A Facile Two-Step Hydrothermal Synthesis of Co(OH)2@NiCo2O4 Nanosheet Nanocomposites for Supercapacitor Electrodes
Abstract
1. Introduction
2. Experimental
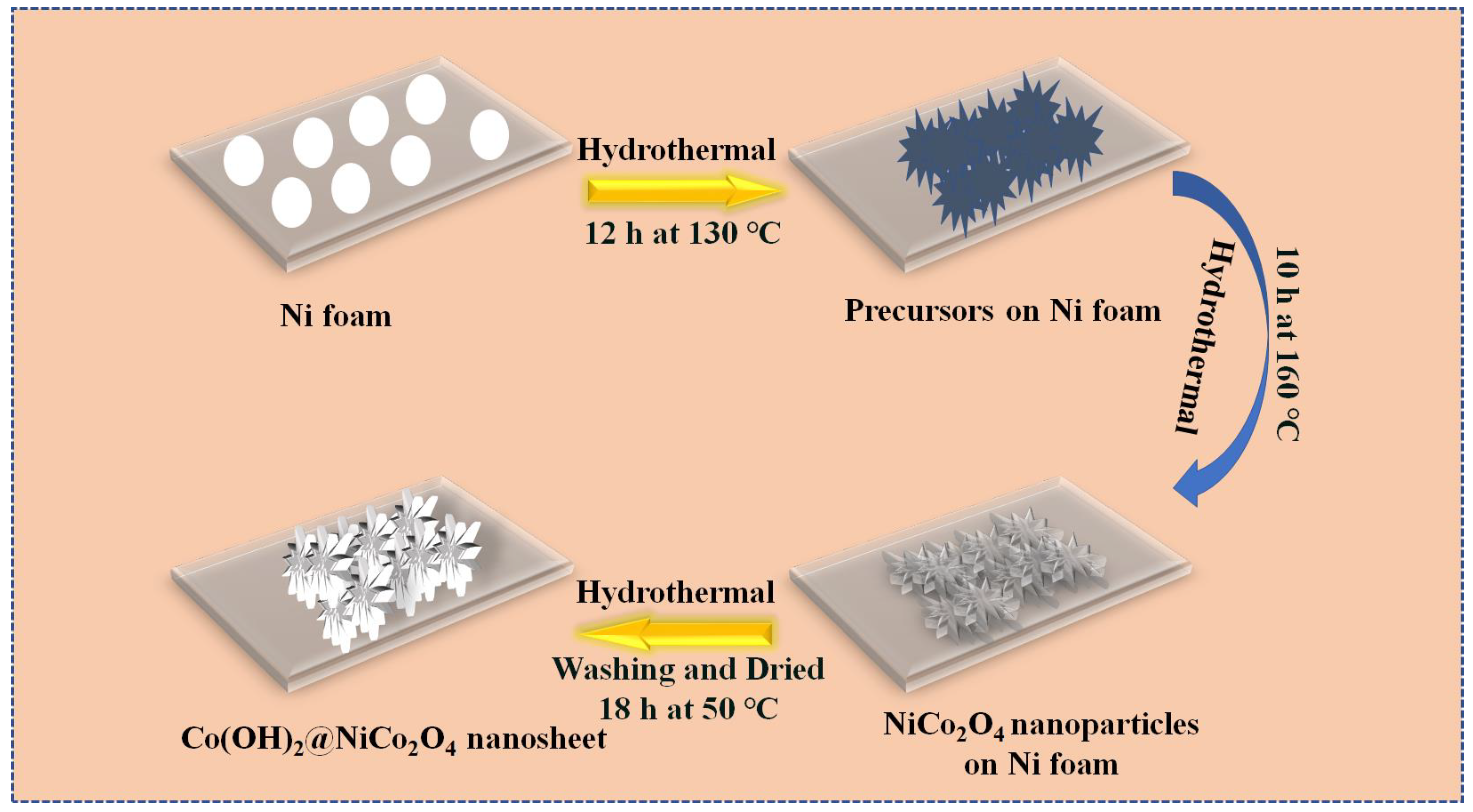
2.1. Synthesis of Co(OH)2@NiCo2O4 Nanosheet Composite
2.2. Physical Characterization
2.3. Electrochemical Characterization
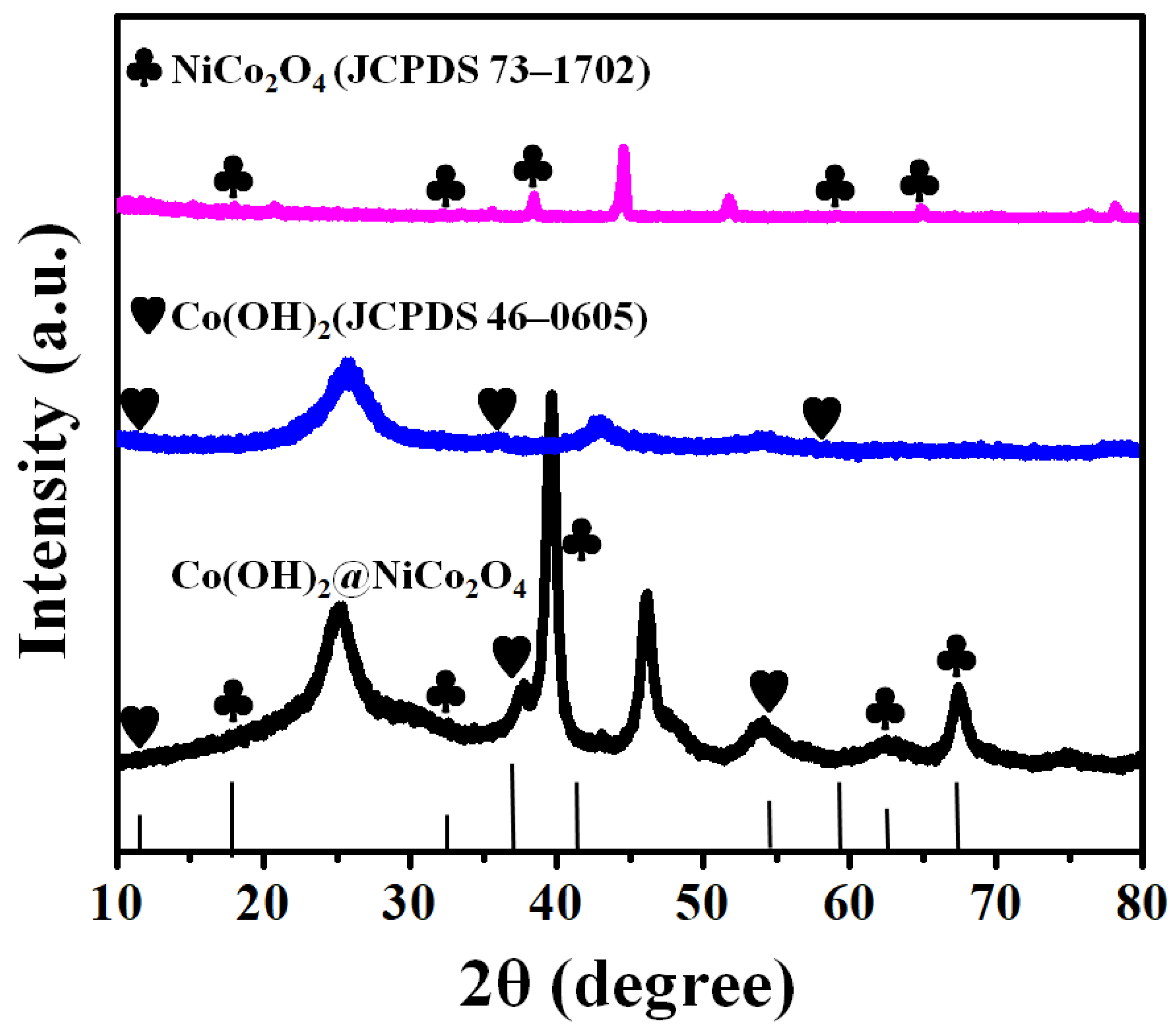
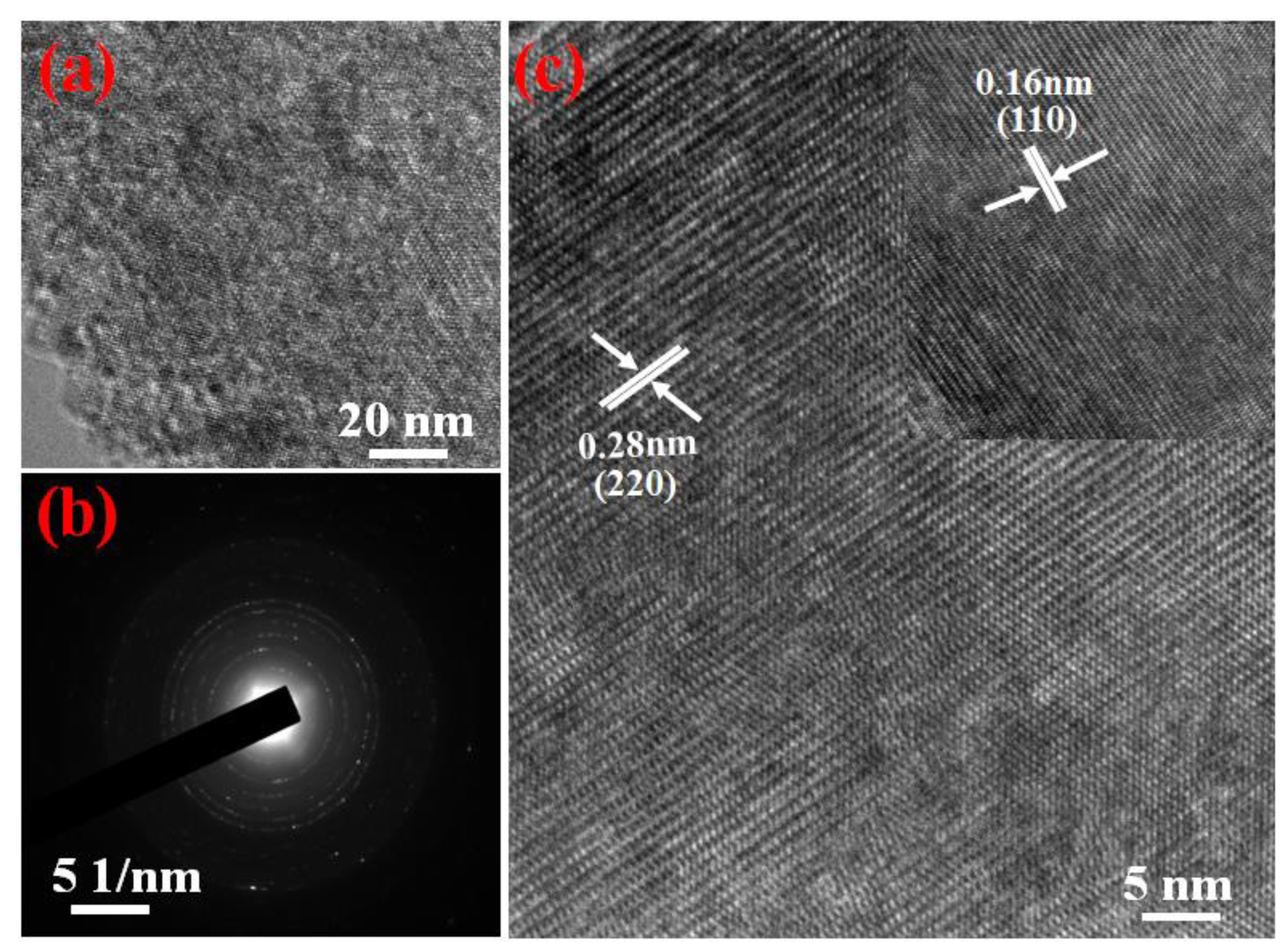
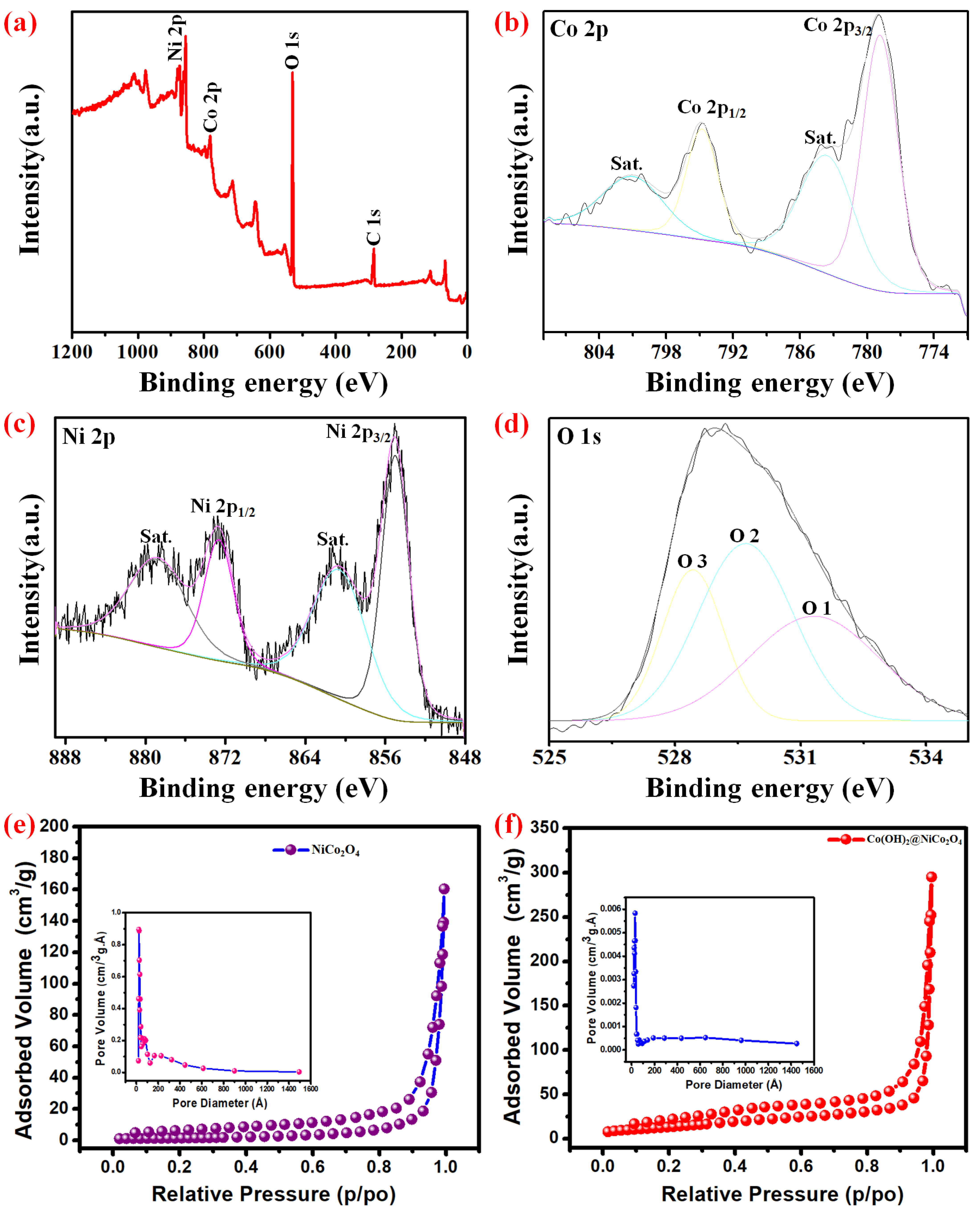
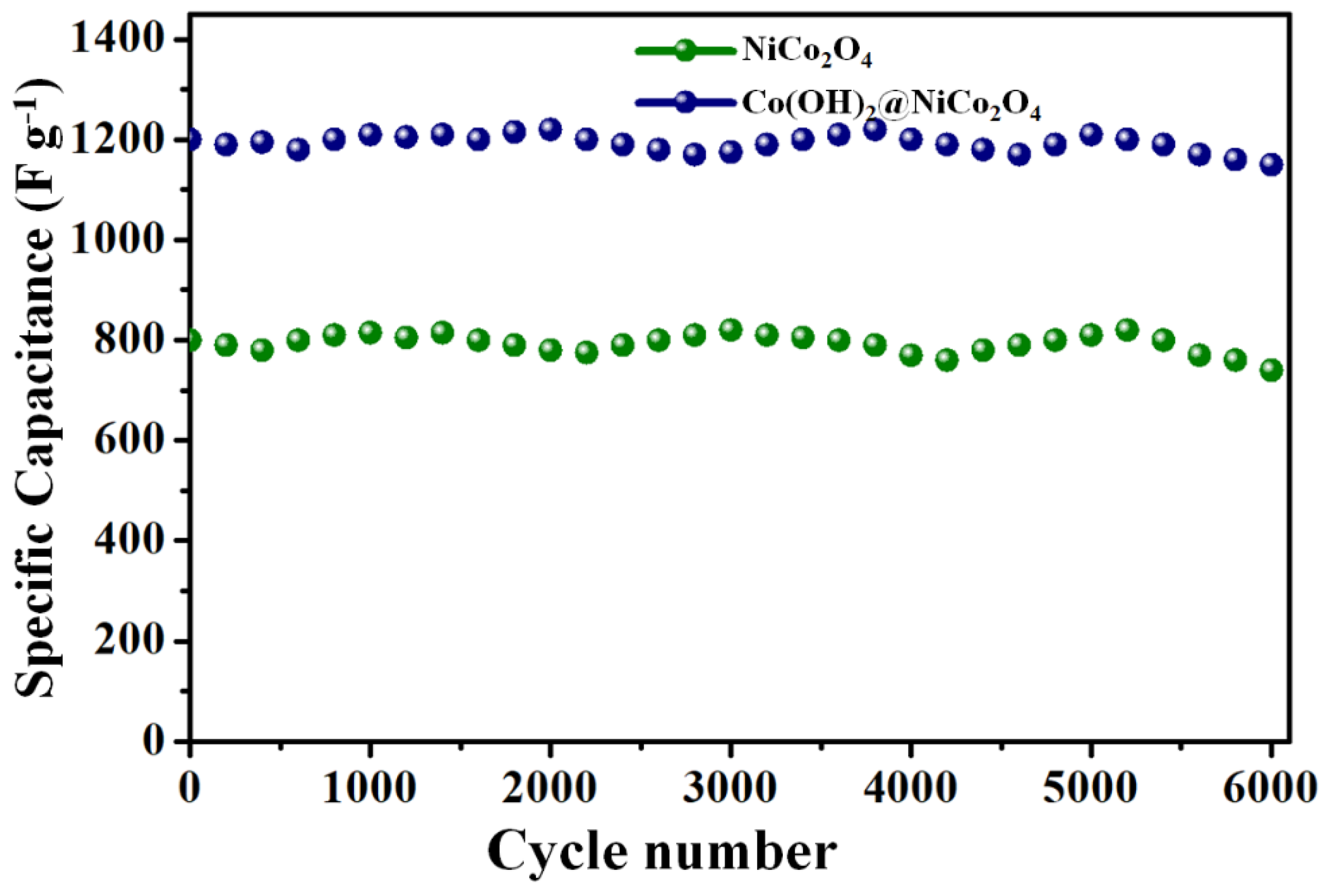
3. Results and Discussions
Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoon, J.H.; Kumar, Y.A.; Sambasivam, S.; Hira, S.A.; Krishna, T.N.V.; Zeb, K.; Uddin, W.; Kumar, K.D.; Obaidat, I.M.; Kim, S.; et al. Highly efficient copper-cobalt sulfide nano-reeds array with simplistic fabrication strategy for battery-type supercapacitors. Energy Storage 2020, 32, 101988. [Google Scholar] [CrossRef]
- Mun, C.H.; Gopi, C.V.V.M.; Vinodh, R.; Sambasivam, S.; Obaidat, I.M.; Kim, H.J. Microflower-like nickel sulfide-lead sulfide hierarchical composites as binder-free electrodes for high-performance supercapacitors. J. Energy Storage 2019, 26, 100925. [Google Scholar] [CrossRef]
- Gopi, C.V.V.M.; Vinodh, R.; Sambasivam, S.; Obaidat, I.M.; Kalla, R.M.N.; Kim, H.J. One-pot synthesis of copper oxide–cobalt oxide core–shell nanocactus-like heterostructures as binder-free electrode materials for high-rate hybrid supercapacitors. Mater. Today Energy 2019, 14, 100358. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Sambasivam, S.; Hira, S.A.; Zeb, K.; Uddin, W.; Krishna, T.N.V.; Kumar, K.D.; Obaidat, I.M.; Kim, H.J. Boosting the energy density of highly efficient flexible hybrid supercapacitors via selective integration of hierarchical nanostructured energy materials. Electrochim. Acta 2020, 364, 137318. [Google Scholar] [CrossRef]
- Yang, J.; Jo, M.R.; Kang, M.; Huh, Y.S.; Jung, H.; Kang, Y.M. Rapid and controlled synthesis of nitrogen doped reduced graphene oxide using microwave-assisted hydrothermal reaction for high power-density supercapacitors. Carbon 2014, 73, 106–113. [Google Scholar] [CrossRef]
- Yang, J.; Park, S.; Lee, S.; Kim, J.; Huang, D.; Gim, J.; Lee, E.; Kim, G.; Park, K.; Kang, Y.-M.; et al. High-voltage deprotonation of layered-type materials as a newly identified cause of electrode degradation. J. Mater. Chem. A 2023, 11, 3018–3027. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Dang, A.; Sun, Y.; Fang, C.; Li, T.; Liu, X.; Xia, Y.; Ye, F.; Zada, A.; Khan, M. Rational design of Ti3C2/carbon nanotubes/MnCo2S4 electrodes for symmetric supercapacitors with high energy storage. Appl. Surf. Sci. 2022, 581, 152432. [Google Scholar] [CrossRef]
- Kumar, K.S.; Choudhary, N.; Jung, Y.; Thomas, J. Recent Advances in Two-Dimensional Nanomaterials for Supercapacitor Electrode Applications. ACS Energy Lett. 2018, 3, 482–495. [Google Scholar] [CrossRef]
- Sharma, P.; Sundaram, M.M.; Watcharatharapong, T.; Jungthawan, S.; Ahuja, R. Tuning the Nanoparticle Interfacial Properties and Stability of the Core–Shell Structure in Zn-Doped NiMoO4@AWO4. ACS Appl. Mater. Interfaces 2021, 13, 56116–56130. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Sundaram, M.M.; Henry, D.J.; Gao, X. Alginate Biopolymer Effect on the Electrodeposition of Manganese Dioxide on Electrodes for Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 7040–7051. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Wu, W.; Qian, J.; Song, S.; Yue, Z.J. Feasibility of activated carbon derived from anaerobic digester residues for supercapacitors. J. Power Sources 2019, 412, 683–688. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Long, J.W. To be or not to be pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185. [Google Scholar] [CrossRef]
- Gu, C.; Ge, X.; Wang, X.; Tu, J.J. Cation–anion double hydrolysis derived layered single metal hydroxide superstructures for boosted supercapacitive energy storage. Mater. Chem. A 2015, 3, 14228–14238. [Google Scholar] [CrossRef]
- Chhetri, K.; Tiwari, A.P.; Dahal, B.; Ojha, G.P.; Mukhiya, T.; Lee, M.; Kim, T.; Chae, S.; Muthurasu, A.; Kim, H.Y. A ZIF-8-derived nanoporous carbon nanocomposite wrapped with Co3O4-polyaniline as an efficient electrode material for an asymmetric supercapacitor. J. Electroanal. Chem. 2020, 856, 113670. [Google Scholar] [CrossRef]
- Chhetri, K.; Dahal, B.; Mukhiya, T.; Tiwari, A.P.; Muthurasu, A.; Kim, H.Y. Integrated hybrid of graphitic carbon-encapsulated CuxO on multilayered mesoporous carbon from copper MOFs and polyaniline for asymmetric supercapacitor and oxygen reduction reactions. Carbon 2021, 179, 89–99. [Google Scholar] [CrossRef]
- Dang, A.; Li, T.; Xiong, C.; Zhao, T.; Shang, Y.; Liu, H.; Chen, X.; Li, H.; Zhuang, Q.; Zhang, S. Long-life electrochemical supercapacitor based on a novel hierarchically carbon foam templated carbon nanotube electrode. Compos. Part B Eng. 2018, 141, 250–257. [Google Scholar] [CrossRef]
- Zheng, J.; Yan, B.; Feng, L.; Zhang, Q.; Zhang, C.; Yang, W.; Han, J.; Jiang, S.; He, S. Potassium citrate assisted synthesis of hierarchical porous carbon materials for high performance supercapacitors. Diam. Relat. Mater. 2022, 128, 109247. [Google Scholar] [CrossRef]
- Wang, M.; Sui, X.; Wang, Y.; Juan, Y.; Lyu, Y.; Peng, H.; Huang, T.; Shen, S.; Guo, C.; Zhang, J.; et al. Manipulate the electronic and magnetic states in NiCo2O4 films through electric-field-induced protonation at elevated temperature. Adv. Mater. 2019, 31, 1900458. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Zhong, Y.; Tade, M.; Zhou, W.; Shao, Z. Molecular design of mesoporous NiCo2O4 and NiCo2S4 with sub-micrometer polyhedron architectures for efficient pseudocapacitive energy storage. Adv. Funct. Mater. 2017, 27, 1701229. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Kim, H.-J. Preparation and electrochemical performance of NiCo2O4@NiCo2O4 composite nanoplates for high performance supercapacitor applications. New J. Chem. 2018, 42, 19971–19978. [Google Scholar] [CrossRef]
- Wang, X.; Fang, Y.; Shi, B.; Huang, F.; Rong, F.; Que, R. Three-dimensional NiCo2O4@NiCo2O4 core–shell nanocones arrays for high-performance supercapacitors. Chem. Eng. J. 2018, 344, 311–319. [Google Scholar] [CrossRef]
- Shao, Y.; Zhao, Y.; Li, H.; Xu, C. Three-dimensional hierarchical NixCo1−xO/NiyCo2−yP@C hybrids on nickel foam for excellent supercapacitors. ACS Appl. Mater. Inter. 2016, 8, 35368–35376. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, N.; Zhang, G.; Xu, M.; Lu, W.; Zhou, L.; Huang, H. Design of Hierarchical NiCo@NiCo Layered Double Hydroxide Core-Shell Structured Nanotube Array for High-Performance Flexible All-Solid-State Battery-Type Supercapacitors. Adv. Func. Mater. 2017, 27, 1605307. [Google Scholar] [CrossRef]
- Liu, B.; Kong, D.; Huang, Z.X.; Mo, R.; Wang, Y.; Han, Z.; Cheng, C.; Yang, H.Y. Three-dimensional hierarchical NiCo2O4 nanowire@Ni3S2 nanosheet core/shell arrays for flexible asymmetric supercapacitors. Nanoscale 2016, 8, 10686–10694. [Google Scholar] [CrossRef]
- Ko, T.H.; Lei, D.; Balasubramaniam, S.; Seo, M.-K.; Chung, Y.-S.; Kim, H.-Y.; Kim, B.-S. Polypyrrole-Decorated Hierarchical NiCo2O4 Nanoneedles/Carbon Fiber Papers for Flexible High-Performance Supercapacitor Applications. Electrochim. Acta 2017, 247, 524–534. [Google Scholar] [CrossRef]
- Mohamed, S.G.; Attia, S.Y.; Hassan, H.H. Spinel-structured FeCo2O4 mesoporous nanosheets as efficient electrode for supercapacitor applications. Microporous Mesoporous Mater. 2017, 251, 26–33. [Google Scholar] [CrossRef]
- Suksomboon, M.; Khuntilo, J.; Kalasina, S.; Suktha, P.; Limtrakul, J.; Sawangphruk, M. High-performance energy storage of Ag-doped Co(OH)2-coated graphene paper: In situ electrochemical X-ray absorption spectroscopy. Electrochim. Acta 2017, 252, 91–100. [Google Scholar] [CrossRef]
- Zhao, T.; Jiang, H.; Ma, J. Surfactant-assisted electrochemical deposition of α-cobalt hydroxide for supercapacitors. J. Power Sources 2011, 196, 860–864. [Google Scholar] [CrossRef]
- Jagadale, A.D.; Guan, G.; Li, X.; Du, X.; Ma, X.; Hao, X.; Abudula, A. Ultrathin nanoflakes of cobalt-manganese layered double hydroxide with high reversibility for asymmetric supercapacitor. J. Power Sources 2016, 306, 526–534. [Google Scholar] [CrossRef]
- Gupta, V.; Gupta, S.; Miura, N. Al-substituted α-cobalt hydroxide synthesized by potentiostatic deposition method as an electrode material for redox-supercapacitors. J. Power Sources 2008, 177, 685–689. [Google Scholar] [CrossRef]
- Casella, I.; Gatta, M. Study of the electrochemical deposition and properties of cobalt oxide species in citrate alkaline solutions. J. Electroanal. Chem. 2002, 534, 31–38. [Google Scholar] [CrossRef]
- Kulurumotlakatla, D.K.; Yedluri, A.K.; Kim, H.J. Hierarchical NiCo2S4 nanostructure as highly efficient electrode material for high-performance supercapacitor applications. J. Energy Storage 2020, 31, 31–38. [Google Scholar] [CrossRef]
- Arbi, H.M.; Yadav, A.A.; Anil Kumar, Y.; Moniruzzaman, M.; Alzahmi, S.; Obaidat, I.M. Polypyrrole-Assisted Ag Doping Strategy to Boost Co(OH)2 Nanosheets on Ni Foam as a Novel Electrode for High-Performance Hybrid Supercapacitors. Nanomaterials 2022, 12, 3982. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Anil Kumar, Y.; Pallavolu, M.R.; Arbi, H.M.; Alzahmi, S.; Obaidat, I.M. Two-Dimensional Core-Shell Structure of Cobalt-Doped@MnO2 Nanosheets Grown on Nickel Foam as a Binder-Free Battery-Type Electrode for Supercapacitor Application. Nanomaterials 2022, 12, 3187. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Z.H.; Wang, Z.G.; Zhang, F.X.; Jin, J. Layered α-Co(OH)2 Nanocones as Electrode Materials for Pseudocapacitors: Understanding the Effect of Interlayer Space on Electrochemical Activity. Adv. Funct. Mater. 2013, 23, 2758–2764. [Google Scholar] [CrossRef]
- Zhou, X.; Yue, X.; Dong, Y.; Zheng, Q.; Lin, D.; Du, X.; Qu, G. Enhancing electrochemical performance of electrode material via combining defect and heterojunction engineering for supercapacitors. J. Colloid Interface Sci. 2021, 599, 68–78. [Google Scholar] [CrossRef]
- Pang, H.; Li, X.; Zhao, Q.; Xue, H.; Lai, W.-Y.; Hu, Z.; Huang, W. One-Pot Synthesis of Heterogeneous Co3O4-Nanocube/Co(OH)2-Nanosheet Hybrids for High-Performance Flexible Asymmetric All-Solid-State Supercapacitors. Nano Energy 2017, 35, 138–145. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Yu, X.; Tu, J.; Ruan, D.; Qiao, Z.J. High-performance discarded separator-based activated carbon for the application of supercapacitors. J. Energy Storage 2021, 44, 103378. [Google Scholar] [CrossRef]
- Kong, D.; Ren, W.; Cheng, C.; Wang, Y.; Huang, Z.; Yang, H. Three-dimensional NiCo2O4@polypyrrole coaxial nanowire arrays on carbon textiles for high-performance flexible asymmetric solid-state supercapacitor. ACS Appl. Mater. Interface 2015, 7, 21334–21346. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Huang, F.; Zhao, J.; Wang, X. Ni(OH)2 nanodot-decorated Co-Co LDH/C hollow nanocages for a high peformance supercapacitor. Dalton Trans. 2020, 49, 17310–17320. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, L.; Cheng, B.; Yu, J. Rationally designed hierarchical NiCo2O4–C@Ni(OH)2 core-shell nanofibers for high performance supercapacitors. Carbon 2019, 152, 652–660. [Google Scholar] [CrossRef]
- Tholkappiyan, R.; Vishista, K. Tuning the composition and magnetostructure of dysprosium iron garnets by Co-substitution: An XRD, FT-IR, XPS and VSM study. Appl. Surf. Sci. 2015, 351, 1016–1024. [Google Scholar] [CrossRef]
- Tholkappiyan, R.; Vishista, K. Combustion synthesis of Mg–Er ferrite nanoparticles: Cation distribution and structural, optical, and magnetic properties. Mater. Sci. Semicond. Process. 2015, 40, 631–642. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Deng, T.; Zhang, W. Ni(OH)2 derived Ni-MOF supported on carbon nanowalls for supercapacitors. Nanotechnology 2021, 32, 195404. [Google Scholar] [CrossRef]
- Xin, Y.; Dai, X.; Lv, G.; Wei, X.; Li, S.; Li, Z.; Xue, T.; Shi, M.; Zou, K.; Chen, Y.; et al. Stability-Enhanced α-Ni(OH)2 Pillared by Metaborate Anions for Pseudocapacitors. ACS Appl. Mater. Interf. 2021, 13, 28118–28128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Ran, W.; He, J.; Huang, Y.Z.; Liu, Z.F.; Liu, W.; Tang, Y.F.; Zhang, L.; Gao, D.W.; Gao, F.M. High-performance asymmetric supercapacitors based on multilayer MnO2/graphene oxide nanoflakes and hierarchical porous carbon with enhanced cycling stability. Small 2015, 11, 1310–1319. [Google Scholar] [CrossRef]
- Tholkappiyan Ramachandran, Fathalla Hamed, Electrochemical performance of plate-like zinc cobaltite electrode material for supercapacitor applications. J. Phys. Chem. Solids 2018, 121, 93–101. [CrossRef]
- Xu, K.; Zou, R.; Li, W.; Liu, Q.; Liu, X.; An, L.; Hu, J. Design and synthesis of 3D interconnected mesoporous NiCo2O4@CoxNi1−x (OH)2 core-shell nanosheet arrays with large areal capacitance and high rate performance for supercapacitors. J. Mater. Chem. A 2014, 2, 10090–10097. [Google Scholar] [CrossRef]
- Liu, W.W.; Lu, C.; Liang, K.; Tay, B.K. A three dimensional vertically aligned multiwall carbon nanotube/NiCo2O4 core/shell structure for novel high-performance supercapacitors. J. Mater. Chem. A 2014, 2, 5100–5107. [Google Scholar] [CrossRef]
- Huang, L.; Chen, D.; Ding, Y.; Wang, Z.L.; Zeng, Z.; Liu, M. Hybrid composite Ni(OH)2@NiCo2O4 grown on carbon fiber paper for high-performance supercapacitors. ACS Appl. Mater. Interface 2013, 5, 11159–11162. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Shim, J.J. Three-dimensional nickel foam/graphene/NiCo2O4 as high-performance electrodes for supercapacitors. J. Power Sources 2015, 273, 110–117. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, S.; Su, D.; Sun, B.; Zhu, J.; Wang, G. 3D mesoporous hybrid NiCo2O4@graphene nanoarchitectures as electrode materials for supercapacitors with enhanced performances. J. Mater. Chem. A 2014, 2, 8103–8109. [Google Scholar] [CrossRef]
- Wang, H.W.; Hu, Z.A.; Chang, Y.Q.; Chen, Y.L.; Wu, H.Y.; Zhang, Z.Y.; Yang, Y.Y. Design and synthesis of NiCo2O4 -reduced graphene oxide composites for high performance supercapacitors. J. Mater. Chem. 2011, 21, 10504–10511. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, P.; Peng, S.; Zou, T.; Yang, Y.; Xing, X.; Wang, Z.; Zhao, R.; Yan, Z.; Wang, Y. Co(OH)2@FeCo2O4 as Electrode Material for High Performance Faradaic Supercapacitor Application. Electrochim. Acta 2019, 299, 312–319. [Google Scholar] [CrossRef]
- Li, G.; Li, W.; Xu, K.; Zou, R.; Chen, Z.; Hu, J. Sponge-like NiCo2O4/MnO2 ultrathin nanoflakes for supercapacitor with high-rate performance and ultra-long cycle life. J. Mater. Chem. A 2014, 2, 7738–7741. [Google Scholar] [CrossRef]


| Electrode | Fabrication Method | Electrolyte | Capacitance (Current Density) | Cycling Stability (No. of Cycles) | Ref. |
|---|---|---|---|---|---|
| NiCo2O4@CoxNi1-x (OH)2 | electrochemical deposition | 1 M KOH | 5.71 F·cm−1 (5.5 mA·cm−2) | 80% (3000 cycles) | [49] |
| carbon nanotube/NiCo2O4 | electrochemical deposition | 6 M KOH | 694 F·g−1 (1 A·g−1) | 91% (1500 cycles) | [50] |
| Ni(OH)2@NiCo2O4 | electrochemical deposition | 1 M KOH | 5.71 F·cm−1 (2 mA·cm−2) | 36% (1000) | [51] |
| graphene/NiCo2O4 | electrochemical deposition | 3 M KOH | 1950 F·g−1 (7.5 A·g−1) | 92.8% (10,000 cycles) | [52] |
| NiCo2O4@graphene nanoarchitectures | Hydrothermal | 2 M KOH | 778 F·g−1 (1 A·g−1) | 90% (10,000 cycles) | [53] |
| NiCo2O4–RGO composite | Self-assembly | 6 M KOH | 835 F·g−1 (1 A·g−1) | 80% (4000 cycles) | [54] |
| Co(OH)2@FeCo2O4 | Hydrothermal | 6 M KOH | 1173.43 F·g−1 (1 A·g−1) | 95.4% (5000) | [55] |
| sponge-like NiCo2O4/MnO2 ultrathin nanoflakes | electrochemical deposition | 1 M KOH | 935 F·g−1 (1 A·g−1) | 103.1% (25,000) | [56] |
| Co(OH)2@NiCo2O4 nanosheet | Hydrothermal | 2 M KOH | 1308 F·g−1 (0.5 A·g−1) | 92.83% (6000) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arbi, H.M.; Vijayalakshmi, L.; Anil Kumar, Y.; Alzahmi, S.; Gopi, C.V.V.M.; Rusydi, A.; Obaidat, I.M. A Facile Two-Step Hydrothermal Synthesis of Co(OH)2@NiCo2O4 Nanosheet Nanocomposites for Supercapacitor Electrodes. Nanomaterials 2023, 13, 1981. https://doi.org/10.3390/nano13131981
Arbi HM, Vijayalakshmi L, Anil Kumar Y, Alzahmi S, Gopi CVVM, Rusydi A, Obaidat IM. A Facile Two-Step Hydrothermal Synthesis of Co(OH)2@NiCo2O4 Nanosheet Nanocomposites for Supercapacitor Electrodes. Nanomaterials. 2023; 13(13):1981. https://doi.org/10.3390/nano13131981
Chicago/Turabian StyleArbi, Hammad Mueen, L. Vijayalakshmi, Yedluri Anil Kumar, Salem Alzahmi, Chandu V. V. Muralee Gopi, Andrivo Rusydi, and Ihab M. Obaidat. 2023. "A Facile Two-Step Hydrothermal Synthesis of Co(OH)2@NiCo2O4 Nanosheet Nanocomposites for Supercapacitor Electrodes" Nanomaterials 13, no. 13: 1981. https://doi.org/10.3390/nano13131981
APA StyleArbi, H. M., Vijayalakshmi, L., Anil Kumar, Y., Alzahmi, S., Gopi, C. V. V. M., Rusydi, A., & Obaidat, I. M. (2023). A Facile Two-Step Hydrothermal Synthesis of Co(OH)2@NiCo2O4 Nanosheet Nanocomposites for Supercapacitor Electrodes. Nanomaterials, 13(13), 1981. https://doi.org/10.3390/nano13131981






