In Situ and Operando Characterization Techniques in Stability Study of Perovskite-Based Devices
Abstract
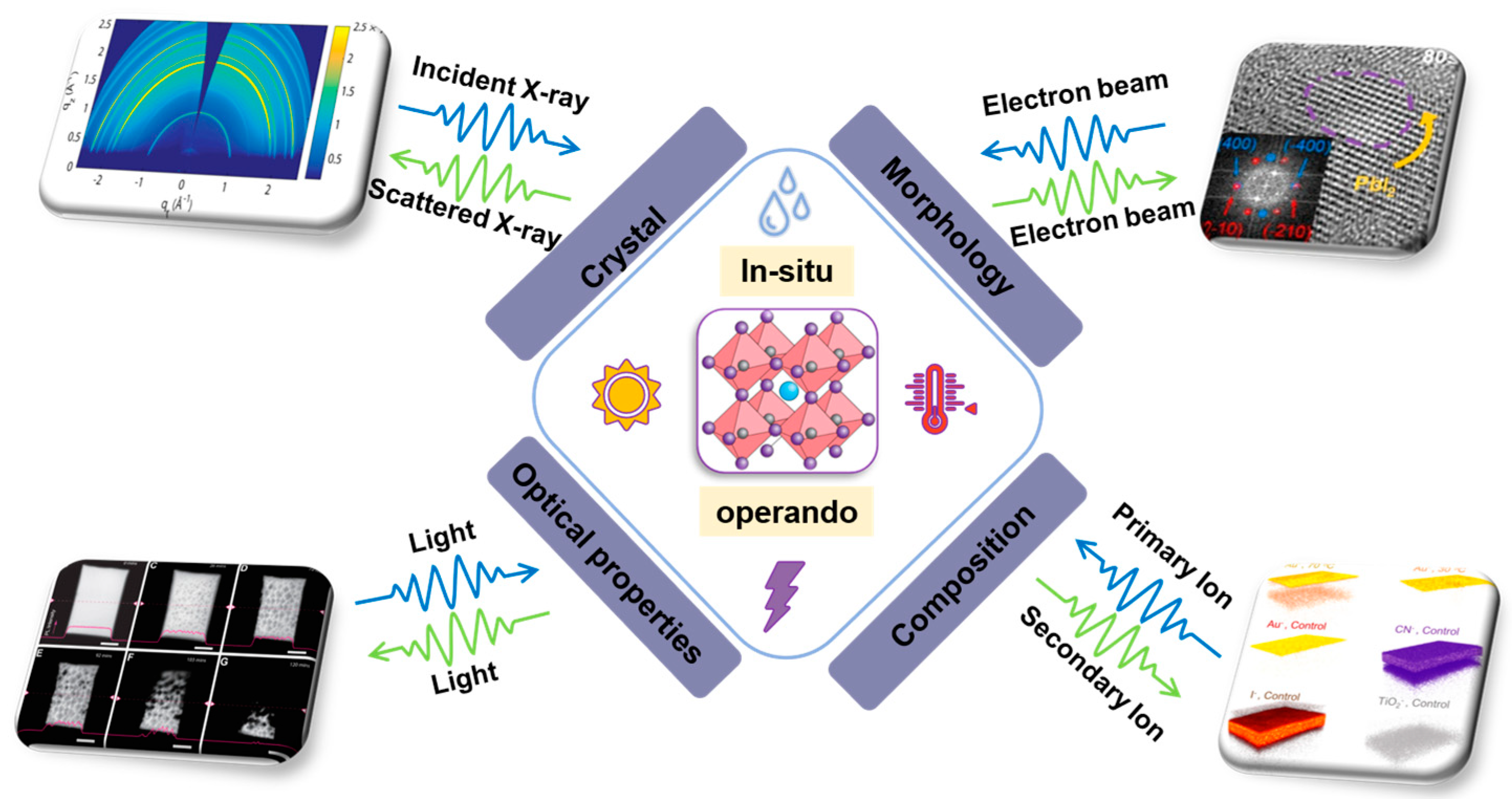
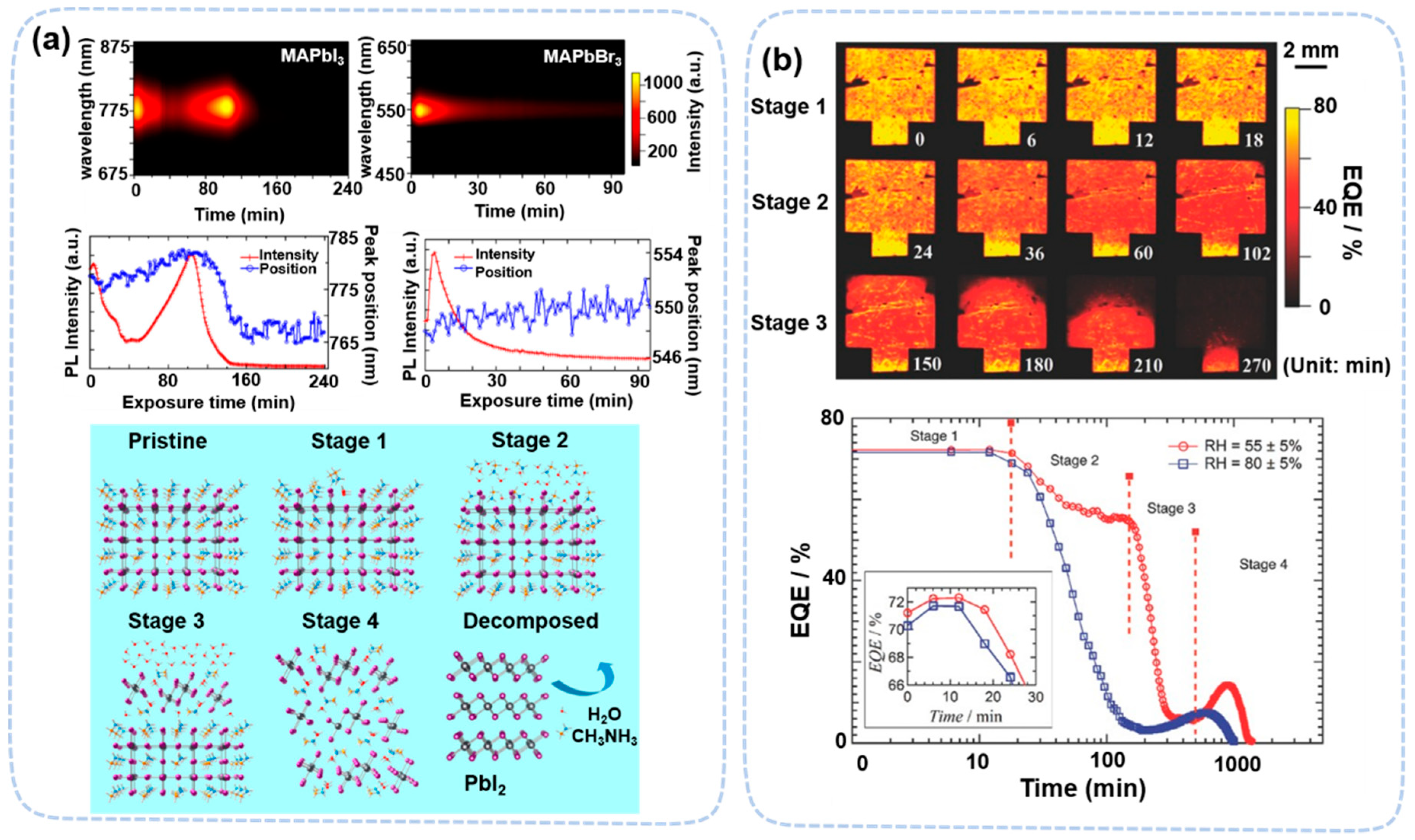
1. Introduction
2. Crystal Structural Characterization Techniques
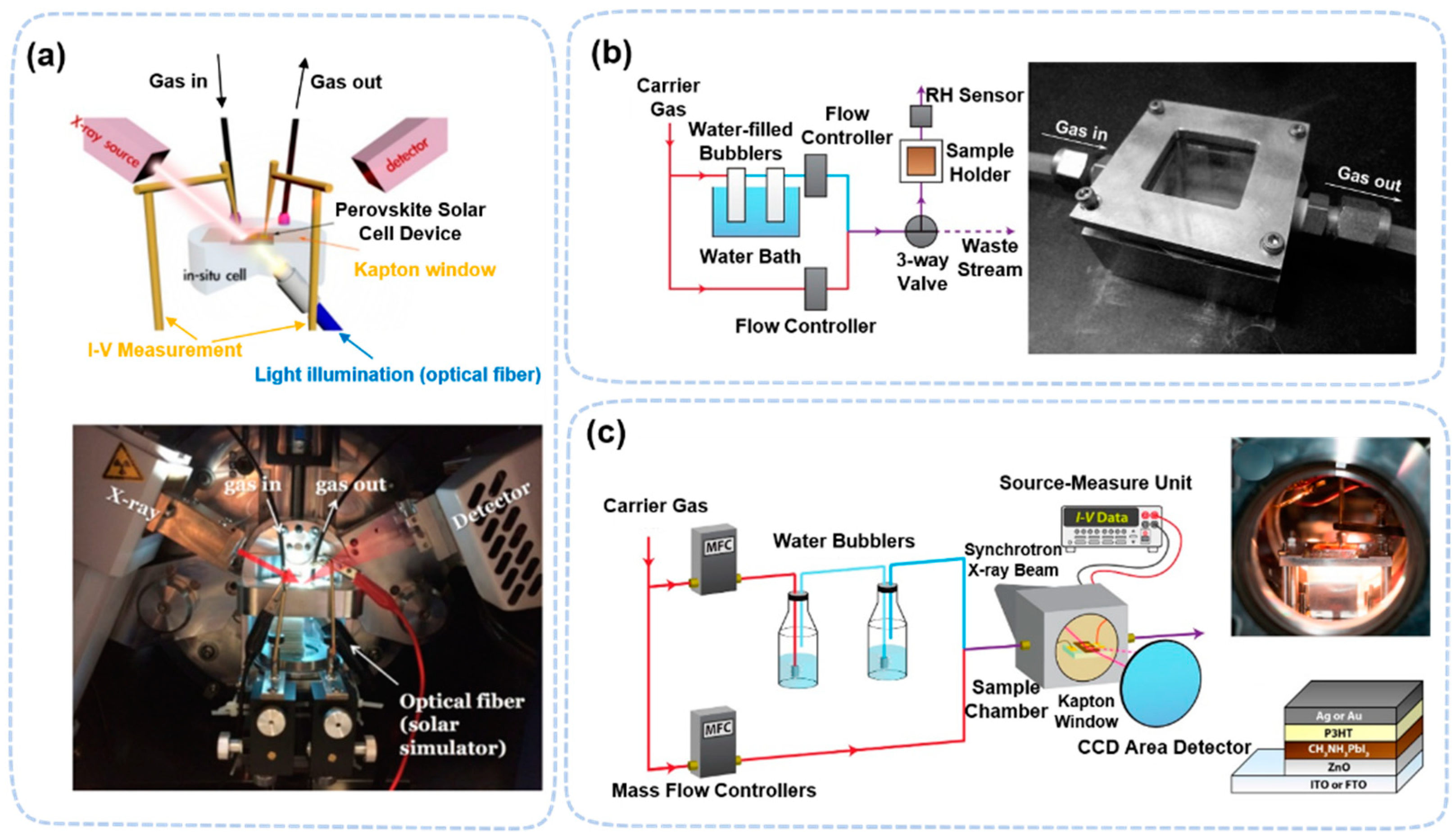
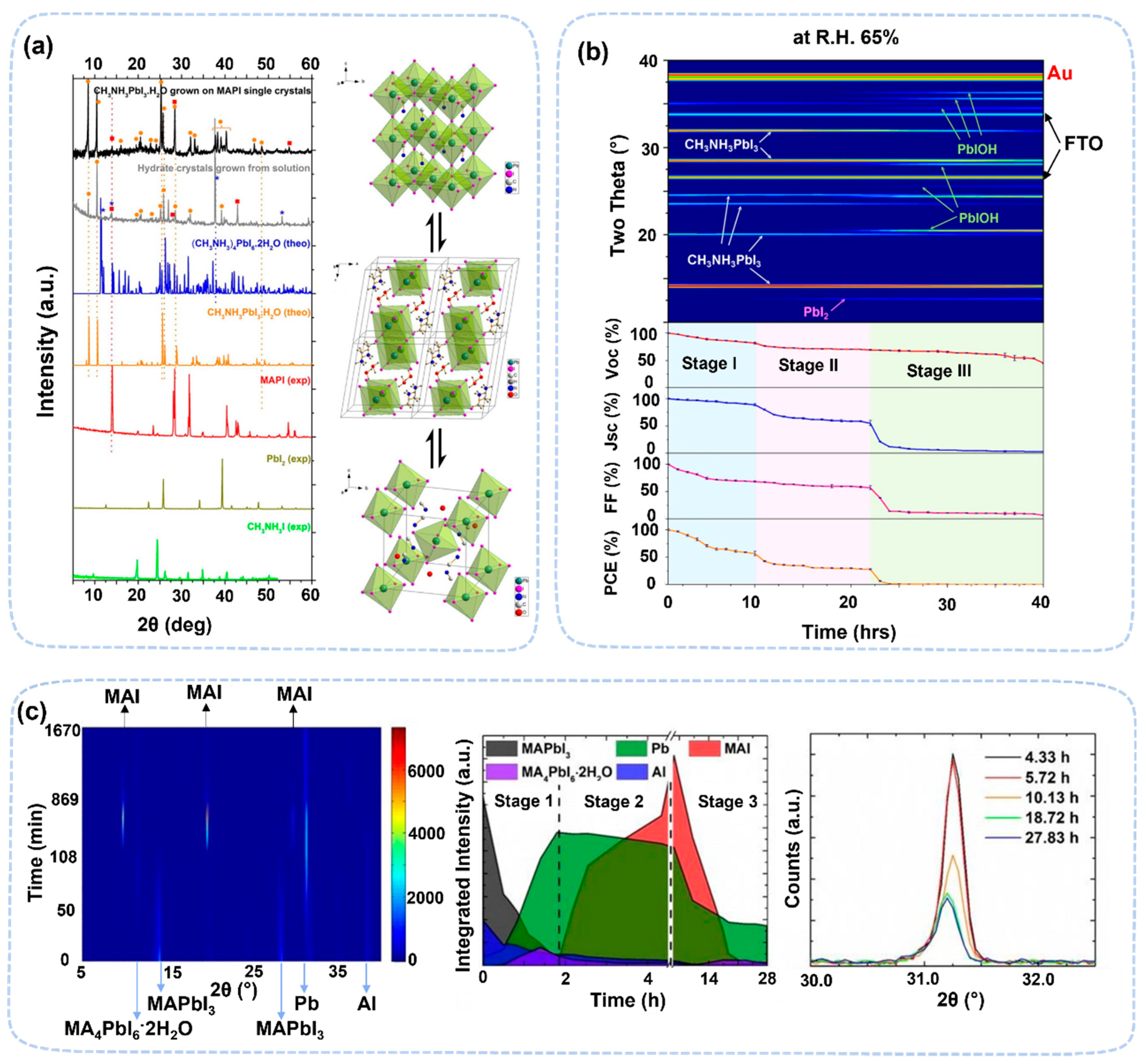
2.1. Perovskite Degradation Studies by In Situ/Operando X-ray Scattering Techniques
2.2. Perovskite Degradation Studies by In Situ/Operando Neutron Scattering Techniques
3. Composition Characterization Techniques
3.1. Perovskite Degradation Studies by Operando Thermogravimetric Analysis Techniques
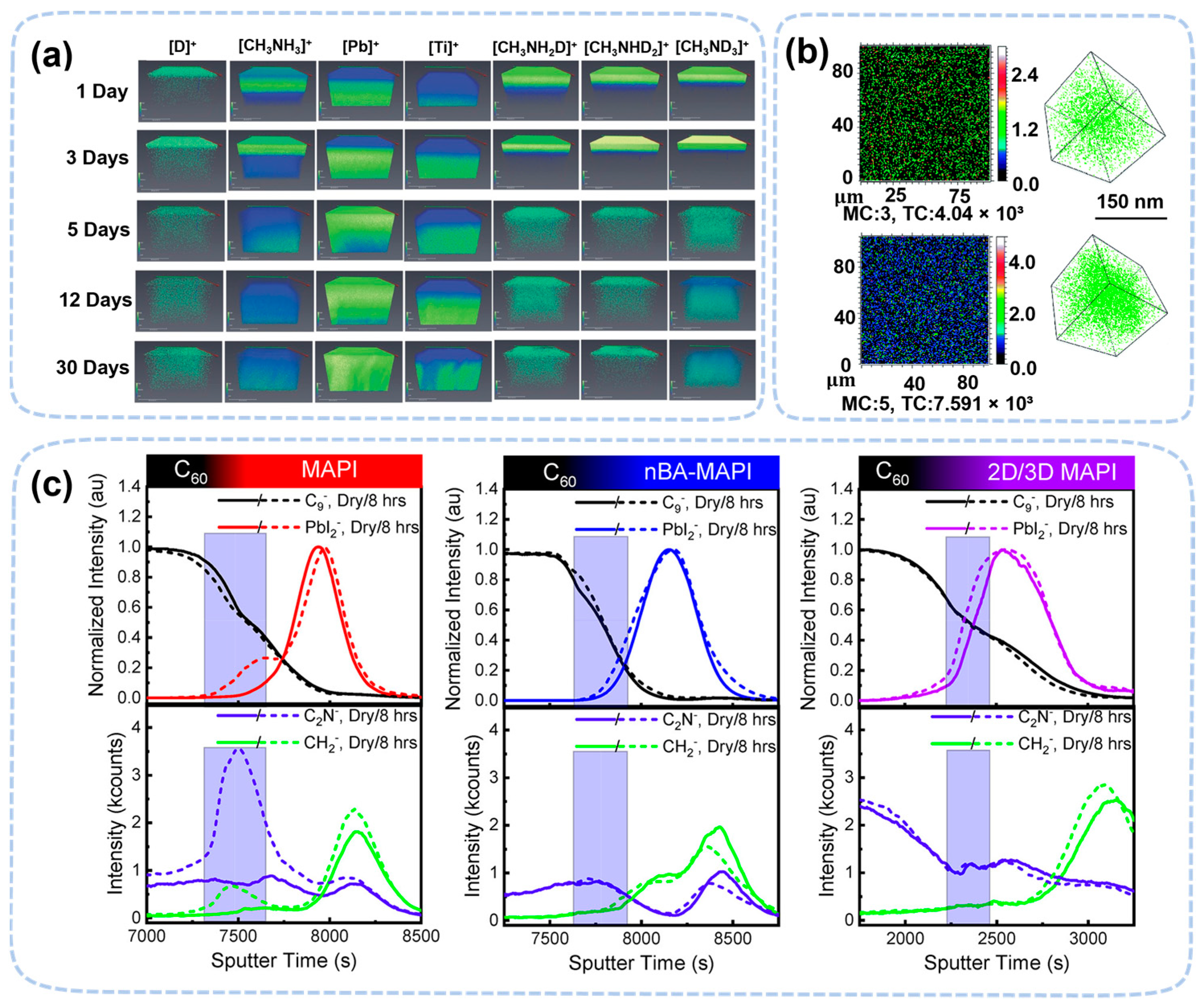
3.2. Perovskite Degradation Studies by In Situ Mass Spectrometry and Time-of-Flight Secondary Ion Mass Spectroscopy
3.3. Perovskite Degradation Studies by In Situ/Operando Photoelectron Spectroscopy Techniques
4. Morphology Structure Characterization Techniques
4.1. Perovskite Degradation Studies by In Situ/Operando Electron Microscopy Techniques
4.2. Perovskite Degradation Studies by Operando Atomic Force Microscopy Techniques
5. Optical Techniques
6. Conclusions and Outlook
6.1. Conclusions
6.2. Outlook
- (1)
- Combine multiple degradation factors in studies.
- (2)
- Extend the measurement range of in situ and operando techniques (higher humidity, higher temperature, and so on).
- (3)
- Integrate multiple techniques.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, S.; Da, P.; Li, C.; Wang, Z.; Yuan, Z.; Fu, F.; Kawecki, M.; Liu, X.; Sakai, N.; Wang, J.T.; et al. Planar perovskite solar cells with long-term stability using ionic liquid additives. Nature 2019, 571, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, N.; Tian, H.; Guo, J.; Wei, Y.; Chen, H.; Miao, Y.; Zou, W.; Pan, K.; He, Y.; et al. Perovskite light-emitting diodes based on spontaneously formed submicrometre-scale structures. Nature 2018, 562, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, K.C.; Li, Y.Q.; Guo, M.; Wang, J.; Ye, Y.; Xie, F.M.; Ren, H.; Gao, X.; Song, F.; et al. Interfacial Potassium-Guided Grain Growth for Efficient Deep-Blue Perovskite Light-Emitting Diodes. Adv. Funct. Mater. 2020, 31, 2006736–2006738. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Ma, J.; Shen, H.; Li, L.; Duan, X.; Li, D. Self-trapped state enabled filterless narrowband photodetections in 2D layered perovskite single crystals. Nat. Commun. 2019, 10, 806. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, C.; Liu, X.; Yao, J.; Zhao, Y.S. Materials chemistry and engineering in metal halide perovskite lasers. Chem. Soc. Rev. 2020, 49, 951–982. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Z.; Cui, D.; Ren, X.; Sun, J.; Liu, X.; Zhang, J.; Wei, Q.; Fan, H.; Yu, F.; et al. Two-Inch-Sized Perovskite CH3 NH3 PbX3 (X = Cl, Br, I) Crystals: Growth and Characterization. Adv. Mater. 2015, 27, 5176–5183. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, Y.; Shao, Y.; Yan, Y. Understanding the physical properties of hybrid perovskites for photovoltaic applications. Nat. Rev. Mater. 2017, 2, 17042. [Google Scholar] [CrossRef]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Solar cells. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Poindexter, J.R.; Hoye, R.L.Z.; Nienhaus, L.; Kurchin, R.C.; Morishige, A.E.; Looney, E.E.; Osherov, A.; Correa-Baena, J.P.; Lai, B.; Bulovic, V.; et al. High Tolerance to Iron Contamination in Lead Halide Perovskite Solar Cells. ACS Nano 2017, 11, 7101–7109. [Google Scholar] [CrossRef]
- Steirer, K.X.; Schulz, P.; Teeter, G.; Stevanovic, V.; Yang, M.; Zhu, K.; Berry, J.J. Defect Tolerance in Methylammonium Lead Triiodide Perovskite. ACS Energy Lett. 2016, 1, 360–366. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T.J. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-halide anion engineering for alpha-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.; Yun, H.S.; Paik, M.J.; Noh, E.; Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S.I. Controlled growth of perovskite layers with volatile alkylammonium chlorides. Nature 2023, 616, 724–730. [Google Scholar] [CrossRef]
- Klampaftis, E.; Richards, B.S. Improvement in multi-crystalline silicon solar cell efficiency via addition of luminescent material to EVA encapsulation layer. Prog. Photovolt. 2011, 19, 345–351. [Google Scholar] [CrossRef]
- Singh, A.N.; Kajal, S.; Kim, J.; Jana, A.; Kim, J.Y.; Kim, K.S. Interface Engineering Driven Stabilization of Halide Perovskites against Moisture, Heat, and Light for Optoelectronic Applications. Adv. Energy Mater. 2020, 10, 2000768. [Google Scholar] [CrossRef]
- Béchu, S.; Ralaiarisoa, M.; Etcheberry, A.; Schulz, P. Photoemission Spectroscopy Characterization of Halide Perovskites. Adv. Energy Mater. 2020, 10, 1904007. [Google Scholar] [CrossRef]
- Kosasih, F.U.; Ducati, C. Characterising degradation of perovskite solar cells through in-situ and operando electron microscopy. Nano Energy 2018, 47, 243–256. [Google Scholar] [CrossRef]
- Wei, J.; Wang, Q.; Huo, J.; Gao, F.; Gan, Z.; Zhao, Q.; Li, H. Mechanisms and Suppression of Photoinduced Degradation in Perovskite Solar Cells. Adv. Energy Mater. 2020, 11, 2002326. [Google Scholar] [CrossRef]
- Hidalgo, J.; Castro-Méndez, A.F.; Correa-Baena, J.P. Imaging and Mapping Characterization Tools for Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1900444. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding Degradation Mechanisms and Improving Stability of Perovskite Photovoltaics. Chem. Rev. 2019, 119, 3418–3451. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Kelly, T.L. In situ studies of the degradation mechanisms of perovskite solar cells. EcoMat 2020, 2, e12025. [Google Scholar] [CrossRef]
- Zhou, Y.; Sternlicht, H.; Padture, N.P. Transmission Electron Microscopy of Halide Perovskite Materials and Devices. Joule 2019, 3, 641–661. [Google Scholar] [CrossRef]
- Szostak, R.; Goncalves, A.d.S.; Freitas, J.N.d.; Marchezi, P.E.; Araujo, F.L.d.; Tolentino, H.C.N.; Toney, M.F.; Marques, F.d.C.; Nogueira, A.F. In Situ and Operando Characterizations of Metal Halide Perovskite and Solar Cells: Insights from Lab-Sized Devices to Upscaling Processes. Chem. Rev. 2023, 123, 3160–3236. [Google Scholar] [CrossRef]
- Meng, X.; Tian, X.; Zhang, S.; Zhou, J.; Zhang, Y.; Liu, Z.; Chen, W. In Situ Characterization for Understanding the Degradation in Perovskite Solar Cells. Sol. RRL 2022, 6, 2200280. [Google Scholar] [CrossRef]
- Dunfield, S.P.; Bliss, L.; Zhang, F.; Luther, J.M.; Zhu, K.; Hest, M.F.A.M.; Reese, M.O.; Berry, J.J. From Defects to Degradation: A Mechanistic Understanding of Degradation in Perovskite Solar Cell Devices and Modules. Adv. Energy Mater. 2020, 10, 1904054. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z.; Zhang, W.; Jiang, Z.; Chen, W.; Chen, R.; Huang, Y.; Yang, Z.; Zhang, Y.; Han, L.; et al. Barrier Designs in Perovskite Solar Cells for Long-Term Stability. Adv. Energy Mater. 2020, 10, 2001610. [Google Scholar] [CrossRef]
- Nguyen, L.; Tao, F.F.; Tang, Y.; Dou, J.; Bao, X.-J. Understanding Catalyst Surfaces during Catalysis through Near Ambient Pressure X-ray Photoelectron Spectroscopy. Chem. Rev. 2019, 119, 6822–6905. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Ford, M.E.; Gregory, D.; Hu, R.; Keturakis, C.J.; Lwin, S.; Tang, Y.; Yang, Z.; Zhu, M.; Bañares, M.A.; et al. A decade+ of operando spectroscopy studies. Catal. Today 2017, 283, 27–53. [Google Scholar] [CrossRef]
- Bañares, M.A.; Guerrero-Pérez, M.O.; Fierro, J.L.G.; Cortez, G.G. Raman spectroscopy during catalytic operations with on-line activity measurement (operando spectroscopy): A method for understanding the active centres of cations supported on porous materials. J. Mater. Chem. 2002, 12, 3337–3342. [Google Scholar] [CrossRef]
- Zhao, L.; Kerner, R.A.; Xiao, Z.; Lin, Y.L.; Lee, K.M.; Schwartz, J.; Rand, B.P. Redox Chemistry Dominates the Degradation and Decomposition of Metal Halide Perovskite Optoelectronic Devices. ACS Energy Lett. 2016, 1, 595–602. [Google Scholar] [CrossRef]
- Staebler, D.L.; Crandall, R.S.; Williams, R. Stability of n-i-p amorphous silicon solar cells Appl. Phys. Lett. 1981, 39, 9. [Google Scholar] [CrossRef]
- Domanski, K.; Correa-Baena, J.P.; Mine, N.; Nazeeruddin, M.K.; Abate, A.; Saliba, M.; Tress, W.; Hagfeldt, A.; Gratzel, M. Not All That Glitters Is Gold: Metal-Migration-Induced Degradation in Perovskite Solar Cells. ACS Nano 2016, 10, 6306–6314. [Google Scholar] [CrossRef]
- Fan, Z.; Xiao, H.; Wang, Y.; Zhao, Z.; Lin, Z.; Cheng, H.-C.; Lee, S.-J.; Wang, G.; Feng, Z.; Goddard, W.A.; et al. Layer-by-Layer Degradation of Methylammonium Lead Tri-iodide Perovskite Microplates. Joule 2017, 1, 548–562. [Google Scholar] [CrossRef]
- Fang, H.-H.; Yang, J.; Tao, S.; Adjokatse, S.; Kamminga, M.E.; Ye, J.; Blake, G.R.; Even, J.; Loi, M.A. Unravelling Light-Induced Degradation of Layered Perovskite Crystals and Design of Efficient Encapsulation for Improved Photostability. Adv. Funct. Mater. 2018, 28, 1800305. [Google Scholar] [CrossRef]
- Yin, J.; Teobaldi, G.; Liu, L.M. The Role of Thermal Fluctuations and Vibrational Entropy: A Theoretical Insight into the delta-to-alpha Transition of FAPbI(3). J. Phys. Chem. Lett. 2022, 13, 3089–3095. [Google Scholar] [CrossRef]
- Niu, T.; Chao, L.; Dong, X.; Fu, L.; Chen, Y. Phase-Pure α-FAPbI3 for Perovskite Solar Cells. J. Phys. Chem. Lett. 2022, 13, 1845–1854. [Google Scholar] [CrossRef]
- Masi, S.; Gualdrón-Reyes, A.F.; Mora-Seró, I. Stabilization of Black Perovskite Phase in FAPbI3 and CsPbI3. ACS Energy Lett. 2020, 5, 1974–1985. [Google Scholar] [CrossRef]
- Su, Z.; Wang, C.; Zheng, G.; Gao, X. Impacts of MAPbBr3 Additive on Crystallization Kinetics of FAPbI3 Perovskite for High Performance Solar Cells. Coatings 2021, 11, 545. [Google Scholar] [CrossRef]
- Li, G.; Su, Z.; Canil, L.; Hughes, D.; Aldamasy, M.H.; Dagar, J.; Trofimov, S.; Wang, L.; Zuo, W.; Jerónimo-Rendon, J.J.; et al. Highly efficient p-i-n perovskite solar cells that endure temperature variations. Science 2023, 379, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Li, W.; Meng, F.; Wang, L.; Dong, H.; Qiu, Y. Study on the stability of CH3NH3PbI3 films and the effect of post-modification by aluminum oxide in all-solid-state hybrid solar cells. J. Mater. Chem. A 2014, 2, 705–710. [Google Scholar] [CrossRef]
- Christians, J.A.; Miranda Herrera, P.A.; Kamat, P.V. Transformation of the excited state and photovoltaic efficiency of CH3NH3PbI3 perovskite upon controlled exposure to humidified air. J. Am. Chem. Soc. 2015, 137, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, F.; Wang, Y.; Zhu, L.; Hu, Y.; Lou, Z.; Hou, Y.; Teng, F. High-Performance Photodiode-Type Photodetectors Based on Polycrystalline Formamidinium Lead Iodide Perovskite Thin Films. Sci. Rep. 2018, 8, 11157. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Siempelkamp, B.D.; Liu, D.; Kelly, T.L. Investigation of CH3NH3PbI3 degradation rates and mechanisms in controlled humidity environments using in situ techniques. ACS Nano 2015, 9, 1955–1963. [Google Scholar] [CrossRef]
- Fransishyn, K.M.; Kundu, S.; Kelly, T.L. Elucidating the Failure Mechanisms of Perovskite Solar Cells in Humid Environments Using In Situ Grazing-Incidence Wide-Angle X-ray Scattering. ACS Energy Lett. 2018, 3, 2127–2133. [Google Scholar] [CrossRef]
- Chen, B.-A.; Lin, J.-T.; Suen, N.-T.; Tsao, C.-W.; Chu, T.-C.; Hsu, Y.-Y.; Chan, T.-S.; Chan, Y.-T.; Yang, J.-S.; Chiu, C.-W.; et al. In Situ Identification of Photo- and Moisture-Dependent Phase Evolution of Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 342–348. [Google Scholar] [CrossRef]
- Leguy, A.M.A.; Hu, Y.; Campoy-Quiles, M.; Alonso, M.I.; Weber, O.J.; Azarhoosh, P.; van Schilfgaarde, M.; Weller, M.T.; Bein, T.; Nelson, J.; et al. Reversible Hydration of CH3NH3PbI3 in Films, Single Crystals, and Solar Cells. Chem. Mater. 2015, 27, 3397–3407. [Google Scholar] [CrossRef]
- Li, D.; Bretschneider, S.A.; Bergmann, V.W.; Hermes, I.M.; Mars, J.; Klasen, A.; Lu, H.; Tremel, W.; Mezger, M.; Butt, H.-J.; et al. Humidity-Induced Grain Boundaries in MAPbI3 Perovskite Films. J. Phys. Chem. C 2016, 120, 6363–6368. [Google Scholar] [CrossRef]
- Schlipf, J.; Biessmann, L.; Oesinghaus, L.; Berger, E.; Metwalli, E.; Lercher, J.A.; Porcar, L.; Muller-Buschbaum, P. In Situ Monitoring the Uptake of Moisture into Hybrid Perovskite Thin Films. J. Phys. Chem. Lett. 2018, 9, 2015–2021. [Google Scholar] [CrossRef]
- Long, M.; Zhang, T.; Liu, M.; Chen, Z.; Wang, C.; Xie, W.; Xie, F.; Chen, J.; Li, G.; Xu, J. Abnormal Synergetic Effect of Organic and Halide Ions on the Stability and Optoelectronic Properties of a Mixed Perovskite via In Situ Characterizations. Adv. Mater. 2018, 30, 1801562. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Bowring, A.R.; Meng, A.C.; McGehee, M.D.; McIntyre, P.C. Thermal Stability of Mixed Cation Metal Halide Perovskites in Air. ACS Appl. Mater. Interfaces 2018, 10, 5485–5491. [Google Scholar] [CrossRef] [PubMed]
- Nenon, D.P.; Christians, J.A.; Wheeler, L.M.; Blackburn, J.L.; Sanehira, E.M.; Dou, B.; Olsen, M.L.; Zhu, K.; Berry, J.J.; Luther, J.M. Structural and chemical evolution of methylammonium lead halide perovskites during thermal processing from solution. Energy Environ. Sci. 2016, 9, 2072–2082. [Google Scholar] [CrossRef]
- Sutter-Fella, C.M.; Ngo, Q.P.; Cefarin, N.; Gardner, K.L.; Tamura, N.; Stan, C.V.; Drisdell, W.S.; Javey, A.; Toma, F.M.; Sharp, I.D. Cation-Dependent Light-Induced Halide Demixing in Hybrid Organic-Inorganic Perovskites. Nano Lett. 2018, 18, 3473–3480. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Z.-K.; Yang, Y.-G.; Hu, Y.; Feng, S.-L.; Wang, J.-M.; Gao, X.-Y.; Liao, L.-S. Copper Salts Doped Spiro-OMeTAD for High-Performance Perovskite Solar Cells. Adv. Energy Mater. 2016, 6, 1601156. [Google Scholar] [CrossRef]
- Miyadera, T.; Shibata, Y.; Koganezawa, T.; Murakami, T.N.; Sugita, T.; Tanigaki, N.; Chikamatsu, M. Crystallization Dynamics of Organolead Halide Perovskite by Real-Time X-ray Diffraction. Nano Lett. 2015, 15, 5630–5634. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dar, M.I.; Ono, L.K.; Zhang, T.; Kan, M.; Li, Y.; Zhang, L.; Wang, X.; Yang, Y.; Gao, X.; et al. Thermodynamically stabilized β-CsPbI3-based perovskite solar cells with efficiencies. Science 2019, 365, 591–595. [Google Scholar] [CrossRef]
- Qin, M.; Tse, K.; Lau, T.K.; Li, Y.; Su, C.J.; Yang, G.; Chen, J.; Zhu, J.; Jeng, U.S.; Li, G.; et al. Manipulating the Mixed-Perovskite Crystallization Pathway Unveiled by In Situ GIWAXS. Adv. Mater. 2019, 31, e1901284. [Google Scholar] [CrossRef]
- Lu, L.; Shen, K.C.; Wang, J.; Su, Z.; Li, Y.; Chen, L.; Luo, Y.; Song, F.; Gao, X.; Tang, J.X. Interaction of the Cation and Vacancy in Hybrid Perovskites Induced by Light Illumination. ACS Appl. Mater. Interfaces 2020, 12, 42369–42377. [Google Scholar] [CrossRef]
- Song, J.; Zhou, G.; Chen, W.; Zhang, Q.; Ali, J.; Hu, Q.; Wang, J.; Wang, C.; Feng, W.; Djurisic, A.B.; et al. Unraveling the Crystallization Kinetics of 2D Perovskites with Sandwich-Type Structure for High-Performance Photovoltaics. Adv. Mater. 2020, 32, e2002784. [Google Scholar] [CrossRef]
- Qin, M.; Chan, P.F.; Lu, X. A Systematic Review of Metal Halide Perovskite Crystallization and Film Formation Mechanism Unveiled by In Situ GIWAXS. Adv. Mater. 2021, 33, e2105290. [Google Scholar] [CrossRef] [PubMed]
- Schlipf, J.; Hu, Y.; Pratap, S.; Bießmann, L.; Hohn, N.; Porcar, L.; Bein, T.; Docampo, P.; Müller-Buschbaum, P. Shedding Light on the Moisture Stability of 3D/2D Hybrid Perovskite Heterojunction Thin Films. ACS Appl. Energy Mater. 2019, 2, 1011–1018. [Google Scholar] [CrossRef]
- Hada, M.; Hasegawa, Y.; Nagaoka, R.; Miyake, T.; Abdullaev, U.; Ota, H.; Nishikawa, T.; Yamashita, Y.; Hayashi, Y. In-situ X-ray diffraction reveals the degradation of crystalline CH3NH3PbI3 by water-molecule collisions at room temperature. Jpn. J. Appl. Phys. 2018, 57, 028001. [Google Scholar] [CrossRef]
- Pistor, P.; Burwig, T.; Brzuska, C.; Weber, B.; Fränzel, W. Thermal stability and miscibility of co-evaporated methyl ammonium lead halide (MAPbX3, X = I, Br, Cl) thin films analysed by in situ X-ray diffraction. J. Mater. Chem. A 2018, 6, 11496–11506. [Google Scholar] [CrossRef]
- Tang, X.; Brandl, M.; May, B.; Levchuk, I.; Hou, Y.; Richter, M.; Chen, H.; Chen, S.; Kahmann, S.; Osvet, A.; et al. Photoinduced degradation of methylammonium lead triiodide perovskite semiconductors. J. Mater. Chem. A 2016, 4, 15896–15903. [Google Scholar] [CrossRef]
- Ruf, F.; Rietz, P.; Aygüler, M.F.; Kelz, I.; Docampo, P.; Kalt, H.; Hetterich, M. The Bandgap as a Moving Target: Reversible Bandgap Instabilities in Multiple-Cation Mixed-Halide Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 2995–3001. [Google Scholar] [CrossRef]
- Barker, A.J.; Sadhanala, A.; Deschler, F.; Gandini, M.; Senanayak, S.P.; Pearce, P.M.; Mosconi, E.; Pearson, A.J.; Wu, Y.; Srimath Kandada, A.R.; et al. Defect-Assisted Photoinduced Halide Segregation in Mixed-Halide Perovskite Thin Films. ACS Energy Lett. 2017, 2, 1416–1424. [Google Scholar] [CrossRef]
- Kim, N.K.; Min, Y.H.; Noh, S.; Cho, E.; Jeong, G.; Joo, M.; Ahn, S.W.; Lee, J.S.; Kim, S.; Ihm, K.; et al. Investigation of Thermally Induced Degradation in CH3NH3PbI3 Perovskite Solar Cells using In-situ Synchrotron Radiation Analysis. Sci. Rep. 2017, 7, 4645. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Hawash, Z.; Raga, S.R.; Ono, L.K.; Qi, Y. Thermal degradation of CH3NH3PbI3 perovskite into NH3 and CH3I gases observed by coupled thermogravimetry–mass spectrometry analysis. Energy Environ. Sci. 2016, 9, 3406–3410. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Ono, L.K.; Qi, Y. Thermal degradation of formamidinium based lead halide perovskites into sym-triazine and hydrogen cyanide observed by coupled thermogravimetry-mass spectrometry analysis. J. Mater. Chem. A 2019, 7, 16912–16919. [Google Scholar] [CrossRef]
- Williams, A.E.; Holliman, P.J.; Carnie, M.J.; Davies, M.L.; Worsley, D.A.; Watson, T.M. Perovskite processing for photovoltaics: A spectro-thermal evaluation. J. Mater. Chem. A 2014, 2, 19338–19346. [Google Scholar] [CrossRef]
- Ciccioli, A.; Latini, A. Thermodynamics and the Intrinsic Stability of Lead Halide Perovskites CH3NH3PbX3. J. Phys. Chem. Lett. 2018, 9, 3756–3765. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hua, X.; Liu, S.C.; Qiao, H.W.; Yang, H.G.; Long, Y.T.; Tian, H. In situ and real-time ToF-SIMS analysis of light-induced chemical changes in perovskite CH3NH3PbI3. Chem. Commun. 2018, 54, 5434–5437. [Google Scholar] [CrossRef]
- Nickel, N.H.; Lang, F.; Brus, V.V.; Shargaieva, O.; Rappich, J. Unraveling the Light-Induced Degradation Mechanisms of CH3NH3PbI3 Perovskite Films. Adv. Electron. Mater. 2017, 3, 1700158. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Ono, L.K.; Maeda, M.; Jiang, Y.; Hawash, Z.; Qi, Y. Photodecomposition and thermal decomposition in methylammonium halide lead perovskites and inferred design principles to increase photovoltaic device stability. J. Mater. Chem. A 2018, 6, 9604–9612. [Google Scholar] [CrossRef]
- Song, Z.; Wang, C.; Phillips, A.B.; Grice, C.R.; Zhao, D.; Yu, Y.; Chen, C.; Li, C.; Yin, X.; Ellingson, R.J.; et al. Probing the origins of photodegradation in organic–inorganic metal halide perovskites with time-resolved mass spectrometry. Sustain. Energy Fuels 2018, 2, 2460–2467. [Google Scholar] [CrossRef]
- Zhang, T.; Cheung, S.H.; Meng, X.; Zhu, L.; Bai, Y.; Ho, C.H.Y.; Xiao, S.; Xue, Q.; So, S.K.; Yang, S. Pinning Down the Anomalous Light Soaking Effect toward High-Performance and Fast-Response Perovskite Solar Cells: The Ion-Migration-Induced Charge Accumulation. J. Phys. Chem. Lett. 2017, 8, 5069–5076. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.; Wu, Y.; Shen, H.; Peng, J.; Zhao, S.; Wu, N.; Lockrey, M.; White, T.; Weber, K.; Catchpole, K. Light and elevated temperature induced degradation (LeTID) in perovskite solar cells and development of stable semi-transparent cells. Sol. Energy Mat. Sol. Cells 2018, 188, 27–36. [Google Scholar] [CrossRef]
- Lin, W.-C.; Chang, H.-Y.; Abbasi, K.; Shyue, J.-J.; Burda, C. 3D In Situ ToF-SIMS Imaging of Perovskite Films under Controlled Humidity Environmental Conditions. Adv. Mater. Interfaces 2017, 4, 1600673. [Google Scholar] [CrossRef]
- Xu, D.; Liu, D.; Xie, T.; Cao, Y.; Wang, J.G.; Ning, Z.J.; Long, Y.T.; Tian, H. Plasmon resonance scattering at perovskite CH3NH3PbI3 coated single gold nanoparticles: Evidence for electron transfer. Chem. Commun. 2016, 52, 9933–9936. [Google Scholar] [CrossRef]
- Aristidou, N.; Eames, C.; Islam, M.S.; Haque, S.A. Insights into the increased degradation rate of CH3NH3PbI3 solar cells in combined water and O2 environments. J. Mater. Chem. A 2017, 5, 25469–25475. [Google Scholar] [CrossRef]
- Aristidou, N.; Eames, C.; Sanchez-Molina, I.; Bu, X.; Kosco, J.; Islam, M.S.; Haque, S.A. Fast oxygen diffusion and iodide defects mediate oxygen-induced degradation of perovskite solar cells. Nat. Commun. 2017, 8, 15218. [Google Scholar] [CrossRef] [PubMed]
- Senocrate, A.; Acartürk, T.; Kim, G.Y.; Merkle, R.; Starke, U.; Grätzel, M.; Maier, J. Interaction of oxygen with halide perovskites. J. Mater. Chem. A 2018, 6, 10847–10855. [Google Scholar] [CrossRef]
- Wygant, B.R.; Ye, A.Z.; Dolocan, A.; Vu, Q.; Abbot, D.M.; Mullins, C.B. Probing the Degradation Chemistry and Enhanced Stability of 2D Organolead Halide Perovskites. J. Am. Chem. Soc. 2019, 141, 18170–18181. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, Q.; Li, N.; Wang, L. Direct Evidence of Ion Diffusion for the Silver-Electrode-Induced Thermal Degradation of Inverted Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1602922. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Zhou, F.; Dahan, J.; Wang, X.; Li, Z.; Shen, W. Temperature Gradient-Induced Instability of Perovskite via Ion Transport. ACS Appl. Mater. Interfaces 2018, 10, 835–844. [Google Scholar] [CrossRef]
- Palma, A.L.; Cina, L.; Busby, Y.; Marsella, A.; Agresti, A.; Pescetelli, S.; Pireaux, J.J.; Di Carlo, A. Mesoscopic Perovskite Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2016, 8, 26989–26997. [Google Scholar] [CrossRef]
- Domanski, K.; Roose, B.; Matsui, T.; Saliba, M.; Turren-Cruz, S.-H.; Correa-Baena, J.-P.; Carmona, C.R.; Richardson, G.; Foster, J.M.; De Angelis, F.; et al. Migration of cations induces reversible performance losses over day/night cycling in perovskite solar cells. Energy Environ. Sci. 2017, 10, 604–613. [Google Scholar] [CrossRef]
- Lee, H.; Ko, D.; Lee, C. Direct Evidence of Ion-Migration-Induced Degradation of Ultrabright Perovskite Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2019, 11, 11667–11673. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, W.; Tan, H.; Fu, R.; Li, Q.; Lin, F.; Yu, D.; Walters, G.; Sargent, E.H.; Zhao, Q. Mobile-Ion-Induced Degradation of Organic Hole-Selective Layers in Perovskite Solar Cells. J. Phys. Chem. C 2017, 121, 14517–14523. [Google Scholar] [CrossRef]
- Lee, H.; Lee, C. Analysis of Ion-Diffusion-Induced Interface Degradation in Inverted Perovskite Solar Cells via Restoration of the Ag Electrode. Adv. Energy Mater. 2018, 8, 1702197. [Google Scholar] [CrossRef]
- Drozdov, M.N.; Yunin, P.A.; Travkin, V.V.; Koptyaev, A.I.; Pakhomov, G.L. Direct Imaging of Current-Induced Transformation of a Perovskite/Electrode Interface. Adv. Mater. Interfaces 2019, 6, 1900364. [Google Scholar] [CrossRef]
- Wang, J.; Senanayak, S.P.; Liu, J.; Hu, Y.; Shi, Y.; Li, Z.; Zhang, C.; Yang, B.; Jiang, L.; Di, D.; et al. Investigation of Electrode Electrochemical Reactions in CH3 NH3 PbBr3 Perovskite Single-Crystal Field-Effect Transistors. Adv. Mater. 2019, 31, 1902618. [Google Scholar] [CrossRef] [PubMed]
- Bridges, C.A.; Sun, X.-G.; Zhao, J.; Paranthaman, M.P.; Dai, S. In Situ Observation of Solid Electrolyte Interphase Formation in Ordered Mesoporous Hard Carbon by Small-Angle Neutron Scattering. J. Phys. Chem. C 2012, 116, 7701–7711. [Google Scholar] [CrossRef]
- Huang, W.; Manser, J.S.; Kamat, P.V.; Ptasinska, S. Evolution of Chemical Composition, Morphology, and Photovoltaic Efficiency of CH3NH3PbI3 Perovskite under Ambient Conditions. Chem. Mater. 2015, 28, 303–311. [Google Scholar] [CrossRef]
- Philippe, B.; Park, B.-W.; Lindblad, R.; Oscarsson, J.; Ahmadi, S.; Johansson, E.M.J.; Rensmo, H. Chemical and Electronic Structure Characterization of Lead Halide Perovskites and Stability Behavior under Different Exposures—A Photoelectron Spectroscopy Investigation. Chem. Mater. 2015, 27, 1720–1731. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Wang, C.; Wang, C.; Xie, F.; Yang, J.; Gao, Y. Degradation by Exposure of Coevaporated CH3NH3PbI3 Thin Films. J. Phys. Chem. C 2015, 119, 23996–24002. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Wang, C.; Ecker, B.; Yang, J.; Huang, J.; Gao, Y. Light-Induced Degradation of CH3NH3PbI3 Hybrid Perovskite Thin Film. J. Phys. Chem. C 2017, 121, 3904–3910. [Google Scholar] [CrossRef]
- Ralaiarisoa, M.; Salzmann, I.; Zu, F.S.; Koch, N. Effect of Water, Oxygen, and Air Exposure on CH3NH3PbI3–xClx Perovskite Surface Electronic Properties. Adv. Electron. Mater. 2018, 4, 1800307. [Google Scholar] [CrossRef]
- Chun-Ren Ke, J.; Walton, A.S.; Lewis, D.J.; Tedstone, A.; O’Brien, P.; Thomas, A.G.; Flavell, W.R. In situ investigation of degradation at organometal halide perovskite surfaces by X-ray photoelectron spectroscopy at realistic water vapour pressure. Chem. Commun. 2017, 53, 5231–5234. [Google Scholar] [CrossRef]
- Chen, S.; Solanki, A.; Pan, J.; Sum, T.C. Compositional and Morphological Changes in Water-Induced Early-Stage Degradation in Lead Halide Perovskites. Coatings 2019, 9, 535. [Google Scholar] [CrossRef]
- Yang, J.; Hong, Q.; Yuan, Z.; Xu, R.; Guo, X.; Xiong, S.; Liu, X.; Braun, S.; Li, Y.; Tang, J.; et al. Unraveling Photostability of Mixed Cation Perovskite Films in Extreme Environment. Adv. Opt. Mater. 2018, 6, 1800262. [Google Scholar] [CrossRef]
- Xu, R.P.; Li, Y.Q.; Jin, T.Y.; Liu, Y.Q.; Bao, Q.Y.; O’Carroll, C.; Tang, J.X. In Situ Observation of Light Illumination-Induced Degradation in Organometal Mixed-Halide Perovskite Films. ACS Appl. Mater. Interfaces 2018, 10, 6737–6746. [Google Scholar] [CrossRef] [PubMed]
- Cappel, U.B.; Svanstrom, S.; Lanzilotto, V.; Johansson, F.O.L.; Aitola, K.; Philippe, B.; Giangrisostomi, E.; Ovsyannikov, R.; Leitner, T.; Fohlisch, A.; et al. Partially Reversible Photoinduced Chemical Changes in a Mixed-Ion Perovskite Material for Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 34970–34978. [Google Scholar] [CrossRef]
- Seo, Y.-H.; Kim, J.H.; Kim, D.-H.; Chung, H.-S.; Na, S.-I. In situ TEM observation of the heat–induced degradation of single—and triple–cation planar perovskite solar cells. Nano Energy 2020, 77, 105164. [Google Scholar] [CrossRef]
- Aguiar, J.A.; Wozny, S.; Alkurd, N.R.; Yang, M.; Kovarik, L.; Holesinger, T.G.; Al-Jassim, M.; Zhu, K.; Zhou, W.; Berry, J.J. Effect of Water Vapor, Temperature, and Rapid Annealing on Formamidinium Lead Triiodide Perovskite Crystallization. ACS Energy Lett. 2016, 1, 155–161. [Google Scholar] [CrossRef]
- Kim, M.-c.; Ahn, N.; Cheng, D.; Xu, M.; Ham, S.-Y.; Pan, X.; Kim, S.J.; Luo, Y.; Fenning, D.P.; Tan, D.H.S.; et al. Imaging Real-Time Amorphization of Hybrid Perovskite Solar Cells under Electrical Biasing. ACS Energy Lett. 2021, 6, 3530–3537. [Google Scholar] [CrossRef]
- Aguiar, J.A.; Wozny, S.; Holesinger, T.G.; Aoki, T.; Patel, M.K.; Yang, M.; Berry, J.J.; Al-Jassim, M.; Zhou, W.; Zhu, K. In situ investigation of the formation and metastability of formamidinium lead tri-iodide perovskite solar cells. Energy Environ. Sci. 2016, 9, 2372–2382. [Google Scholar] [CrossRef]
- Qin, F.; Wang, Z.; Wang, Z.L. Anomalous Growth and Coalescence Dynamics of Hybrid Perovskite Nanoparticles Observed by Liquid-Cell Transmission Electron Microscopy. ACS Nano 2016, 10, 9787–9793. [Google Scholar] [CrossRef]
- Kim, T.W.; Shibayama, N.; Cojocaru, L.; Uchida, S.; Kondo, T.; Segawa, H. Real-Time In Situ Observation of Microstructural Change in Organometal Halide Perovskite Induced by Thermal Degradation. Adv. Funct. Mater. 2018, 28, 1804039. [Google Scholar] [CrossRef]
- Divitini, G.; Cacovich, S.; Matteocci, F.; Cinà, L.; Di Carlo, A.; Ducati, C. In situ observation of heat-induced degradation of perovskite solar cells. Nat. Energy 2016, 1, 1. [Google Scholar] [CrossRef]
- Yang, B.; Dyck, O.; Ming, W.; Du, M.H.; Das, S.; Rouleau, C.M.; Duscher, G.; Geohegan, D.B.; Xiao, K. Observation of Nanoscale Morphological and Structural Degradation in Perovskite Solar Cells by in Situ TEM. ACS Appl. Mater. Interfaces 2016, 8, 32333–32340. [Google Scholar] [CrossRef] [PubMed]
- Azpiroz, J.M.; Mosconi, E.; Bisquert, J.; De Angelis, F. Defect migration in methylammonium lead iodide and its role in perovskite solar cell operation. Energy Environ. Sci. 2015, 8, 2118–2127. [Google Scholar] [CrossRef]
- Eames, C.; Frost, J.M.; Barnes, P.R.; O’Regan, B.C.; Walsh, A.; Islam, M.S. Ionic transport in hybrid lead iodide perovskite solar cells. Nat. Commun. 2015, 6, 7497. [Google Scholar] [CrossRef]
- Haruyama, J.; Sodeyama, K.; Han, L.; Tateyama, Y. First-Principles Study of Ion Diffusion in Perovskite Solar Cell Sensitizers. J. Am. Chem. Soc. 2015, 137, 10048–10051. [Google Scholar] [CrossRef]
- Jeangros, Q.; Duchamp, M.; Werner, J.; Kruth, M.; Dunin-Borkowski, R.E.; Niesen, B.; Ballif, C.; Hessler-Wyser, A. In Situ TEM Analysis of Organic-Inorganic Metal-Halide Perovskite Solar Cells under Electrical Bias. Nano Lett. 2016, 16, 7013–7018. [Google Scholar] [CrossRef]
- Jung, H.J.; Kim, D.; Kim, S.; Park, J.; Dravid, V.P.; Shin, B. Stability of Halide Perovskite Solar Cell Devices: In Situ Observation of Oxygen Diffusion under Biasing. Adv. Mater. 2018, 30, 1802769. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, J.; Ge, Y.; Tian, B.; Zhang, Z.; Sui, M. Decisive influence of amorphous PbI2−x on the photodegradation of halide perovskites. J. Mater. Chem. A 2021, 9, 15059–15067. [Google Scholar] [CrossRef]
- Ran, J.; Dyck, O.; Wang, X.; Yang, B.; Geohegan, D.B.; Xiao, K. Electron-Beam-Related Studies of Halide Perovskites: Challenges and Opportunities. Adv. Energy Mater. 2020, 10, 1903191. [Google Scholar] [CrossRef]
- Kutes, Y.; Zhou, Y.; Bosse, J.L.; Steffes, J.; Padture, N.P.; Huey, B.D. Mapping the Photoresponse of CH3NH3PbI3 Hybrid Perovskite Thin Films at the Nanoscale. Nano Lett. 2016, 16, 3434–3441. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, B.; Liu, Y.; Deng, Y.; Bai, Y.; Dong, Q.; Huang, J. Scaling behavior of moisture-induced grain degradation in polycrystalline hybrid perovskite thin films. Energy Environ. Sci. 2017, 10, 516–522. [Google Scholar] [CrossRef]
- Ji, F.; Pang, S.; Zhang, L.; Zong, Y.; Cui, G.; Padture, N.P.; Zhou, Y. Simultaneous Evolution of Uniaxially Oriented Grains and Ultralow-Density Grain-Boundary Network in CH3NH3PbI3 Perovskite Thin Films Mediated by Precursor Phase Metastability. ACS Energy Lett. 2017, 2, 2727–2733. [Google Scholar] [CrossRef]
- Song, Z.; Shrestha, N.; Watthage, S.C.; Liyanage, G.K.; Almutawah, Z.S.; Ahangharnejhad, R.H.; Phillips, A.B.; Ellingson, R.J.; Heben, M.J. Impact of Moisture on Photoexcited Charge Carrier Dynamics in Methylammonium Lead Halide Perovskites. J. Phys. Chem. Lett. 2018, 9, 6312–6320. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gan, Z.; Green, M.A.; Jia, B.; Wen, X. Revealing Dynamic Effects of Mobile Ions in Halide Perovskite Solar Cells Using Time-Resolved Microspectroscopy. Small Methods 2020, 5, 2000731. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Abate, A.; Watthage, S.C.; Liyanage, G.K.; Phillips, A.B.; Steiner, U.; Graetzel, M.; Heben, M.J. Perovskite Solar Cell Stability in Humid Air: Partially Reversible Phase Transitions in the PbI2-CH3NH3H2O System. Adv. Energy Mater. 2016, 6, 1600846. [Google Scholar] [CrossRef]
- Yin, X.; Guo, Y.; Liu, J.; Que, W.; Ma, F.; Xu, K. Photoinduced Phase Segregation Leading to Evident Open-Circuit Voltage Loss in Efficient Inorganic CsPbIBr2 Solar Cells. J. Phys. Chem. Lett. 2020, 11, 7035–7041. [Google Scholar] [CrossRef]
- Liu, S.; Guan, Y.; Sheng, Y.; Hu, Y.; Rong, Y.; Mei, A.; Han, H. A Review on Additives for Halide Perovskite Solar Cells. Adv. Energy Mater. 2019, 10, 1902492. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, Y.; Zhang, X.; You, J. Recent Progresses on Defect Passivation toward Efficient Perovskite Solar Cells. Adv. Energy Mater. 2019, 10, 1902650. [Google Scholar] [CrossRef]
- Akin, S.; Arora, N.; Zakeeruddin, S.M.; Grätzel, M.; Friend, R.H.; Dar, M.I. New Strategies for Defect Passivation in High-Efficiency Perovskite Solar Cells. Adv. Energy Mater. 2019, 10, 1903090. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Duong, T.; Peng, J.; Wu, Y.; Shen, H.; Walter, D.; Nguyen, H.T.; Mozaffari, N.; Tabi, G.D.; Catchpole, K.R.; et al. Origin of Efficiency and Stability Enhancement in High-Performing Mixed Dimensional 2D-3D Perovskite Solar Cells: A Review. Adv. Funct. Mater. 2021, 32, 2009164. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Tang, Z.; Su, C.; Huang, W.; Li, Y.; Xing, G. Polymer strategies for high-efficiency and stable perovskite solar cells. Nano Energy 2021, 82, 105712. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, K. Additive Engineering for Efficient and Stable Perovskite Solar Cells. Adv. Energy Mater. 2019, 10, 1902579. [Google Scholar] [CrossRef]
- Grancini, G.; Roldan-Carmona, C.; Zimmermann, I.; Mosconi, E.; Lee, X.; Martineau, D.; Narbey, S.; Oswald, F.; De Angelis, F.; Graetzel, M.; et al. One-Year stable perovskite solar cells by 2D/3D interface engineering. Nat. Commun. 2017, 8, 15684. [Google Scholar] [CrossRef] [PubMed]




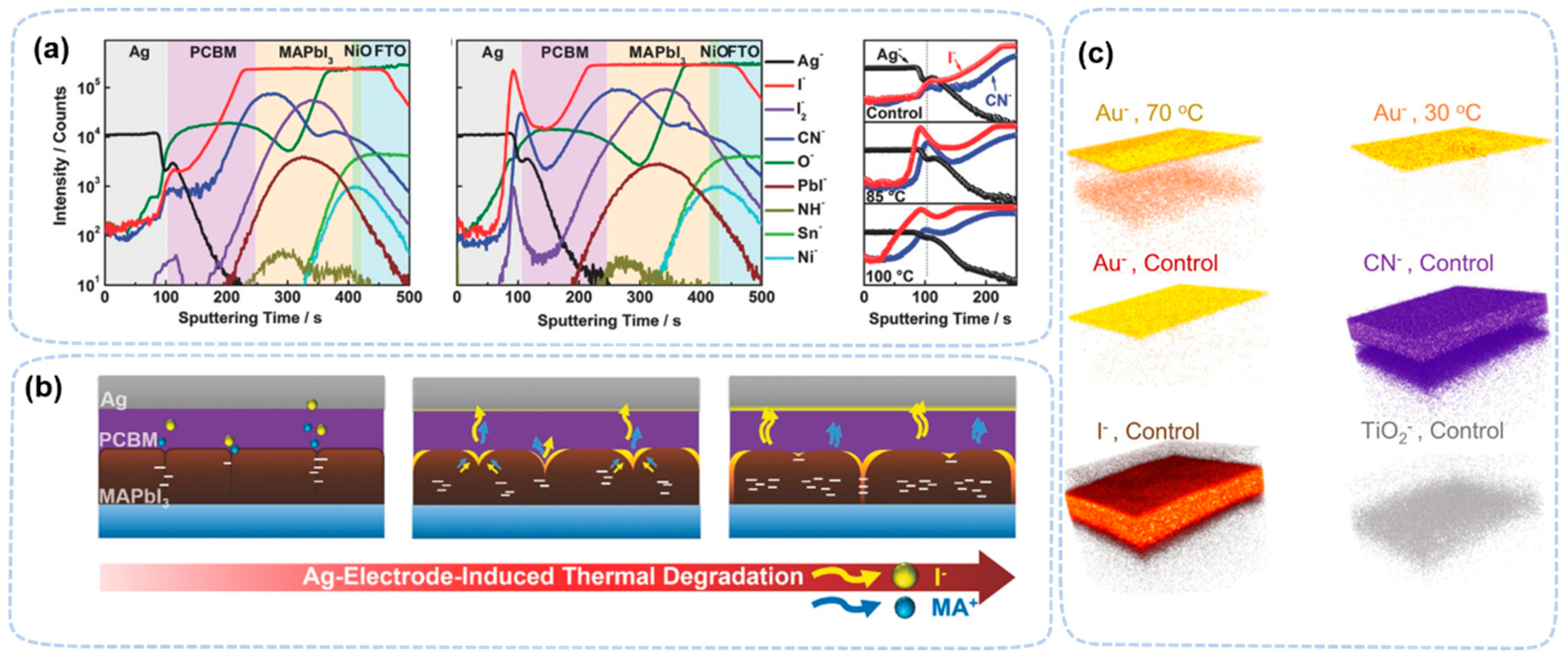
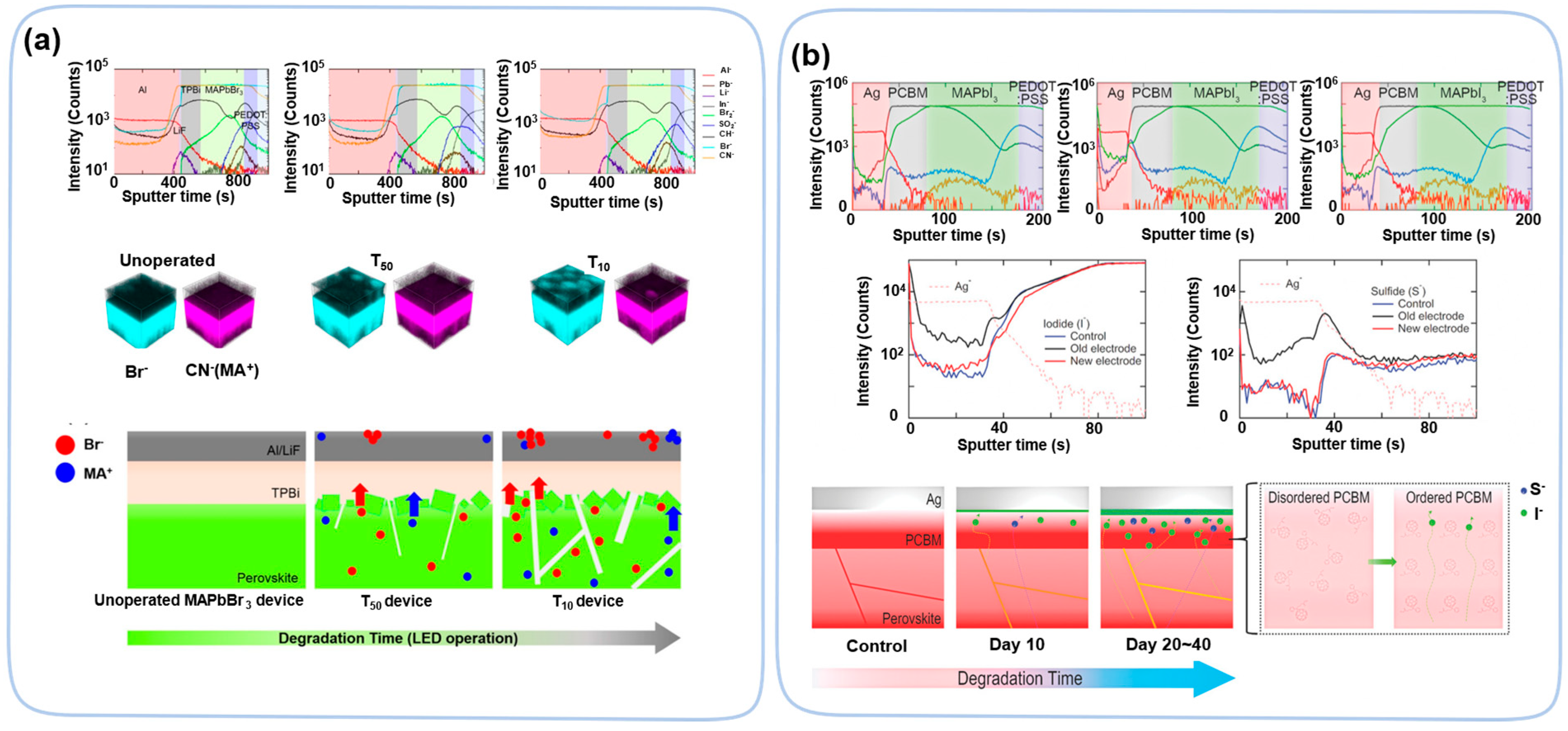
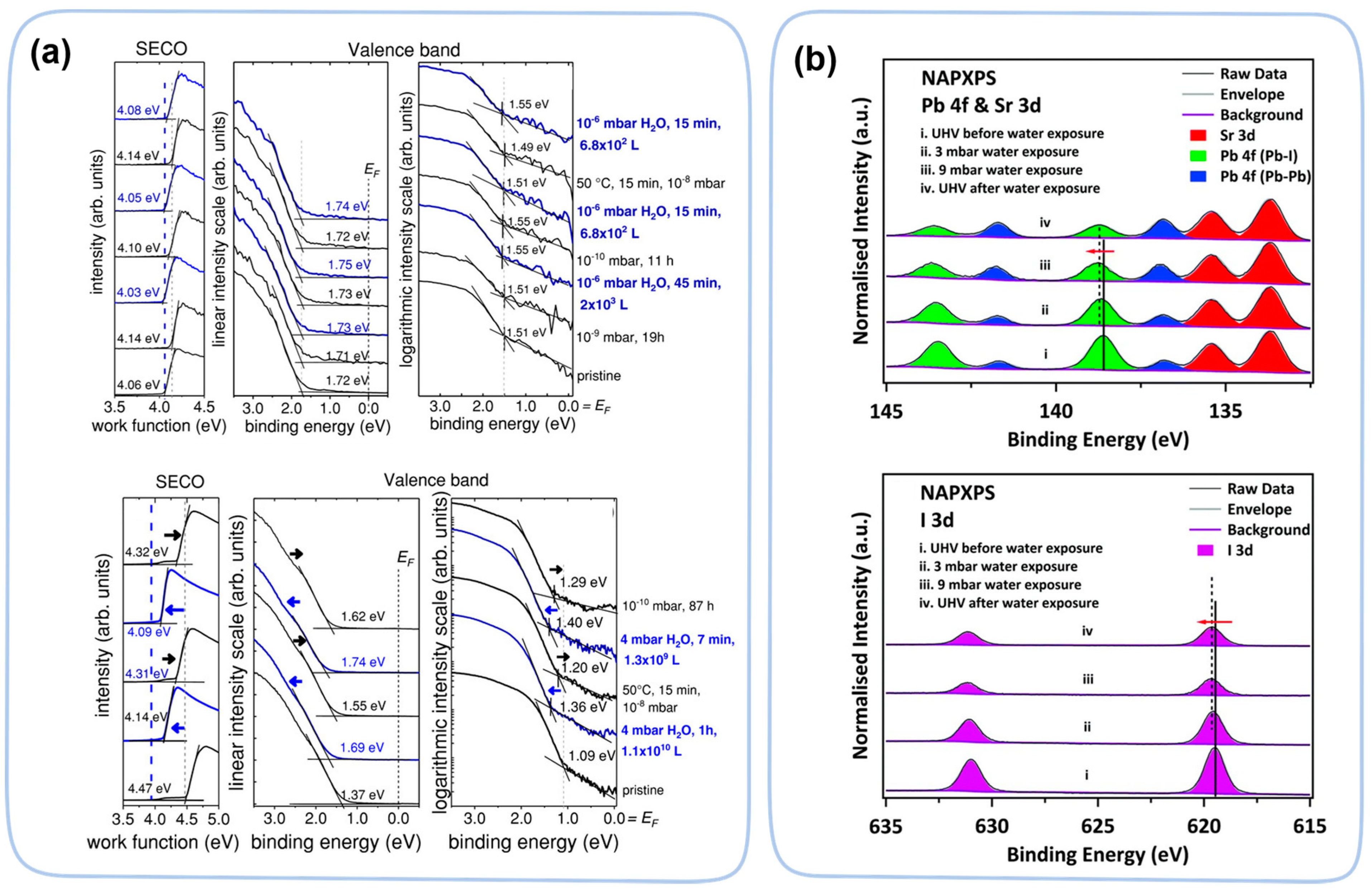

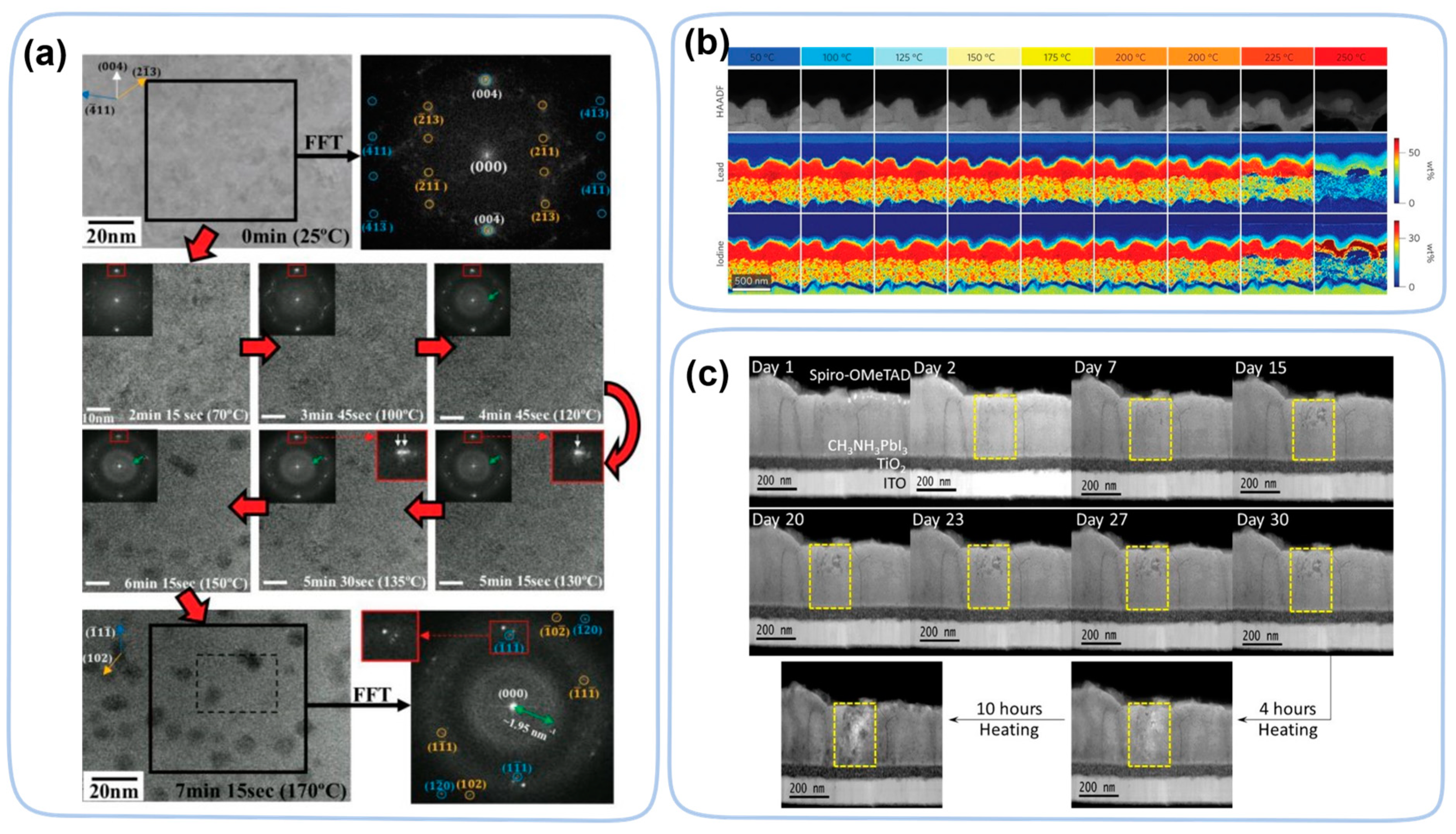

| Perovskite Films | Exposure Condition | Techniques | Ref. |
|---|---|---|---|
| MAPbI3 | Water (27~90% RH) | XRD | [32] |
| MAPbI3 | Water (80% RH) | XRD | [48] |
| MAPbI3 | Water (80% RH) | XRD | [49] |
| MAPbI3 | Water (65% RH) | XRD | [47] |
| CsFAPb(IBr)3 | Heat (80~320 °C) | XRD | [51] |
| MAPbI3−xClx | Heat (28~400 °C) | XRD | [53] |
| FACsPb(IBr)3, MAPb(IBr)3 | Light | XRD | [54] |
| 3D/2D | Water (90% RH) | XRD | [62] |
| MAPbI3 | Liquid water | XRD | [63] |
| MAPbX3(X = I, Br, Cl) | Heat (30~320 °C) | XRD | [64] |
| MAPbI3 | Vacuum/light | XRD | [65] |
| (CsFAMA)Pb(IBr)3 | Light and bias | XRD | [66] |
| MAPb(IBr)3 | Light | XRD | [67] |
| MAPbI3 | Water (80% RH) | GIXRD | [45] |
| MAPbI3 | Water (85% RH) | GIWAXS | [46] |
| MAPbI3 | Heat (25~130 °C) | GIWAXS | [68] |
| MAPbI3 | D2O (73% and 93% RH) | GISANS | [50] |
| 3D/2D | Water (90% RH) | GISANS | [62] |
| Perovskite Films | Exposure Condition | Techniques | Ref. |
|---|---|---|---|
| MAPbI3 | Heat (20~600 °C) | TGA, MS | [69] |
| FAPbI3, MAPbI3 | Heat (25~700 °C) | TGA, MS | [70] |
| MAPbI3 | Heat (30~390 °C) | TGA | [71] |
| MAPbI3 | Light (5 × 1017 s−1 cm−2), (blue and UV LEDs) | MS | [74] |
| MAPbI3 | Light and heat (white LED/80 mW·cm−2, blue LED/71 mW·cm−2, Xe lamp/55 mW·cm−2; 35~80 °C) | MS | [75] |
| MAPbI3 | Light (100 mW·cm−2) | MS | [76] |
| MAPbI3 | Light (white LED/55 mW·cm−2, red laser 80 mW·cm−2) | ToF-SIMS | [73] |
| MAPbI3 | Light (AM 1.5, 100 mW·cm−2) | ToF-SIMS | [77] |
| Rb0.03Cs0.07FA0.765MA0.135PbI2.55Br0.45 | Heat and light (50~70 °C; white LED illumination at 1 Sun intensity) | ToF-SIMS | [78] |
| MAPbI3 | 84% RH | ToF-SIMS | [79] |
| MAPbI3 | Water (25% RH and 85% RH) and O2 | ToF-SIMS | [81] |
| MAPbI3 | O2 | ToF-SIMS | [82] |
| MAPbI3 | O2 | ToF-SIMS | [83] |
| MAPbI3, nBA-MAPbI3, 2D/3D MAPbI3 | Water (D2O, 15 Torr) | ToF-SIMS | [84] |
| MAPbI3 | Heat (85 °C and 100 °C) | ToF-SIMS | [85] |
| FA0.83MA0.17Pb(I0.83Br017)3 | Heat (30 °C and 70 °C) | ToF-SIMS | [34] |
| MAPbI3 | Heat (Oven and plate) | ToF-SIMS | [86] |
| MAPbBr3 | Heat | ToF-SIMS | [87] |
| MAPbBr3 | Bias (−2~2 V) | ToF-SIMS | [88] |
| MAPbBr3 | Bias (0~2 V) | ToF-SIMS | [89] |
| MAPbI3 | Bias (~0.5 V) | ToF-SIMS | [90] |
| MAPbI3 | Bias | ToF-SIMS | [91] |
| MAPbI3 | Water (0 to 1013 Langmuir) | XPS, UPS | [97] |
| MAPbI3 | Light (408 nm, blue laser, 6.8 mW·cm−2) | XPS | [98] |
| MAPbI3−xClx | Water (10−6 mbar,4 mbar), Oxygen (50 mbar), air | UPS | [99] |
| MAPbI3 | Water (3 mbar (10% RH) and 9 mbar (30% RH)) | NAPXPS | [100] |
| FAPbI3, MAPbI3 | Water (23 mbar (80% RH)) | NAPXPS | [101] |
| MAPb(I0.83Cl0.17)3 | Light (white) | XPS | [102] |
| (MAFA)PbIBr | Light (515 nm laser, 280 to 2800 mW·cm−2) | XPS | [104] |
| Perovskite | Exposure Condition | Techniques | Ref. |
|---|---|---|---|
| MAPbI3 | Heat (25~170 °C) | TEM, SEM | [110] |
| MAPbI3 | Heat (85 °C) | TEM | [35] |
| MAPbI3 | Heat (50~250 °C) | TEM | [111] |
| MAPbI3 | Heat (50~60 °C) | TEM | [112] |
| (CsFAMA)1Pb(IBr)3 | Heat (85~170 °C) | TEM | [105] |
| MAPbI3 | Light (white LED) | TEM | [118] |
| (MAFA)1Pb(IBr)3 | Bias (1 V) | TEM | [107] |
| MAPbI3 | Bias (−6~6 V) | TEM | [116] |
| MAPbI3 | Bias (−1~1 V) | TEM | [117] |
| MAPbI3 | Humidity (80%) | AFM | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, B.; Wang, C.; Li, J.; Su, Z.; Xing, G.; Gao, X.; Chen, S. In Situ and Operando Characterization Techniques in Stability Study of Perovskite-Based Devices. Nanomaterials 2023, 13, 1983. https://doi.org/10.3390/nano13131983
He B, Wang C, Li J, Su Z, Xing G, Gao X, Chen S. In Situ and Operando Characterization Techniques in Stability Study of Perovskite-Based Devices. Nanomaterials. 2023; 13(13):1983. https://doi.org/10.3390/nano13131983
Chicago/Turabian StyleHe, Bingchen, Chenyue Wang, Jielei Li, Zhenhuang Su, Guichuan Xing, Xingyu Gao, and Shi Chen. 2023. "In Situ and Operando Characterization Techniques in Stability Study of Perovskite-Based Devices" Nanomaterials 13, no. 13: 1983. https://doi.org/10.3390/nano13131983
APA StyleHe, B., Wang, C., Li, J., Su, Z., Xing, G., Gao, X., & Chen, S. (2023). In Situ and Operando Characterization Techniques in Stability Study of Perovskite-Based Devices. Nanomaterials, 13(13), 1983. https://doi.org/10.3390/nano13131983






