An Up-to-Date Review on the Remediation of Dyes and Phenolic Compounds from Wastewaters Using Enzymes Immobilized on Emerging and Nanostructured Materials: Promises and Challenges
Abstract
1. Introduction
2. Enzyme Immobilization Techniques
2.1. Physical Techniques
2.1.1. Adsorption
| Immobilization Technique | Advantages | Drawbacks | Approaches to Address the Limitations | Ref. |
|---|---|---|---|---|
| Adsorption |
|
|
| [121,122,123] |
| Entrapment |
|
|
| [124,125,126] |
| Encapsulation |
|
|
| [126,127,128,129] |
| Covalent binding |
|
|
| [130,131,132] |
| Cross-linking |
|
|
| [130,133,134,135] |
2.1.2. Entrapment
2.1.3. Encapsulation
2.2. Chemical Techniques
2.2.1. Covalent Binding
2.2.2. Cross-Linking
2.2.3. Affinity Immobilization
3. Wastewater Treatment Using Enzymes Immobilized on Graphene Materials
3.1. The Synthesis of Graphene-Based Materials
3.1.1. Graphene Oxide
3.1.2. Reduced Graphene Oxide
3.1.3. Graphene Nanoplatelets
3.2. Enzyme Immobilization on Graphene-Based Materials
3.3. The Remediation of Dyes by Enzymes Immobilized on Graphene-Based Materials
| Dye | ||||||
|---|---|---|---|---|---|---|
| Media | Enzyme | Techniques | Optimum Condition | Pollutant | Removal (%) | Ref. |
| GO nanosheets | Genetically Modified Aspergillus Laccase | Covalent binding | 60 min, pH 5, 45 °C | Acid Blue 92 Direct Red 23 | 75 75 | [208] |
| Polypyrrole-cellulose- GO nanocomposite | Peroxidase | Non-covalent binding | 100 min, pH 4, 40 °C | Reactive Blue 4 | 99% | [211] |
| GO | Porcine pancreas lipase | Adsorption | 240 min, pH 8, 40 °C | Azo dyes | 89.47 | [212] |
| GO | Laccase | Adsorption | 24 h, pH 5, 50 °C | Crystal Violet Reactive Brilliant Blue Methyl Orange Reactive Brilliant Blue 14 | Better than 40% | [203] |
| GO | Manganese Peroxidase | Covalent binding | 5 h, pH 5, 35 °C | Azo dye Triphenylmethane dye Anthraquinone dye | Better than simple enzyme | [213] |
| GO | Laccase | Cross-linking | 60 min, pH 3, 45 °C | Direct Red 23 | 91 | [214] |
| CNTs/GO | Laccase from trametes versicolor | Adsorption | 20 °C | Methylene Blue | 80 | [215] |
| CNTs | Laccase from trametes versicolor | Cross-linking | 24 h, pH 5, 25 °C | Methylene Blue Orange II dye | 96 74 | [216] |
| CNTs | Laccase from trametes versicolor | Adsorption | 3 h, pH 7, 35 °C | Congo Red | 96 | [94] |
| CNTs | Ganoderma lucidum’s LiP | Covalent binding | 24 h, pH 3.5, 25 °C | Remazol Brilliant Blue R | 78 | [217] |
| Fe3O4-MWCNTs@SiO2 | Laccase from Trametes versicolor | Covalent binding | 3.5 h, pH 3, 60 °C | Acid Red 88 Reactive Black 5 Eriochrome Black T | 98, 99, 66 | [218] |
| MWCNTs | Laccase from myceliophthora Thermophile | Covalent binding | 24 h, pH 5, 25 °C | Reactive Black 5 | 84 | [219] |
| Cu-PABA (MOFs) | Laccase | Encapsulation | 6 h, pH 4.5, 40 °C | Direct Red 31 | 92 | [220] |
| Cu-MOFs Co-MOFs Cu-MOFs Co-MOFs | Laccase | Encapsulation | 1 h, pH 4.5, 50 °C 1 h, pH 5, 50 °C 1 h, pH 4.5, 50 °C 1 h, pH 5, 50 °C | Reactive Blue 171 Reactive Blue 171 Reactive Blue 198 Reactive Blue 198 | 89 88 39 77 | [221] |
| Fe-BTC/NiFe2O4 (MOFs) | Laccase | Coprecipitation | 1 h, pH 3, 22 °C | Methylene blue | 100 | [222] |
| Fe3O4@ZIF-8 (MOFs) | Laccase | Coprecipitation | 30 min, pH 4.5, 70 °C | Crystal Violet Methylene Blue | 93 91 | [223] |
| NH2-MIL88 (Fe) (MOFs) | Laccase | Cross-linking | 2 h, 30 °C | Remazol Brilliant Blue R | 92 | [224] |
| Fe3O4@ZIF-8 (MOFs) | Laccase | Coprecipitation | 15 min, pH 7, 40 °C | Indigo Carmine | 100 | [225] |
| ZIF-8 (MOFs) | Laccase | Covalent binding | 2 h, pH 3, 40 °C | Acid Blue 92 | 90 | [226] |
| Fe3O4-NH2@MIL-101 (MOFs) | Laccase | Covalent binding and adsorption | 2 h, pH 3, 25 °C | Alizarin Red S Reactive Black 5 | 100 81 | [227] |
| Phenolic compound | ||||||
| GO | HRP | Covalent binding | pH 5, 40 °C | Phenol | 100 | [194] |
| Nanostructure GO | HRP | Adsorption | 30 min, pH 6 | 3-aminophenol Catechol 2-methoxy phenol Phenol 4-methoxy phenol 2,4-dimetheoxyphenol 2-CP | 87.6 72.7 68 64 69 34.4 20.4 | [201] |
| GO/Fe3O4 | HRP | Covalent binding | 3 h, pH 6, 25 °C | Phenol 2,4-DCP | 70 100 | [228] |
| Fe3O4/GO | HRP | Covalent binding | 2 h, pH 6.4, 25 °C | 2-CP 4-CP 2,4-DCP | 23 44 83 | [202] |
| rGO | Ochrobactrum sp. FJ1 | Adsorption | 10 days, pH 7, 25 °C | BPA | 64.6 | [229] |
| Functionalized MWCNTs | Laccase | Covalent binding | 60 min, pH 5.6, 23 °C | Phenol Resorcinol 4-Methoxyphenol 4-CP | 90 90 100 45 | [230] |
| MWCNTs | Laccase | Cross-linking | 300 min, pH 5, 35–45 °C | BPA | 90 | [231] |
| PAN-MIL-101 (Cr) (MOFs) | Laccase | Electrostatic adsorption | 5 h, pH 5, 23 °C | BPA | 92 | [232] |
| NH2-MIL-53(Al) (MOFs) | Laccase | Non-covalent immobilization | 0.5 h, pH 4.5, 21 °C | BPA | 99 | [233] |
| HKUST-1 (MOFs) | Laccase | Encapsulation | 4 h, pH 6.5, 40 °C | BPA | 98.2 | [234] |
| Cu3(BTC)2 @P1 (MOFs) | Laccase | Encapsulation | 12 h, pH 5, 40 °C | BPA | 99.6 | [235] |
| Cu-PABA (MOFs) | Laccase | Coprecipitation | 12 h, pH 5.5, 35 °C | BPA | 84.7 | [95] |
| Graphene aerogel-Zr-MOFs | Laccase | Adsorption | 24 h, pH 4, 40 °C | Hydroquinone | 79 | [236] |
| BC/c-MWCNTs/ZIF-90 (MOFs) | Laccase | Encapsulation | 2 h, pH 4, 50 °C | Catechol | 93.4 | [237] |
| Fe3O4-NH2@MIL-101(Cr) (MOFs) | Laccase | Adsorption and covalent binding | 2 h, pH 4, 25 °C | 2,4-DCP | 87 | [238] |
| Fe3O4-NH2@MIL-100(Fe) (MOFs) | Laccase | Adsorption and covalent binding | 200 min, pH 5, 50 °C | Nonylphenol polyethoxylated Octylphenol polyethoxylated | 98.16 100 | [239] |
3.4. The Remediation of Phenols by Enzymes Immobilized on Graphene-Based Materials
4. Wastewater Treatment Using Enzymes Immobilized on CNTs
4.1. Synthesis of CNTs
4.2. Enzyme Immobilization on CNTs
4.3. The Remediation of Dyes by Enzymes Immobilized on CNTs
4.4. The Remediation of Phenols by Enzymes Immobilized on CNTs
5. Wastewater Treatment Using Enzymes Immobilized on MOFs
5.1. Synthesis of MOFs
5.2. Enzyme Immobilization on MOFs
5.3. The Remediation of Dyes by Enzymes Immobilized on MOFs
5.4. The Remediation of Phenols by Enzymes Immobilized on MOFs
6. Challenges and Limitations of Wastewater Treatment Using Immobilized Enzymes
6.1. Costs of Enzyme Immobilization
6.2. The Production of Obstacles That Hinder the Access of Substrates to the Active Site of the Enzyme
6.3. Using Unstable Supports
6.4. The Process of Extrapolating from One Support to Another Is Not Always Straightforward
- Different supports may allow varying degrees of activation, influencing the enzyme immobilization rate and multi-point covalent linkage. Comparing supports with diverse surface densities of reactive groups may not be fair. The highest activation level should be considered for each support [297].
- Support surfaces can possess unique physical properties, leading to unwanted enzyme–support interactions [313]. Proper blocking can minimize these effects, but physically active supports can never be completely inert. These interactions can impact enzyme function, stability, and the inactivation process [313,314].
6.5. Difficulties in Co-Immobilizing Multiple Enzymes
6.6. The Protocols of Immobilization Are Not Complete When All the Enzyme Activity Is Incorporated into the Support
6.7. The Utilization of Weakened-Loading Supports
6.8. Enzyme Release from the Support
7. Conclusions
- Hybrid Materials: Given the unique properties of graphene, CNTs, and MOFs, the development of hybrid materials that combine their strengths could lead to superior supports for enzyme immobilization [221,222]. Future research could explore this avenue and potentially unveil highly efficient, tailor-made materials for wastewater treatment.
- Enzyme-Substrate Dynamics: While we have discussed the enzyme-substrate interactions in the context of the physicochemical properties of graphene, CNTs, and MOFs, further understanding of these dynamics in various operational conditions will enhance the efficacy of the treatment process [83,100,102]. Unraveling these complex interactions could provide critical insights into the design of advanced immobilization techniques.
- Environmental Impacts: Long-term environmental studies are needed to ensure the sustainability of using these emerging materials in the enzyme immobilization process. The environmental fate of these materials, once they complete their operational lifecycle, is still not well understood and requires thorough investigation.
- Cost-Effectiveness: The economic aspect of implementing these emerging materials in real-world scenarios is another research gap that needs to be addressed. It will be important to develop techniques to lower the cost of producing and using these materials, ensuring their feasibility for industrial applications.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farhan Hanafi, M.; Sapawe, N. A Review on the Water Problem Associate with Organic Pollutants Derived from Phenol, Methyl Orange, and Remazol Brilliant Blue Dyes. Mater. Today Proc. 2020, 31, A141–A150. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Asmaly, H.A.; Abussaud, B.; Ihsanullah; Saleh, T.A.; Bukhari, A.A.; Laoui, T.; Shemsi, A.M.; Gupta, V.K.; Atieh, M.A. Evaluation of Micro- and Nano-Carbon-Based Adsorbents for the Removal of Phenol from Aqueous Solutions. Toxicol. Environ. Chem. 2015, 97, 1164–1179. [Google Scholar] [CrossRef]
- Asmaly, H.A.; Ihsanullah; Abussaud, B.; Saleh, T.A.; Laoui, T.; Gupta, V.K.; Atieh, M.A. Adsorption of Phenol on Aluminum Oxide Impregnated Fly Ash. Desalin. Water Treat. 2016, 57, 6801–6808. [Google Scholar] [CrossRef]
- Ahmed, J.; Thakur, A.; Goyal, A. Industrial Wastewater and Its Toxic Effects. In Biological Treatment of Industrial Wastewater; The Royal Society of Chemistry: London, UK, 2021; pp. 1–14. [Google Scholar] [CrossRef]
- Muthukumaran, M. Advances in Bioremediation of Nonaqueous Phase Liquid Pollution in Soil and Water. In Biological Approaches to Controlling Pollutants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 191–231. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Stegeman, J.J.; Fleming, L.E.; Allemand, D.; Anderson, D.M.; Backer, L.C.; Brucker-Davis, F.; Chevalier, N.; Corra, L.; Czerucka, D.; et al. Human Health and Ocean Pollution. Ann. Glob. Health 2020, 86, 151. [Google Scholar] [CrossRef]
- Anku, W.W.; Mamo, M.A.; Govender, P.P. Phenolic Compounds in Water: Sources, Reactivity, Toxicity and Treatment Methods. In Phenolic Compounds—Natural Sources, Importance and Applications; InTech: Rijeka, Romania, 2017. [Google Scholar] [CrossRef]
- Manasa, R.L.; Mehta, A. Wastewater: Sources of Pollutants and Its Remediation. Environ. Chem. A Sustain. World 2020, 2, 197–219. [Google Scholar] [CrossRef]
- Akpor, O.B.; Ohiobor, G.O.; Olaolu, T.D.; Oghenerobor, B.; Akpor, G.O.; Ohiobor, T.; Debby, O. Heavy Metal Pollutants in Wastewater Effluents: Sources, Effects and Remediation. Adv. Biosci. Bioeng. 2014, 2, 37. [Google Scholar] [CrossRef]
- Ahmed, A.; Forster, M.; Jin, J.; Myers, P.; Zhang, H. Tuning Morphology of Nanostructured ZIF-8 on Silica Microspheres and Applications in Liquid Chromatography and Dye Degradation. ACS Appl. Mater. Interfaces 2015, 7, 18054–18063. [Google Scholar] [CrossRef]
- Gao, W.; Fatehi, P. Fly Ash Based Adsorbent for Treating Bleaching Effluent of Kraft Pulping Process. Sep. Purif. Technol. 2018, 195, 60–69. [Google Scholar] [CrossRef]
- Li, G.; Xu, Q.; Jin, X.; Li, R.; Dharmarajan, R.; Chen, Z. Enhanced Adsorption and Fenton Oxidation of 2,4-Dichlorophenol in Aqueous Solution Using Organobentonite Supported NZVI. Sep. Purif. Technol. 2018, 197, 401–406. [Google Scholar] [CrossRef]
- Abussaud, B.; Asmaly, H.A.; Ihsanullah; Saleh, T.A.; Gupta, V.K.; Laoui, T.; Atieh, M.A. Sorption of Phenol from Waters on Activated Carbon Impregnated with Iron Oxide, Aluminum Oxide and Titanium Oxide. J. Mol. Liq. 2016, 213, 351–359. [Google Scholar] [CrossRef]
- Bahadi, S.A.; Iddrisu, M.; Al-Sakkaf, M.K.; Elgzoly, M.A.A.; Drmosh, Q.A.; Al-Amrani, W.A.; Ahmed, U.; Zahid, U.; Onaizi, S.A. Optimization of Methyl Orange Adsorption on MgFeAl-LTH through the Manipulation of Solution Chemistry and Synthesis Conditions. Emerg. Mater. 2023. [Google Scholar] [CrossRef]
- Bahadi, S.A.; Iddrisu, M.; Al-Sakkaf, M.K.; Elgzoly, M.A.A.; Al-Amrani, W.A.; Ahmed, U.; Zahid, U.; Drmosh, Q.A.; Onaizi, S.A. Chemically versus Thermally Reduced Graphene Oxide: Effects of Reduction Methods and Reducing Agents on the Adsorption of Phenolic Compounds from Wastewater. Emerg. Mater. 2023. [Google Scholar] [CrossRef]
- Ismail, U.M.; Onaizi, S.A.; Vohra, M.S. Novel MgCuAl-Layered Triple Hydroxide for Aqueous Selenite and Selenate Treatment. Emerg. Mater. 2023. [Google Scholar] [CrossRef]
- Fortunato, L.; Elcik, H.; Blankert, B.; Ghaffour, N.; Vrouwenvelder, J. Textile Dye Wastewater Treatment by Direct Contact Membrane Distillation: Membrane Performance and Detailed Fouling Analysis. J. Memb. Sci. 2021, 636, 119552. [Google Scholar] [CrossRef]
- Criscuoli, A.; Zhong, J.; Figoli, A.; Carnevale, M.C.; Huang, R.; Drioli, E. Treatment of Dye Solutions by Vacuum Membrane Distillation. Water Res. 2008, 42, 5031–5037. [Google Scholar] [CrossRef]
- Jaradat, A.Q.; Gharaibeh, S.; Abu Irjei, M. The Application of Solar Distillation Technique as a Mean for Olive Mill Wastewater Management. Water Environ. J. 2018, 32, 134–140. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Wastewater Treatment: An Overview. In Green Adsorbents for Pollutant Removal; Springer: Cham, Switzerlands, 2018; pp. 1–21. [Google Scholar] [CrossRef]
- Shikuku, V.O.; Nyairo, W.N. Advanced Oxidation Processes for Dye Removal from Wastewater. In Impact of Textile Dyes on Public Health and the Environment; IGI Global: Hershey, PA, USA, 2022; pp. 205–238. [Google Scholar] [CrossRef]
- Loos, G.; Scheers, T.; Van Eyck, K.; Van Schepdael, A.; Adams, E.; Van der Bruggen, B.; Cabooter, D.; Dewil, R. Electrochemical Oxidation of Key Pharmaceuticals Using a Boron Doped Diamond Electrode. Sep. Purif. Technol. 2018, 195, 184–191. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, H.; Zhang, H.; Cao, J.; Zhou, K.; Lian, J. Highly Efficient Degradation of Phenol Wastewater by Microwave Induced H2O2-CuOx/GAC Catalytic Oxidation Process. Sep. Purif. Technol. 2018, 193, 49–57. [Google Scholar] [CrossRef]
- Pandit, P.; Basu, S. Dye and Solvent Recovery in Solvent Extraction Using Reverse Micelles for the Removal of Ionic Dyes. Ind. Eng. Chem. Res. 2004, 43, 7861–7864. [Google Scholar] [CrossRef]
- Pandit, P.; Basu, S. Removal of Ionic Dyes from Water by Solvent Extraction Using Reverse Micelles. Environ. Sci. Technol. 2004, 38, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Asrami, M.R.; Saien, J. Salting-out Effect on Extraction of Phenol from Aqueous Solutions by [Hmim][NTf2] Ionic Liquid: Experimental Investigations and Modeling. Sep. Purif. Technol. 2018, 204, 175–184. [Google Scholar] [CrossRef]
- González, E.J.; Díaz, I.; Gonzalez-Miquel, M.; Rodríguez, M.; Sueiras, A. On the Behavior of Imidazolium versus Pyrrolidinium Ionic Liquids as Extractants of Phenolic Compounds from Water: Experimental and Computational Analysis. Sep. Purif. Technol. 2018, 201, 214–222. [Google Scholar] [CrossRef]
- Kadhim, R.J.; Al-Ani, F.H.; Al-Shaeli, M.; Alsalhy, Q.F.; Figoli, A. Removal of Dyes Using Graphene Oxide (GO) Mixed Matrix Membranes. Membranes 2020, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Huang, Z.; Tang, X.; Xiong, C.; Tang, M.; Lu, Y. A Dually Charged Nanofiltration Membrane by PH-Responsive Polydopamine for Pharmaceuticals and Personal Care Products Removal. Sep. Purif. Technol. 2019, 211, 90–97. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, W.; Li, R.; Xu, Y.; Shen, L.; Lin, H.; Liao, B.Q.; Wu, G. Novel Conductive Membranes Breaking through the Selectivity-Permeability Trade-off for Congo Red Removal. Sep. Purif. Technol. 2019, 211, 368–376. [Google Scholar] [CrossRef]
- Sunil, K.; Sherugar, P.; Rao, S.; Lavanya, C.; Balakrishna, G.R.; Arthanareeswaran, G.; Padaki, M. Prolific Approach for the Removal of Dyes by an Effective Interaction with Polymer Matrix Using Ultrafiltration Membrane. J. Environ. Chem. Eng. 2021, 9, 106328. [Google Scholar] [CrossRef]
- Badvi, K.; Javanbakht, V. Enhanced Photocatalytic Degradation of Dye Contaminants with TiO2 Immobilized on ZSM-5 Zeolite Modified with Nickel Nanoparticles. J. Clean. Prod. 2021, 280, 124518. [Google Scholar] [CrossRef]
- Sirirerkratana, K.; Kemacheevakul, P.; Chuangchote, S. Color Removal from Wastewater by Photocatalytic Process Using Titanium Dioxide-Coated Glass, Ceramic Tile, and Stainless Steel Sheets. J. Clean. Prod. 2019, 215, 123–130. [Google Scholar] [CrossRef]
- Nguyen, D.C.T.; Cho, K.Y.; Oh, W.C. Mesoporous CuO-Graphene Coating of Mesoporous TiO2 for Enhanced Visible-Light Photocatalytic Activity of Organic Dyes. Sep. Purif. Technol. 2019, 211, 646–657. [Google Scholar] [CrossRef]
- Lin, J.C.T.; Sopajaree, K.; Jitjanesuwan, T.; Lu, M.C. Application of Visible Light on Copper-Doped Titanium Dioxide Catalyzing Degradation of Chlorophenols. Sep. Purif. Technol. 2018, 191, 233–243. [Google Scholar] [CrossRef]
- Alkadhem, A.M.; Elgzoly, M.A.A.; Onaizi, S.A. Novel Amine-Functionalized Magnesium Oxide Adsorbents for CO2 Capture at Ambient Conditions. J. Environ. Chem. Eng. 2020, 8, 103968. [Google Scholar] [CrossRef]
- Hezam, A.; Drmosh, Q.A.; Ponnamma, D.; Bajiri, M.A.; Qamar, M.; Namratha, K.; Zare, M.; Nayan, M.B.; Onaizi, S.A.; Byrappa, K. Strategies to Enhance ZnO Photocatalyst’s Performance for Water Treatment: A Comprehensive Review. Chem. Rec. 2022, 22, e202100299. [Google Scholar] [CrossRef] [PubMed]
- Al Lagtah, N.M.A.; Onaizi, S.A.; Albadarin, A.B.; Ghaith, F.A.; Nour, M.I. Techno-Economic Analysis of the Effects of Heat Integration and Different Carbon Capture Technologies on the Performance of Coal-Based IGCC Power Plants. J. Environ. Chem. Eng. 2019, 7, 103471. [Google Scholar] [CrossRef]
- Almarouf, H.S.; Nasser, M.S.; Al-Marri, M.J.; Khraisheh, M.; Onaizi, S.A. Demulsification of Stable Emulsions from Produced Water Using a Phase Separator with Inclined Parallel Arc Coalescing Plates. J. Pet. Sci. Eng. 2015, 135, 16–21. [Google Scholar] [CrossRef]
- Al-Sakkaf, M.K.; Onaizi, S.A. Crude Oil/Water Nanoemulsions Stabilized by Rhamnolipid Biosurfactant: Effects of Acidity/Basicity and Salinity on Emulsion Characteristics, Stability, and Demulsification. Fuel 2023, 344, 128052. [Google Scholar] [CrossRef]
- Lateef, S.A.; Ajumobi, O.O.; Onaizi, S.A. Enzymatic Desulfurization of Crude Oil and Its Fractions: A Mini Review on the Recent Progresses and Challenges. Arab. J. Sci. Eng. 2019, 44, 5181–5193. [Google Scholar] [CrossRef]
- Al-Sakkaf, M.K.; Onaizi, S.A. Rheology, Characteristics, Stability, and PH-Responsiveness of Biosurfactant-Stabilized Crude Oil/Water Nanoemulsions. Fuel 2022, 307, 121845. [Google Scholar] [CrossRef]
- Onaizi, S.A.; Alsulaimani, M.; Al-Sakkaf, M.K.; Bahadi, S.A.; Mahmoud, M.; Alshami, A. Crude Oil/Water Nanoemulsions Stabilized by Biosurfactant: Stability and PH-Switchability. J. Pet. Sci. Eng. 2021, 198, 108173. [Google Scholar] [CrossRef]
- Onaizi, S.A. Demulsification of Crude Oil/Water Nanoemulsions Stabilized by Rhamnolipid Biosurfactant Using Enzymes and PH-Swing. Sep. Purif. Technol. 2021, 259, 118060. [Google Scholar] [CrossRef]
- Onaizi, S.A.; He, L.; Middelberg, A.P.J. The Construction, Fouling and Enzymatic Cleaning of a Textile Dye Surface. J. Colloid Interface Sci. 2010, 351, 203–209. [Google Scholar] [CrossRef]
- Onaizi, S.A. Dynamic Surface Tension and Adsorption Mechanism of Surfactin Biosurfactant at the Air–Water Interface. Eur. Biophys. J. 2018, 47, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Onaizi, S.A.; Malcolm, A.S.; He, L.; Middelberg, A.P.J. Directed Disassembly of an Interfacial Rubisco Protein Network. Langmuir 2007, 23, 6336–6341. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singh, J. An Enzymatic Method for Removal of Phenol from Industrial Effluent. Prep. Biochem. Biotechnol. 2002, 32, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ariaeenejad, S.; Motamedi, E.; Salekdeh, G.H. Highly Efficient Removal of Dyes from Wastewater Using Nanocellulose from Quinoa Husk as a Carrier for Immobilization of Laccase. Bioresour. Technol. 2022, 349, 126833. [Google Scholar] [CrossRef]
- Sariaslani, F.S.; Dalton, H. Microbial Enzymes for Oxidation of Organic Molecules. Crit. Rev. Biotechnol. 1989, 9, 171–257. [Google Scholar] [CrossRef]
- Misal, S.A.; Gawai, K.R. Azoreductase: A Key Player of Xenobiotic Metabolism. Bioresour. Bioprocess. 2018, 5, 17. [Google Scholar] [CrossRef]
- Gaur, G.; Gänzle, M.G. Conversion of (Poly)Phenolic Compounds in Food Fermentations by Lactic Acid Bacteria: Novel Insights into Metabolic Pathways and Functional Metabolites. Curr. Res. Food Sci. 2023, 6, 100448. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial Applications of Immobilized Enzymes—A Review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme Immobilization by Adsorption: A Review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Alshabib, M.; Onaizi, S.A. A Review on Phenolic Wastewater Remediation Using Homogeneous and Heterogeneous Enzymatic Processes: Current Status and Potential Challenges. Sep. Purif. Technol. 2019, 219, 186–207. [Google Scholar] [CrossRef]
- Torres, J.A.; Nogueira, F.G.E.; Silva, M.C.; Lopes, J.H.; Tavares, T.S.; Ramalho, T.C.; Corrêa, A.D. Novel Eco-Friendly Biocatalyst: Soybean Peroxidase Immobilized onto Activated Carbon Obtained from Agricultural Waste. RSC Adv. 2017, 7, 16460–16466. [Google Scholar] [CrossRef]
- Gholami-Borujeni, F.; Mahvi, A.H.; Naseri, S.; Faramarzi, M.A.; Nabizadeh, R.; Alimohammadi, M. Application of Immobilized Horseradish Peroxidase for Removal and Detoxification of Azo Dye from Aqueous Solution. Res. J. Chem. Environ. 2011, 15, 217–222. [Google Scholar]
- Silva, M.C.; Torres, J.A.; Vasconcelos De Sá, L.R.; Chagas, P.M.B.; Ferreira-Leitão, V.S.; Corrêa, A.D. The Use of Soybean Peroxidase in the Decolourization of Remazol Brilliant Blue R and Toxicological Evaluation of Its Degradation Products. J. Mol. Catal. B Enzym. 2013, 89, 122–129. [Google Scholar] [CrossRef]
- Chiong, T.; Lau, S.Y.; Lek, Z.H.; Koh, B.Y.; Danquah, M.K. Enzymatic Treatment of Methyl Orange Dye in Synthetic Wastewater by Plant-Based Peroxidase Enzymes. J. Environ. Chem. Eng. 2016, 4, 2500–2509. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological Methods for Textile Dye Removal from Wastewater: A Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Forootanfar, H.; Moezzi, A.; Aghaie-Khozani, M.; Mahmoudjanlou, Y.; Ameri, A.; Niknejad, F.; Ali Faramarzi, M. Synthetic Dye Decolorization by Three Sources of Fungal Laccase. Iran. J. Environ. Health Sci. Eng. 2012, 9, 27. [Google Scholar] [CrossRef]
- Onaizi, S.A.; Alshabib, M. The Degradation of Bisphenol A by Laccase: Effect of Biosurfactant Addition on the Reaction Kinetics under Various Conditions. Sep. Purif. Technol. 2021, 257, 117785. [Google Scholar] [CrossRef]
- Silva, M.C.; Torres, J.A.; Castro, A.A.; da Cunha, E.F.F.; Alves de Oliveira, L.C.; Corrêa, A.D.; Ramalho, T.C. Combined Experimental and Theoretical Study on the Removal of Pollutant Compounds by Peroxidases: Affinity and Reactivity toward a Bioremediation Catalyst. J. Biomol. Struct. Dyn. 2016, 34, 1839–1848. [Google Scholar] [CrossRef]
- Veitch, N.C. Horseradish Peroxidase: A Modern View of a Classic Enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef]
- Jaiswal, N.; Pandey, V.P.; Dwivedi, U.N. Immobilization of Papaya Laccase in Chitosan Led to Improved Multipronged Stability and Dye Discoloration. Int. J. Biol. Macromol. 2016, 86, 288–295. [Google Scholar] [CrossRef]
- Effron, D.; De La Horra, A.M.; Defrieri, R.L.; Fontanive, V.; Palma, R.M. Effect of Cadmium, Copper, and Lead on Different Enzyme Activities in a Native Forest Soil. Commun. Soil Sci. Plant Anal. 2006, 35, 1309–1321. [Google Scholar] [CrossRef]
- Torres, J.A.; Silva, M.C.; Lopes, J.H.; Nogueira, A.E.; Nogueira, F.G.E.; Corrêa, A.D. Development of a Reusable and Sustainable Biocatalyst by Immobilization of Soybean Peroxidase onto Magnetic Adsorbent. Int. J. Biol. Macromol. 2018, 114, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, H.; Ren, D.; Li, Q.; Zhang, S.; Feng, T. Effect of Direct-Current Electric Field on Enzymatic Activity and the Concentration of Laccase. Indian. J. Microbiol. 2015, 55, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Alshabib, M.; Onaizi, S.A. Enzymatic Remediation of Bisphenol A from Wastewaters: Effects of Biosurfactant, Anionic, Cationic, Nonionic, and Polymeric Additives. Water Air Soil. Pollut. 2020, 231, 428. [Google Scholar] [CrossRef]
- Alshabib, M.; Onaizi, S.A. Effects of Surface Active Additives on the Enzymatic Treatment of Phenol and Its Derivatives: A Mini Review. Curr. Pollut. Rep. 2019, 5, 52–65. [Google Scholar] [CrossRef]
- Sellami, K.; Couvert, A.; Nasrallah, N.; Maachi, R.; Tandjaoui, N.; Abouseoud, M.; Amrane, A. Bio-Based and Cost Effective Method for Phenolic Compounds Removal Using Cross-Linked Enzyme Aggregates. J. Hazard. Mater. 2021, 403, 124021. [Google Scholar] [CrossRef]
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A Short Review of Techniques for Phenol Removal from Wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef]
- Onaizi, S.A. Statistical Analyses of the Effect of Rhamnolipid Biosurfactant Addition on the Enzymatic Removal of Bisphenol A from Wastewater. Biocatal. Agric. Biotechnol. 2021, 32, 101929. [Google Scholar] [CrossRef]
- Onaizi, S.A. Enzymatic Treatment of Phenolic Wastewater: Effects of Salinity and Biosurfactant Addition. In Proceedings of the International Petroleum Technology Conference, Virtual, 23 March–1 April 2021. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kumari, S.; Rath, S.; Das, S. Prospects and Scope of Microbial Bioremediation for the Restoration of the Contaminated Sites. In Microbial Biodegradation and Bioremediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–31. [Google Scholar] [CrossRef]
- Kanaujiya, D.K.; Paul, T.; Sinharoy, A.; Pakshirajan, K. Biological Treatment Processes for the Removal of Organic Micropollutants from Wastewater: A Review. Curr. Pollut. Rep. 2019, 5, 112–128. [Google Scholar] [CrossRef]
- Ramirez, E.; de la Luz Asunción, M.; Rivalcoba, V.S.; Hernández, A.; Santos, C.V. Removal of Phenolic Compounds from Water by Adsorption and Photocatalysis. In Phenolic Compounds—Natural Sources, Importance and Applications; InTech: Rijeka, Romania, 2017. [Google Scholar] [CrossRef]
- Betancur-Ramírez, K.J.; Meneses-Jácome, A.; Ruiz-Colorado, A.A.; Gallego-Suárez, D. Life Cycle Assessment of an Alternative Enzymatic-Biological Treatment for Effluents from Industrial Processing of Potatoes. J. Clean. Prod. 2021, 324, 129151. [Google Scholar] [CrossRef]
- Sirisha, V.L.; Jain, A.; Jain, A. Enzyme Immobilization: An Overview on Methods, Support Material, and Applications of Immobilized Enzymes. Adv. Food Nutr. Res. 2016, 79, 179–211. [Google Scholar] [CrossRef] [PubMed]
- Lou, W.Y.; Fernández-Lucas, J.; Ge, J.; Wu, C. Enzyme or Whole Cell Immobilization for Efficient Biocatalysis: Focusing on Novel Supporting Platforms and Immobilization Techniques. Front. Bioeng. Biotechnol. 2021, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.S.; Vo, D.V.N.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P.R. A Review on Catalytic-Enzyme Degradation of Toxic Environmental Pollutants: Microbial Enzymes. J. Hazard. Mater. 2021, 419, 126451. [Google Scholar] [CrossRef]
- Xu, K.; Chen, X.; Zheng, R.; Zheng, Y. Immobilization of Multi-Enzymes on Support Materials for Efficient Biocatalysis. Front. Bioeng. Biotechnol. 2020, 8, 660. [Google Scholar] [CrossRef]
- Irfan, M.; Ghazanfar, M.; Ur Rehman, A.; Siddique, A. Strategies to Reuse Cellulase: Immobilization of Enzymes (Part II). In Approaches to Enhance Industrial Production of Fungal Cellulases; Springer: Berlin/Heidelberg, Germany, 2019; pp. 137–151. [Google Scholar] [CrossRef]
- Sepahvand, H.; Heravi, M.M.; Saber, M.; Hooshmand, S.E. Techniques and Support Materials for Enzyme Immobilization Using Ugi Multicomponent Reaction: An Overview. J. Iran. Chem. Soc. 2022, 19, 2115–2130. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Y.; Yang, S. Developing a Novel Strategy for Light-Triggered Reversible Enzyme Immobilization and Reuse of Support. Alex. Eng. J. 2022, 61, 6949–6957. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying Enzyme Activity and Selectivity by Immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Lanie, M.; Ab, H. Enzymatic Strategies for Asymmetric Synthesis. RSC Chem. Biol. 2021, 2, 958–989. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Dosseto, A.; Richardson, C.; Price, W.E.; Nghiem, L.D. Continuous Adsorption and Biotransformation of Micropollutants by Granular Activated Carbon-Bound Laccase in a Packed-Bed Enzyme Reactor. Bioresour. Technol. 2016, 210, 108–116. [Google Scholar] [CrossRef]
- Isanapong, J.; Lohawet, K.; Kumnorkaew, P. Optimization and Characterization of Immobilized Laccase on Titanium Dioxide Nanostructure and Its Application in Removal of Remazol Brilliant Blue R. Biocatal. Agric. Biotechnol. 2021, 37, 102186. [Google Scholar] [CrossRef]
- Ponmudi, K.; Cherian, A.R.; Varghese, A. Carbon Dots as an Effective Material in Enzyme Immobilization for Sensing Applications. In Carbon Dots in Analytical Chemistry Detection and Imaging; Elsevier: Amsterdam, The Netherlands, 2023; pp. 241–253. [Google Scholar] [CrossRef]
- Oliveira, F.L.; França, A.d.S.; de Castro, A.M.; Alves de Souza, R.O.M.; Esteves, P.M.; Gonçalves, R.S.B. Enzyme Immobilization in Covalent Organic Frameworks: Strategies and Applications in Biocatalysis. Chempluschem 2020, 85, 2051–2066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Zhang, F.; Yang, H.; Huang, X.; Liu, H.; Guo, S. Graphene Oxide as a Matrix for Enzyme Immobilization. Langmuir 2010, 26, 6083–6085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, Q.; Luo, Q.; Shi, L.; Meng, S. Laccase-Carbon Nanotube Nanocomposites for Enhancing Dyes Removal. J. Clean. Prod. 2020, 242, 118425. [Google Scholar] [CrossRef]
- Yuan, Y.; Cai, W.; Xu, J.; Cheng, J.; Du, K.S. Recyclable Laccase by Coprecipitation with Aciduric Cu-Based MOFs for Bisphenol A Degradation in an Aqueous Environment. Colloids Surf. B Biointerfaces 2021, 204, 111792. [Google Scholar] [CrossRef]
- Nawaz, A.F.; Zafar, S.; Fatim, S.L.; Shahzadi, K.; Fatima, Z.; Siddique, I. Use of Nanomaterials for the Immobilization of Industrially Important Enzymes. J. Nanotechnol. Res. 2020, 3, 45–57. [Google Scholar]
- Soares, A.M.B.F.; Gonçalves, L.M.O.; Ferreira, R.D.S.; de Souza, J.M.; Fangueiro, R.; Alves, M.M.M.; Carvalho, F.A.A.; Mendes, A.N.; Cantanhêde, W. Immobilization of Papain Enzyme on a Hybrid Support Containing Zinc Oxide Nanoparticles and Chitosan for Clinical Applications. Carbohydr. Polym. 2020, 243, 116498. [Google Scholar] [CrossRef]
- Ramakrishna, T.R.B.; Nalder, T.D.; Yang, W.; Marshall, S.N.; Barrow, C.J. Controlling Enzyme Function through Immobilisation on Graphene, Graphene Derivatives and Other Two Dimensional Nanomaterials. J. Mater. Chem. B 2018, 6, 3200–3218. [Google Scholar] [CrossRef]
- Wen, H.; Nallathambi, V.; Chakraborty, D.; Barton, S.C. Carbon Fiber Microelectrodes Modified with Carbon Nanotubes as a New Support for Immobilization of Glucose Oxidase. Microchim. Acta 2011, 175, 283–289. [Google Scholar] [CrossRef]
- Thakur, K.; Attri, C.; Seth, A. Nanocarriers-Based Immobilization of Enzymes for Industrial Application. 3 Biotech. 2021, 11, 427. [Google Scholar] [CrossRef]
- Li, S.F.; Zhai, X.J.; Zhang, C.; Mo, H.L.; Zang, S.Q. Enzyme Immobilization in Highly Ordered Macro–Microporous Metal–Organic Frameworks for Rapid Biodegradation of Hazardous Dyes. Inorg. Chem. Front. 2020, 7, 3146–3153. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.Y.; Lv, X.L.; Yan, T.H.; Zhou, H.C. Hierarchically Porous Metal–Organic Frameworks: Synthetic Strategies and Applications. Natl. Sci. Rev. 2020, 7, 1743–1758. [Google Scholar] [CrossRef] [PubMed]
- Guisan, J.M.; López-Gallego, F.; Bolivar, J.M.; Rocha-Martín, J.; Fernandez-Lorente, G. The Science of Enzyme Immobilization. Methods Mol. Biol. 2020, 2100, 1–26. [Google Scholar] [CrossRef]
- Khan, M.R. Immobilized Enzymes: A Comprehensive Review. Bull. Natl. Res. Cent. 2021, 45, 207. [Google Scholar] [CrossRef]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme Entrapment, Biocatalyst Immobilization without Covalent Attachment. Green. Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Brena, B.; González-Pombo, P.; Batista-Viera, F. Immobilization of Enzymes and Cells, 3rd ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 1051, pp. 15–31. [Google Scholar]
- Spahn, C.; Minteer, S.D. Enzyme Immobilization in Biotechnology. Recent. Pat. Eng. 2008, 2, 195–200. [Google Scholar] [CrossRef]
- Dey, G.; Nagpal, V.; Banerjee, R. Immobilization of Alpha-Amylase from Bacillus Circulans GRS 313 on Coconut Fiber. Appl. Biochem. Biotechnol. 2002, 102–103, 303–313. [Google Scholar] [CrossRef]
- Rosales-Hernández, M.; Kispert, L.; Torres-Ramírez, E.; Ramírez-Rosales, D.; Zamorano-Ulloa, R.; Trujillo-Ferrara, J. Electron Paramagnetic Resonance Analyses of Biotransformation Reactions with Cytochrome P-450 Immobilized on Mesoporous Molecular Sieves. Biotechnol. Lett. 2007, 29, 919–924. [Google Scholar] [CrossRef]
- Karagulyan, H.K.; Gasparyan, V.K.; Decker, S.R. Immobilization of Fungal Beta-Glucosidase on Silica Gel and Kaolin Carriers. Appl. Biochem. Biotechnol. 2008, 146, 39–47. [Google Scholar] [CrossRef]
- Brígida, A.I.S.; Calado, V.M.A.; Gonçalves, L.R.B.; Coelho, M.A.Z. Effect of Chemical Treatments on Properties of Green Coconut Fiber. Carbohydr. Polym. 2010, 79, 832–838. [Google Scholar] [CrossRef]
- Huang, X.J.; Chen, P.C.; Huang, F.; Ou, Y.; Chen, M.R.; Xu, Z.K. Immobilization of Candida Rugosa Lipase on Electrospun Cellulose Nanofiber Membrane. J. Mol. Catal. B Enzym. 2011, 70, 95–100. [Google Scholar] [CrossRef]
- Mitchell, S.; Pérez-Ramírez, J. Mesoporous Zeolites as Enzyme Carriers: Synthesis, Characterization, and Application in Biocatalysis. Catal. Today 2011, 168, 28–37. [Google Scholar] [CrossRef]
- Díaz, J.F.; Balkus, K.J. Enzyme Immobilization in MCM-41 Molecular Sieve. J. Mol. Catal. B Enzym. 1996, 2, 115–126. [Google Scholar] [CrossRef]
- Persson, M.; Wehtje, E.; Adlercreutz, P. Immobilisation of Lipases by Adsorption and Deposition: High Protein Loading Gives Lower Water Activity Optimum. Biotechnol. Lett. 2000, 22, 1571–1575. [Google Scholar] [CrossRef]
- Sabbani, S.; Hedenström, E.; Nordin, O. The Enantioselectivity of Candida Rugosa Lipase Is Influenced by the Particle Size of the Immobilising Support Material Accurel. J. Mol. Catal. B Enzym. 2006, 42, 1–9. [Google Scholar] [CrossRef]
- Cunha, A.G.; Fernández-Lorente, G.; Bevilaqua, J.V.; Destain, J.; Paiva, L.M.C.; Freire, D.M.G.; Fernández-Lafuente, R.; Guisán, J.M. Immobilization of Yarrowia Lipolytica Lipase—A Comparison of Stability of Physical Adsorption and Covalent Attachment Techniques. Appl. Biochem. Biotechnol. 2008, 146, 49–56. [Google Scholar] [CrossRef]
- Cabrera-Padilla, R.Y.; Lisboa, M.C.; Fricks, A.T.; Franceschi, E.; Lima, A.S.; Silva, D.P.; Soares, C.M.F. Immobilization of Candida Rugosa Lipase on Poly(3-hydroxybutyrate-co-hydroxyvalerate): A New Eco-Friendly Support. J. Ind. Microbiol. Biotechnol. 2012, 39, 289–298. [Google Scholar] [CrossRef]
- Mishra, N.; Pithawala, K.; Bahadur, A. Byssus Thread: A Novel Support Material for Urease Immobilization. Appl. Biochem. Biotechnol. 2011, 165, 1568–1576. [Google Scholar] [CrossRef]
- Popat, A.; Hartono, S.B.; Stahr, F.; Liu, J.; Qiao, S.Z.; Lu, G.Q. Mesoporous Silica Nanoparticles for Bioadsorption, Enzyme Immobilisation, and Delivery Carriers. Nanoscale 2011, 3, 2801–2818. [Google Scholar] [CrossRef]
- Magner, E. Immobilisation of Enzymes on Mesoporous Silicate Materials. Chem. Soc. Rev. 2013, 42, 6213–6222. [Google Scholar] [CrossRef]
- Zucca, P.; Sanjust, E. Inorganic Materials as Supports for Covalent Enzyme Immobilization: Methods and Mechanisms. Molecules 2014, 19, 14139–14194. [Google Scholar] [CrossRef] [PubMed]
- Azodi, M.; Falamaki, C.; Mohsenifar, A. Sucrose Hydrolysis by Invertase Immobilized on Functionalized Porous Silicon. J. Mol. Catal. B Enzym. 2011, 69, 154–160. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme Immobilization: An Update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding Enzyme Immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Betancor, L.; Luckarift, H.R. Bioinspired Enzyme Encapsulation for Biocatalysis. Trends Biotechnol. 2008, 26, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Kurzbaum, E.; Raizner, Y.; Kuc, M.E.; Kulikov, A.; Hakimi, B.; Kruh, L.I.; Menashe, O. Phenol Biodegradation by Bacterial Cultures Encapsulated in 3D Microfiltration-Membrane Capsules. Environ. Technol. 2019, 41, 2875–2883. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.S. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
- Song, J.; He, W.; Shen, H.; Zhou, Z.; Li, M.; Su, P.; Yang, Y. Construction of Multiple Enzyme Metal–Organic Frameworks Biocatalyst via DNA Scaffold: A Promising Strategy for Enzyme Encapsulation. Chem. Eng. J. 2019, 363, 174–182. [Google Scholar] [CrossRef]
- Tran, D.N.; Balkus, K.J. Perspective of Recent Progress in Immobilization of Enzymes. ACS Catal. 2011, 1, 956–968. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme Immobilization: An Overview on Techniques and Support Materials. 3 Biotech. 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Hartmann, M.; Jung, D. Biocatalysis with Enzymes Immobilized on Mesoporous Hosts: The Status Quo and Future Trends. J. Mater. Chem. 2010, 20, 844–857. [Google Scholar] [CrossRef]
- Lôpez-Serrano, P.; Cao, L.; Van Rantwijk, F.; Sheldon, R.A. Cross-Linked Enzyme Aggregates with Enhanced Activity: Application to Lipases. Biotechnol. Lett. 2002, 24, 1379–1383. [Google Scholar] [CrossRef]
- Aytar, B.S.; Bakir, U. Preparation of Cross-Linked Tyrosinase Aggregates. Process Biochem. 2008, 43, 125–131. [Google Scholar] [CrossRef]
- Roessl, U.; Nahálka, J.; Nidetzky, B. Carrier-Free Immobilized Enzymes for Biocatalysis. Biotechnol. Lett. 2010, 32, 341–350. [Google Scholar] [CrossRef]
- Singh, B.D. Biotechnology Expanding Horizons, 4th ed.; Kalyani Publishers: Delhi, India, 2012; ISBN 9789327222982. [Google Scholar]
- Shen, Q.; Yang, R.; Hua, X.; Ye, F.; Zhang, W.; Zhao, W. Gelatin-Templated Biomimetic Calcification for β-Galactosidase Immobilization. Process Biochem. 2011, 46, 1565–1571. [Google Scholar] [CrossRef]
- Ispas, C.; Sokolov, I.; Andreescu, S. Enzyme-Functionalized Mesoporous Silica for Bioanalytical Applications. Anal. Bioanal. Chem. 2009, 393, 543–554. [Google Scholar] [CrossRef]
- Chen, G.C.; Kuan, I.C.; Hong, J.R.; Tsai, B.H.; Lee, S.L.; Yu, C.Y. Activity Enhancement and Stabilization of Lipase from Pseudomonas Cepacia in Polyallylamine-Mediated Biomimetic Silica. Biotechnol. Lett. 2011, 33, 525–529. [Google Scholar] [CrossRef]
- Tümtürk, H.; Karaca, N.; Demirel, G.; Şahin, F. Preparation and Application of Poly(N,N-Dimethylacrylamide-Co-Acrylamide) and Poly(N-Isopropylacrylamide-Co-Acrylamide)/Kappa-Carrageenan Hydrogels for Immobilization of Lipase. Int. J. Biol. Macromol. 2007, 40, 281–285. [Google Scholar] [CrossRef]
- Jegannathan, K.R.; Jun-Yee, L.; Chan, E.S.; Ravindra, P. Production of Biodiesel from Palm Oil Using Liquid Core Lipase Encapsulated in κ-Carrageenan. Fuel 2010, 89, 2272–2277. [Google Scholar] [CrossRef]
- Brena, B.; González-Pombo, P.; Batista-Viera, F. Immobilization of Enzymes: A Literature Survey. Methods Mol. Biol. 2013, 1051, 15–31. [Google Scholar] [CrossRef]
- Patil, J.S.; Kamalapur, M.V.; Marapur, S.C.; Kadam, D.V. Ionotropic gelation and polyelectrolyte complexation: The novel techniques to design hydrogel particulate sustained, modulated drug delivery system: A review. Dig. J. Nanomater. Biostruct. 2010, 5, 241–248. [Google Scholar]
- Rother, C.; Nidetzky, B. Enzyme Immobilization by Microencapsulation: Methods, Materials, and Technological Applications. In Encyclopedia of Industrial Biotechnology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 1–21. [Google Scholar] [CrossRef]
- Honda, T.; Miyazaki, M.; Nakamura, H.; Maeda, H. Immobilization of Enzymes on a Microchannel Surface through Cross-Linking Polymerization. Chem. Commun. 2005, 40, 5062–5064. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Reinhold, J.; Woodbury, N.W. Peptide-Modified Surfaces for Enzyme Immobilization. PLoS ONE 2011, 6, e18692. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.J.; Liu, P.C.; Liao, W.J. Immobilization of Invertase via Carbohydrate Moiety on Chitosan to Enhance Its Thermal Stability. Biotechnol. Lett. 2000, 22, 1459–1464. [Google Scholar] [CrossRef]
- Szymańska, K.; Bryjak, J.; Jarzębski, A.B. Immobilization of Invertase on Mesoporous Silicas to Obtain Hyper Active Biocatalysts. Top. Catal. 2009, 52, 1030–1036. [Google Scholar] [CrossRef]
- Kim, J.; Jia, H.; Wang, P. Challenges in Biocatalysis for Enzyme-Based Biofuel Cells. Biotechnol. Adv. 2006, 24, 296–308. [Google Scholar] [CrossRef]
- Huang, X.J.; Yu, A.G.; Xu, Z.K. Covalent Immobilization of Lipase from Candida Rugosa onto Poly(acrylonitrile-co-2-hydroxyethyl methacrylate) Electrospun Fibrous Membranes for Potential Bioreactor Application. Bioresour. Technol. 2008, 99, 5459–5465. [Google Scholar] [CrossRef]
- Sakai, S.; Liu, Y.; Yamaguchi, T.; Watanabe, R.; Kawabe, M.; Kawakami, K. Immobilization of Pseudomonas Cepacia Lipase onto Electrospun Polyacrylonitrile Fibers through Physical Adsorption and Application to Transesterification in Nonaqueous Solvent. Biotechnol. Lett. 2010, 32, 1059–1062. [Google Scholar] [CrossRef]
- Wu, L.; Yuan, X.; Sheng, J. Immobilization of Cellulase in Nanofibrous PVA Membranes by Electrospinning. J. Memb. Sci. 2005, 250, 167–173. [Google Scholar] [CrossRef]
- Ren, G.; Xu, X.; Liu, Q.; Cheng, J.; Yuan, X.; Wu, L.; Wan, Y. Electrospun Poly(vinyl Alcohol)/Glucose Oxidase Biocomposite Membranes for Biosensor Applications. React. Funct. Polym. 2006, 66, 1559–1564. [Google Scholar] [CrossRef]
- Hilal, N.; Kochkodan, V.; Nigmatullin, R.; Goncharuk, V.; Al-Khatib, L. Lipase-Immobilized Biocatalytic Membranes for Enzymatic Esterification: Comparison of Various Approaches to Membrane Preparation. J. Memb. Sci. 2006, 268, 198–207. [Google Scholar] [CrossRef]
- Sheldon, R.A. Characteristic Features and Biotechnological Applications of Cross-Linked Enzyme Aggregates (CLEAs). Appl. Microbiol. Biotechnol. 2011, 92, 467. [Google Scholar] [CrossRef] [PubMed]
- Yusdy; Patel, S.R.; Yap, M.G.S.; Wang, D.I.C. Immobilization of L-Lactate Dehydrogenase on Magnetic Nanoclusters for Chiral Synthesis of Pharmaceutical Compounds. Biochem. Eng. J. 2009, 48, 13–21. [Google Scholar] [CrossRef]
- Wang, A.; Wang, H.; Zhu, S.; Zhou, C.; Du, Z.; Shen, S. An Efficient Immobilizing Technique of Penicillin Acylase with Combining Mesocellular Silica Foams Support and P-Benzoquinone Cross Linker. Bioprocess. Biosyst. Eng. 2008, 31, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Kennel, S.J.; Oden, P.I.; Jacobson, K.B.; Woodward, J.; Doktycz, M.J. Comparison of Techniques for Enzyme Immobilization on Silicon Supports. Enzym. Microb. Technol. 1999, 24, 26–34. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-Linked Enzyme Aggregates (CLEAs): Stable and Recyclable Biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef]
- Górecka, E.; Jastrzębska, M. Immobilization Techniques and Biopolymer Carriers. Biotechnol. Food Sci. 2011, 75, 65–86. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in Aqueous Solution, Reaction with Proteins, and Application to Enzyme Crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef]
- Öztürk, B. Immobilization of Lipase from Candida Rugosa on Hydrophobic and Hydrophilic Supports; Izmir Institute of Technology: Urla, Türkiye, 2001. [Google Scholar]
- Lee, C.K.; Au-Duong, A.N. Enzyme Immobilization on Nanoparticles: Recent Applications. Emerg. Areas Bioeng. 2017, 1, 67–80. [Google Scholar] [CrossRef]
- Sharifi, M.; Sohrabi, M.J.; Hosseinali, S.H.; Hasan, A.; Kani, P.H.; Talaei, A.J.; Karim, A.Y.; Nanakali, N.M.Q.; Salihi, A.; Aziz, F.M.; et al. Enzyme Immobilization onto the Nanomaterials: Application in Enzyme Stability and Prodrug-Activated Cancer Therapy. Int. J. Biol. Macromol. 2020, 143, 665–676. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, M.; Park, H.S.; Jang, A.; Min, J.; Kim, Y.H. Immobilization of Lysozyme-CLEA onto Electrospun Chitosan Nanofiber for Effective Antibacterial Applications. Int. J. Biol. Macromol. 2013, 54, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Sardar, M.; Roy, I.; Gupta, M.N. Simultaneous Purification and Immobilization of Aspergillus Niger Xylanase on the Reversibly Soluble Polymer Eudragit(TM) L-100. Enzym. Microb. Technol. 2000, 27, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.F.; Li, S.Y.; Lin, S.C.; Hsu, W.H. Integrated Enzyme Purification and Immobilization Processes with Immobilized Metal Affinity Adsorbents. Process Biochem. 2004, 39, 1573–1581. [Google Scholar] [CrossRef]
- Shi, Q.H.; Tian, Y.; Dong, X.Y.; Bai, S.; Sun, Y. Chitosan-Coated Silica Beads as Immobilized Metal Affinity Support for Protein Adsorption. Biochem. Eng. J. 2003, 16, 317–322. [Google Scholar] [CrossRef]
- Sardar, M.; Gupta, M.N. Immobilization of Tomato Pectinase on Con A–Seralose 4B by Bioaffinity Layering. Enzym. Microb. Technol. 2005, 37, 355–359. [Google Scholar] [CrossRef]
- Haider, T.; Husain, Q. Concanavalin A Layered Calcium Alginate-Starch Beads Immobilized Beta Galactosidase as a Therapeutic Agent for Lactose Intolerant Patients. Int. J. Pharm. 2008, 359, 1–6. [Google Scholar] [CrossRef]
- Faruque, M.A.A.; Syduzzaman, M.; Sarkar, J.; Bilisik, K.; Naebe, M. A Review on the Production Methods and Applications of Graphene-Based Materials. Nanomaterials 2021, 11, 2414. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical Vapour Deposition. Nat. Rev. Methods Primers 2021, 1, 5. [Google Scholar] [CrossRef]
- Devi, M.; Rawat, S. A Comprehensive Review of the Pyrolysis Process: From Carbon Nanomaterial Synthesis to Waste Treatment. Oxf. Open Mater. Sci. 2020, 1, itab014. [Google Scholar] [CrossRef]
- Drogowska-Horná, K.; Frank, O.; Kalbac, M. Chemical Vapor Deposition (CVD) Growth of Graphene Films. In Graphene: Properties, Preparation, Characterization and Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 199–222. [Google Scholar] [CrossRef]
- Chen, X.; Qu, Z.; Liu, Z.; Ren, G. Mechanism of Oxidization of Graphite to Graphene Oxide by the Hummers Method. ACS Omega 2022, 7, 23503–23510. [Google Scholar] [CrossRef]
- Chen, C.H.; Hu, S.; Shih, J.F.; Yang, C.Y.; Luo, Y.W.; Jhang, R.H.; Chiang, C.M.; Hung, Y.J. Effective Synthesis of Highly Oxidized Graphene Oxide That Enables Wafer-Scale Nanopatterning: Preformed Acidic Oxidizing Medium Approach. Sci. Rep. 2017, 7, 3908. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, R.; Shafiekhani, A. Ultrasonic Waves and Temperature Effects on Graphene Structure Fabricated by Electrochemical Exfoliation Method. Mater. Chem. Phys. 2018, 212, 95–102. [Google Scholar] [CrossRef]
- Hong, G.; Han, Y.; Schutzius, T.M.; Wang, Y.; Pan, Y.; Hu, M.; Jie, J.; Sharma, C.S.; Müller, U.; Poulikakos, D. On the Mechanism of Hydrophilicity of Graphene. Nano Lett. 2016, 16, 4447–4453. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.E.F.; Braga, G.B.; Tarley, C.R.T.; Pereira, A.C. Thermally Reduced Graphene Oxide: Synthesis, Studies and Characterization. J. Mater. Sci. 2018, 53, 12005–12015. [Google Scholar] [CrossRef]
- Some, S.; Kim, Y.; Yoon, Y.; Yoo, H.; Lee, S.; Park, Y.; Lee, H. High-Quality Reduced Graphene Oxide by a Dual-Function Chemical Reduction and Healing Process. Sci. Rep. 2013, 3, 1929. [Google Scholar] [CrossRef]
- Hidayat, R.; Wahyuningsih, S.; Ramelan, A.H. Simple Synthesis of RGO (Reduced Graphene Oxide) by Thermal Reduction of GO (Graphene Oxide). IOP Conf. Ser. Mater. Sci. Eng. 2020, 858, 012009. [Google Scholar] [CrossRef]
- Guex, L.G.; Sacchi, B.; Peuvot, K.F.; Andersson, R.L.; Pourrahimi, A.M.; Ström, V.; Farris, S.; Olsson, R.T. Experimental Review: Chemical Reduction of Graphene Oxide (GO) to Reduced Graphene Oxide (RGO) by Aqueous Chemistry. Nanoscale 2017, 9, 9562–9571. [Google Scholar] [CrossRef]
- Lesiak, B.; Trykowski, G.; Tóth, J.; Biniak, S.; Kövér, L.; Rangam, N.; Stobinski, L.; Malolepszy, A. Chemical and Structural Properties of Reduced Graphene Oxide—Dependence on the Reducing Agent. J. Mater. Sci. 2021, 56, 3738–3754. [Google Scholar] [CrossRef]
- Du, W.; Geng, H.; Yang, Y.; Zhang, Y.; Rui, X.; Li, C.C. Pristine Graphene for Advanced Electrochemical Energy Applications. J. Power Sources 2019, 437, 226899. [Google Scholar] [CrossRef]
- Balci, E.; Yavas, B.; Goller, G. Investigation of the Effects of Varying Amount of Graphene Nanoplatelets’ (GNPs) Addition on Carbon Nanotubes (CNTs) Reinforced Boron Carbide Produced by Spark Plasma Sintering. J. Aust. Ceram. Soc. 2021, 57, 1435–1444. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Zhang, J. Interactions of Graphene and Graphene Oxide with Proteins and Peptides. Nanotechnol. Rev. 2013, 2, 27–45. [Google Scholar] [CrossRef]
- Al-Qadri, A.A.Q.; Drmosh, Q.A.; Onaizi, S.A. Enhancement of Bisphenol a Removal from Wastewater via the Covalent Functionalization of Graphene Oxide with Short Amine Molecules. Case Stud. Chem. Environ. Eng. 2022, 6, 100233. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, S.; Alvarez, P.J.J.; Chen, W. Reduced Graphene Oxide Enhances Horseradish Peroxidase Stability by Serving as Radical Scavenger and Redox Mediator. Carbon 2015, 94, 531–538. [Google Scholar] [CrossRef]
- Pavlidis, I.V.; Vorhaben, T.; Tsoufis, T.; Rudolf, P.; Bornscheuer, U.T.; Gournis, D.; Stamatis, H. Development of Effective Nanobiocatalytic Systems through the Immobilization of Hydrolases on Functionalized Carbon-Based Nanomaterials. Bioresour. Technol. 2012, 115, 164–171. [Google Scholar] [CrossRef]
- Tseng, C.W.; Liao, C.Y.; Sun, Y.; Peng, C.C.; Tzen, J.T.C.; Guo, R.T.; Liu, J.R. Immobilization of Clostridium Cellulolyticum D-Psicose 3-Epimerase on Artificial Oil Bodies. J. Agric. Food Chem. 2014, 62, 6771–6776. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, B.; Hwang, S.J.; Lee, J.U.; Cheong, H.; Kwon, O.S.; Shin, K.; Hur, N.H. Large Scale Production of Highly Conductive Reduced Graphene Oxide Sheets by a Solvent-Free Low Temperature Reduction. Carbon 2014, 69, 327–335. [Google Scholar] [CrossRef]
- Li, W.; Wen, H.; Shi, Q.; Zheng, G. Study on Immobilization of (+) γ-Lactamase Using a New Type of Epoxy Graphene Oxide Carrier. Process Biochem. 2016, 51, 270–276. [Google Scholar] [CrossRef]
- Hernández-Cancel, G.; Suazo-Dávila, D.; Ojeda-Cruzado, A.J.; García-Torres, D.; Cabrera, C.R.; Griebenow, K. Graphene Oxide as a Protein Matrix: Influence on Protein Biophysical Properties. J. Nanobiotechnol. 2015, 13, 70. [Google Scholar] [CrossRef]
- Besharati Vineh, M.; Saboury, A.A.; Poostchi, A.A.; Rashidi, A.M.; Parivar, K. Stability and Activity Improvement of Horseradish Peroxidase by Covalent Immobilization on Functionalized Reduced Graphene Oxide and Biodegradation of High Phenol Concentration. Int. J. Biol. Macromol. 2018, 106, 1314–1322. [Google Scholar] [CrossRef]
- Dedania, S.R.; Patel, M.J.; Patel, D.M.; Akhani, R.C.; Patel, D.H. Immobilization on Graphene Oxide Improves the Thermal Stability and Bioconversion Efficiency of D-Psicose 3-Epimerase for Rare Sugar Production. Enzym. Microb. Technol. 2017, 107, 49–56. [Google Scholar] [CrossRef]
- Johnson, B.J.; Russ Algar, W.; Malanoski, A.P.; Ancona, M.G.; Medintz, I.L. Understanding Enzymatic Acceleration at Nanoparticle Interfaces: Approaches and Challenges. Nano Today 2014, 9, 102–131. [Google Scholar] [CrossRef]
- Ding, S.; Cargill, A.A.; Medintz, I.L.; Claussen, J.C. Increasing the Activity of Immobilized Enzymes with Nanoparticle Conjugation. Curr. Opin. Biotechnol. 2015, 34, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, I.V.; Vorhaben, T.; Gournis, D.; Papadopoulos, G.K.; Bornscheuer, U.T.; Stamatis, H. Regulation of Catalytic Behaviour of Hydrolases through Interactions with Functionalized Carbon-Based Nanomaterials. J. Nanopart. Res. 2012, 14, 842. [Google Scholar] [CrossRef]
- Jin, L.; Yang, K.; Yao, K.; Zhang, S.; Tao, H.; Lee, S.T.; Liu, Z.; Peng, R. Functionalized Graphene Oxide in Enzyme Engineering: A Selective Modulator for Enzyme Activity and Thermostability. ACS Nano 2012, 6, 4864–4875. [Google Scholar] [CrossRef]
- Wei, X.L.; Ge, Z.Q. Effect of Graphene Oxide on Conformation and Activity of Catalase. Carbon 2013, 60, 401–409. [Google Scholar] [CrossRef]
- Zhang, F.; Zheng, B.; Zhang, J.; Huang, X.; Liu, H.; Guo, S.; Zhang, J. Horseradish Peroxidase Immobilized on Graphene Oxide: Physical Properties and Applications in Phenolic Compound Removal. J. Phys. Chem. C 2010, 114, 8469–8473. [Google Scholar] [CrossRef]
- Chang, Q.; Jiang, G.; Tang, H.; Li, N.; Huang, J.; Wu, L. Enzymatic Removal of Chlorophenols Using Horseradish Peroxidase Immobilized on Superparamagnetic Fe3O4/Graphene Oxide Nanocomposite. Chin. J. Catal. 2015, 36, 961–968. [Google Scholar] [CrossRef]
- Xu, H.M.; Sun, X.F.; Wang, S.Y.; Song, C.; Wang, S.G. Development of Laccase/Graphene Oxide Membrane for Enhanced Synthetic Dyes Separation and Degradation. Sep. Purif. Technol. 2018, 204, 255–260. [Google Scholar] [CrossRef]
- Wang, X.; Hou, C.; Qiu, W.; Ke, Y.; Xu, Q.; Liu, X.Y.; Lin, Y. Protein-Directed Synthesis of Bifunctional Adsorbent-Catalytic Hemin-Graphene Nanosheets for Highly Efficient Removal of Dye Pollutants via Synergistic Adsorption and Degradation. ACS Appl. Mater. Interfaces 2017, 9, 684–692. [Google Scholar] [CrossRef]
- Begum, R.; Najeeb, J.; Sattar, A.; Naseem, K.; Irfan, A.; Al-Sehemi, A.G.; Farooqi, Z.H. Chemical Reduction of Methylene Blue in the Presence of Nanocatalysts: A Critical Review. Rev. Chem. Eng. 2020, 36, 749–770. [Google Scholar] [CrossRef]
- Kumar Sahoo, P.; Panigrahy, B.; Thakur, D.; Bahadur, D. Ice-Templating Synthesis of Macroporous Noble Metal/3D-Graphene Nanocomposites: Their Fluorescence Lifetimes and Catalytic Study. New J. Chem. 2017, 41, 7861–7869. [Google Scholar] [CrossRef]
- Patila, M.; Kouloumpis, A.; Gournis, D.; Rudolf, P.; Stamatis, H. Laccase-Functionalized Graphene Oxide Assemblies as Efficient Nanobiocatalysts for Oxidation Reactions. Sensors 2016, 16, 287. [Google Scholar] [CrossRef]
- Kashefi, S.; Borghei, S.M.; Mahmoodi, N.M. Covalently Immobilized Laccase onto Graphene Oxide Nanosheets: Preparation, Characterization, and Biodegradation of Azo Dyes in Colored Wastewater. J. Mol. Liq. 2019, 276, 153–162. [Google Scholar] [CrossRef]
- Ariaeenejad, S.; Motamedi, E.; Hosseini Salekdeh, G. Application of the Immobilized Enzyme on Magnetic Graphene Oxide Nano-Carrier as a Versatile Bi-Functional Tool for Efficient Removal of Dye from Water. Bioresour. Technol. 2021, 319, 124228. [Google Scholar] [CrossRef]
- Vineh, M.B.; Saboury, A.A.; Poostchi, A.A.; Ghasemi, A. Biodegradation of Phenol and Dyes with Horseradish Peroxidase Covalently Immobilized on Functionalized RGO-SiO2 Nanocomposite. Int. J. Biol. Macromol. 2020, 164, 4403–4414. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Husain, Q.; Sultana, S.; Ahmad, M. Immobilization of Peroxidase on Polypyrrole-Cellulose-Graphene Oxide Nanocomposite via Non-Covalent Interactions for the Degradation of Reactive Blue 4 Dye. Chemosphere 2018, 202, 198–207. [Google Scholar] [CrossRef]
- Yao, L.W.; Ahmed Khan, F.S.; Mubarak, N.M.; Karri, R.R.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Mazari, S.A.; Ahmad, A.; Dehghani, M.H. Insight into Immobilization Efficiency of Lipase Enzyme as a Biocatalyst on the Graphene Oxide for Adsorption of Azo Dyes from Industrial Wastewater Effluent. J. Mol. Liq. 2022, 354, 118849. [Google Scholar] [CrossRef]
- Yang, S.; Yang, J.; Wang, T.; Li, L.; Yu, S.; Jia, R.; Chen, P. Construction of a Combined Enzyme System of Graphene Oxide and Manganese Peroxidase for Efficient Oxidation of Aromatic Compounds. Nanoscale 2020, 12, 7976–7985. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Saffar-Dastgerdi, M.H. Clean Laccase Immobilized Nanobiocatalysts (Graphene Oxide—Zeolite Nanocomposites): From Production to Detailed Biocatalytic Degradation of Organic Pollutant. Appl. Catal. B 2020, 268, 118443. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Z.; Luo, X.; Liang, W.; Li, S.; He, J.; Zhang, W.; Hao, T.; Yang, Z. Biomimetic Dynamic Membrane (BDM): Fabrication Method and Roles of Carriers and Laccase. Chemosphere 2020, 240, 124882. [Google Scholar] [CrossRef]
- Lai, Y.; Wang, F.; Zhang, Y.; Ou, P.; Wu, P.; Fang, Q.; Li, S.; Chen, Z. Effective Removal of Methylene Blue and Orange II by Subsequent Immobilized Laccase Decolorization on Crosslinked Polymethacrylate/Carbon Nanotubes. Mater. Res. Express 2019, 6, 085541. [Google Scholar] [CrossRef]
- Oliveira, S.F.; da Luz, J.M.R.; Kasuya, M.C.M.; Ladeira, L.O.; Correa Junior, A. Enzymatic Extract Containing Lignin Peroxidase Immobilized on Carbon Nanotubes: Potential Biocatalyst in Dye Decolourization. Saudi J. Biol. Sci. 2018, 25, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Habimana, P.; Gao, J.; Mwizerwa, J.P.; Ndayambaje, J.B.; Liu, H.; Luan, P.; Ma, L.; Jiang, Y. Improvement of Laccase Activity Via Covalent Immobilization over Mesoporous Silica Coated Magnetic Multiwalled Carbon Nanotubes for the Discoloration of Synthetic Dyes. ACS Omega 2021, 6, 2777–2789. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.M.; González-Domínguez, E.; Sanromán, Á.; Correa-Duarte, M.; Moldes, D. Immobilization of Laccase on Functionalized Multiwalled Carbon Nanotube Membranes and Application for Dye Decolorization. RSC Adv. 2016, 6, 114690–114697. [Google Scholar] [CrossRef]
- Jiang, S.; Ren, D.; Wang, Z.; Zhang, S.; Zhang, X.; Chen, W. Improved Stability and Promoted Activity of Laccase by One-Pot Encapsulation with Cu (PABA) Nanoarchitectonics and Its Application for Removal of Azo Dyes. Ecotoxicol. Environ. Saf. 2022, 234, 113366. [Google Scholar] [CrossRef]
- Birhanlı, E.; Noma, S.A.A.; Boran, F.; Ulu, A.; Yeşilada, Ö.; Ateş, B. Design of Laccase–Metal–Organic Framework Hybrid Constructs for Biocatalytic Removal of Textile Dyes. Chemosphere 2022, 292, 133382. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Ng, D.H.L.; Yang, P.; Yang, W.; Liu, Y. Micromotor-Assisted Highly Efficient Fenton Catalysis by a Laccase/Fe-BTC-NiFe2O4 Nanozyme Hybrid with a 3D Hierarchical Structure. Environ. Sci. Nano 2020, 7, 2573–2583. [Google Scholar] [CrossRef]
- Ladole, M.R.; Pokale, P.B.; Patil, S.S.; Belokar, P.G.; Pandit, A.B. Laccase Immobilized Peroxidase Mimicking Magnetic Metal Organic Frameworks for Industrial Dye Degradation. Bioresour. Technol. 2020, 317, 124035. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, P.; Muhammad, Y.; Tang, Y.; Shao, S.; Gao, Z.; Wang, J.; Wang, R.; Hu, Y.; Kuang, L.; et al. High-Density Immobilization of Laccase on Hollow Nano-Sphere NH2-MIL88(Fe) Host with Interfacial Defects to Improve Enzyme Activity and Stability for Remazol Brilliant Blue R Decolorization. Chem. Eng. J. 2021, 405, 127003. [Google Scholar] [CrossRef]
- Wang, J.; Yu, S.; Feng, F.; Lu, L. Simultaneous Purification and Immobilization of Laccase on Magnetic Zeolitic Imidazolate Frameworks: Recyclable Biocatalysts with Enhanced Stability for Dye Decolorization. Biochem. Eng. J. 2019, 150, 107285. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J. Metal-Organic Framework as a Platform of the Enzyme to Prepare Novel Environmentally Friendly Nanobiocatalyst for Degrading Pollutant in Water. J. Ind. Eng. Chem. 2019, 80, 606–613. [Google Scholar] [CrossRef]
- Amari, A.; Alzahrani, F.M.; Alsaiari, N.S.; Katubi, K.M.; Rebah, F.B.; Tahoon, M.A. Magnetic Metal Organic Framework Immobilized Laccase for Wastewater Decolorization. Processes 2021, 9, 774. [Google Scholar] [CrossRef]
- Chang, Q.; Huang, J.; Ding, Y.; Tang, H. Catalytic Oxidation of Phenol and 2,4-Dichlorophenol by Using Horseradish Peroxidase Immobilized on Graphene Oxide/Fe3O4. Molecules 2016, 21, 1044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xue, C.; Owens, G.; Chen, Z. Preparation of Bionanomaterial Based on Green Reduced Graphene Immobilized Ochrobactrum Sp. FJ1: Optimization, Characterization and Its Application. Sep. Purif. Technol. 2023, 310, 123144. [Google Scholar] [CrossRef]
- Costa, J.B.; Lima, M.J.; Sampaio, M.J.; Neves, M.C.; Faria, J.L.; Morales-Torres, S.; Tavares, A.P.M.; Silva, C.G. Enhanced Biocatalytic Sustainability of Laccase by Immobilization on Functionalized Carbon Nanotubes/Polysulfone Membranes. Chem. Eng. J. 2019, 355, 974–985. [Google Scholar] [CrossRef]
- Dai, Y.; Yao, J.; Song, Y.; Wang, S.; Yuan, Y. Enhanced Adsorption and Degradation of Phenolic Pollutants in Water by Carbon Nanotube Modified Laccase-Carrying Electrospun Fibrous Membranes. Environ. Sci. Nano 2016, 3, 857–868. [Google Scholar] [CrossRef]
- Ren, Z.; Luo, J.; Wan, Y. Highly Permeable Biocatalytic Membrane Prepared by 3D Modification: Metal-Organic Frameworks Ameliorate Its Stability for Micropollutants Removal. Chem. Eng. J. 2018, 348, 389–398. [Google Scholar] [CrossRef]
- Molina, M.A.; Díez-Jaén, J.; Sánchez-Sánchez, M.; Blanco, R.M. One-Pot Laccase@MOF Biocatalysts Efficiently Remove Bisphenol A from Water. Catal. Today 2022, 390–391, 265–271. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, L.; Han, J.; Wu, J.; Li, C.; Ni, L.; Wang, Y. Improving Laccase Activity and Stability by HKUST-1 with Cofactor via One-Pot Encapsulation and Its Application for Degradation of Bisphenol A. J. Hazard. Mater. 2020, 383, 121130. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New Synthetic Routes towards MOF Production at Scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef]
- Li, G.; Pang, S.; Wu, Y.; Ouyang, J. Enhanced Removal of Hydroquinone by Graphene Aerogel-Zr-MOF with Immobilized Laccase. Chem. Eng. Commun. 2018, 205, 698–705. [Google Scholar] [CrossRef]
- Li, D.; Cheng, Y.; Zuo, H.; Zhang, W.; Pan, G.; Fu, Y.; Wei, Q. Dual-Functional Biocatalytic Membrane Containing Laccase-Embedded Metal-Organic Frameworks for Detection and Degradation of Phenolic Pollutant. J. Colloid Interface Sci. 2021, 603, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.; Li, Y.; Huang, Q.; Yang, Z.; Wei, A.; Hu, Q. Laccase Immobilization on Amino-Functionalized Magnetic Metal Organic Framework for Phenolic Compound Removal. Chemosphere 2019, 233, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lou, J.; Yuan, J.; Xu, J.; Zhu, R.; Wang, Q.; Fan, X. Laccase Immobilization on Core-Shell Magnetic Metal-Organic Framework Microspheres for Alkylphenol Ethoxylate Compound Removal. J. Environ. Chem. Eng. 2021, 9, 105000. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y.; Han, T.; Wu, H.; Guo, S.; Zhang, J. Composite of Graphene Quantum Dots and Fe3O4 Nanoparticles: Peroxidase Activity and Application in Phenolic Compound Removal. RSC Adv. 2013, 4, 3299–3305. [Google Scholar] [CrossRef]
- Lim, X.X.; Low, S.C.; Oh, W. Da A Critical Review of Heterogeneous Catalyst Design for Carbon Nanotubes Synthesis: Functionalities, Performances, and Prospects. Fuel Process. Technol. 2023, 241, 107624. [Google Scholar] [CrossRef]
- Guo, T.; Nikolaev, P.; Rinzler, A.G.; Tomanek, D.; Colbert, D.T.; Smalley, R.E. Self-Assembly of Tubular Fullerenes. J. Phys. Chem. 1995, 99, 10694–10697. [Google Scholar] [CrossRef]
- Han, F.; Qian, L.; Wu, Q.; Li, D.; Hao, S.; Feng, L.; Xin, L.; Yang, T.; Zhang, J.; He, M. Narrow-Chirality Distributed Single-Walled Carbon Nanotube Synthesized from Oxide Promoted Fe–SiC Catalyst. Carbon 2022, 191, 146–152. [Google Scholar] [CrossRef]
- Schwandt, C.; Dimitrov, A.T.; Fray, D.J. High-Yield Synthesis of Multi-Walled Carbon Nanotubes from Graphite by Molten Salt Electrolysis. Carbon 2012, 50, 1311–1315. [Google Scholar] [CrossRef]
- Notarianni, M.; Liu, J.; Vernon, K.; Motta, N. Synthesis and Applications of Carbon Nanomaterials for Energy Generation and Storage. Beilstein J. Nanotechnol. 2016, 7, 149–196. [Google Scholar] [CrossRef]
- Xu, R.; Tang, R.; Zhou, Q.; Li, F.; Zhang, B. Enhancement of Catalytic Activity of Immobilized Laccase for Diclofenac Biodegradation by Carbon Nanotubes. Chem. Eng. J. 2015, 262, 88–95. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Yang, Y.; Sun, H. How Cushion Communities Are Maintained in Alpine Ecosystems: A Review and Case Study on Alpine Cushion Plant Reproduction. Plant Divers. 2017, 39, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.; Li, M.; Zhang, C. Degradation of Phenolic Compounds by Laccase Immobilized on Carbon Nanomaterials: Diffusional Limitation Investigation. Talanta 2015, 131, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Pang, S.; Wu, Y.; Jiang, S.; Ouyang, J. Synthesis and Characterization of Mesoporous Cu–MOF for Laccase Immobilization. J. Chem. Technol. Biotechnol. 2017, 92, 1841–1847. [Google Scholar] [CrossRef]
- Ismail, U.M.; Onaizi, S.A.; Vohra, M.S. Aqueous Pb(II) Removal Using ZIF-60: Adsorption Studies, Response Surface Methodology and Machine Learning Predictions. Nanomaterials 2023, 13, 1402. [Google Scholar] [CrossRef]
- Gascón, V.; Márquez-Álvarez, C.; Blanco, R.M. Efficient Retention of Laccase by Non-Covalent Immobilization on Amino-Functionalized Ordered Mesoporous Silica. Appl. Catal. A Gen. 2014, 482, 116–126. [Google Scholar] [CrossRef]
- Garg, A.; Jain, A. Hydrogen Storage in Metal-Organic Frameworks: A Review. SGVU Int. J. Environ. 2014, 128, 368–392. [Google Scholar]
- Han, Y.; Yang, H.; Guo, X.; Han, Y.; Yang, H.; Guo, X. Synthesis Methods and Crystallization of MOFs; IntechOpen: Rijeka, Romania, 2020. [Google Scholar] [CrossRef]
- Han, Z.; Fan, X.; Yu, S.; Li, X.; Wang, S.; Lu, L. Metal-Organic Frameworks (MOFs): A Novel Platform for Laccase Immobilization and Application. J. Environ. Chem. Eng. 2022, 10, 108795. [Google Scholar] [CrossRef]
- Silva, A.R.M.; Alexandre, J.Y.N.H.; Souza, J.E.S.; Neto, J.G.L.; De, S.; Júnior, P.G.; Rocha, M.V.P.; Dos Santos, J.C.S.; Silva, A.R.M.; Alexandre, J.Y.N.H.; et al. The Chemistry and Applications of Metal–Organic Frameworks (MOFs) as Industrial Enzyme Immobilization Systems. Molecules 2022, 27, 4529. [Google Scholar] [CrossRef]
- Tao, A.R.; Habas, S.; Yang, P. Shape Control of Colloidal Metal Nanocrystals. Small 2008, 4, 310–325. [Google Scholar] [CrossRef]
- Li, R.; Zhang, W.; Zhou, K.; Li, R.; Zhang, W.; Zhou, K. Metal–Organic-Framework-Based Catalysts for Photoreduction of CO2. Adv. Mater. 2018, 30, 1705512. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Z.; Chen, L.; Liu, G.; Yuan, Z.Y.; Li, B.F.; Zhang, X.; Wei, J.Q. Porous Metal–Organic Frameworks for Methane Storage and Capture: Status and Challenges. New Carbon. Mater. 2021, 36, 468–496. [Google Scholar] [CrossRef]
- Chuhadiya, S.; Himanshu; Suthar, D.; Patel, S.L.; Dhaka, M.S. Metal Organic Frameworks as Hybrid Porous Materials for Energy Storage and Conversion Devices: A Review. Coord. Chem. Rev. 2021, 446, 214115. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Q. Metal-Organic Framework Composites for Catalysis. Matter 2019, 1, 57–89. [Google Scholar] [CrossRef]
- Ibrahim, A.; Vohra, M.S.; Bahadi, S.A.; Onaizi, S.A.; Essa, M.H.; Mohammed, T. Heavy Metals Adsorption onto Graphene Oxide: Effect of Mixed Systems Anresponse Surface Methodology Modeling. Desalin. Water Treat. 2022, 266, 78–90. [Google Scholar] [CrossRef]
- Ganiyu, S.A.; Awwal Suleiman, M.; Ahmed Al-Amrani, W.; Kilaco Usman, A.; Onaizi, S.A. Adsorptive Removal of Organic Pollutants from Contaminated Waters Using Zeolitic Imidazolate Framework Composites: A Comprehensive and Up-to-Date Review. Sep. Purif. Technol. 2023, 318, 123765. [Google Scholar] [CrossRef]
- Tocco, D.; Carucci, C.; Todde, D.; Shortall, K.; Otero, F.; Sanjust, E.; Magner, E.; Salis, A. Enzyme Immobilization on Metal Organic Frameworks: Laccase from Aspergillus Sp. Is Better Adapted to ZIF-Zni Rather than Fe-BTC. Colloids Surf. B Biointerfaces 2021, 208, 112147. [Google Scholar] [CrossRef]
- Pang, S.; Wu, Y.; Zhang, X.; Li, B.; Ouyang, J.; Ding, M. Immobilization of Laccase via Adsorption onto Bimodal Mesoporous Zr-MOF. Process Biochem. 2016, 51, 229–239. [Google Scholar] [CrossRef]
- Kong, X.J.; Li, J.R. An Overview of Metal–Organic Frameworks for Green Chemical Engineering. Engineering 2021, 7, 1115–1139. [Google Scholar] [CrossRef]
- Qin, Y.; Wan, Y.; Guo, J.; Zhao, M. Two-Dimensional Metal-Organic Framework Nanosheet Composites: Preparations and Applications. Chin. Chem. Lett. 2022, 33, 693–702. [Google Scholar] [CrossRef]
- Qi, L.; Luo, Z.; Lu, X. Biomimetic Mineralization Inducing Lipase-Metal-Organic Framework Nanocomposite for Pickering Interfacial Biocatalytic System. ACS Sustain. Chem. Eng. 2019, 7, 7127–7139. [Google Scholar] [CrossRef]
- Nemiwal, M.; Gosu, V.; Zhang, T.C.; Kumar, D. Metal Organic Frameworks as Electrocatalysts: Hydrogen Evolution Reactions and Overall Water Splitting. Int. J. Hydrogen Energy 2021, 46, 10216–10238. [Google Scholar] [CrossRef]
- Konnerth, H.; Matsagar, B.M.; Chen, S.S.; Prechtl, M.H.G.; Shieh, F.K.; Wu, K.C.W. Metal-Organic Framework (MOF)-Derived Catalysts for Fine Chemical Production. Coord. Chem. Rev. 2020, 416, 213319. [Google Scholar] [CrossRef]
- Lin, C.; Xu, K.; Zheng, R.; Zheng, Y. Immobilization of Amidase into a Magnetic Hierarchically Porous Metal–Organic Framework for Efficient Biocatalysis. Chem. Commun. 2019, 55, 5697–5700. [Google Scholar] [CrossRef]
- He, J.; Sun, S.; Zhou, Z.; Yuan, Q.; Liu, Y.; Liang, H. Thermostable Enzyme-Immobilized Magnetic Responsive Ni-Based Metal–Organic Framework Nanorods as Recyclable Biocatalysts for Efficient Biosynthesis of S-Adenosylmethionine. Dalton Trans. 2019, 48, 2077–2085. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, H.; Wang, Z.; Du, Y.; Zhong, L.; Zhang, C.; Jiang, Y.; Jia, S.; Cui, J. Three-Dimensional Ordered Magnetic Macroporous Metal-Organic Frameworks for Enzyme Immobilization. J. Colloid Interface Sci. 2021, 590, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Tranchemontagne, D.J.; Tranchemontagne, J.L.; O’keeffe, M.; Yaghi, O.M. Secondary Building Units, Nets and Bonding in the Chemistry of Metal-Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wu, C. De Designed Fabrication of Biomimetic Metal–Organic Frameworks for Catalytic Applications. Coord. Chem. Rev. 2019, 378, 445–465. [Google Scholar] [CrossRef]
- Farmakes, J.; Schuster, I.; Overby, A.; Alhalhooly, L.; Lenertz, M.; Li, Q.; Ugrinov, A.; Choi, Y.; Pan, Y.; Yang, Z. Enzyme Immobilization on Graphite Oxide (GO) Surface via One-Pot Synthesis of GO/Metal-Organic Framework Composites for Large-Substrate Biocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 23119–23126. [Google Scholar] [CrossRef]
- Gkaniatsou, E.; Sicard, C.; Ricoux, R.; Benahmed, L.; Bourdreux, F.; Zhang, Q.; Serre, C.; ean-Pierre Mahy, J.; Steunou, N.; Gkaniatsou, E.; et al. Enzyme Encapsulation in Mesoporous Metal–Organic Frameworks for Selective Biodegradation of Harmful Dye Molecules. Angew. Chem. Int. Ed. 2018, 57, 16141–16146. [Google Scholar] [CrossRef]
- Cui, J.; Feng, Y.; Jia, S. Silica Encapsulated Catalase@metal-Organic Framework Composite: A Highly Stable and Recyclable Biocatalyst. Chem. Eng. J. 2018, 351, 506–514. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ampiaw, R.E.; Lee, W. Adsorptive Removal of Dyes from Wastewater Using a Metal-Organic Framework: A Review. Chemosphere 2021, 284, 131314. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wu, E.; Lou, X.; Deng, Q.; Hou, X.; Lv, C.; Hu, Q. Anthraquinone Removal by a Metal-Organic Framework/Polyvinyl Alcohol Cryogel-Immobilized Laccase: Effect and Mechanism Exploration. Chem. Eng. J. 2021, 418, 129473. [Google Scholar] [CrossRef]
- Unuofin, J.O. Sustainability Potentials of Novel Laccase Tinctures from Stenotrophomonas Maltophilia BIJ16 and Bordetella Bronchiseptica HSO16: From Dye Decolourization to Denim Bioscouring. Biotechnol. Rep. 2020, 25, e00409. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Varghese, L.M.; Battan, B.; Patra, A.K.; Mandhan, R.P.; Mahajan, R. Environmental Pollution Reducing Strategy for Scouring of Undegummed Sisal Fibers Using Xylanase and Pectinase Enzymes. Bioprocess. Biosyst. Eng. 2021, 44, 607–615. [Google Scholar] [CrossRef]
- Mojsov, K. Enzymatic Scouring and Bleaching of Cotton Terry Fabrics—Opportunity of the Improvement on Some Physicochemical and Mechanical Properties of the Fabrics. J. Nat. Fibers 2017, 15, 740–751. [Google Scholar] [CrossRef]
- Tülek, A.; Yıldırım, D.; Aydın, D.; Binay, B. Highly-Stable Madurella Mycetomatis Laccase Immobilized in Silica-Coated ZIF-8 Nanocomposites for Environmentally Friendly Cotton Bleaching Process. Colloids Surf. B Biointerfaces 2021, 202, 111672. [Google Scholar] [CrossRef]
- Greca, S.C.d.A.; Kyrou, I.; Pink, R.; Randeva, H.; Grammatopoulos, D.; Silva, E.; Karteris, E. Involvement of the Endocrine-Disrupting Chemical Bisphenol A (BPA) in Human Placentation. J. Clin. Med. 2020, 9, 405. [Google Scholar] [CrossRef]
- Ohore, O.E.; Songhe, Z. Endocrine Disrupting Effects of Bisphenol A Exposure and Recent Advances on Its Removal by Water Treatment Systems. A Review. Sci. Afr. 2019, 5, e00135. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N.; Barceló, D. Mitigation of Bisphenol A Using an Array of Laccase-Based Robust Bio-Catalytic Cues—A Review. Sci. Total Environ. 2019, 689, 160–177. [Google Scholar] [CrossRef]
- Cañas, A.I.; Camarero, S. Laccases and Their Natural Mediators: Biotechnological Tools for Sustainable Eco-Friendly Processes. Biotechnol. Adv. 2010, 28, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Han, J.; Liu, Y.; Lan, H.; Li, C.; Wang, Y. Morphology-Dependent Intelligent Biocatalysts with Automatic Functionality Regulation for Activity Enhancement and Controllable Recycling. Chem. Eng. J. 2021, 409, 127985. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Multi-Faceted Strategy Based on Enzyme Immobilization with Reactant Adsorption and Membrane Technology for Biocatalytic Removal of Pollutants: A Critical Review. Biotechnol. Adv. 2019, 37, 107401. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Saharan, V.; Kumar, S.; Gulati, P.; Kapoor, R.K. Laccase Grafted Membranes for Advanced Water Filtration Systems: A Green Approach to Water Purification Technology. Crit. Rev. Biotechnol. 2017, 38, 883–901. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Basso, A.; Brady, D. New Frontiers in Enzyme Immobilisation: Robust Biocatalysts for a Circular Bio-Based Economy. Chem. Soc. Rev. 2021, 50, 5850–5862. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme Immobilisation in Biocatalysis: Why, What and How. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Di Cosimo, R.; Mc Auliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial Use of Immobilized Enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient Immobilisation of Industrial Biocatalysts: Criteria and Constraints for the Selection of Organic Polymeric Carriers and Immobilisation Methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef]
- Zucca, P.; Fernandez-Lafuente, R.; Sanjust, E. Agarose and Its Derivatives as Supports for Enzyme Immobilization. Molecules 2016, 21, 1577. [Google Scholar] [CrossRef]
- Kulshrestha, Y.; Husain, Q. Bioaffinity-Based an Inexpensive and High Yield Procedure for the Immobilization of Turnip (Brassica rapa) Peroxidase. Biomol. Eng. 2006, 23, 291–297. [Google Scholar] [CrossRef]
- Santos, J.C.S.D.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the Support Properties for Immobilization or Purification of Enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef]
- Siar, E.H.; Zaak, H.; Kornecki, J.F.; Zidoune, M.N.; Barbosa, O.; Fernandez-Lafuente, R. Stabilization of Ficin Extract by Immobilization on Glyoxyl Agarose. Preliminary Characterization of the Biocatalyst Performance in Hydrolysis of Proteins. Process Biochem. 2017, 58, 98–104. [Google Scholar] [CrossRef]
- Siar, E.H.; Morellon-Sterling, R.; Zidoune, M.N.; Fernandez-Lafuente, R. Amination of Ficin Extract to Improve Its Immobilization on Glyoxyl-Agarose: Improved Stability and Activity versus Casein. Int. J. Biol. Macromol. 2019, 133, 412–419. [Google Scholar] [CrossRef]
- Marsden, S.R.; Mestrom, L.; McMillan, D.G.G.; Hanefeld, U. Thermodynamically and Kinetically Controlled Reactions in Biocatalysis—From Concepts to Perspectives. ChemCatChem 2020, 12, 426–437. [Google Scholar] [CrossRef]
- Kasche, V. Mechanism and Yields in Enzyme Catalysed Equilibrium and Kinetically Controlled Synthesis of β-Lactam Antibiotics, Peptides and Other Condensation Products. Enzym. Microb. Technol. 1986, 8, 4–16. [Google Scholar] [CrossRef]
- Guajardo, N.; de María, P.D. Production of Bulk Chemicals with Biocatalysis: Drivers and Challenges Reflected in Recent Industrial Granted Patents (2015–2020). Molecules 2021, 26, 736. [Google Scholar] [CrossRef] [PubMed]
- Woodley, J.M. Towards the Sustainable Production of Bulk-Chemicals Using Biotechnology. New Biotechnol. 2020, 59, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Asmaly, H.A.; Abussaud, B.; Ihsanullah; Saleh, T.A.; Gupta, V.K.; Atieh, M.A. Ferric Oxide Nanoparticles Decorated Carbon Nanotubes and Carbon Nanofibers: From Synthesis to Enhanced Removal of Phenol. J. Saudi Chem. Soc. 2015, 19, 511–520. [Google Scholar] [CrossRef]
- Del Arco, J.; Alcántara, A.R.; Fernández-Lafuente, R.; Fernández-Lucas, J. Magnetic Micro-Macro Biocatalysts Applied to Industrial Bioprocesses. Bioresour. Technol. 2021, 322, 124547. [Google Scholar] [CrossRef]
- Tavano, O.L.; Berenguer-Murcia, A.; Secundo, F.; Fernandez-Lafuente, R. Biotechnological Applications of Proteases in Food Technology. Compr. Rev. Food Sci. Food Saf. 2018, 17, 412–436. [Google Scholar] [CrossRef]
- Santi, M.; Sancineto, L.; Nascimento, V.; Azeredo, J.B.; Orozco, E.V.M.; Andrade, L.H.; Gröger, H.; Santi, C. Flow Biocatalysis: A Challenging Alternative for the Synthesis of APIs and Natural Compounds. Int. J. Mol. Sci. 2021, 22, 990. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Mateos, A.I.; Huber, C.; Nidetzky, B.; Bolivar, J.M.; López-Gallego, F. Design of the Enzyme-Carrier Interface to Overcome the O2 and NADH Mass Transfer Limitations of an Immobilized Flavin Oxidase. ACS Appl. Mater. Interfaces 2020, 12, 56027–56038. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Rocha-Martín, J.; De Las Rivas, B.; Muñoz, R.; Guisán, J.M.; López-Gallego, F. Rational Co-Immobilization of Bi-Enzyme Cascades on Porous Supports and Their Applications in Bio-Redox Reactions with In Situ Recycling of Soluble Cofactors. ChemCatChem 2012, 4, 1279–1288. [Google Scholar] [CrossRef]
- López-Gallego, F.; Acebrón, I.; Mancheño, J.M.; Raja, S.; Lillo, M.P.; Guisán Seijas, J.M. Directed, Strong, and Reversible Immobilization of Proteins Tagged with a β-Trefoil Lectin Domain: A Simple Method to Immobilize Biomolecules on Plain Agarose Matrixes. Bioconjug. Chem. 2012, 23, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Kastantin, M.; Langdon, B.B.; Schwartz, D.K. A Bottom-up Approach to Understanding Protein Layer Formation at Solid–Liquid Interfaces. Adv. Colloid Interface Sci. 2014, 207, 240–252. [Google Scholar] [CrossRef]
- Souza, P.M.P.; Carballares, D.; Lopez-Carrobles, N.; Gonçalves, L.R.B.; Lopez-Gallego, F.; Rodrigues, S.; Fernandez-Lafuente, R. Enzyme-Support Interactions and Inactivation Conditions Determine Thermomyces Lanuginosus Lipase Inactivation Pathways: Functional and Florescence Studies. Int. J. Biol. Macromol. 2021, 191, 79–91. [Google Scholar] [CrossRef]
- Mateo, C.; Abian, O.; Fernández-Lorente, G.; Pedroche, J.; Fernández-Lafuente, R.; Guisan, J.M.; Tam, A.; Daminati, M. Epoxy Sepabeads: A Novel Epoxy Support for Stabilization of Industrial Enzymes via Very Intense Multipoint Covalent Attachment. Biotechnol. Prog. 2002, 18, 629–634. [Google Scholar] [CrossRef]
- Vranish, J.N.; Ancona, M.G.; Oh, E.; Susumu, K.; Lasarte Aragonés, G.; Breger, J.C.; Walper, S.A.; Medintz, I.L. Enhancing Coupled Enzymatic Activity by Colocalization on Nanoparticle Surfaces: Kinetic Evidence for Directed Channeling of Intermediates. ACS Nano 2018, 12, 7911–7926. [Google Scholar] [CrossRef]
- Jäger, V.D.; Lamm, R.; Kloß, R.; Kaganovitch, E.; Grünberger, A.; Pohl, M.; Büchs, J.; Jaeger, K.E.; Krauss, U. A Synthetic Reaction Cascade Implemented by Colocalization of Two Proteins within Catalytically Active Inclusion Bodies. ACS Synth. Biol. 2018, 7, 2282–2295. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; da Silva, E.S.; Llop, J.; López-Gallego, F. Sustainable and Continuous Synthesis of Enantiopure L-Amino Acids by Using a Versatile Immobilised Multienzyme System. ChemBioChem 2018, 19, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.S.; Rueda, N.; Barbosa, O.; Fernández-Sánchez, J.F.; Medina-Castillo, A.L.; Ramón-Márquez, T.; Arias-Martos, M.C.; Millán-Linares, M.C.; Pedroche, J.; Yust, M.D.M.; et al. Characterization of Supports Activated with Divinyl Sulfone as a Tool to Immobilize and Stabilize Enzymes via Multipoint Covalent Attachment. Application to Chymotrypsin. RSC Adv. 2015, 5, 20639–20649. [Google Scholar] [CrossRef]
- Andrés-Sanz, D.; Diamanti, E.; Di Silvo, D.; Gurauskis, J.; López-Gallego, F. Selective Coimmobilization of His-Tagged Enzymes on Yttrium-Stabilized Zirconia-Based Membranes for Continuous Asymmetric Bioreductions. ACS Appl. Mater. Interfaces 2022, 14, 4285–4296. [Google Scholar] [CrossRef] [PubMed]
- Velasco Abadia, A.; Herbert, K.M.; Matavulj, V.M.; White, T.J.; Schwartz, D.K.; Kaar, J.L. Chemically Triggered Changes in Mechanical Properties of Responsive Liquid Crystal Polymer Networks with Immobilized Urease. J. Am. Chem. Soc. 2021, 143, 16740–16749. [Google Scholar] [CrossRef]
- Zeballos, N.; Diamanti, E.; Benítez-Mateos, A.I.; Schmidt-Dannert, C.; López-Gallego, F. Solid-Phase Assembly of Multienzyme Systems into Artificial Cellulosomes. Bioconjug. Chem. 2021, 32, 1966–1972. [Google Scholar] [CrossRef]
- Pedroche, J.; del Mar Yust, M.; Mateo, C.; Fernández-Lafuente, R.; Girón-Calle, J.; Alaiz, M.; Vioque, J.; Guisán, J.M.; Millán, F. Effect of the Support and Experimental Conditions in the Intensity of the Multipoint Covalent Attachment of Proteins on Glyoxyl-Agarose Supports: Correlation between Enzyme–Support Linkages and Thermal Stability. Enzym. Microb. Technol. 2007, 40, 1160–1166. [Google Scholar] [CrossRef]
- Bolivar, J.M.; López-Gallego, F. Characterization and Evaluation of Immobilized Enzymes for Applications in Flow Reactors. Curr. Opin. Green Sustain. Chem. 2020, 25, 100349. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Eisl, I.; Nidetzky, B. Advanced Characterization of Immobilized Enzymes as Heterogeneous Biocatalysts. Catal. Today 2016, 259, 66–80. [Google Scholar] [CrossRef]
- Barbosa, O.; Torres, R.; Ortiz, C.; Berenguer-Murcia, Á.; Rodrigues, R.C.; Fernandez-Lafuente, R. Heterofunctional Supports in Enzyme Immobilization: From Traditional Immobilization Protocols to Opportunities in Tuning Enzyme Properties. Biomacromolecules 2013, 14, 2433–2462. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of Enzymes via Immobilization: Multipoint Covalent Attachment and Other Stabilization Strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef]
- Rocchietti, S.; Ubiali, D.; Terreni, M.; Albertini, A.M.; Fernández-Lafuente, R.; Guisán, J.M.; Pregnolato, M. Immobilization and Stabilization of Recombinant Multimeric Uridine and Purine Nucleoside Phosphorylases from Bacillus subtilis. Biomacromolecules 2004, 5, 2195–2200. [Google Scholar] [CrossRef] [PubMed]
- Ubiali, D.; Rocchietti, S.; Scaramozzino, F.; Terreni, M.; Albertini, A.M.; Fernández-Lafuente, R.; Guisán, J.M.; Pregnolato, M. Synthesis of 2′-Deoxynucleosides by Transglycosylation with New Immobilized and Stabilized Uridine Phosphorylase and Purine Nucleoside Phosphorylase. Adv. Synth. Catal. 2004, 346, 1361–1366. [Google Scholar] [CrossRef]
- De Santis, P.; Meyer, L.E.; Kara, S. The Rise of Continuous Flow Biocatalysis—Fundamentals, Very Recent Developments and Future Perspectives. React. Chem. Eng. 2020, 5, 2155–2184. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Wiesbauer, J.; Nidetzky, B. Biotransformations in Microstructured Reactors: More than Flowing with the Stream? Trends Biotechnol. 2011, 29, 333–342. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of Lipases on Hydrophobic Supports: Immobilization Mechanism, Advantages, Problems, and Solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef]
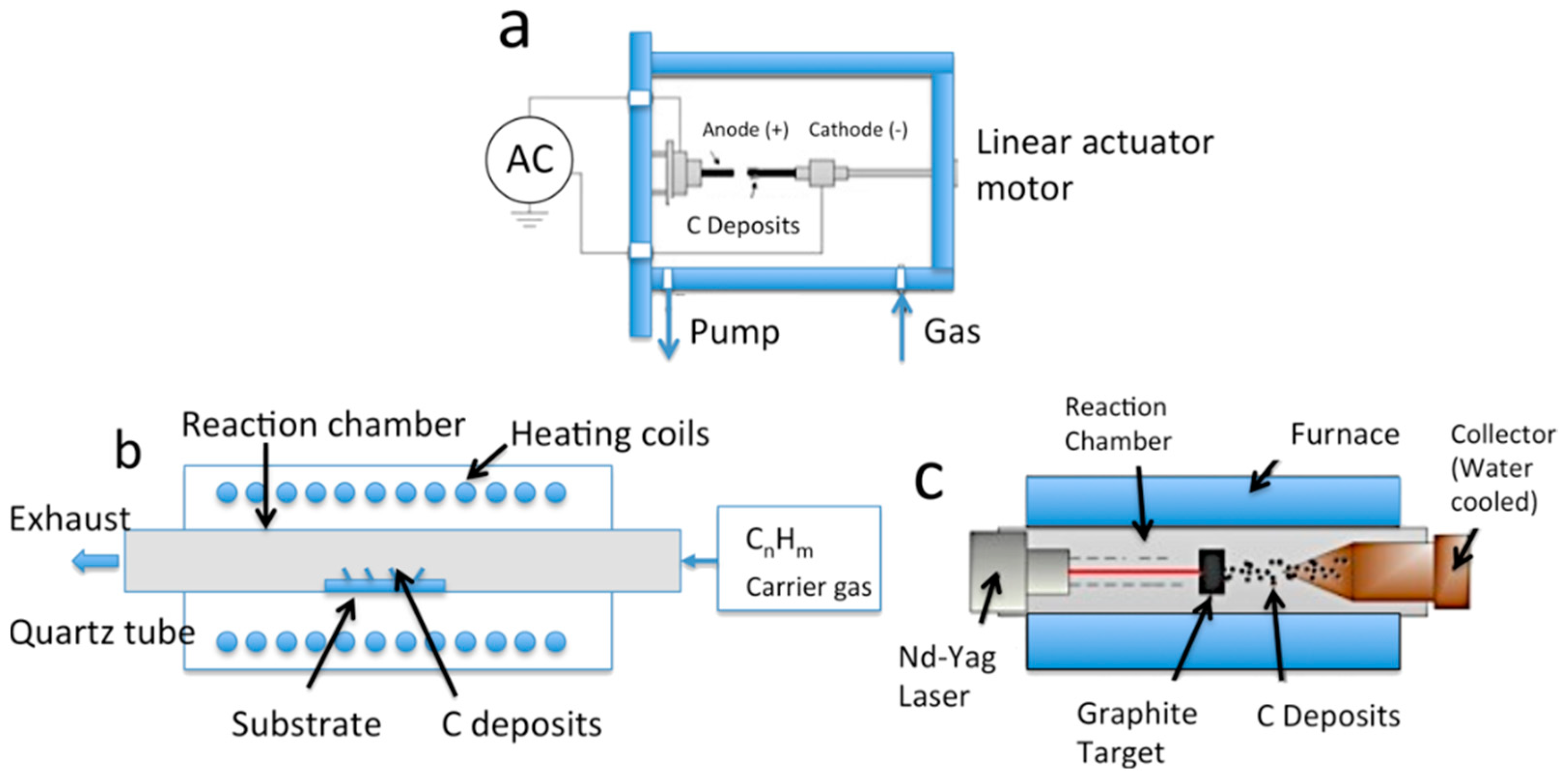

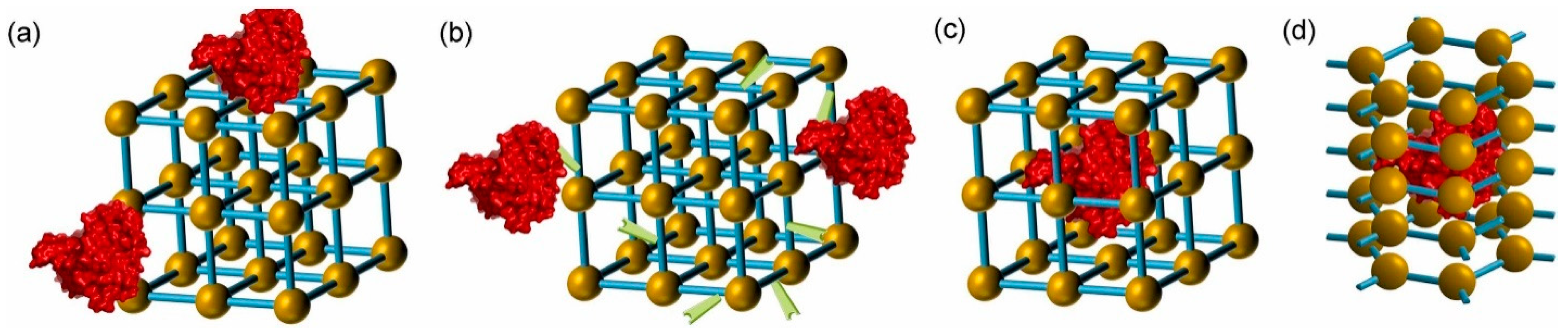
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Sakkaf, M.K.; Basfer, I.; Iddrisu, M.; Bahadi, S.A.; Nasser, M.S.; Abussaud, B.; Drmosh, Q.A.; Onaizi, S.A. An Up-to-Date Review on the Remediation of Dyes and Phenolic Compounds from Wastewaters Using Enzymes Immobilized on Emerging and Nanostructured Materials: Promises and Challenges. Nanomaterials 2023, 13, 2152. https://doi.org/10.3390/nano13152152
Al-Sakkaf MK, Basfer I, Iddrisu M, Bahadi SA, Nasser MS, Abussaud B, Drmosh QA, Onaizi SA. An Up-to-Date Review on the Remediation of Dyes and Phenolic Compounds from Wastewaters Using Enzymes Immobilized on Emerging and Nanostructured Materials: Promises and Challenges. Nanomaterials. 2023; 13(15):2152. https://doi.org/10.3390/nano13152152
Chicago/Turabian StyleAl-Sakkaf, Mohammed K., Ibrahim Basfer, Mustapha Iddrisu, Salem A. Bahadi, Mustafa S. Nasser, Basim Abussaud, Qasem A. Drmosh, and Sagheer A. Onaizi. 2023. "An Up-to-Date Review on the Remediation of Dyes and Phenolic Compounds from Wastewaters Using Enzymes Immobilized on Emerging and Nanostructured Materials: Promises and Challenges" Nanomaterials 13, no. 15: 2152. https://doi.org/10.3390/nano13152152
APA StyleAl-Sakkaf, M. K., Basfer, I., Iddrisu, M., Bahadi, S. A., Nasser, M. S., Abussaud, B., Drmosh, Q. A., & Onaizi, S. A. (2023). An Up-to-Date Review on the Remediation of Dyes and Phenolic Compounds from Wastewaters Using Enzymes Immobilized on Emerging and Nanostructured Materials: Promises and Challenges. Nanomaterials, 13(15), 2152. https://doi.org/10.3390/nano13152152







