Biofunctionalization of HMX with Peptides via Polydopamine Crosslinking for Assembling an HMX@Al@CuO Nanoenergetic Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterization
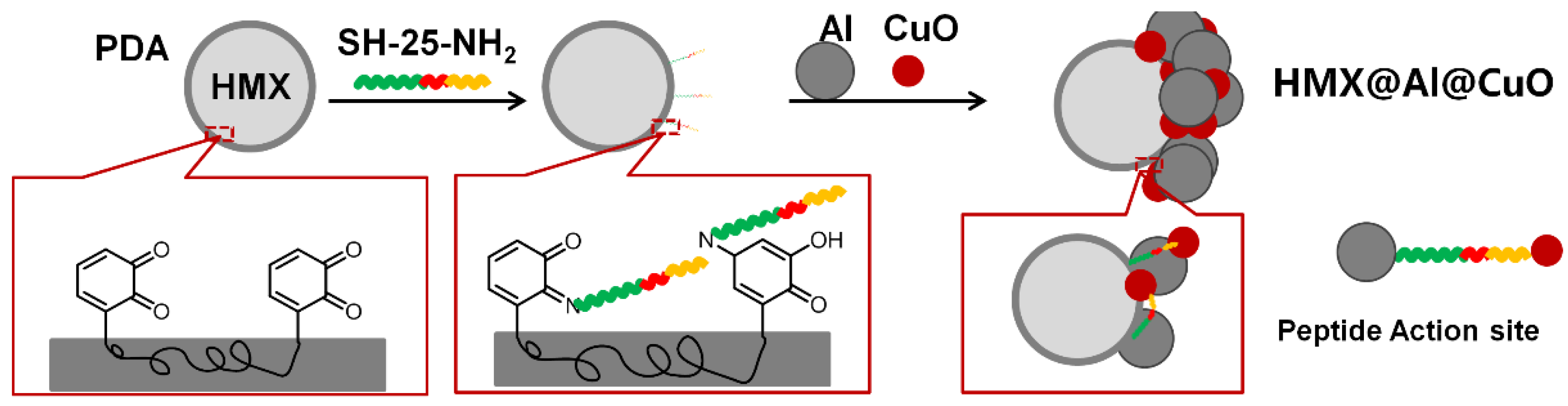
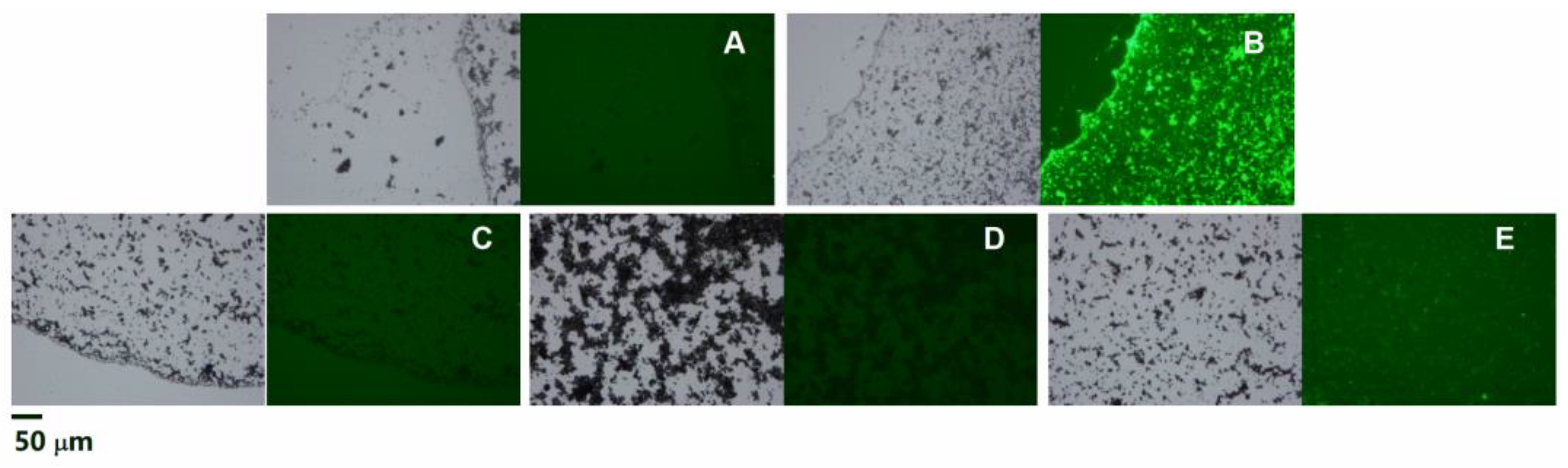
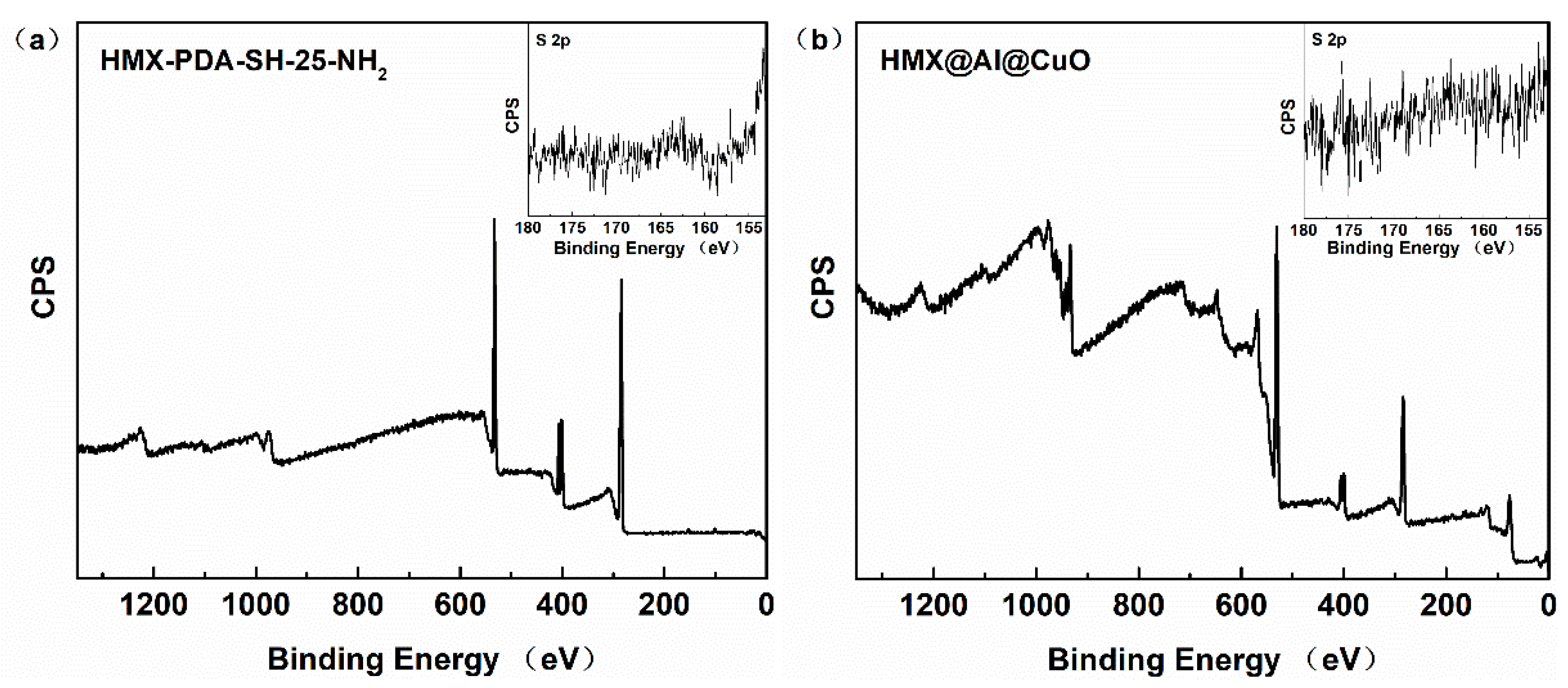
3. Results
3.1. Morphological and Compositional Characterization
3.2. Thermal Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Ren, W.; Zheng, Z.; Wu, G.; Hu, B.; Chen, J.; Wang, J.; Yu, C.; Ma, K.; Zhou, X.; et al. Reactivity adjustment from the contact extent between CuO and Al phases in nanothermites. Chem. Eng. J. 2020, 402, 126288–126297. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, W.; Yu, C.; Zheng, Z.; Chen, Y.; Wang, J.; Liu, J.; Ma, K.; Ren, W. Electrochemical Synthesis of Al/CuO Thermite Films on Copper Substrates. Ind. Eng. Chem. Res. 2019, 58, 7131–7138. [Google Scholar] [CrossRef]
- Xue, Z.-H.; Zhang, X.-X.; Huang, B.-B.; Bai, X.; Zhu, L.-Y.; Chen, S.; Yan, Q.-L. The structural diversity of hybrid qy-HMX crystals with constraint of 2D dopants and the resulted changes in thermal reactivity. Chem. Eng. J. 2020, 390, 124565–124577. [Google Scholar] [CrossRef]
- Yan, T.; Ren, H.; Liu, J.; Jiao, Q. Facile preparation and synergetic energy releasing of nano-Al@RDX@Viton hollow microspheres. Chem. Eng. J. 2020, 379, 122333–122342. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, X.; Xu, J.; Ma, X.; Ye, Y.; Yang, G.; Zhang, K. In situ preparation of explosive embedded CuO/Al/CL20 nanoenergetic composite with enhanced reactivity. Chem. Eng. J. 2018, 354, 885–895. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Liu, L.-L.; Xiao, L.-Y.; Wang, Z.-X. Thermal decomposition of HTPB/AP and HTPB/HMX mixtures with low content of oxidizer. J. Therm. Anal. Calorim. 2015, 119, 1673–1678. [Google Scholar] [CrossRef]
- Tillotson, T.M.; Gash, A.E.; Simpson, R.L.; Hrubesh, L.W.; Satcher, J.H.; Poco, J.F. Nanostructured energetic materials using sol-gel methodologies. J. Non-Cryst. Solids 2001, 285, 338–345. [Google Scholar] [CrossRef]
- Cheng, J.L.; Hng, H.H.; Ng, H.Y.; Soon, P.C.; Lee, Y.W. Synthesis and characterization of self-assembled nanoenergetic Al-Fe2O3 thermite system. J. Phys. Chem. Solids 2010, 71, 90–94. [Google Scholar] [CrossRef]
- Séverac, F.; Alphonse, P.; Estève, A.; Bancaud, A.; Rossi, C. High-Energy Al/CuO Nanocomposites Obtained by DNA-Directed Assembly. Adv. Funct. Mater. 2012, 22, 323–329. [Google Scholar] [CrossRef]
- Sullivan, K.T.; Worsley, M.A.; Kuntz, J.D.; Gash, A.E. Electrophoretic deposition of binary energetic composites. Combust. Flame 2012, 159, 2210–2218. [Google Scholar] [CrossRef]
- Dong, Z.; Al-Sharab, J.F.; Kear, B.H.; Tse, S.D. Combined flame and electrodeposition synthesis of energetic coaxial tungsten-oxide/aluminum nanowire arrays. Nano Lett. 2013, 13, 4346–4350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, X.; Qin, B.; Lai, C.; Guo, X. Electrophoretic deposition and characterization of nano-Al/Fe2O3 thermites. Mater. Lett. 2014, 120, 224–227. [Google Scholar] [CrossRef]
- Yi, Z.; Cao, Y.; Yuan, J.; Mary, C.; Wan, Z.; Li, Y.; Zhu, C.; Zhang, L.; Zhu, S. Functionalized carbon fibers assembly with Al/Bi2O3: A new strategy for high-reliability ignition. Chem. Eng. J. 2020, 389, 124254–124261. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Huang, H.; Zhao, Y.; Song, K.; Wang, H.; Xie, W.; Cheng, Y.; Fan, X. Preparation and characterization of the Al/Fe2O3/RDX/NC nanocomposites by electrospray. J. Therm. Anal. Calorim. 2019, 137, 1615–1620. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Guo, Z. Fabrication of inorganic-organic hybrid TiO2@PDA@CuO composite nanoparticles and its special wettable, gas sensing and photocatalytic behaviors. Mater. Lett. 2018, 217, 320–323. [Google Scholar] [CrossRef]
- Lin, C.; Zeng, C.; Wen, Y.; Gong, F.; He, G.; Li, Y.; Yang, Z.; Ding, L.; Li, J.; Guo, S. Litchi-like Core-Shell HMX@HPW@PDA Microparticles for Polymer-Bonded Energetic Composites with Low Sensitivity and High Mechanical Properties. ACS Appl. Mater. Interfaces 2020, 12, 4002–4013. [Google Scholar] [CrossRef]
- Perlman, O.; Borodetsky, A.; Kauffmann, Y.; Shamay, Y.; Azhari, H.; Weitz, I.S. Gold/Copper@Polydopamine Nanocomposite for Contrast-Enhanced Dual Modal Computed Tomography–Magnetic Resonance Imaging. ACS Appl. Nano Mater. 2019, 2, 6124–6134. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Niu, H.; Ma, Y.; Zeng, T.; Cai, Y.; Meng, Z. Preparation of polydopamine coated Fe3O4 nanoparticles and their application for enrichment of polycyclic aromatic hydrocarbons from environmental water samples. J. Chromatogr. A 2013, 1283, 20–26. [Google Scholar] [CrossRef]
- He, W.; Liu, P.J.; Gong, F.; Tao, B.; Gu, J.; Yang, Z.; Yan, Q.L. Tuning the Reactivity of Metastable Intermixed Composite n-Al/PTFE by Polydopamine Interfacial Control. ACS Appl. Mater. Interfaces 2018, 10, 32849–32858. [Google Scholar] [CrossRef]
- He, W.; Tao, B.; Yang, Z.; Yang, G.; Guo, X.; Liu, P.-J.; Yan, Q.-L. Mussel-inspired polydopamine-directed crystal growth of core-shell n-Al@PDA@CuO metastable intermixed composites. Chem. Eng. J. 2019, 369, 1093–1101. [Google Scholar] [CrossRef]
- Lin, C.; Gong, F.; Yang, Z.; Zhao, X.; Li, Y.; Zeng, C.; Li, J.; Guo, S. Core-Shell Structured HMX@Polydopamine Energetic Microspheres: Synergistically Enhanced Mechanical, Thermal, and Safety Performances. Polymers 2019, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.-Y.; Yu, J.-H.; Tang, D.-Y.; He, W.; Tao, B.-W.; Guo, X.; Yan, Q.-L. Unexpected burning rate independence of composite propellants on the pressure by fine interfacial control of fuel/oxidizer. Chem. Eng. J. 2020, 388, 124320–124331. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Wang, P.; He, W.; Duan, H. Robust Nanoparticle-DNA Conjugates Based on Mussel-Inspired Polydopamine Coating for Cell Imaging and Tailored Self-Assembly. Bioconjug. Chem. 2016, 27, 815–823. [Google Scholar] [CrossRef]
- Zhu, L.P.; Jiang, J.H.; Zhu, B.K.; Xu, Y.Y. Immobilization of bovine serum albumin onto porous polyethylene membranes using strongly attached polydopamine as a spacer. Colloids Surf. B 2011, 86, 111–118. [Google Scholar] [CrossRef]
- Cui, J.; Ju, Y.; Liang, K.; Ejima, H.; Lorcher, S.; Gause, K.T.; Richardson, J.J.; Caruso, F. Nanoscale engineering of low-fouling surfaces through polydopamine immobilisation of zwitterionic peptides. Soft Matter 2014, 10, 2656–2663. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Faraj, Y.; Wei, J.; Wang, W.; Xie, R.; Liu, Z.; Ju, X.-J.; Chu, L.-Y. Antimicrobial peptide-functionalized magnetic nanoparticles for rapid capture and removal of pathogenic bacteria. Microchem. J. 2020, 159, 105493–105500. [Google Scholar] [CrossRef]
- Song, Z.; Jin, M.; Xian, M.; Wang, C. Peptide-driven assembly of Al/CuO energetic nanocomposite material. Chem. Eng. J. 2020, 388, 124225–124233. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, N.; Zheng, W.; Chen, J.; Song, X.; Wang, J.; Cui, C.; Zhang, D.; Zhao, F. Application and Properties of CL-20/HMX Cocrystal in Composite Modified Double Base Propellants. Propellants Explos. Pyrotech. 2019, 45, 92–100. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Q.; Chen, J.; Luo, R.; Maitz, M.F.; Huang, N. Immobilization of serum albumin and peptide aptamer for EPC on polydopamine coated titanium surface for enhanced in-situ self-endothelialization. Mater. Sci. Eng. C 2016, 60, 219–229. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, M.; Yang, Y.; Zheng, M.; Yang, Y.; Liu, X.; Tan, J. Biofunctionalization of zirconia with cell-adhesion peptides via polydopamine crosslinking for soft tissue engineering: Effects on the biological behaviors of human gingival fibroblasts and oral bacteria. RSC Adv. 2020, 10, 6200–6212. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Guo, X.; Chen, L.; Huang, S.; Zhao, L.; Ji, J.; Zhou, X. Alcohol-thermal synthesis of approximately core-shell structured Al@CuO nanothermite with improved heat-release and combustion characteristics. Combust. Flame 2021, 228, 331–339. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, S.; Zhao, F.; Chen, P.; Yin, C.M.; Li, S.W. Effect of nano metal powder on the thermal decomposition characteristics of HMX. J. Propuls. Technol. 2002, 23, 258–261. [Google Scholar]


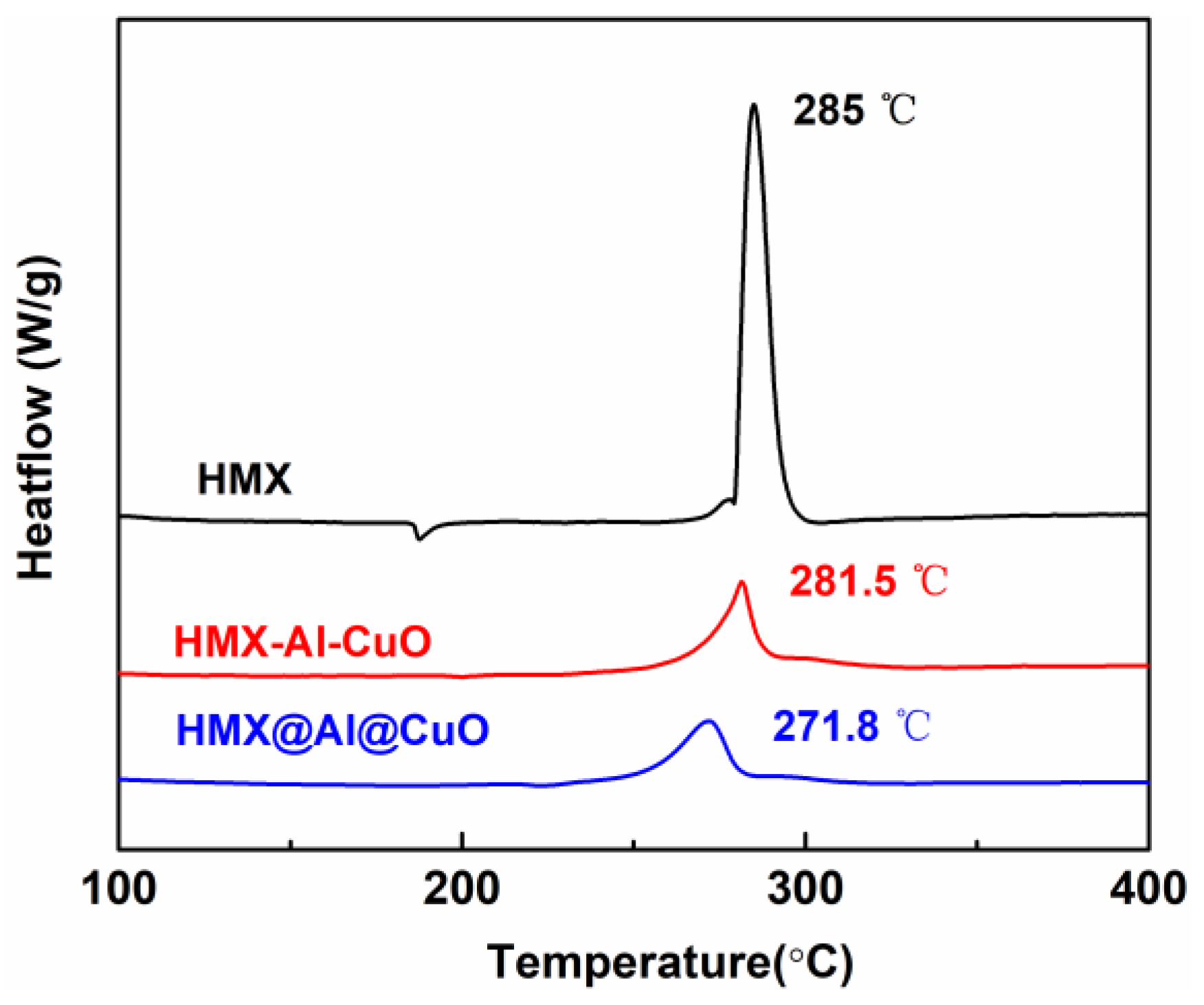
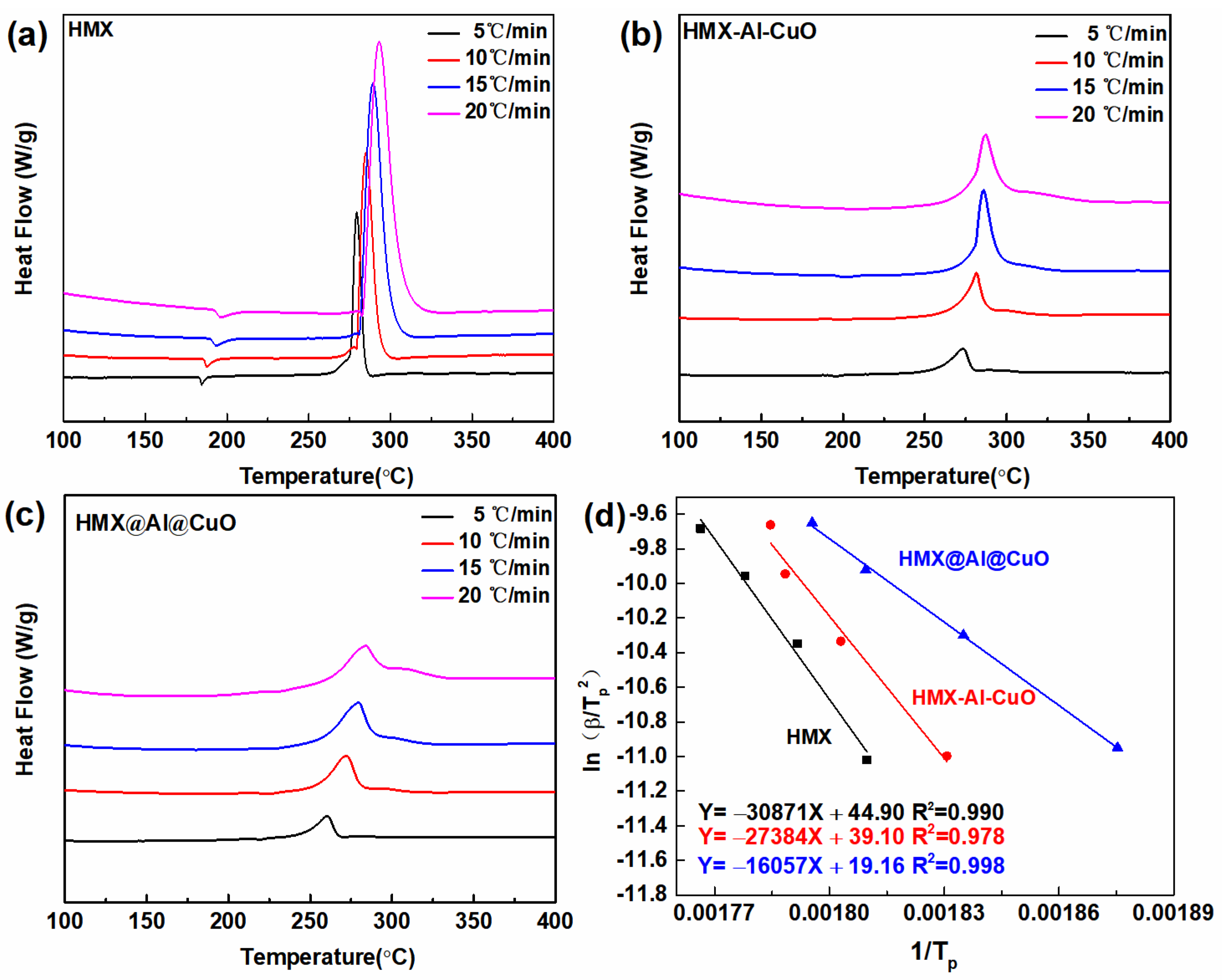
| Samples | Tp (°C) | |||
|---|---|---|---|---|
| 5 °C/min | 10 °C/min | 15 °C/min | 20 °C/min | |
| HMX | 279.4 | 285.0 | 289.3 | 293.0 |
| HMX-Al-CuO | 273.1 | 281.5 | 286.0 | 287.2 |
| HMX@Al@CuO | 260.1 | 271.8 | 279.5 | 283.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, M.; Song, Z.; Liu, W.; Wang, G.; Xian, M. Biofunctionalization of HMX with Peptides via Polydopamine Crosslinking for Assembling an HMX@Al@CuO Nanoenergetic Composite. Nanomaterials 2023, 13, 1837. https://doi.org/10.3390/nano13121837
Jin M, Song Z, Liu W, Wang G, Xian M. Biofunctionalization of HMX with Peptides via Polydopamine Crosslinking for Assembling an HMX@Al@CuO Nanoenergetic Composite. Nanomaterials. 2023; 13(12):1837. https://doi.org/10.3390/nano13121837
Chicago/Turabian StyleJin, Miaomiao, Zhanxin Song, Wei Liu, Guozhen Wang, and Mo Xian. 2023. "Biofunctionalization of HMX with Peptides via Polydopamine Crosslinking for Assembling an HMX@Al@CuO Nanoenergetic Composite" Nanomaterials 13, no. 12: 1837. https://doi.org/10.3390/nano13121837
APA StyleJin, M., Song, Z., Liu, W., Wang, G., & Xian, M. (2023). Biofunctionalization of HMX with Peptides via Polydopamine Crosslinking for Assembling an HMX@Al@CuO Nanoenergetic Composite. Nanomaterials, 13(12), 1837. https://doi.org/10.3390/nano13121837






