Photoluminescence of Two-Dimensional MoS2 Nanosheets Produced by Liquid Exfoliation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
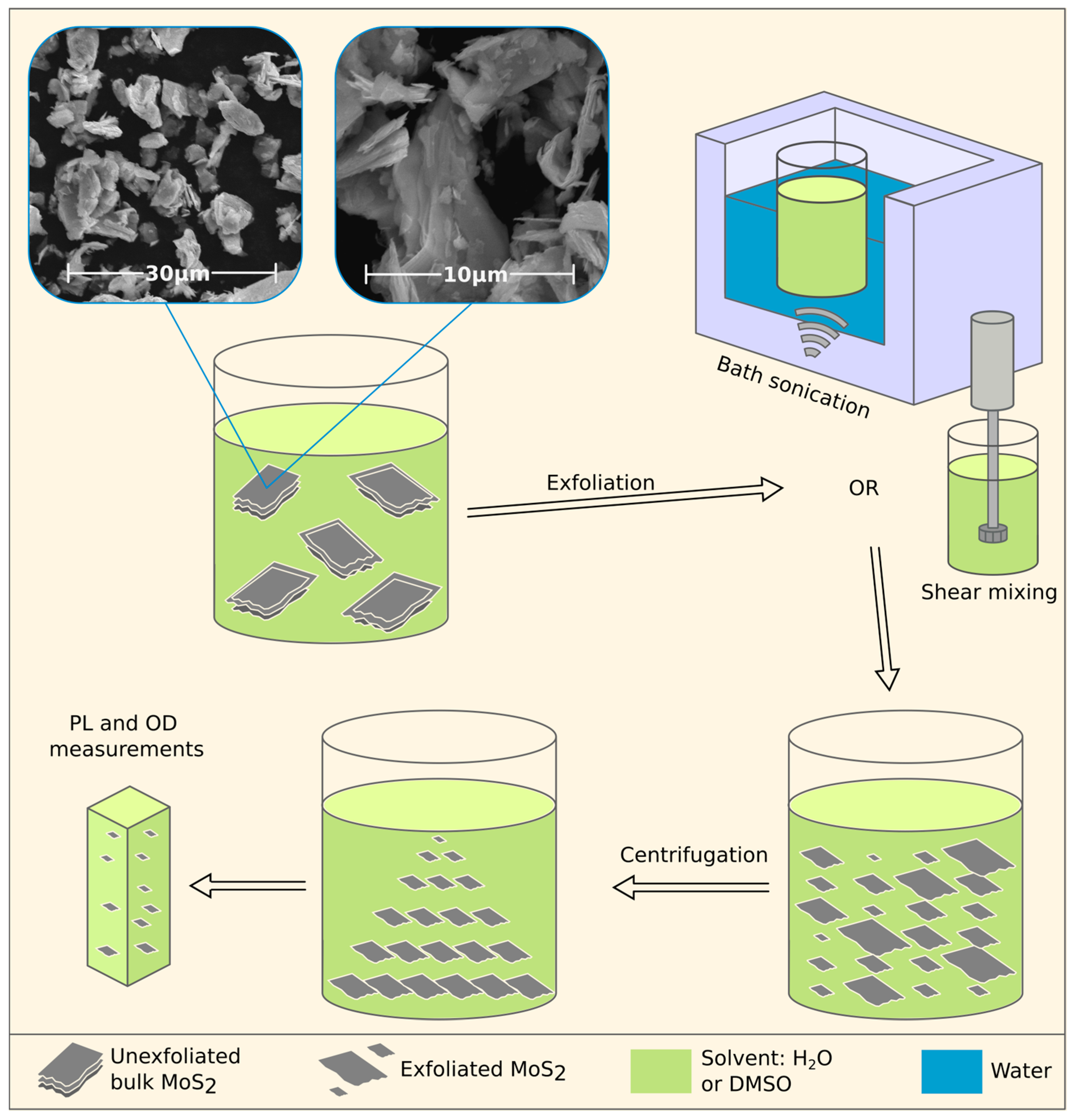
2.2. MoS2 Liquid Exfoliation
2.3. Characterization
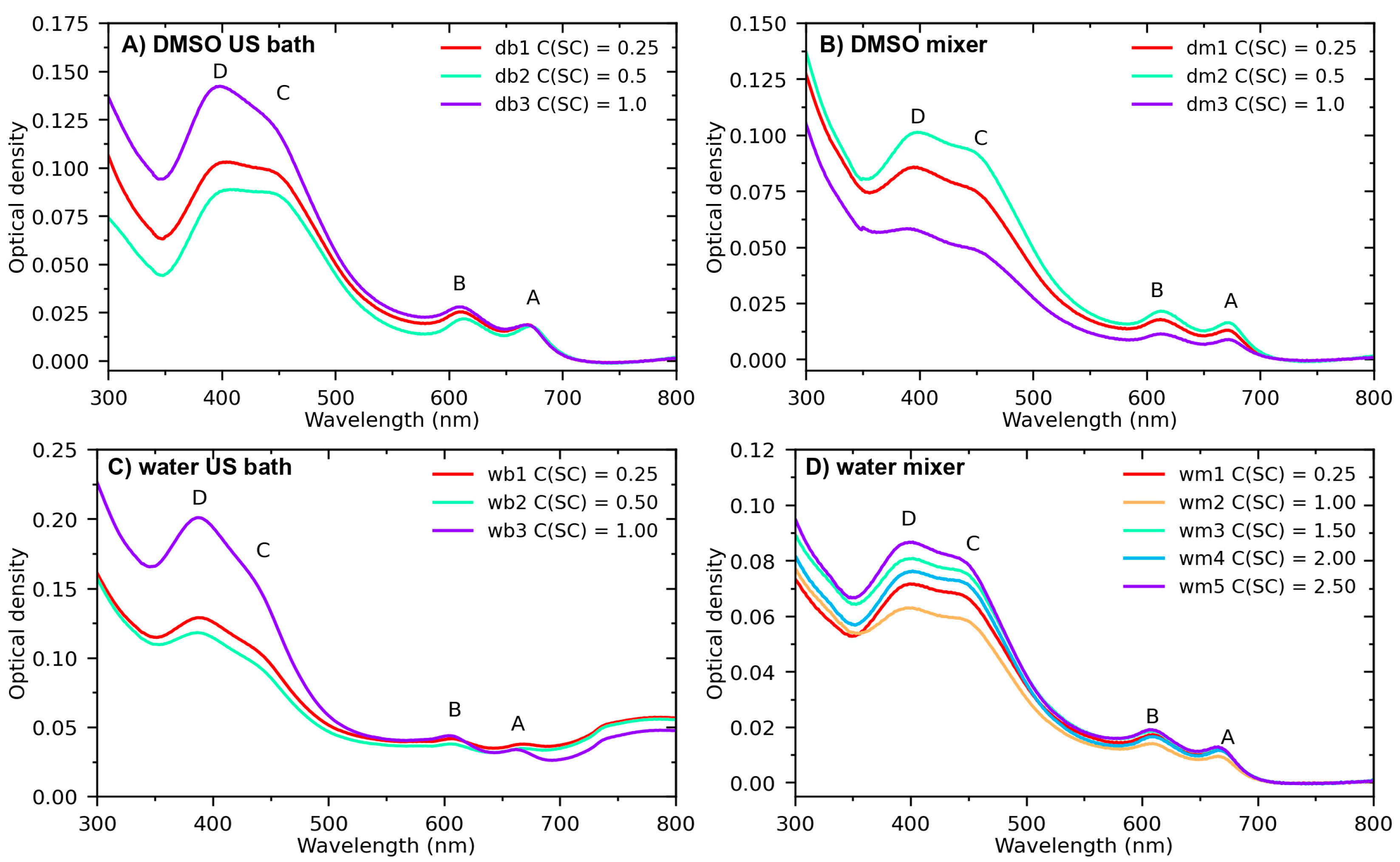
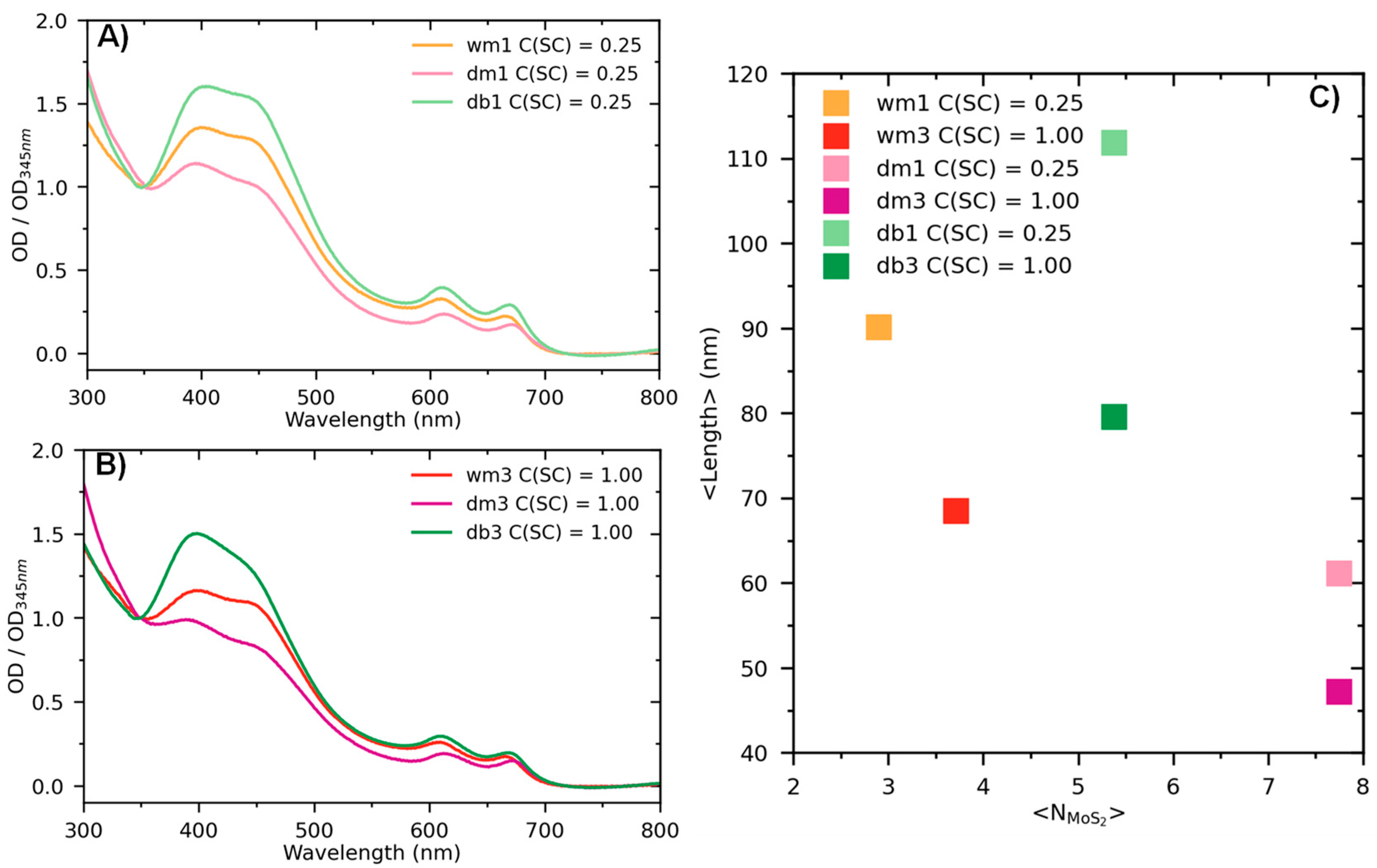
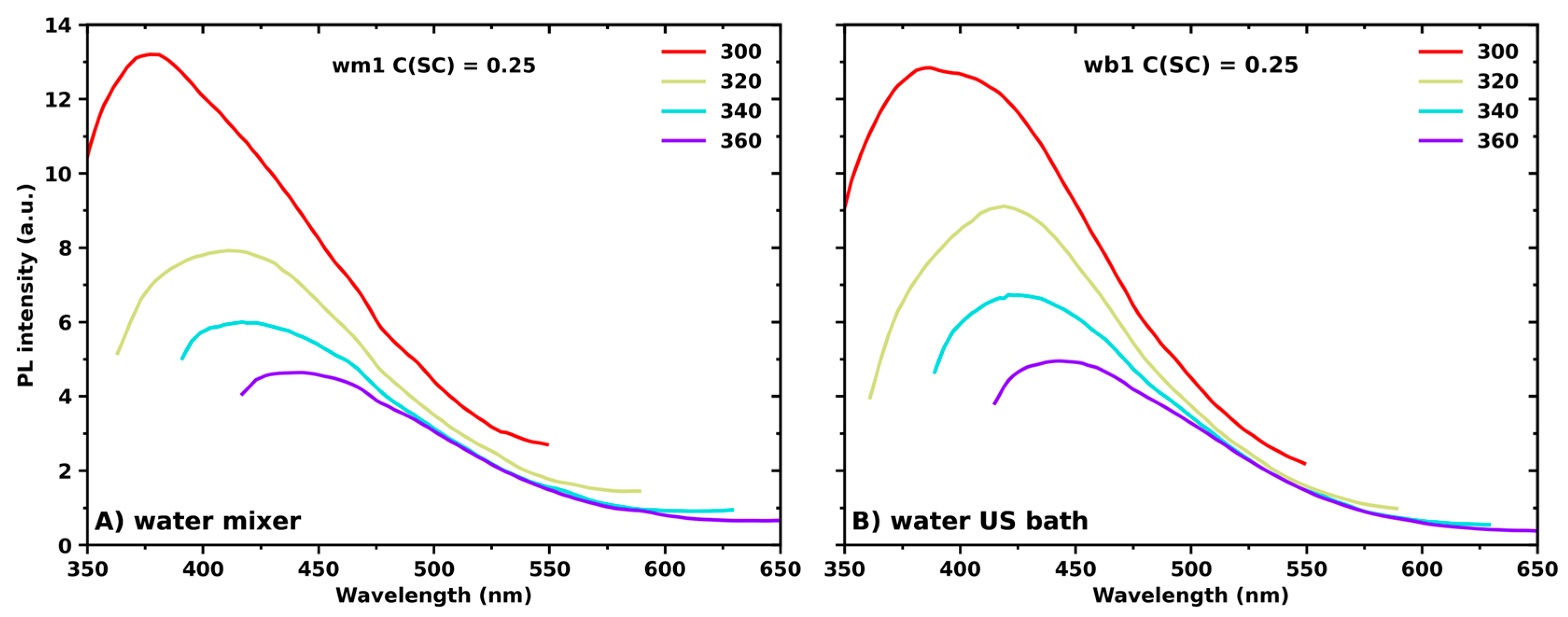
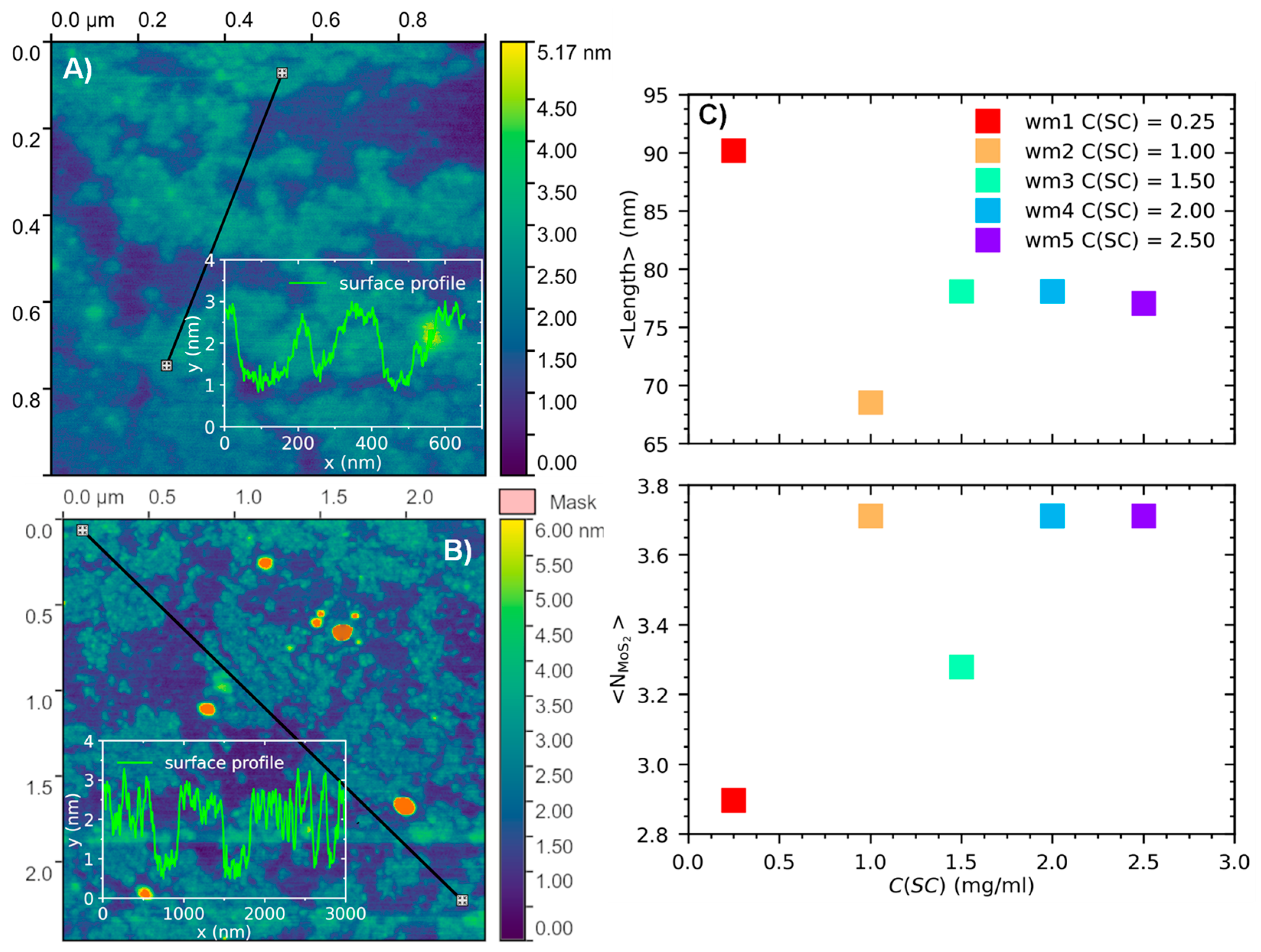
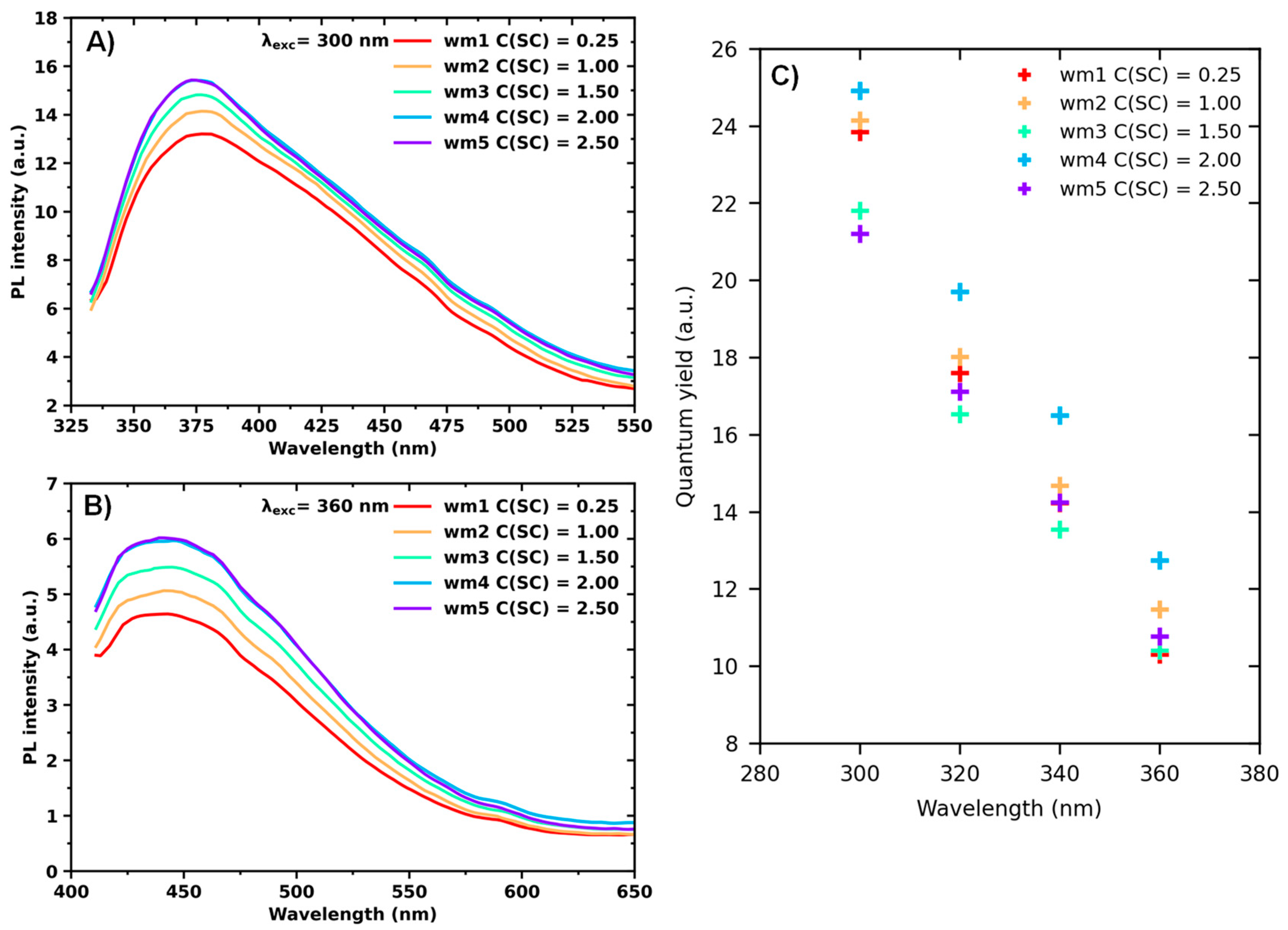
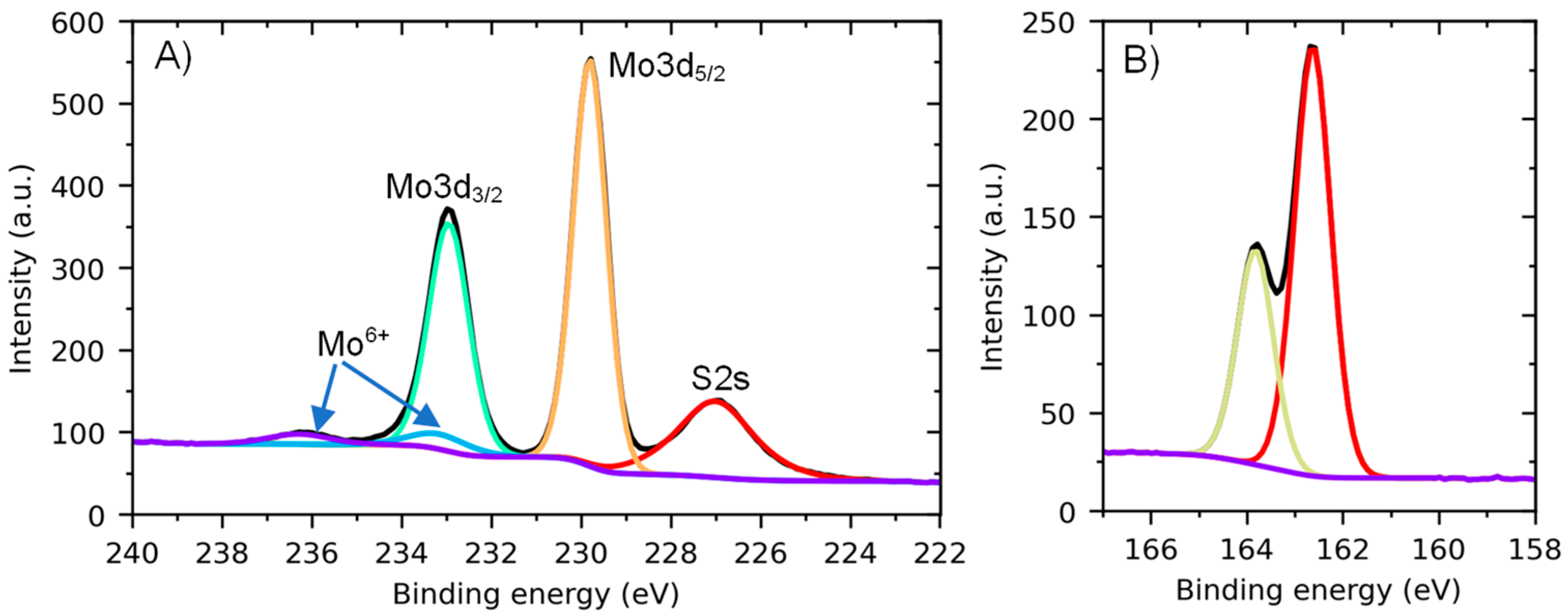
3. Results and Discussion
Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Tao, L.; Chen, Z.; Fang, H.; Li, X.; Wang, X.; Xu, J.-B.; Zhu, H. Graphene and Related Two-Dimensional Materials: Structure-Property Relationships for Electronics and Optoelectronics. Appl. Phys. Rev. 2017, 4, 021306. [Google Scholar] [CrossRef] [Green Version]
- Castellanos-Gomez, A.; Poot, M.; Steele, G.A.; van der Zant, H.S.J.; Agraït, N.; Rubio-Bollinger, G. Elastic Properties of Freely Suspended MoS2 Nanosheets. Adv. Mater. 2012, 24, 772–775. [Google Scholar] [CrossRef] [Green Version]
- Bertolazzi, S.; Brivio, J.; Kis, A. Stretching and Breaking of Ultrathin MoS2. ACS Nano 2011, 5, 9703–9709. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Dai, J.; Yao, W.; Xiao, D.; Cui, X. Valley Polarization in MoS2 Monolayers by Optical Pumping. Nat. Nanotechnol. 2012, 7, 490–493. [Google Scholar] [CrossRef]
- Zhang, W.; Chuu, C.-P.; Huang, J.-K.; Chen, C.-H.; Tsai, M.-L.; Chang, Y.-H.; Liang, C.-T.; Chen, Y.-Z.; Chueh, Y.-L.; He, J.-H.; et al. Ultrahigh-Gain Photodetectors Based on Atomically Thin Graphene-MoS2 Heterostructures. Sci. Rep. 2014, 4, 3826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cong, C.; Shang, J.; Wu, X.; Cao, B.; Peimyoo, N.; Qiu, C.; Sun, L.; Yu, T. Synthesis and Optical Properties of Large-Area Single-Crystalline 2D Semiconductor WS2 Monolayer from Chemical Vapor Deposition. Adv. Opt. Mater. 2014, 2, 131–136. [Google Scholar] [CrossRef]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.-Y.; Galli, G.; Wang, F. Emerging Photoluminescence in Monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically Thin MoS2: A New Direct-Gap Semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Galli, G. Electronic Properties of MoS2 Nanoparticles. J. Phys. Chem. C 2007, 111, 16192–16196. [Google Scholar] [CrossRef]
- Li, H.; Lu, G.; Yin, Z.; He, Q.; Li, H.; Zhang, Q.; Zhang, H. Optical Identification of Single- and Few-Layer MoS2 Sheets. Small 2012, 8, 682–686. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-Layer MoS2 Transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sanchez, O.; Lembke, D.; Kayci, M.; Radenovic, A.; Kis, A. Ultrasensitive Photodetectors Based on Monolayer MoS2. Nat. Nanotechnol. 2013, 8, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The Chemistry of Two-Dimensional Layered Transition Metal Dichalcogenide Nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Monga, D.; Sharma, S.; Shetti, N.P.; Basu, S.; Reddy, K.R.; Aminabhavi, T.M. Advances in Transition Metal Dichalcogenide-Based Two-Dimensional Nanomaterials. Mater. Today Chem. 2021, 19, 100399. [Google Scholar] [CrossRef]
- Tongay, S.; Zhou, J.; Ataca, C.; Lo, K.; Matthews, T.S.; Li, J.; Grossman, J.C.; Wu, J. Thermally Driven Crossover from Indirect toward Direct Bandgap in 2D Semiconductors: MoSe2 versus MoS2. Nano Lett. 2012, 12, 5576–5580. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Sup Choi, M.; Lee, G.-H.; Yu, Y.-J.; Lee, D.-Y.; Hwan Lee, S.; Kim, P.; Hone, J.; Jong Yoo, W. Controlled Charge Trapping by Molybdenum Disulphide and Graphene in Ultrathin Heterostructured Memory Devices. Nat. Commun. 2013, 4, 1624. [Google Scholar] [CrossRef] [Green Version]
- Zhang, E.; Wang, W.; Zhang, C.; Jin, Y.; Zhu, G.; Sun, Q.; Zhang, D.W.; Zhou, P.; Xiu, F. Tunable Charge-Trap Memory Based on Few-Layer MoS2. ACS Nano 2015, 9, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Klein, J.; Sigl, L.; Gyger, S.; Barthelmi, K.; Florian, M.; Rey, S.; Taniguchi, T.; Watanabe, K.; Jahnke, F.; Kastl, C.; et al. Scalable Single-Photon Sources in Atomically Thin MoS2. arXiv 2020, arXiv:2002.08819. [Google Scholar]
- Klein, J.; Lorke, M.; Florian, M.; Sigger, F.; Sigl, L.; Rey, S.; Wierzbowski, J.; Cerne, J.; Müller, K.; Mitterreiter, E.; et al. Site-Selectively Generated Photon Emitters in Monolayer MoS2 via Local Helium Ion Irradiation. Nat. Commun. 2019, 10, 2755. [Google Scholar] [CrossRef] [Green Version]
- Klein, J.; Sigl, L.; Gyger, S.; Barthelmi, K.; Florian, M.; Rey, S.; Taniguchi, T.; Watanabe, K.; Jahnke, F.; Kastl, C.; et al. Engineering the Luminescence and Generation of Individual Defect Emitters in Atomically Thin MoS2. ACS Photonics 2021, 8, 669–677. [Google Scholar] [CrossRef]
- Wu, W.; Wang, L.; Yu, R.; Liu, Y.; Wei, S.-H.; Hone, J.; Wang, Z.L. Piezophototronic Effect in Single-Atomic-Layer MoS2 for Strain-Gated Flexible Optoelectronics. Adv. Mater. 2016, 28, 8463–8468. [Google Scholar] [CrossRef]
- Yang, X.; Li, B. Monolayer MoS2 for Nanoscale Photonics. Nanophotonics 2020, 9, 1557–1577. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Pan, Y.-H.; Yang, R.; Bao, L.-H.; Meng, L.; Luo, H.-L.; Cai, Y.-Q.; Liu, G.-D.; Zhao, W.-J.; Zhou, Z.; et al. Universal Mechanical Exfoliation of Large-Area 2D Crystals. Nat. Commun. 2020, 11, 2453. [Google Scholar] [CrossRef] [PubMed]
- Huo, C.; Yan, Z.; Song, X.F.; Zeng, H. 2D Materials via Liquid Exfoliation: A Review on Fabrication and Applications. Sci. Bull. 2015, 60, 1994–2008. [Google Scholar] [CrossRef]
- Varrla, E.; Backes, C.; Paton, K.R.; Harvey, A.; Gholamvand, Z.; McCauley, J.; Coleman, J.N. Large-Scale Production of Size-Controlled MoS2 Nanosheets by Shear Exfoliation. Chem. Mater. 2015, 27, 1129–1139. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, Z.; Tan, C.; Zhang, H. Solution-Processed Two-Dimensional MoS2 Nanosheets: Preparation, Hybridization, and Applications. Angew. Chem. Int. Ed. 2016, 55, 8816–8838. [Google Scholar] [CrossRef]
- Cunningham, G.; Lotya, M.; Cucinotta, C.S.; Sanvito, S.; Bergin, S.D.; Menzel, R.; Shaffer, M.S.; Coleman, J.N. Solvent Exfoliation of Transition Metal Dichalcogenides: Dispersibility of Exfoliated Nanosheets Varies Only Weakly between Compounds. ACS Nano 2012, 6, 3468–3480. [Google Scholar] [CrossRef] [PubMed]
- Biccai, S.; Barwich, S.; Boland, D.; Harvey, A.; Hanlon, D.; McEvoy, N.; Coleman, J.N. Exfoliation of 2D Materials by High Shear Mixing. 2D Mater. 2018, 6, 015008. [Google Scholar] [CrossRef]
- Varrla, E.; Paton, K.R.; Backes, C.; Harvey, A.; Smith, R.J.; McCauley, J.; Coleman, J.N. Turbulence-Assisted Shear Exfoliation of Graphene Using Household Detergent and a Kitchen Blender. Nanoscale 2014, 6, 11810–11819. [Google Scholar] [CrossRef] [Green Version]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid Exfoliation of Layered Materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef] [Green Version]
- Griffin, A.; Nisi, K.; Pepper, J.; Harvey, A.; Szyd\lowska, B.M.; Coleman, J.N.; Backes, C. Effect of Surfactant Choice and Concentration on the Dimensions and Yield of Liquid-Phase-Exfoliated Nanosheets. Chem. Mater. 2020, 32, 2852–2862. [Google Scholar] [CrossRef]
- Zhou, K.-G.; Mao, N.-N.; Wang, H.-X.; Peng, Y.; Zhang, H.-L. A Mixed-Solvent Strategy for Efficient Exfoliation of Inorganic Graphene Analogues. Angew. Chem. Int. Ed. 2011, 50, 10839–10842. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Naraghi, M.; Ahmadi, G.; Soleymaniha, M.; Shanbedi, M. A Review on Liquid-Phase Exfoliation for Scalable Production of Pure Graphene, Wrinkled, Crumpled and Functionalized Graphene and Challenges. FlatChem 2018, 8, 40–71. [Google Scholar] [CrossRef]
- Güler, Ö.; Tekeli, M.; Taşkın, M.; Güler, S.H.; Yahia, I.S. The Production of Graphene by Direct Liquid Phase Exfoliation of Graphite at Moderate Sonication Power by Using Low Boiling Liquid Media: The Effect of Liquid Media on Yield and Optimization. Ceram. Int. 2021, 47, 521–533. [Google Scholar] [CrossRef]
- Fernandes, J.; Nemala, S.S.; De Bellis, G.; Capasso, A. Green Solvents for the Liquid Phase Exfoliation Production of Graphene: The Promising Case of Cyrene. Front. Chem. 2022, 10, 878799. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.N. Liquid Exfoliation of Defect-Free Graphene. Acc. Chem. Res. 2013, 46, 14–22. [Google Scholar] [CrossRef]
- Trusova, E.A.; Klimenko, I.V.; Afzal, A.M.; Shchegolikhin, A.N.; Jurina, L.V. Comparison of Oxygen-Free Graphene Sheets Obtained in DMF and DMF-Aqua Media. New J. Chem. 2021, 45, 10448–10458. [Google Scholar] [CrossRef]
- Green, A.A.; Hersam, M.C. Solution Phase Production of Graphene with Controlled Thickness via Density Differentiation. Nano Lett. 2009, 9, 4031–4036. [Google Scholar] [CrossRef]
- Hasan, T.; Torrisi, F.; Sun, Z.; Popa, D.; Nicolosi, V.; Privitera, G.; Bonaccorso, F.; Ferrari, A.C. Solution-Phase Exfoliation of Graphite for Ultrafast Photonics. Phys. Status Solidi B 2010, 247, 2953–2957. [Google Scholar] [CrossRef]
- Seo, J.-W.T.; Green, A.A.; Antaris, A.L.; Hersam, M.C. High-Concentration Aqueous Dispersions of Graphene Using Nonionic, Biocompatible Block Copolymers. J. Phys. Chem. Lett. 2011, 2, 1004–1008. [Google Scholar] [CrossRef]
- Gobbi, M.; Orgiu, E.; Samorì, P. When 2D Materials Meet Molecules: Opportunities and Challenges of Hybrid Organic/Inorganic van Der Waals Heterostructures. Adv. Mater. 2018, 30, 1706103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amani, M.; Lien, D.-H.; Kiriya, D.; Xiao, J.; Azcatl, A.; Noh, J.; Madhvapathy, S.R.; Addou, R.; Kc, S.; Dubey, M.; et al. Near-Unity Photoluminescence Quantum Yield in MoS2. Science 2015, 350, 1065–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Gong, K.; Zhao, G.; Lou, W.; Wang, X.; Liu, W. Surface Modification of MoS2 Nanosheets as Effective Lubricant Additives for Reducing Friction and Wear in Poly-α-Olefin. Ind. Eng. Chem. Res. 2018, 57, 8105–8114. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, M.; Wang, J.; Liu, G.; Liu, H.; Jiang, Y. Bioinspired Modification of Layer-Stacked Molybdenum Disulfide (MoS2) Membranes for Enhanced Nanofiltration Performance. ACS Omega 2019, 4, 4012–4022. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Zhang, B.; Liu, Z.; Chen, Y. MoS2 Quantum Dots Chemically Modified with Porphyrin for Solid-State Broadband Optical Limiters. Nanoscale 2019, 11, 20449–20455. [Google Scholar] [CrossRef]
- Amani, M.; Burke, R.A.; Ji, X.; Zhao, P.; Lien, D.-H.; Taheri, P.; Ahn, G.H.; Kirya, D.; Ager III, J.W.; Yablonovitch, E.; et al. High Luminescence Efficiency in MoS2 Grown by Chemical Vapor Deposition. ACS Nano 2016, 10, 6535–6541. [Google Scholar] [CrossRef]
- Tantardini, C.; Kvashnin, A.G.; Azizi, M.; Gonze, X.; Gatti, C.; Altalhi, T.; Yakobson, B.I. Electronic Properties of Functionalized Diamanes for Field-Emission Displays. ACS Appl. Mater. Interfaces 2023, 15, 16317–16326. [Google Scholar] [CrossRef]
- Tantardini, C.; Kvashnin, A.G.; Gatti, C.; Yakobson, B.I.; Gonze, X. Computational Modeling of 2D Materials under High Pressure and Their Chemical Bonding: Silicene as Possible Field-Effect Transistor. ACS Nano 2021, 15, 6861–6871. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.J.; King, P.J.; Lotya, M.; Wirtz, C.; Khan, U.; De, S.; O’Neill, A.; Duesberg, G.S.; Grunlan, J.C.; Moriarty, G.; et al. Large-Scale Exfoliation of Inorganic Layered Compounds in Aqueous Surfactant Solutions. Adv. Mater. 2011, 23, 3944–3948. [Google Scholar] [CrossRef]
- Krishnan, U.; Kaur, M.; Singh, K.; Kumar, M.; Kumar, A. A Synoptic Review of MoS2: Synthesis to Applications. Superlattices Microstruct. 2019, 128, 274–297. [Google Scholar] [CrossRef]
- Wilson, J.A.; Yoffe, A. The Transition Metal Dichalcogenides Discussion and Interpretation of the Observed Optical, Electrical and Structural Properties. Adv. Phys. 1969, 18, 193–335. [Google Scholar] [CrossRef]
- Kappera, R.; Voiry, D.; Yalcin, S.E.; Branch, B.; Gupta, G.; Mohite, A.D.; Chhowalla, M. Phase-Engineered Low-Resistance Contacts for Ultrathin MoS2 Transistors. Nat. Mater. 2014, 13, 1128–1134. [Google Scholar] [CrossRef]
- Wilcoxon, J.; Newcomer, P.; Samara, G. Synthesis and Optical Properties of MoS2 Nanoclusters. MRS Online Proc. Libr. OPL 1996, 452, 371. [Google Scholar] [CrossRef]
- Wilcoxon, J.; Samara, G. Strong Quantum-Size Effects in a Layered Semiconductor: MoS2 Nanoclusters. Phys. Rev. B 1995, 51, 7299. [Google Scholar] [CrossRef]
- Backes, C.; Smith, R.J.; McEvoy, N.; Berner, N.C.; McCloskey, D.; Nerl, H.C.; O’Neill, A.; King, P.J.; Higgins, T.; Hanlon, D.; et al. Edge and Confinement Effects Allow in Situ Measurement of Size and Thickness of Liquid-Exfoliated Nanosheets. Nat. Commun. 2014, 5, 4576. [Google Scholar] [CrossRef] [Green Version]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from Chemically Exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ghorannevis, Z.; Chu, L.; Toh, M.; Kloc, C.; Tan, P.-H.; Eda, G. Evolution of Electronic Structure in Atomically Thin Sheets of WS2 and WSe2. ACS Nano 2013, 7, 791–797. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Ji, A.; Zhu, C.; Cui, L.; Mao, L.-F. Layer-Dependent Bandgap and Electrical Engineering of Molybdenum Disulfide. J. Phys. Chem. Solids 2020, 139, 109331. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous Lattice Vibrations of Single-and Few-Layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, V.M.; Dhumal, N.R.; Zehentbauer, F.M.; Kim, H.J.; Kiefer, J. Revisiting the Aqueous Solutions of Dimethyl Sulfoxide by Spectroscopy in the Mid-and near-Infrared: Experiments and Car–Parrinello Simulations. J. Phys. Chem. B 2015, 119, 14780–14789. [Google Scholar] [CrossRef] [PubMed]
- Štengl, V.; Henych, J. Strongly Luminescent Monolayered MoS2 Prepared by Effective Ultrasound Exfoliation. Nanoscale 2013, 5, 3387–3394. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.; Kumar, B.; Sinha, J.; Ghosh, S.; Roy, S.S.; Kaviraj, B. Cost Effective Liquid Phase Exfoliation of MoS2 Nanosheets and Photocatalytic Activity for Wastewater Treatment Enforced by Visible Light. Sci. Rep. 2020, 10, 10759. [Google Scholar] [CrossRef] [PubMed]
- Saigal, N.; Ghosh, S. Evidence for Two Distinct Defect Related Luminescence Features in Monolayer MoS2. Appl. Phys. Lett. 2016, 109, 122105. [Google Scholar] [CrossRef]
- Fisk, P.R.; Ford, M.G.; Watson, P. The Kinetics of the Partitioning of Compounds between Octanol and Water, and Its Relationship to the Movement of Molecules in Biological Systems. Compr. Chem. Kinet. 1999, 37, 161–194. [Google Scholar]
- Cheknlyuk, A.; Fadeev, V.; Georgiev, G.; Kalkanjiev, T.; Nickolov, Z. Determination of Fluorescence Quantum Yields Using a Spontaneous Raman Scattering Line of the Solvent as Internal Standard. Spectrosc. Lett. 1982, 15, 355–365. [Google Scholar] [CrossRef]


| Solvent Type | Exfoliation Approach | Sample Name | C(SC), mg/mL | C (MoS2), mg/mL | Production Time, h | Centrifugation #1 |
|---|---|---|---|---|---|---|
| Water | Shear force promoted | wm1 | 0.25 | 2.5 | 2 | 1500 rpm × 45 min |
| wm2 | 1.0 | |||||
| wm3 | 1.5 | |||||
| wm4 | 2.0 | |||||
| wm5 | 2.5 | |||||
| Sonication promoted | wb1 | 0.25 | 1 | |||
| wb2 | 0.5 | |||||
| wb3 | 1 | |||||
| DMSO | Shear force promoted | dm1 | 0.25 | 2 | ||
| dm2 | 0.5 | |||||
| dm3 | 1 | |||||
| Sonication promoted | db1 | 0.25 | 1 | |||
| db2 | 0.5 | |||||
| db3 | 1 |
| Sample Name | Ci(SC), mg/mL | Cf(MoS2) mg/mL | Centrifugation #2 |
|---|---|---|---|
| wm1 | 0.25 | 2.5 | 10 krpm × 6 min |
| wm2 | 1.0 | ||
| wm3 | 1.5 | ||
| wm4 | 2.0 | ||
| wm5 | 2.5 | ||
| wb1 | 0.25 | 1.25 | 12 krpm × 30 min |
| wb2 | 0.5 | 2.5 | |
| wb3 | 0.5 | 2.5 | |
| dm1 | 0.17 | 1.67 | 10 krpm × 5 min |
| dm2 | 0.33 | ||
| dm3 | 0.67 | ||
| db1 | 0.015 | 0.153 | 10 krpm × 5 min |
| db2 | 0.031 | ||
| db3 | 0.061 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukianov, M.Y.; Rubekina, A.A.; Bondareva, J.V.; Sybachin, A.V.; Diudbin, G.D.; Maslakov, K.I.; Kvashnin, D.G.; Klimova-Korsmik, O.G.; Shirshin, E.A.; Evlashin, S.A. Photoluminescence of Two-Dimensional MoS2 Nanosheets Produced by Liquid Exfoliation. Nanomaterials 2023, 13, 1982. https://doi.org/10.3390/nano13131982
Lukianov MY, Rubekina AA, Bondareva JV, Sybachin AV, Diudbin GD, Maslakov KI, Kvashnin DG, Klimova-Korsmik OG, Shirshin EA, Evlashin SA. Photoluminescence of Two-Dimensional MoS2 Nanosheets Produced by Liquid Exfoliation. Nanomaterials. 2023; 13(13):1982. https://doi.org/10.3390/nano13131982
Chicago/Turabian StyleLukianov, Mikhail Y., Anna A. Rubekina, Julia V. Bondareva, Andrey V. Sybachin, George D. Diudbin, Konstantin I. Maslakov, Dmitry G. Kvashnin, Olga G. Klimova-Korsmik, Evgeny A. Shirshin, and Stanislav A. Evlashin. 2023. "Photoluminescence of Two-Dimensional MoS2 Nanosheets Produced by Liquid Exfoliation" Nanomaterials 13, no. 13: 1982. https://doi.org/10.3390/nano13131982
APA StyleLukianov, M. Y., Rubekina, A. A., Bondareva, J. V., Sybachin, A. V., Diudbin, G. D., Maslakov, K. I., Kvashnin, D. G., Klimova-Korsmik, O. G., Shirshin, E. A., & Evlashin, S. A. (2023). Photoluminescence of Two-Dimensional MoS2 Nanosheets Produced by Liquid Exfoliation. Nanomaterials, 13(13), 1982. https://doi.org/10.3390/nano13131982










