Synthesis of TiO2-Cu2+/CuI Nanocomposites and Evaluation of Antifungal and Cytotoxic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Nanostructured Materials (NMs)
2.3. Characterization of Nanomaterials
2.4. Antifungal Activity of CuI-Based Nanostructured Materials
2.5. Cyto-Toxicity Test
3. Results
3.1. Synthesis and Characterization of NMs
3.2. Antifungal Activity of CuI-Based Nanostructured Materials
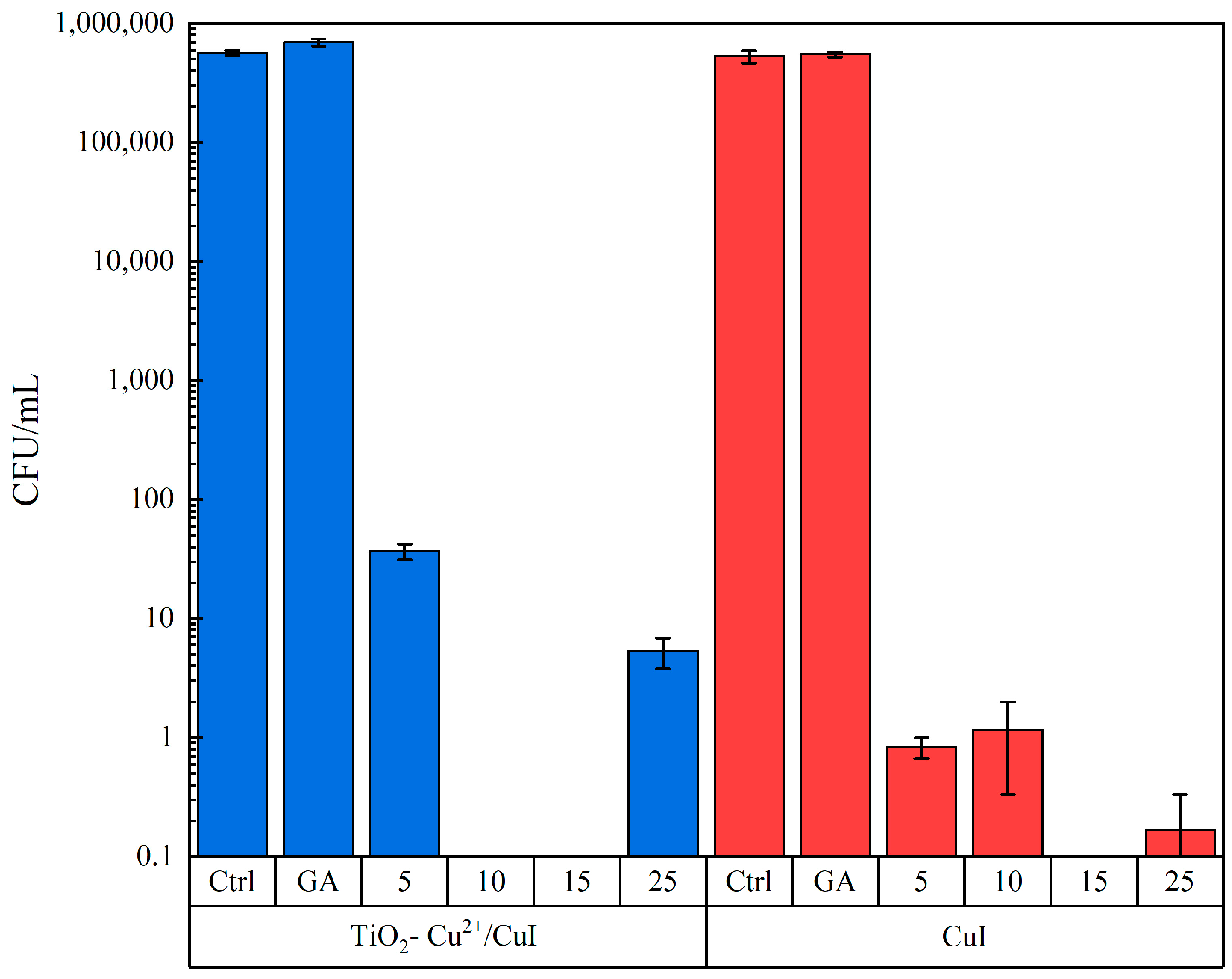
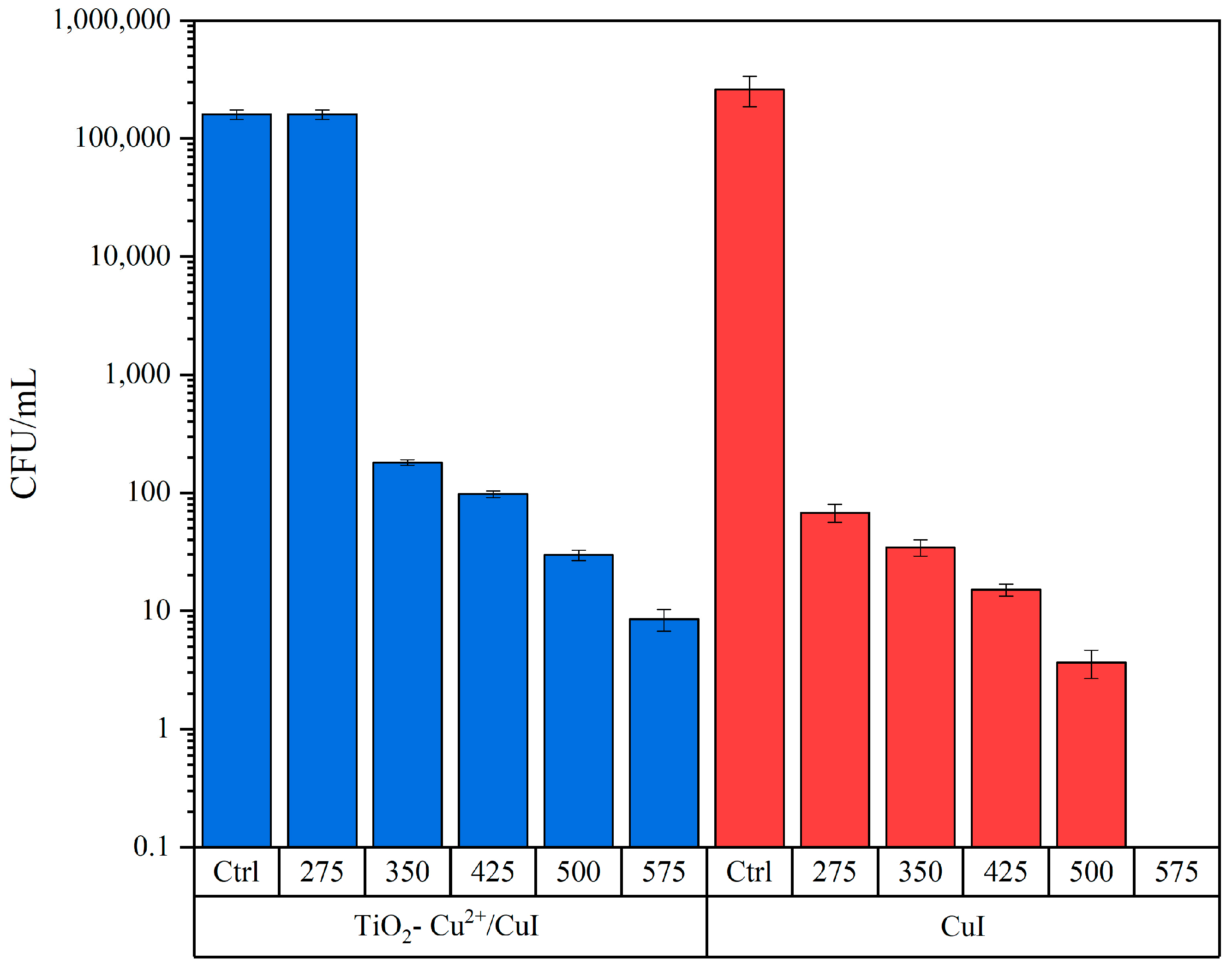
3.2.1. Candida Parapsilosis Interaction Tests
3.2.2. Aspergillus Niger Interaction Tests
3.2.3. AFM and HT Evaluation of the Interaction of Fungi and NMs
3.3. Toxicity Tests of CuI and TiO2-Cu2+/CuI Composite
HT Microscopy Evaluation of the Interaction of BEAS-2B and NMs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedman, D.Z.P.; Schwartz, I.S. Emerging Fungal Infections: New Patients, New Patterns, and New Pathogens. J. Fungi 2019, 5, 67. [Google Scholar] [CrossRef] [Green Version]
- Thomaz, D.Y.; Del Negro, G.M.B.; Ribeiro, L.B.; da Silva, M.; Carvalho, G.O.M.H.; Camargo, C.H.; de Almeida, J.N.; Motta, A.L.; Siciliano, R.F.; Sejas, O.N.E.; et al. A Brazilian Inter-Hospital Candidemia Outbreak Caused by Fluconazole-Resistant Candida Parapsilosis in the COVID-19 Era. J. Fungi 2022, 8, 100. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a Therapeutic Tool to Combat Microbial Resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.; Ferreira, D.; Reis, S.; Costa, P. Current Insights on Antifungal Therapy: Novel Nanotechnology Approaches for Drug Delivery Systems and New Drugs from Natural Sources. Pharmaceuticals 2020, 13, 248. [Google Scholar] [CrossRef]
- Pianalto, K.M.; Alspaugh, J.A. New Horizons in Antifungal Therapy. J. Fungi 2016, 2, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Salazar, E.; Acosta-Altamirano, G.; Betancourt-Cisneros, P.; Reyes-Montes, M.D.R.; Rosas-De-paz, E.; Duarte-Escalante, E.; Sánchez-Conejo, A.R.; Hernández, E.O.; Frías-De-león, M.G. Detection and Molecular Identification of Eight Candida Species in Clinical Samples by Simplex PCR. Microorganisms 2022, 10, 374. [Google Scholar] [CrossRef] [PubMed]
- Awed, A.S.; El-Sayyad, G.S.; El-ghandour, A.; Hameed, M.F.O.; Abdel Maksoud, M.I.A.; El-Batal, A.I.; Obayya, S.S.A. Unveiling Antimicrobial Activity of Metal Iodide (CuI, AgI, and PbI2) Nanoparticles: Towards Biomedical Surfaces Applications. J. Clust. Sci. 2019, 32, 1–16. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial Activity of the Metals and Metal Oxide Nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Ren, Y.; Han, Y.; Li, Z.; Liu, X.; Zhu, S.; Liang, Y.; Yeung, K.W.K.; Wu, S. Ce and Er Co-Doped TiO2 for Rapid Bacteria- Killing Using Visible Light. Bioact. Mater. 2020, 5, 201–209. [Google Scholar] [CrossRef]
- Birkett, M.; Dover, L.; Cherian Lukose, C.; Wasy Zia, A.; Tambuwala, M.M.; Serrano-Aroca, Á. Recent Advances in Metal-Based Antimicrobial Coatings for High-Touch Surfaces. Int. J. Mol. Sci. 2022, 23, 1162. [Google Scholar] [CrossRef]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.J.; Bartlett, J.; Nolan, M.; Pillai, S.C. Cu-Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef] [Green Version]
- Ahmed Shehab, M.; Szőri-Dorogházi, E.; Szabó, S.; Valsesia, A.; Chauhan, T.; Koós, T.; Muránszky, G.; Szabó, T.; Hernadi, K.; Németh, Z. Virus and Bacterial Removal Ability of TiO2 Nanowire-Based Self-Supported Hybrid Membranes. Arab. J. Chem. 2023, 16, 104388. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Q.; Yang, H.; Shi, D.; Qian, J. Photocatalytic Antibacterial Properties of Copper Doped TiO2 Prepared by High-Energy Ball Milling. Ceram. Int. 2020, 46, 16716–16724. [Google Scholar] [CrossRef]
- Natsir, M.; Maulidiyah, M.; Watoni, A.H.; Arif, J.; Sari, A.; Salim, L.O.A.; Sarjuna, S.; Irwan, I.; Nurdin, M. Synthesis and Charcterization of Cu-Doped TiO2(Cu/TiO2) Nanoparticle as Antifungal Phytophthora Palmivora. J. Phys. Conf. Ser. 2021, 1899, 012039. [Google Scholar] [CrossRef]
- Archana, K.M.; Rajagopal, R.; Krishnaswamy, V.G.; Aishwarya, S. Application of Green Synthesised Copper Iodide Particles on Cotton Fabric-Protective Face Mask Material against COVID-19 Pandemic. J. Mater. Res. Technol. 2021, 15, 2102–2116. [Google Scholar] [CrossRef]
- Archana, K.M.; Rajalakshmi, S.; Kumar, P.S.; Krishnaswamy, V.G.; Rajagopal, R.; Kumar, D.T.; Priya Doss, C.G. Effect of Shape and Anthocyanin Capping on Antibacterial Activity of CuI Particles. Environ. Res. 2021, 200, 111759. [Google Scholar] [CrossRef]
- Martínez-Montelongo, J.H.; Medina-Ramírez, I.E.; Romo-Lozano, Y.; González-Gutiérrez, A.; Macías-Díaz, J.E. Development of Nano-Antifungal Therapy for Systemic and Endemic Mycoses. J. Fungi 2021, 7, 158. [Google Scholar] [CrossRef]
- Fernstrom, A.; Goldblatt, M. Aerobiology and Its Role in the Transmission of Infectious Diseases. J. Pathog. 2013, 2013, 493960. [Google Scholar] [CrossRef] [Green Version]
- Dillon, C.F.; Dillon, M.B. Multiscale Airborne Infectious Disease Transmission. Appl. Environ. Microbiol. 2021, 87, 1–13. [Google Scholar] [CrossRef]
- Molina-Hernández, J.B.; Scroccarello, A.; Della Pelle, F.; De Flaviis, R.; Compagnone, D.; Del Carlo, M.; Paparella, A.; Chaves Lόpez, C. Synergistic Antifungal Activity of Catechin and Silver Nanoparticles on Aspergillus Niger Isolated from Coffee Seeds. Lwt 2022, 169, 113990. [Google Scholar] [CrossRef]
- Thomas-Rüddel, D.O.; Schlattmann, P.; Pletz, M.; Kurzai, O.; Bloos, F. Risk Factors for Invasive Candida Infection in Critically Ill Patients: A Systematic Review and Meta-Analysis. Chest 2022, 161, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Pedroza-Herrera, G.; Medina-Ramírez, I.E.; Lozano-Álvarez, J.A.; Rodil, S.E. Evaluation of the Photocatalytic Activity of Copper Doped TiO2 Nanoparticles for the Purification and/or Disinfection of Industrial Effluents. Catal. Today 2020, 341, 37–48. [Google Scholar] [CrossRef]
- Oussou-Azo, A.F.; Nakama, T.; Nakamura, M.; Futagami, T.; Vestergaard, M.C.M. Antifungal Potential of Nanostructured Crystalline Copper and Its Oxide Forms. Nanomaterials 2020, 10, 1003. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Laha, D.; Bhattacharya, D.; Pramanik, P.; Karmakar, P. A Novel Study of Antibacterial Activity of Copper Iodide Nanoparticle Mediated by DNA and Membrane Damage. Colloids Surf. B Biointerfaces 2012, 96, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Montelongo, J.H.; Medina-Ramírez, I.E.; Romo-Lozano, Y.; Zapien, J.A. Development of a Sustainable Photocatalytic Process for Air Purification. Chemosphere 2020, 257, 127236. [Google Scholar] [CrossRef]
- Sahu, S.C.; Hayes, A.W. Toxicity of Nanomaterials Found in Human Environment. Toxicol. Res. Appl. 2017, 1, 239784731772635. [Google Scholar] [CrossRef]
- Medina-Ramirez, I.E.; Jimenez-Chavez, A.; De Vizcaya-Ruiz, A. Toxicity of Nanoparticles. In Antimicrobial Activity of Nanoparticles; Guisbiers, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 249–284. ISBN 9780128216378. [Google Scholar]
- Bai, R.; Zhang, L.; Liu, Y.; Li, B.; Wang, L.; Wang, P.; Autrup, H.; Beer, C.; Chen, C. Integrated Analytical Techniques with High Sensitivity for Studying Brain Translocation and Potential Impairment Induced by Intranasally Instilled Copper Nanoparticles. Toxicol. Lett. 2014, 226, 70–80. [Google Scholar] [CrossRef]
- Chen, R.; Hu, B.; Liu, Y.; Xu, J.; Yang, G.; Xu, D.; Chen, C. Beyond PM2.5: The Role of Ultrafine Particles on Adverse Health Effects of Air Pollution. Biochim. Biophys. Acta-Gen. Subj. 2016, 1860, 2844–2855. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dhar, B.; Sen, S. Aerobic Oxidative Amidation of Alkynes Using Titanium Oxide Encapsulated Cuprous Iodide Nanoparticles (CuI@TiO2). New J. Chem. 2018, 42, 12062–12071. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, R.; Xu, R.; Fang, L.; Zhou, J.; Chen, Y.; Ruan, S. Visible-Blind Self-Powered Ultraviolet Photodetector Based on CuI/TiO2Nanostructured Heterojunctions. ACS Appl. Nano Mater. 2022, 5, 16804–16811. [Google Scholar] [CrossRef]
- Sun, M.; Hu, J.; Zhai, C.; Zhu, M.; Pan, J. A P-n Heterojunction of CuI/TiO2 with Enhanced Photoelectrocatalytic Activity for Methanol Electro-Oxidation. Electrochim. Acta 2017, 245, 863–871. [Google Scholar] [CrossRef]
- Raj, V.; Lu, T.; Lockrey, M.; Liu, R.; Kremer, F.; Li, L.; Liu, Y.; Tan, H.H.; Jagadish, C. Introduction of TiO2 in CuI for Its Improved Performance as a P-Type Transparent Conductor. ACS Appl. Mater. Interfaces 2019, 11, 24254–24263. [Google Scholar] [CrossRef] [PubMed]
- Allison, T. Doctor Blades. Gravure 2007, 21, 32–35. [Google Scholar] [CrossRef]
- Fernandez, A.C.; Archana, K.M. Green Synthesis, Characterization, Catalytic and Antibacterial Studies of Copper Iodide Nanoparticles Synthesized Using Brassica Oleracea Var. Capitata f. Rubra Extract. Chem. Data Collect. 2020, 29, 100538. [Google Scholar] [CrossRef]
- Li, J.; Wang, R.; Zhang, D.; Su, Z.; Li, H.; Yan, Y. Copper Iodide (CuI) Coating as a Self-Cleaning Adsorbent for Highly Efficient Dye Removal. J. Alloys Compd. 2019, 774, 191–200. [Google Scholar] [CrossRef]
- Nasiri, S.; Hosseinnezhad, M.; Rabiei, M.; Palevicius, A.; Janusas, G. The Effect of Calcination Temperature on the Photophysical and Mechanical Properties of Copper Iodide (5 Mol%)–Doped Hydroxyapatite. Opt. Mater. 2021, 121, 111559. [Google Scholar] [CrossRef]
- Zak, A.K.; Majid, W.A.; Abrishami, M.E.; Yousefi, R. X-Ray Analysis of ZnO Nanoparticles by Williamson-Hall and Size-Strain Plot Methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar] [CrossRef]
- Mote, V.; Purushotham, Y.; Dole, B. Williamson-Hall Analysis in Estimation of Lattice Strain in Nanometer-Sized ZnO Particles. J. Theor. Appl. Phys. 2012, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Das, R. Determination of Structural Elements of Synthesized Silver Nano-Hexagon from X-Ray Diffraction Analysis. Indian J. Pure Appl. Phys. 2018, 56, 765–772. [Google Scholar] [CrossRef]
- Garcidueñas-Piña, C.; Medina-Ramírez, I.E.; Guzmán, P.; Rico-Martínez, R.; Morales-Domínguez, J.F.; Rubio-Franchini, I. Evaluation of the Antimicrobial Activity of Nanostructured Materials of Titanium Dioxide Doped with Silver and/or Copper and Their Effects on Arabidopsis Thaliana. Int. J. Photoenergy 2016, 2016, 8060847. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari, M.; Salavati-Niasari, M. Copper Iodide Decorated Graphitic Carbon Nitride Sheets with Enhanced Visible-Light Response for Photocatalytic Organic Pollutant Removal and Antibacterial Activities. Ecotoxicol. Environ. Saf. 2021, 208, 111712. [Google Scholar] [CrossRef]
- Sawangphruk, M.; Srimuk, P.; Chiochan, P.; Sangsri, T.; Siwayaprahm, P. Synthesis and Antifungal Activity of Reduced Graphene Oxide Nanosheets. Carbon 2012, 50, 5156–5161. [Google Scholar] [CrossRef]
- Sayed, M.A.; Abdelsalam, H.K.; El-Bassuony, A.A.H. Antimicrobial Activity of Novel Spinel Nanoferrites against Pathogenic Fungi and Bacteria. World J. Microbiol. Biotechnol. 2020, 36, 25. [Google Scholar] [CrossRef]
- Medina-Ramírez, I.E.; Marroquin-Zamudio, A.; Martínez-Montelongo, J.H.; Romo-Lozano, Y.; Zapien, J.A.; Perez-Larios, A. Enhanced Photocatalytic and Antifungal Activity of ZnO–Cu2+ and Ag@ZnO–Cu2+ Materials. Ceram. Int. 2022, 48, 12660–12674. [Google Scholar] [CrossRef]
- Nosek, J.; Holesova, Z.; Kosa, P.; Gacser, A.; Tomaska, L. Biology and Genetics of the Pathogenic Yeast Candida Parapsilosis. Curr. Genet. 2009, 55, 497–509. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [Green Version]
- Mu, Q.; Jiang, G.; Chen, L.; Zhou, H.; Fourches, D.; Tropsha, A.; Yan, B. Chemical Basis of Interactions Between Engineered Nanoparticles and Biological Systems. Chem. Rev. 2014, 114, 7740–7781. [Google Scholar] [CrossRef] [Green Version]
- Fujimori, Y.; Sato, T.; Hayata, T.; Nagao, T.; Nakayama, M.; Nakayama, T.; Sugamata, R.; Suzuki, K. Novel Antiviral Characteristics of Nanosized Copper(I) Iodide Particles Showing Inactivation Activity against 2009 Pandemic H1N1 Influenza Virus. Appl. Environ. Microbiol. 2012, 78, 951–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Mu, L.; Zhang, J.; Han, X.; Shi, H. TiO2/Cu2(OH)2CO3 Nanocomposite as Efficient Antimicrobials for Inactivation of Crop Pathogens in Agriculture. Mater. Sci. Eng. C 2020, 107, 110344. [Google Scholar] [CrossRef] [PubMed]
- Murugadoss, S.; Mülhopt, S.; Diabaté, S.; Ghosh, M.; Paur, H.R.; Stapf, D.; Weiss, C.; Hoet, P.H. Agglomeration State of Titanium-Dioxide (TiO2) Nanomaterials Influences the Dose Deposition and Cytotoxic Responses in Human Bronchial Epithelial Cells at the Air-Liquid Interface. Nanomaterials 2021, 11, 3226. [Google Scholar] [CrossRef] [PubMed]
- Diabaté, S.; Armand, L.; Murugadoss, S.; Dilger, M.; Fritsch-Decker, S.; Schlager, C.; Béal, D.; Arnal, M.E.; Biola-Clier, M.; Ambrose, S.; et al. Air–Liquid Interface Exposure of Lung Epithelial Cells to Low Doses of Nanoparticles to Assess Pulmonary Adverse Effects. Nanomaterials 2020, 11, 65. [Google Scholar] [CrossRef]
- Hufnagel, M.; Schoch, S.; Wall, J.; Strauch, B.M.; Hartwig, A. Toxicity and Gene Expression Profiling of Copper- And Titanium-Based Nanoparticles Using Air-Liquid Interface Exposure. Chem. Res. Toxicol. 2020, 33, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Yi, J.; Chung, K.H.; Ryu, D.Y.; Choi, J.; Park, K. Oxidative Stress and Apoptosis Induced by Titanium Dioxide Nanoparticles in Cultured BEAS-2B Cells. Toxicol. Lett. 2008, 180, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Choi, J.; Park, Y.K.; Park, K. Oxidative Stress Induced by Cerium Oxide Nanoparticles in Cultured BEAS-2B Cells. Toxicology 2008, 245, 90–100. [Google Scholar] [CrossRef] [PubMed]













| TiO2-Cu2+b | CuI | ||
|---|---|---|---|
| d Spacing (Å) | Plane | d Spacing (Å) | Plane |
| 3.68 | (1 0 1) | 3.70 | (1 1 1) |
| 2.47 | (0 0 4) | 2.26 | (2 2 0) |
| 1.98 | (2 0 0) | 1.93 | (3 1 1) |
| 1.78 | (1 0 5) | 1.44 | (3 3 1) |
| 1.53 | (2 1 1) | 1.23 | (4 2 2) |
| NPs | Hydrodynamic Diameter (nm) | Polydispersity Index | Zeta Potential (mV) |
|---|---|---|---|
| CuI | 85.41 ± 2.37 | 0.434 | −51.9 ± 10.71 |
| TiO2-Cu2+/CuI | 234. 07 ± 3.33 | 0.455 | −35.87 ± 1.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, R.; Jimenez-Chávez, A.; De Vizcaya, A.; Lozano-Alvarez, J.A.; Esquivel, K.; Medina-Ramírez, I.E. Synthesis of TiO2-Cu2+/CuI Nanocomposites and Evaluation of Antifungal and Cytotoxic Activity. Nanomaterials 2023, 13, 1900. https://doi.org/10.3390/nano13131900
Hernandez R, Jimenez-Chávez A, De Vizcaya A, Lozano-Alvarez JA, Esquivel K, Medina-Ramírez IE. Synthesis of TiO2-Cu2+/CuI Nanocomposites and Evaluation of Antifungal and Cytotoxic Activity. Nanomaterials. 2023; 13(13):1900. https://doi.org/10.3390/nano13131900
Chicago/Turabian StyleHernandez, Rafael, Arturo Jimenez-Chávez, Andrea De Vizcaya, Juan Antonio Lozano-Alvarez, Karen Esquivel, and Iliana E. Medina-Ramírez. 2023. "Synthesis of TiO2-Cu2+/CuI Nanocomposites and Evaluation of Antifungal and Cytotoxic Activity" Nanomaterials 13, no. 13: 1900. https://doi.org/10.3390/nano13131900
APA StyleHernandez, R., Jimenez-Chávez, A., De Vizcaya, A., Lozano-Alvarez, J. A., Esquivel, K., & Medina-Ramírez, I. E. (2023). Synthesis of TiO2-Cu2+/CuI Nanocomposites and Evaluation of Antifungal and Cytotoxic Activity. Nanomaterials, 13(13), 1900. https://doi.org/10.3390/nano13131900







