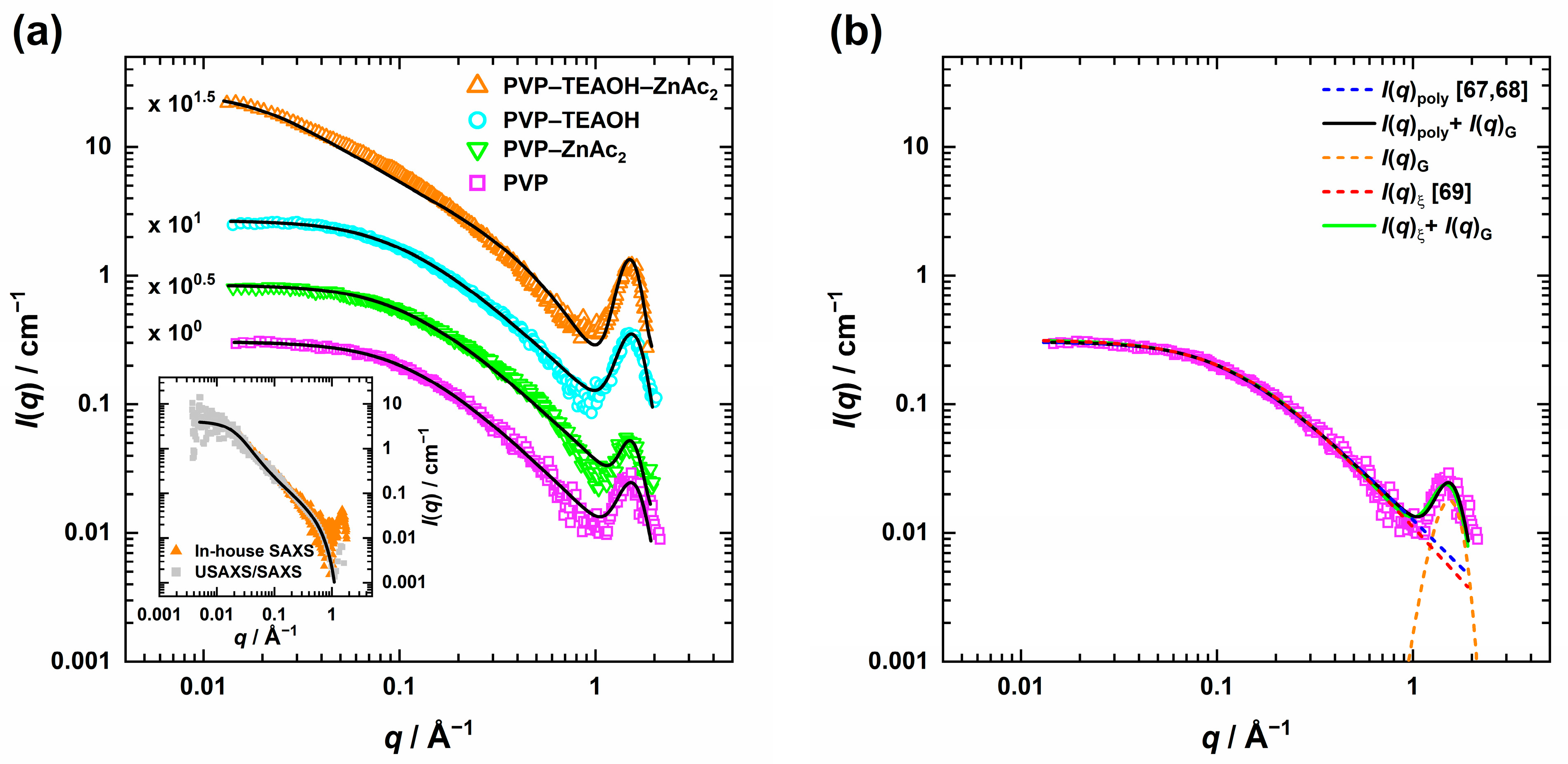
 ).
). ) in Figure 1b.
) in Figure 1b.  ) with Equation (3) revealed a high q power law decay exponent of m ≈ 1.5, suggesting that the polymer chains are no longer in an ideal (m = 2) or swollen (m = 5/3) state but already approach a rod-like state (m = 1) [68,72]. A similar behavior was found by Sapir et al. [73] once the overlap concentration c* of PVP-10k in D2O (ϕPVP ~ 4.8 × 10−2) was exceeded and the semidilute polymer regime began. This regime refers to a state in which the polymer forms a network with overlapping and entangled chains. When PVP was studied at lower volume fractions (ϕPVP = 1.0, 2.0, and 4.0 × 10−2) in methanol, shown in the Supplementary Materials Figures S1 and S2 and Tables S3 and S4, ideal chain behavior was observed exclusively for the lowest PVP volume fraction ϕPVP = 1.0 × 10−2. At ϕPVP = 2.0 × 10−2, the exponent of the power law decay drops steeply and then slowly decreases with increasing PVP amount, while Rg(PVP) decreases continuously. Furthermore, the change of the scaling dependency of the correlation length, observed between ϕPVP = 4.0 and 7.1 × 10−2, confirms that the PVP concentration in the methanolic precursor solution can be assigned to the semidilute regime [74].
) with Equation (3) revealed a high q power law decay exponent of m ≈ 1.5, suggesting that the polymer chains are no longer in an ideal (m = 2) or swollen (m = 5/3) state but already approach a rod-like state (m = 1) [68,72]. A similar behavior was found by Sapir et al. [73] once the overlap concentration c* of PVP-10k in D2O (ϕPVP ~ 4.8 × 10−2) was exceeded and the semidilute polymer regime began. This regime refers to a state in which the polymer forms a network with overlapping and entangled chains. When PVP was studied at lower volume fractions (ϕPVP = 1.0, 2.0, and 4.0 × 10−2) in methanol, shown in the Supplementary Materials Figures S1 and S2 and Tables S3 and S4, ideal chain behavior was observed exclusively for the lowest PVP volume fraction ϕPVP = 1.0 × 10−2. At ϕPVP = 2.0 × 10−2, the exponent of the power law decay drops steeply and then slowly decreases with increasing PVP amount, while Rg(PVP) decreases continuously. Furthermore, the change of the scaling dependency of the correlation length, observed between ϕPVP = 4.0 and 7.1 × 10−2, confirms that the PVP concentration in the methanolic precursor solution can be assigned to the semidilute regime [74]. ) or TEAOH (
) or TEAOH ( ) was added revealed a slight increase in Rg(PVP) (see Table 1) while the decrease in the exponent of the power law decayindicates that the polymer becomes more rod-like [72]. Though PVP is a nonionic polymer, the pyrrolidone head group is polar and interacts with the ions of the ZnO precursor and the organic base [33,75]. Their accumulation along the polymer chains, leading to repulsion of the segments within the polymer chain, can explain why the polymer chain becomes slightly stiffer and more swollen compared to the ion-free solution. This effect is slightly more pronounced with TEAOH, whose concentration is more than twice of ZnAc2.
) was added revealed a slight increase in Rg(PVP) (see Table 1) while the decrease in the exponent of the power law decayindicates that the polymer becomes more rod-like [72]. Though PVP is a nonionic polymer, the pyrrolidone head group is polar and interacts with the ions of the ZnO precursor and the organic base [33,75]. Their accumulation along the polymer chains, leading to repulsion of the segments within the polymer chain, can explain why the polymer chain becomes slightly stiffer and more swollen compared to the ion-free solution. This effect is slightly more pronounced with TEAOH, whose concentration is more than twice of ZnAc2.
| Sample | Rg(PVP)/nm | m | m′ | ξPVP/nm |
|---|---|---|---|---|
| PVP | 1.5 ± 0.1 | 1.52 ± 0.01 | - | 1.1 ± 0.1 |
| PVP–ZnAc2 | 1.7 ± 0.1 | 1.46 ± 0.01 | - | 1.1 ± 0.1 |
| PVP–TEAOH | 1.8 ± 0.1 | 1.45 ± 0.01 | - | 1.3 ± 0.1 |
| PVP–TEAOH–ZnAc2 | 6.3 ± 0.3 a | - | 1.55 ± 0.01 | 2.9 ± 0.2 |
 ) (where both ZnAc2 and TEAOH are present) in more detail, the power law decay (I(q) ≈ q−1.4) recorded in the low q limit of the in-house SAXS instrument provides little information on the global structure and size of the polymer. This information can be obtained by extending the q-range towards much smaller values using the USAXS/SAXS setup at the 9-ID-C beamline at the Advanced Photon Source at the Argonne National Laboratory [58,59]. The inset in Figure 1 shows a good agreement between the desmeared in-house (
) (where both ZnAc2 and TEAOH are present) in more detail, the power law decay (I(q) ≈ q−1.4) recorded in the low q limit of the in-house SAXS instrument provides little information on the global structure and size of the polymer. This information can be obtained by extending the q-range towards much smaller values using the USAXS/SAXS setup at the 9-ID-C beamline at the Advanced Photon Source at the Argonne National Laboratory [58,59]. The inset in Figure 1 shows a good agreement between the desmeared in-house (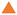 ) and USAXS/SAXS (
) and USAXS/SAXS ( ) data. In addition, the combination of the data clearly shows the transition of the power law into a Guinier region at q < 0.01 Å−1. Utilizing Guinier’s approximation for low q (ln I(q) ≈ ln I0 − (Rg2/3) q2), we obtained Rg(PVP) = 6.3 ± 0.3 nm, which corresponds to a fourfold increase compared to Rg(PVP) in pure methanol and in solutions containing only ZnAc2 or TEAOH. This significant change in the PVP structure caused by the increased ion concentration due to the presence of both ZnO precursor and base is most likely related to a stronger repulsion of the segments within the polymer chain, leading to a further increase in the chain stiffness. This hypothesis is supported by the increase in mesh size determined from the analysis at medium q’s to ξPVP = 2.9 ± 0.2 nm. Moreover, ZnAc2 might start to hydrolyze even at 25 °C, changing its chain flexibility so that the first particle nuclei could have formed within the PVP. However, this reaction is significantly accelerated only by increasing the temperature.
) data. In addition, the combination of the data clearly shows the transition of the power law into a Guinier region at q < 0.01 Å−1. Utilizing Guinier’s approximation for low q (ln I(q) ≈ ln I0 − (Rg2/3) q2), we obtained Rg(PVP) = 6.3 ± 0.3 nm, which corresponds to a fourfold increase compared to Rg(PVP) in pure methanol and in solutions containing only ZnAc2 or TEAOH. This significant change in the PVP structure caused by the increased ion concentration due to the presence of both ZnO precursor and base is most likely related to a stronger repulsion of the segments within the polymer chain, leading to a further increase in the chain stiffness. This hypothesis is supported by the increase in mesh size determined from the analysis at medium q’s to ξPVP = 2.9 ± 0.2 nm. Moreover, ZnAc2 might start to hydrolyze even at 25 °C, changing its chain flexibility so that the first particle nuclei could have formed within the PVP. However, this reaction is significantly accelerated only by increasing the temperature. 3.2. In Situ Study of ZnO Particle Formation and Growth
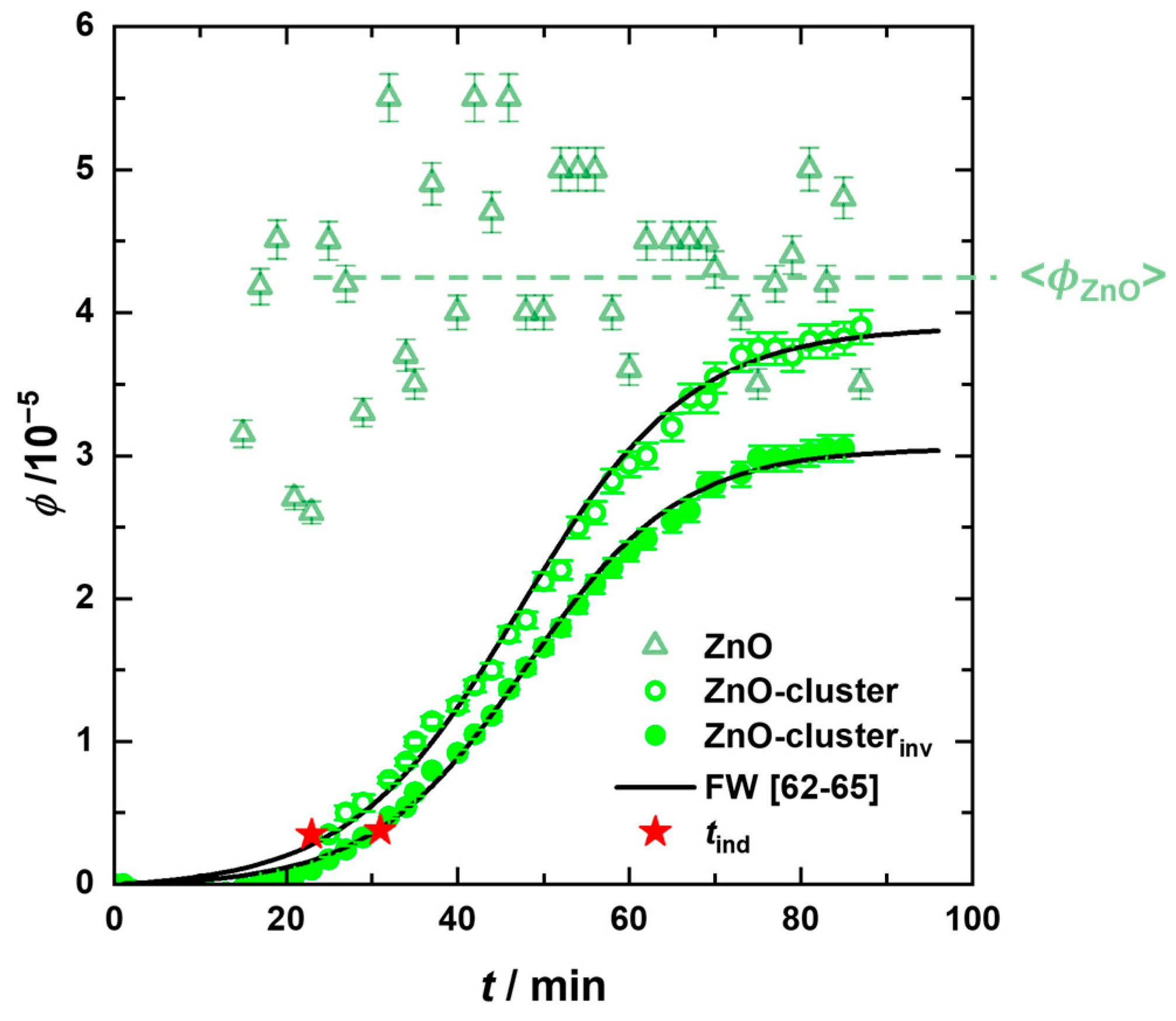
 ) for T = 58 °C in Figure 6, where an initial increase is followed by reaching an almost constant value, while the volume fraction of the cluster (
) for T = 58 °C in Figure 6, where an initial increase is followed by reaching an almost constant value, while the volume fraction of the cluster ( ) follows a sigmoidal profile. These results suggest that new ZnO particles are formed continuously throughout the reaction, feeding the clusters, which in turn grow simultaneously. Hence, at intermediate times, an equilibrium is established between ZnO particles’ formation and consumption.
) follows a sigmoidal profile. These results suggest that new ZnO particles are formed continuously throughout the reaction, feeding the clusters, which in turn grow simultaneously. Hence, at intermediate times, an equilibrium is established between ZnO particles’ formation and consumption. ) (see solid line in Figure 6), yielding nearly identical values for the FW parameters as compared to the R3(t)-data with k1(T = 58 °C) = 8.5 × 10−4 1/s, k2[A]0 (T = 58 °C) = 0.11 1/s with tind(T = 58 °C) = 29 min.
) (see solid line in Figure 6), yielding nearly identical values for the FW parameters as compared to the R3(t)-data with k1(T = 58 °C) = 8.5 × 10−4 1/s, k2[A]0 (T = 58 °C) = 0.11 1/s with tind(T = 58 °C) = 29 min. ) for T = 58 °C is plotted as a function of time. Comparing the cluster volume fractions obtained from the model analysis of the USAXS data and the invariant, i.e., ϕZnO-cluster,I(q) and ϕZnO-cluster,inv, slightly lower values are found for the latter, which might be a consequence of the finite q-range used for the invariant determination as well as the approximation of spherical clusters with compact packing of ZnO particles and a homogeneous scattering length density distribution.
) for T = 58 °C is plotted as a function of time. Comparing the cluster volume fractions obtained from the model analysis of the USAXS data and the invariant, i.e., ϕZnO-cluster,I(q) and ϕZnO-cluster,inv, slightly lower values are found for the latter, which might be a consequence of the finite q-range used for the invariant determination as well as the approximation of spherical clusters with compact packing of ZnO particles and a homogeneous scattering length density distribution.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic–inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Mir, S.H.; Nagahara, L.A.; Thundat, T.; Mokarian-Tabari, P.; Furukawa, H.; Khosla, A. Review—Organic-Inorganic Hybrid Functional Materials: An Integrated Platform for Applied Technologies. J. Electrochem. Soc. 2018, 165, B3137–B3156. [Google Scholar] [CrossRef]
- Eder, D. Carbon Nanotube−Inorganic Hybrids. Chem. Rev. 2010, 110, 1348–1385. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Khan, M.M.; Tan, Y.H.; Walvekar, R.; Abdullah, E.C.; Karri, R.R.; Rahman, M.E. Comprehensive review on carbon nanotubes embedded in different metal and polymer matrix: Fabrications and applications. Crit. Rev. Solid State Mater. Sci. 2021, 47, 837–864. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Adnan, M.M.; Dalod, A.R.M.; Balci, M.H.; Glaum, J.; Einarsrud, M.-A. In Situ Synthesis of Hybrid Inorganic–Polymer Nanocomposites. Polymers 2018, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Shenton, W.; Douglas, T.; Young, M.; Stubbs, G.; Mann, S. Inorganic-Organic Nanotube Composites from Template Mineralization of Tobacco Mosaic Virus. Adv. Mater. 1999, 11, 253–256. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lim, J.-S.; Harris, M.T. Synthesis and application of virus-based hybrid nanomaterials. Biotechnol. Bioeng. 2012, 109, 16–30. [Google Scholar] [CrossRef]
- Caruso, R.A.; Antonietti, M. Sol−Gel Nanocoating: An Approach to the Preparation of Structured Materials. Chem. Mater. 2001, 13, 3272–3282. [Google Scholar] [CrossRef]
- Lei, Q.; Guo, J.; Kong, F.; Cao, J.; Wang, L.; Zhu, W.; Brinker, C.J. Bioinspired Cell Silicification: From Extracellular to Intracellular. J. Am. Chem. Soc. 2021, 143, 6305–6322. [Google Scholar] [CrossRef] [PubMed]
- Parikh, H.; de Guire, M.R. Recent progress in the synthesis of oxide films from liquid solutions. J. Ceram. Soc. Jpn. 2009, 117, 228–235. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Carreón-Moncada, I.; González, L.; Rodríguez-Galicia, J.; Rendón-Angeles, J. Chemical deposition of CdS films by an ammonia-free process with amino acids as complexing agents. Thin Solid Film. 2016, 599, 166–173. [Google Scholar] [CrossRef]
- Enríquez, J.P.; Mathew, X. Influence of the thickness on structural, optical and electrical properties of chemical bath deposited CdS thin films. Sol. Energy Mater. Sol. Cells 2003, 76, 313–322. [Google Scholar] [CrossRef]
- Lee, J.-H. Structural and optical properties of CdS thin films on organic substrates for flexible solar cell applications. J. Electroceramics 2006, 17, 1103–1108. [Google Scholar] [CrossRef]
- Preda, N.; Enculescu, M.; Gherendi, F.; Matei, E.; Toimil-Molares, M.; Enculescu, I. Synthesis of CdS nanostructures using template-assisted ammonia-free chemical bath deposition. J. Phys. Chem. Solids 2012, 73, 1082–1089. [Google Scholar] [CrossRef]
- Shimizu, K.; Imai, H.; Hirashima, H.; Tsukuma, K. Low-temperature synthesis of anatase thin films on glass and organic substrates by direct deposition from aqueous solutions. Thin Solid Film. 1999, 351, 220–224. [Google Scholar] [CrossRef]
- Santhiya, D.; Burghard, Z.; Greiner, C.; Jeurgens, L.P.H.; Subkowski, T.; Bill, J. Bioinspired Deposition of TiO2 Thin Films Induced by Hydrophobins. Langmuir 2010, 26, 6494–6502. [Google Scholar] [CrossRef]
- Dussan, A.; Bohórquez, A.; Quiroz, H.P. Effect of annealing process in TiO2 thin films: Structural, morphological, and optical properties. Appl. Surf. Sci. 2017, 424, 111–114. [Google Scholar] [CrossRef]
- O’Brien, P.; Saeed, T.; Knowles, J. Speciation and the nature of ZnO thin films from chemical bath deposition. J. Mater. Chem. 1996, 6, 1135–1139. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Lin, H.-Y.; Liu, M.-H.; Chen, Y.-F.; Mou, C.-Y. Biomimetic Synthesis of Nacrelike Faceted Mesocrystals of ZnO−Gelatin Composite. J. Phys. Chem. C 2009, 113, 18053–18061. [Google Scholar] [CrossRef]
- Shi, Z.; Walker, A.V. Chemical Bath Deposition of ZnO on Functionalized Self-Assembled Monolayers: Selective Deposition and Control of Deposit Morphology. Langmuir 2015, 31, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, E.; Barquinha, P.; Pimentel, A.; Gonçalves, A.; Marques, A.; Pereira, L.; Martins, R. Recent advances in ZnO transparent thin film transistors. Thin Solid Film. 2005, 487, 205–211. [Google Scholar] [CrossRef]
- Gordillo, G.; Pena, J. Development of system to grow ZnO films by plasma assisted reactive evaporation with improved thickness homogeneity for using in solar cells. J. Mater. Res. Technol. 2022, 19, 1191–1202. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Wang, X.; Liu, Y.; Dai, J.; Chen, C. Highly Integrated In Situ Photoenergy Gas Sensor with Deep Ultraviolet LED. ACS Omega 2020, 5, 9985–9990. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-W.; Park, K.-S.; Heo, J.-H.; Park, J.-G.; Kim, D.-W.; Choi, K.J.; Lee, J.-H.; Hong, S.-H. Gas sensing properties of defect-controlled ZnO-nanowire gas sensor. Appl. Phys. Lett. 2008, 93, 263103. [Google Scholar] [CrossRef]
- Ryu, Y.R.; Lubguban, J.A.; Lee, T.S.; White, H.W.; Jeong, T.S.; Youn, C.J.; Kim, B.J. Excitonic ultraviolet lasing in ZnO-based light emitting devices. Appl. Phys. Lett. 2007, 90, 131115. [Google Scholar] [CrossRef]
- Dong, H.; Zhou, B.; Li, J.; Zhan, J.; Zhang, L. Ultraviolet lasing behavior in ZnO optical microcavities. J. Mater. 2017, 3, 255–266. [Google Scholar] [CrossRef]
- Lipowsky, P.; Jia, S.; Hoffmann, R.C.; Jin-Phillipp, N.Y.; Bill, J.; Rühle, M. Thin film formation by oriented attachment of polymer-capped nanocrystalline ZnO. Int. J. Mater. Res. 2006, 97, 607–613. [Google Scholar] [CrossRef]
- Lipowsky, P.; Hoffmann, R.C.; Welzel, U.; Bill, J.; Aldinger, F. Site-Selective Deposition of Nanostructured ZnO Thin Films from Solutions Containing Polyvinylpyrrolidone. Adv. Funct. Mater. 2007, 17, 2151–2159. [Google Scholar] [CrossRef]
- Lipowsky, P.; Burghard, Ž.; Jeurgens, L.P.H.; Bill, J.; Aldinger, F. Laminates of zinc oxide and poly(amino acid) layers with enhanced mechanical performance. Nanotechnology 2007, 18, 345707. [Google Scholar] [CrossRef]
- Lipowsky, P.; Hedin, N.; Bill, J.; Hoffmann, R.C.; Ahniyaz, A.; Aldinger, F.; Bergström, L. Controlling the Assembly of Nanocrystalline ZnO Films by a Transient Amorphous Phase in Solution. J. Phys. Chem. C 2008, 112, 5373–5383. [Google Scholar] [CrossRef]
- Lipowsky, P.; Hirscher, M.; Hoffmann, R.C.; Bill, J.; Aldinger, F. Zinc oxide microcapsules obtained via a bio-inspired approach. Nanotechnology 2007, 18, 165603. [Google Scholar] [CrossRef]
- Qawasmi, Y.; Atanasova, P.; Jahnke, T.; Burghard, Z.; Müller, A.; Grassberger, L.; Strey, R.; Bill, J.; Sottmann, T. Synthesis of nanoporous organic/inorganic hybrid materials with adjustable pore size. Colloid Polym. Sci. 2018, 296, 1805–1816. [Google Scholar] [CrossRef]
- Atanasova, P.; Weitz, R.T.; Gerstel, P.; Srot, V.; Kopold, P.; van Aken, P.A.; Burghard, M.; Bill, J. DNA-templated synthesis of ZnO thin layers and nanowires. Nanotechnology 2009, 20, 365302. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, P.; Rothenstein, D.; Schneider, J.J.; Hoffmann, R.C.; Dilfer, S.; Eiben, S.; Wege, C.; Jeske, H.; Bill, J. Virus-Templated Synthesis of ZnO Nanostructures and Formation of Field-Effect Transistors. Adv. Mater. 2011, 23, 4918–4922. [Google Scholar] [CrossRef]
- Atanasova, P.; Stitz, N.; Sanctis, S.; Maurer, J.H.M.; Hoffmann, R.C.; Eiben, S.; Jeske, H.; Schneider, J.J.; Bill, J. Genetically Improved Monolayer-Forming Tobacco Mosaic Viruses to Generate Nanostructured Semiconducting Bio/Inorganic Hybrids. Langmuir 2015, 31, 3897–3903. [Google Scholar] [CrossRef]
- Stitz, N.; Eiben, S.; Atanasova, P.; Domingo, N.; Leineweber, A.; Burghard, Z.; Bill, J. Piezoelectric Templates—New Views on Biomineralization and Biomimetics. Sci. Rep. 2016, 6, 26518. [Google Scholar] [CrossRef]
- Atanasova, P.; Hoffmann, R.C.; Stitz, N.; Sanctis, S.; Burghard, Z.; Bill, J.; Schneider, J.J.; Eiben, S. Engineered nanostructured virus/ZnO hybrid materials with dedicated functional properties. Bioinspired Biomim. Nanobiomaterials 2019, 8, 2–15. [Google Scholar] [CrossRef]
- Abitaev, K.; Qawasmi, Y.; Atanasova, P.; Dargel, C.; Bill, J.; Hellweg, T.; Sottmann, T. Adjustable polystyrene nanoparticle templates for the production of mesoporous foams and ZnO inverse opals. Colloid Polym. Sci. 2021, 299, 243–258. [Google Scholar] [CrossRef]
- Kousik, S.R.; Sipp, D.; Abitaev, K.; Li, Y.; Sottmann, T.; Koynov, K.; Atanasova, P. From Macro to Mesoporous ZnO Inverse Opals: Synthesis, Characterization and Tracer Diffusion Properties. Nanomaterials 2021, 11, 196. [Google Scholar] [CrossRef]
- Lin, X.; Chen, M. Fabrication and Photo-Detecting Performance of 2D ZnO Inverse Opal Films. Appl. Sci. 2016, 6, 259. [Google Scholar] [CrossRef]
- Kiyomi, Y.; Shiraiwa, N.; Nakazawa, T.; Fukawa, A.; Oshio, K.; Takase, K.; Ito, T.; Shingubara, S.; Shimizu, T. Fabrication and UV photoresponse of ordered ZnO nanonets using monolayer colloidal crystal template. Micro Nano Eng. 2022, 16, 100160. [Google Scholar] [CrossRef]
- Li, Q.; Yang, C. Facile fabrication of Ag3PO4 supported on ZnO inverse opals for enhancement of solar-driven photocatalysis. Mater. Lett. 2017, 199, 168–171. [Google Scholar] [CrossRef]
- Meldrum, F.C.; Cölfen, H. Controlling Mineral Morphologies and Structures in Biological and Synthetic Systems. Chem. Rev. 2008, 108, 4332–4432. [Google Scholar] [CrossRef] [PubMed]
- Rieger, J.; Kellermeier, M.; Nicoleau, L. Die Bildung von Nanopartikeln und Nanostrukturen—CaCO3, Zement und Polymere aus Sicht der Industrie. Angew. Chem. 2014, 126, 12586–12603. [Google Scholar] [CrossRef]
- Caetano, B.L.; Santilli, C.V.; Pulcinelli, S.H.; Briois, V. SAXS and UV–Vis combined to Quick-XAFS monitoring of ZnO nanoparticles formation and growth. Phase Transit. 2011, 84, 714–725. [Google Scholar] [CrossRef]
- Caetano, B.L.; Santilli, C.V.; Meneau, F.; Briois, V.; Pulcinelli, S.H. In Situ and Simultaneous UV−vis/SAXS and UV−vis/XAFS Time-Resolved Monitoring of ZnO Quantum Dots Formation and Growth. J. Phys. Chem. C 2011, 115, 4404–4412. [Google Scholar] [CrossRef]
- Caetano, B.L.; Briois, V.; Pulcinelli, S.H.; Meneau, F.; Santilli, C.V. Revisiting the ZnO Q-dot Formation Toward an Integrated Growth Model: From Coupled Time Resolved UV–Vis/SAXS/XAS Data to Multivariate Analysis. J. Phys. Chem. C 2017, 121, 886–895. [Google Scholar] [CrossRef]
- Penn, R.L.; Banfield, J.F. Imperfect Oriented Attachment: Dislocation Generation in Defect-Free Nanocrystals. Science 1998, 281, 969–971. [Google Scholar] [CrossRef]
- Ribeiro, C.; Lee, E.J.H.; Longo, E.; Leite, E.R. A Kinetic Model to Describe Nanocrystal Growth by the Oriented Attachment Mechanism. ChemPhysChem 2005, 6, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, I.; Slyozov, V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Wagner, C. Theorie der Alterung von Niederschlägen durch Umlösen (Ostwald-Reifung). Z. Electrochem. 1961, 65, 581–591. [Google Scholar] [CrossRef]
- Herbst, M.; Hofmann, E.; Förster, S. Nucleation and Growth Kinetics of ZnO Nanoparticles Studied by in Situ Microfluidic SAXS/WAXS/UV–Vis Experiments. Langmuir 2019, 35, 11702–11709. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Das, B.; Rao, C.N.R. Growth Kinetics of ZnO Nanorods: Capping-Dependent Mechanism and Other Interesting Features. J. Phys. Chem. C 2008, 112, 2404–2411. [Google Scholar] [CrossRef]
- Orthaber, D.; Bergmann, A.; Glatter, O. SAXS experiments on absolute scale with Kratky systems using water as a secondary standard. J. Appl. Crystallogr. 2000, 33, 218–225. [Google Scholar] [CrossRef]
- Ilavsky, J.; Jemian, P.R.; Allen, A.J.; Zhang, F.; Levine, L.E.; Long, G.G. Ultra-small-angle X-ray scattering at the Advanced Photon Source. J. Appl. Crystallogr. 2009, 42, 469–479. [Google Scholar] [CrossRef]
- Ilavsky, J.; Zhang, F.; Andrews, R.N.; Kuzmenko, I.; Jemian, P.R.; Levine, L.E.; Allen, A.J. Development of combined microstructure and structure characterization facility for in situ and operando studies at the Advanced Photon Source. J. Appl. Crystallogr. 2018, 51, 867–882. [Google Scholar] [CrossRef]
- Ilavsky, J. Nika: Software for two-dimensional data reduction. J. Appl. Crystallogr. 2012, 45, 324–328. [Google Scholar] [CrossRef]
- Ilavsky, J.; Jemian, P.R. Irena: Tool suite for modeling and analysis of small-angle scattering. J. Appl. Crystallogr. 2009, 42, 347–353. [Google Scholar] [CrossRef]
- Watzky, M.A.; Finke, R.G. Transition Metal Nanocluster Formation Kinetic and Mechanistic Studies. A New Mechanism When Hydrogen Is the Reductant: Slow, Continuous Nucleation and Fast Autocatalytic Surface Growth. J. Am. Chem. Soc. 1997, 119, 10382–10400. [Google Scholar] [CrossRef]
- Finney, E.E.; Finke, R.G. Nanocluster nucleation and growth kinetic and mechanistic studies: A review emphasizing transition-metal nanoclusters. J. Colloid Interface Sci. 2008, 317, 351–374. [Google Scholar] [CrossRef]
- Finney, E.E.; Finke, R.G. Is There a Minimal Chemical Mechanism Underlying Classical Avrami-Erofe’ev Treatments of Phase-Transformation Kinetic Data? Chem. Mater. 2009, 21, 4692–4705. [Google Scholar] [CrossRef]
- Bentea, L.; Watzky, M.A.; Finke, R.G. Sigmoidal Nucleation and Growth Curves Across Nature Fit by the Finke–Watzky Model of Slow Continuous Nucleation and Autocatalytic Growth: Explicit Formulas for the Lag and Growth Times Plus Other Key Insights. J. Phys. Chem. C 2017, 121, 5302–5312. [Google Scholar] [CrossRef]
- Timaeva, O.; Pashkin, I.; Mulakov, S.; Kuzmicheva, G.; Konarev, P.; Terekhova, R.; Sadovskaya, N.; Czakkel, O.; Prevost, S. Synthesis and physico-chemical properties of poly(N-vinyl pyrrolidone)-based hydrogels with titania nanoparticles. J. Mater. Sci. 2020, 55, 3005–3021. [Google Scholar] [CrossRef]
- Benoit, H. The diffusion of light by polymers dissolved in a good solvent. Comptes Rendus 1957, 245, 2244–2247. [Google Scholar]
- Hammouda, B. SANS from homogeneous polymer mixtures: A unified overview. In Polymer Characteristics; Springer: Berlin/Heidelberg, Germany, 1993; pp. 87–133. [Google Scholar]
- Hammouda, B.; Ho, D.L.; Kline, S. Insight into Clustering in Poly(ethylene oxide) Solutions. Macromolecules 2004, 37, 6932–6937. [Google Scholar] [CrossRef]
- Pedersen, J.S.; Schurtenberger, P. Scattering Functions of Semiflexible Polymers with and without Excluded Volume Effects. Macromolecules 1996, 29, 7602–7612. [Google Scholar] [CrossRef]
- Chen, W.-R.; Butler, P.D.; Magid, L.J. Incorporating Intermicellar Interactions in the Fitting of SANS Data from Cationic Wormlike Micelles. Langmuir 2006, 22, 6539–6548. [Google Scholar] [CrossRef]
- Porod, G. Die Röntgenkleinwinkelstreuung von dichtgepackten kolloiden Systemen. Kolloid Zeit. 1951, 124, 83–114. [Google Scholar] [CrossRef]
- Sapir, L.; Stanley, C.B.; Harries, D. Properties of Polyvinylpyrrolidone in a Deep Eutectic Solvent. J. Phys. Chem. A 2016, 120, 3253–3259. [Google Scholar] [CrossRef] [PubMed]
- Hamada, F.; Kinugasa, S.; Hayashi, H.; Nakajima, A. Small-angle x-ray scattering from semidilute polymer solutions. I. Polystyrene in toluene. Macromolecules 1985, 18, 2290–2294. [Google Scholar] [CrossRef]
- Distaso, M.; Taylor, R.N.K.; Taccardi, N.; Wasserscheid, P.; Peukert, W. Influence of the Counterion on the Synthesis of ZnO Mesocrystals under Solvothermal Conditions. Chem.-A Eur. J. 2011, 17, 2923–2930. [Google Scholar] [CrossRef] [PubMed]
- Knappe, P.; Bienert, R.; Weidner, S.; Thünemann, A.F. Characterization of poly(N-vinyl-2-pyrrolidone)s with broad size distributions. Polymer 2010, 51, 1723–1727. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Panarin, E.F.; Korneeva, E.V.; Kurochkin, C.V.; Baikov, V.E.; Ushakova, V.N. Hydrodynamic properties of poly(1-vinyl-2-pyrrolidone) molecules in dilute solution. Makromol. Chem. 1990, 191, 2889–2899. [Google Scholar] [CrossRef]
- Benoit, H.; Doty, P. Light Scattering from Non-Gaussian Chains. J. Phys. Chem. 1953, 57, 958–963. [Google Scholar] [CrossRef]
- Narayanan, T.; Sztucki, M.; Van Vaerenbergh, P.; Léonardon, J.; Gorini, J.; Claustre, L.; Sever, F.; Morse, J.; Boesecke, P. A multipurpose instrument for time-resolved ultra-small-angle and coherent X-ray scattering. J. Appl. Crystallogr. 2018, 51, 1511–1524. [Google Scholar] [CrossRef]
- Guinier, A.; Fournet, G. Small-Angle Scattering of X-rays; John Wiley & Sons: New York, NY, USA, 1955. [Google Scholar]
- Jouault, N.; Crawford, M.K.; Chi, C.; Smalley, R.J.; Wood, B.; Jestin, J.; Melnichenko, Y.B.; He, L.; Guise, W.E.; Kumar, S.K. Polymer Chain Behavior in Polymer Nanocomposites with Attractive Interactions. ACS Macro Lett. 2016, 5, 523–527. [Google Scholar] [CrossRef]
- Wong, E.M.; Bonevich, J.E.; Searson, P.C. Growth Kinetics of Nanocrystalline ZnO Particles from Colloidal Suspensions. J. Phys. Chem. B 1998, 102, 7770–7775. [Google Scholar] [CrossRef]
- Hu, Z.; Santos, J.F.H.; Oskam, G.; Searson, P.C. Influence of the reactant concentrations on the synthesis of ZnO nanoparticles. J. Colloid Interface Sci. 2005, 288, 313–316. [Google Scholar] [CrossRef]
- Asakura, S.; Oosawa, F. On Interaction between Two Bodies Immersed in a Solution of Macromolecules. J. Chem. Phys. 1954, 22, 1255–1256. [Google Scholar] [CrossRef]
- Asakura, S.; Oosawa, F. Interaction between particles suspended in solutions of macromolecules. J. Polym. Sci. 1958, 33, 183–192. [Google Scholar] [CrossRef]
- Ghosh, G.; Naskar, M.K.; Patra, A.; Chatterjee, M. Synthesis and characterization of PVP-encapsulated ZnS nanoparticles. Opt. Mater. 2006, 28, 1047–1053. [Google Scholar] [CrossRef]
- Watzky, M.A.; Finke, R.G. Nanocluster Size-Control and “Magic Number” Investigations. Experimental Tests of the “Living-Metal Polymer” Concept and of Mechanism-Based Size-Control Predictions Leading to the Syntheses of Iridium(0) Nanoclusters Centering about Four Sequential Magic Numbers. Chem. Mater. 1997, 9, 3083–3095. [Google Scholar] [CrossRef]
- Hornstein, B.J.; Finke, R.G. Transition-Metal Nanocluster Kinetic and Mechanistic Studies Emphasizing Nanocluster Agglomeration: Demonstration of a Kinetic Method That Allows Monitoring of All Three Phases of Nanocluster Formation and Aging. Chem. Mater. 2004, 16, 139–150. [Google Scholar] [CrossRef]
- Besson, C.; Finney, E.E.; Finke, R.G. A Mechanism for Transition-Metal Nanoparticle Self-Assembly. J. Am. Chem. Soc. 2005, 127, 8179–8184. [Google Scholar] [CrossRef] [PubMed]
- Besson, C.; Finney, E.E.; Finke, R.G. Nanocluster Nucleation, Growth, and Then Agglomeration Kinetic and Mechanistic Studies: A More General, Four-Step Mechanism Involving Double Autocatalysis. Chem. Mater. 2005, 17, 4925–4938. [Google Scholar] [CrossRef]

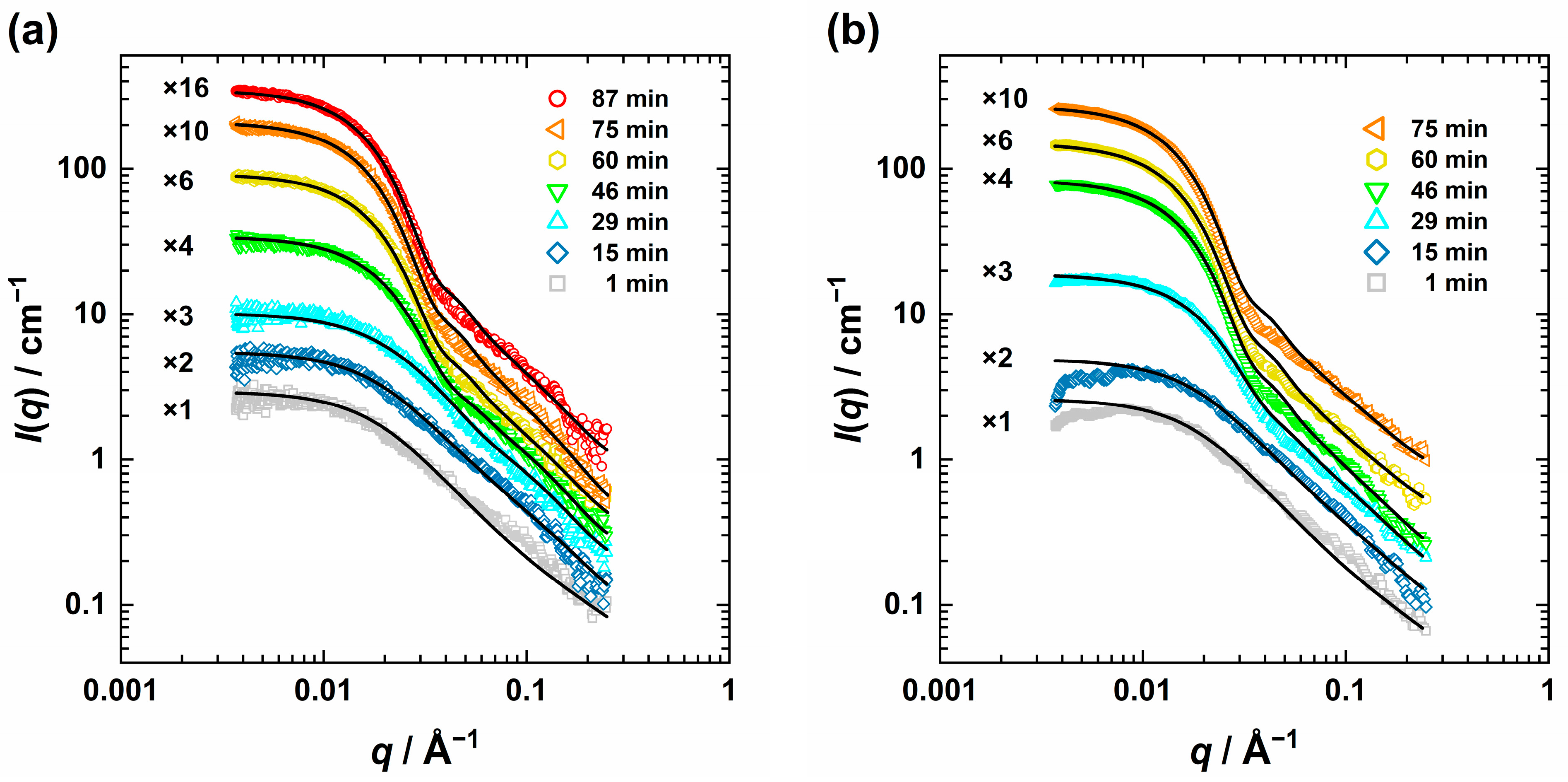
 ) and T = 63 °C (
) and T = 63 °C ( ) with ZnO nanoparticles (dashed line) held constant (a) obtained from the USAXS curve analysis, as well as the corresponding cube of radii (b). A phenomenological approach can describe the growth period (dotted lines), yet to fit the nucleation and growth phase the Finke–Watzky model [62,63,64,65] (solid lines) is more suitable and also provides the induction time tind (red stars).
) with ZnO nanoparticles (dashed line) held constant (a) obtained from the USAXS curve analysis, as well as the corresponding cube of radii (b). A phenomenological approach can describe the growth period (dotted lines), yet to fit the nucleation and growth phase the Finke–Watzky model [62,63,64,65] (solid lines) is more suitable and also provides the induction time tind (red stars).
 ) and T = 63 °C (
) and T = 63 °C ( ) with ZnO nanoparticles (dashed line) held constant (a) obtained from the USAXS curve analysis, as well as the corresponding cube of radii (b). A phenomenological approach can describe the growth period (dotted lines), yet to fit the nucleation and growth phase the Finke–Watzky model [62,63,64,65] (solid lines) is more suitable and also provides the induction time tind (red stars).
) with ZnO nanoparticles (dashed line) held constant (a) obtained from the USAXS curve analysis, as well as the corresponding cube of radii (b). A phenomenological approach can describe the growth period (dotted lines), yet to fit the nucleation and growth phase the Finke–Watzky model [62,63,64,65] (solid lines) is more suitable and also provides the induction time tind (red stars).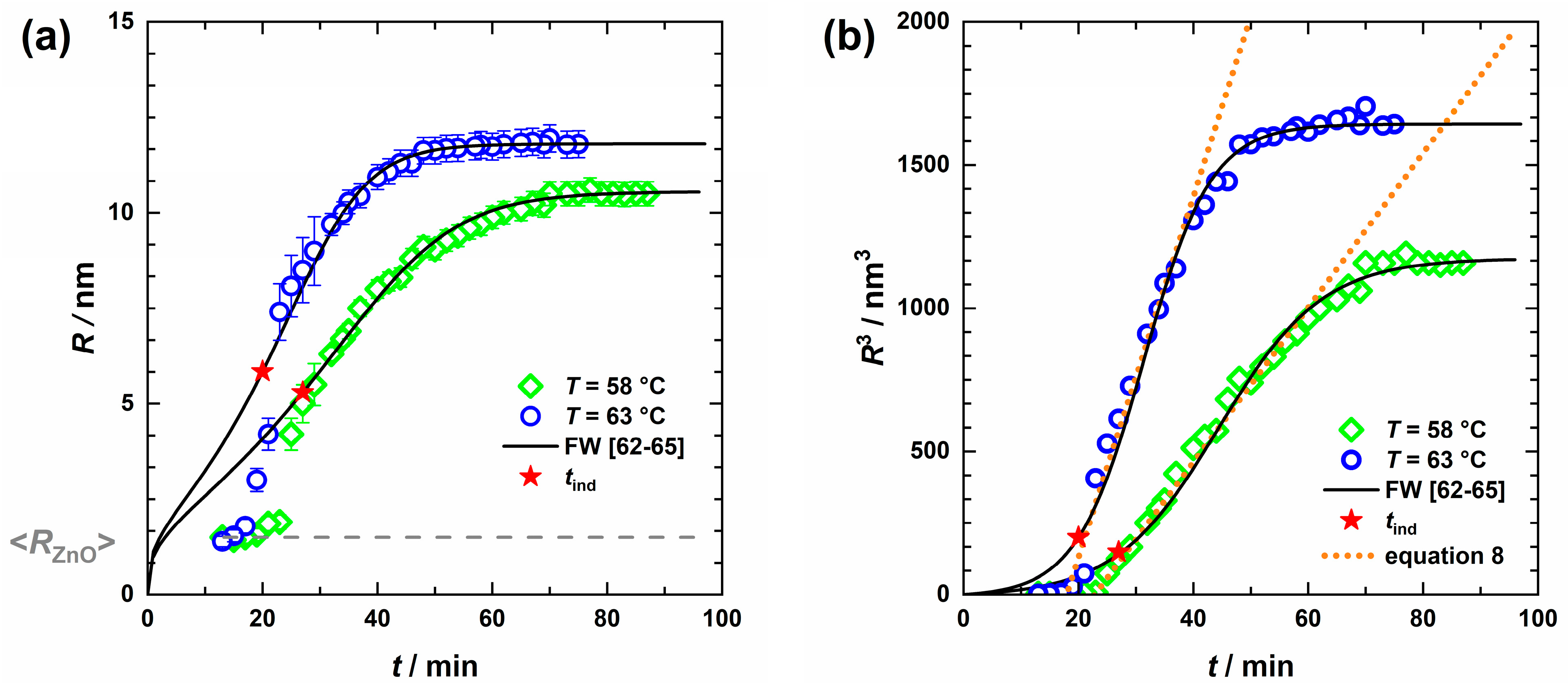
| R3(t)FW | ϕZnO-cluster, inv(t)FW | |||||||
|---|---|---|---|---|---|---|---|---|
| k1/s−1 | k2[A]0/s−1 | tind/min | S/M−1 | k1/s−1 | k2[A]0/s−1 | tind/min | S/M−1 | |
| 58 °C | 8.0 × 10−4 | 0.11 | 27 | 141 | 5.5 × 10−4 | 0.11 | 31 | 200 |
| 63 °C | 8.0 × 10−4 | 0.17 | 20 | 213 | 5.0 × 10−4 | 0.17 | 23 | 340 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abitaev, K.; Atanasova, P.; Bill, J.; Preisig, N.; Kuzmenko, I.; Ilavsky, J.; Liu, Y.; Sottmann, T. In Situ Ultra-Small- and Small-Angle X-ray Scattering Study of ZnO Nanoparticle Formation and Growth through Chemical Bath Deposition in the Presence of Polyvinylpyrrolidone. Nanomaterials 2023, 13, 2180. https://doi.org/10.3390/nano13152180
Abitaev K, Atanasova P, Bill J, Preisig N, Kuzmenko I, Ilavsky J, Liu Y, Sottmann T. In Situ Ultra-Small- and Small-Angle X-ray Scattering Study of ZnO Nanoparticle Formation and Growth through Chemical Bath Deposition in the Presence of Polyvinylpyrrolidone. Nanomaterials. 2023; 13(15):2180. https://doi.org/10.3390/nano13152180
Chicago/Turabian StyleAbitaev, Karina, Petia Atanasova, Joachim Bill, Natalie Preisig, Ivan Kuzmenko, Jan Ilavsky, Yun Liu, and Thomas Sottmann. 2023. "In Situ Ultra-Small- and Small-Angle X-ray Scattering Study of ZnO Nanoparticle Formation and Growth through Chemical Bath Deposition in the Presence of Polyvinylpyrrolidone" Nanomaterials 13, no. 15: 2180. https://doi.org/10.3390/nano13152180
APA StyleAbitaev, K., Atanasova, P., Bill, J., Preisig, N., Kuzmenko, I., Ilavsky, J., Liu, Y., & Sottmann, T. (2023). In Situ Ultra-Small- and Small-Angle X-ray Scattering Study of ZnO Nanoparticle Formation and Growth through Chemical Bath Deposition in the Presence of Polyvinylpyrrolidone. Nanomaterials, 13(15), 2180. https://doi.org/10.3390/nano13152180





