A Review of the Relationship between Gel Polymer Electrolytes and Solid Electrolyte Interfaces in Lithium Metal Batteries
Abstract
1. Introduction
2. GPE Builds the Dendrite-Free Lithium Metal Anode
2.1. The Development of GPE
2.2. Effect of GPE Composition on Lithium Ionic Conductivity
2.2.1. Lithium Salts
2.2.2. Polymer Substrates
2.2.3. GPE Additives
2.3. GPE Design
2.3.1. Structural Design
2.3.2. Functional Design
3. SEIs Formed by GPEs and Their Advantages
3.1. The Brief Overview of SEIs
3.1.1. SEI Formation
3.1.2. Composition and Structure of SEIs
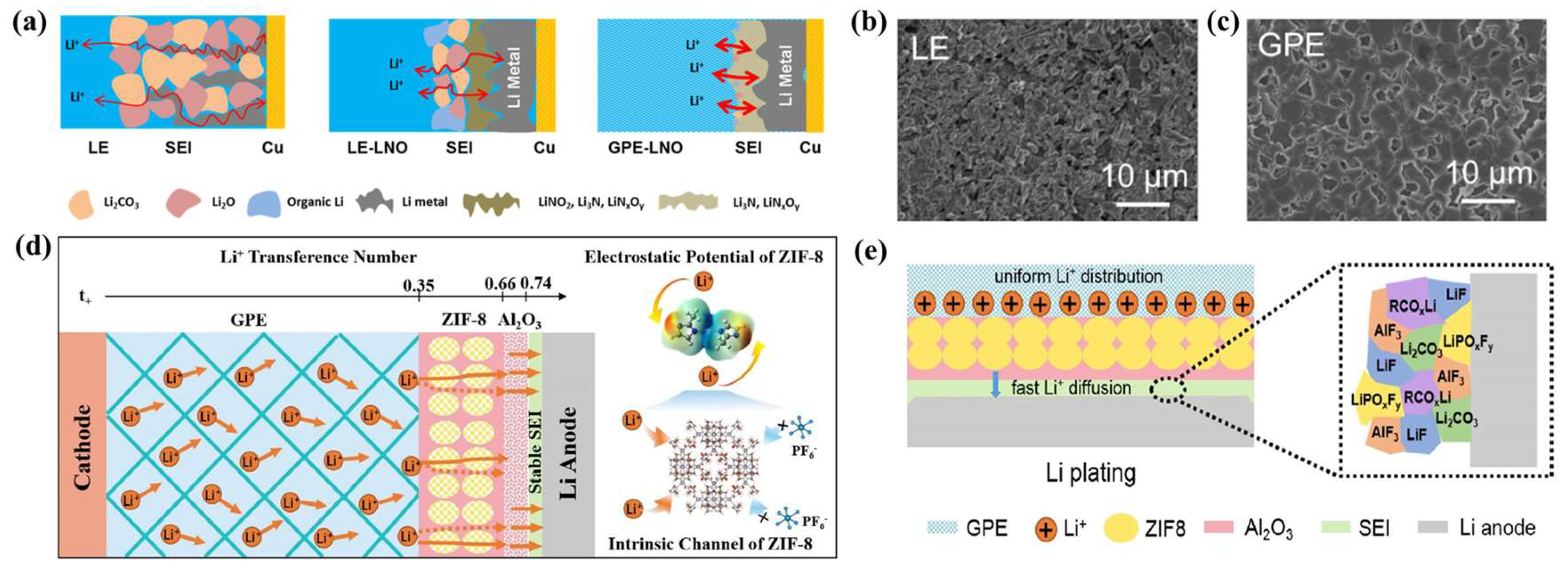
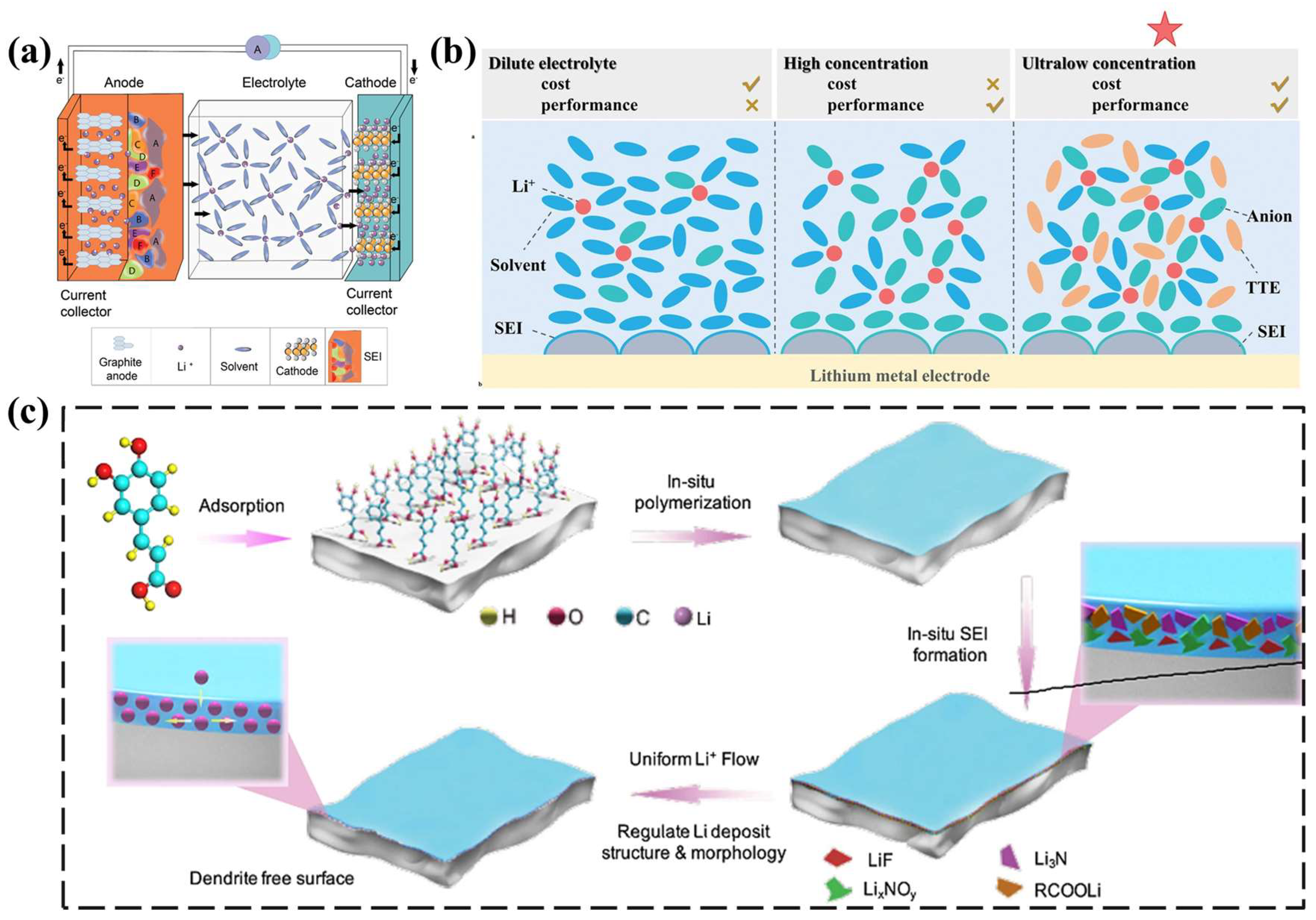
3.2. The Relationship between GPEs and SEIs
4. The Effect of GPE Performance on SEIs
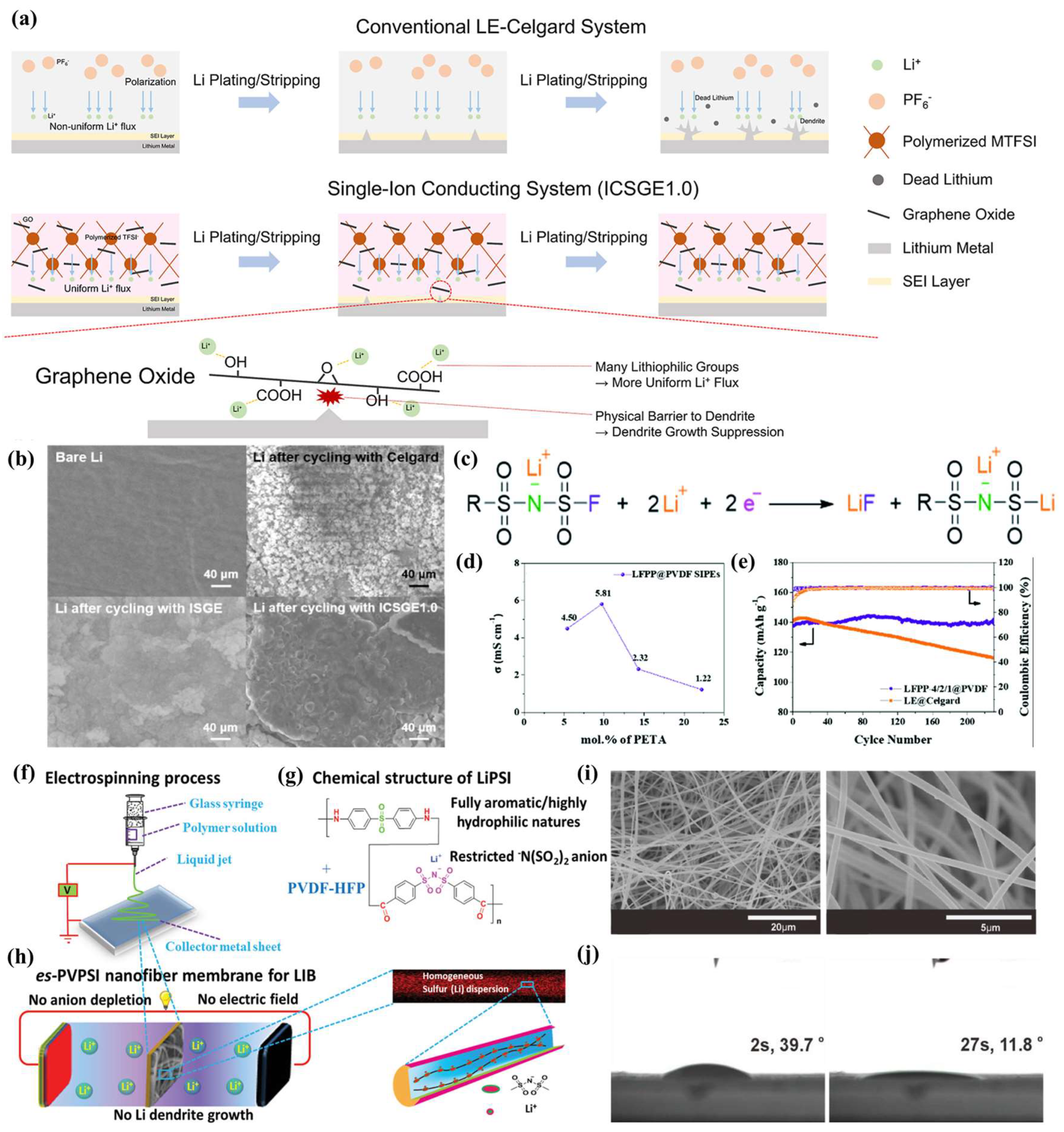
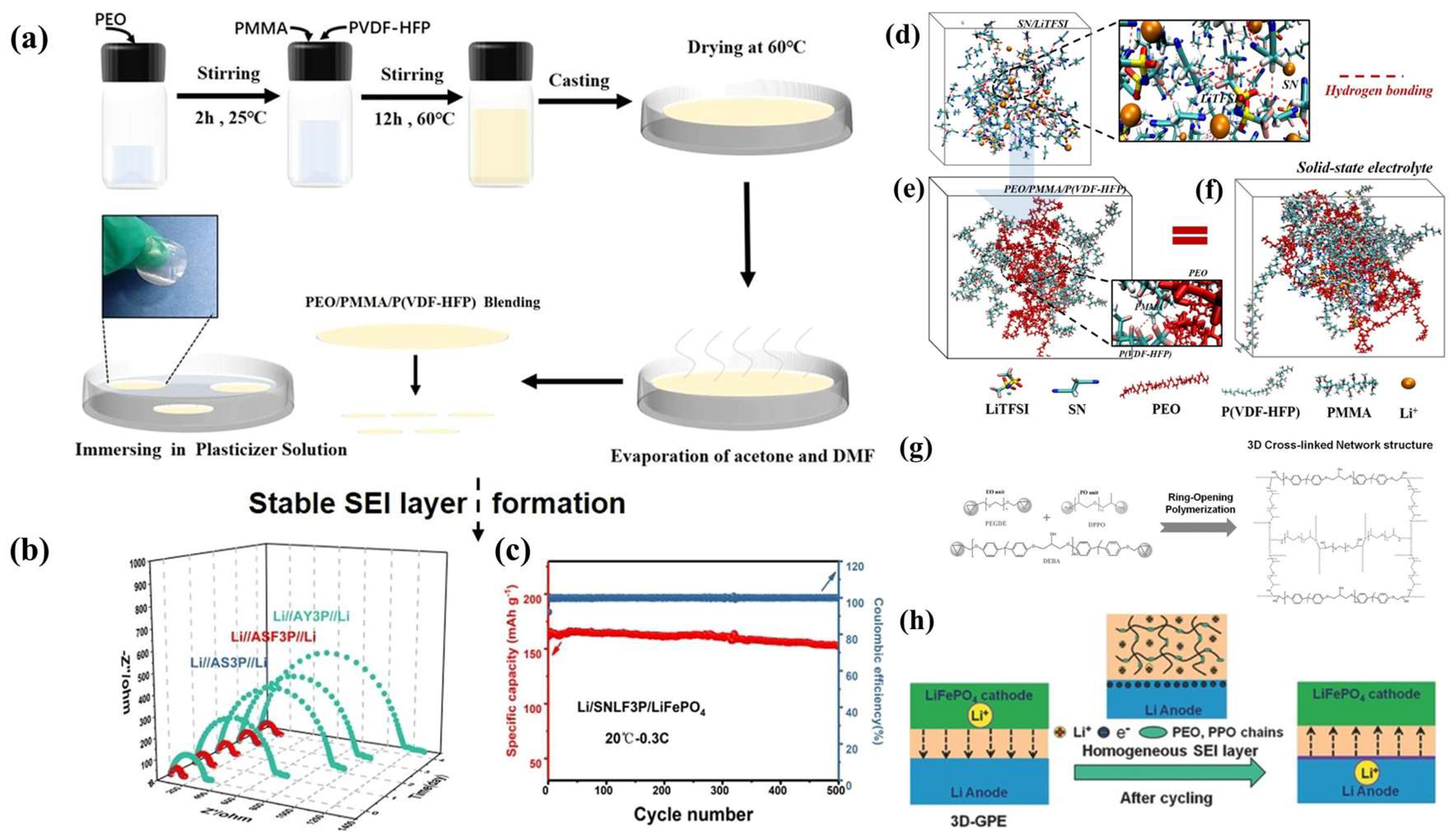
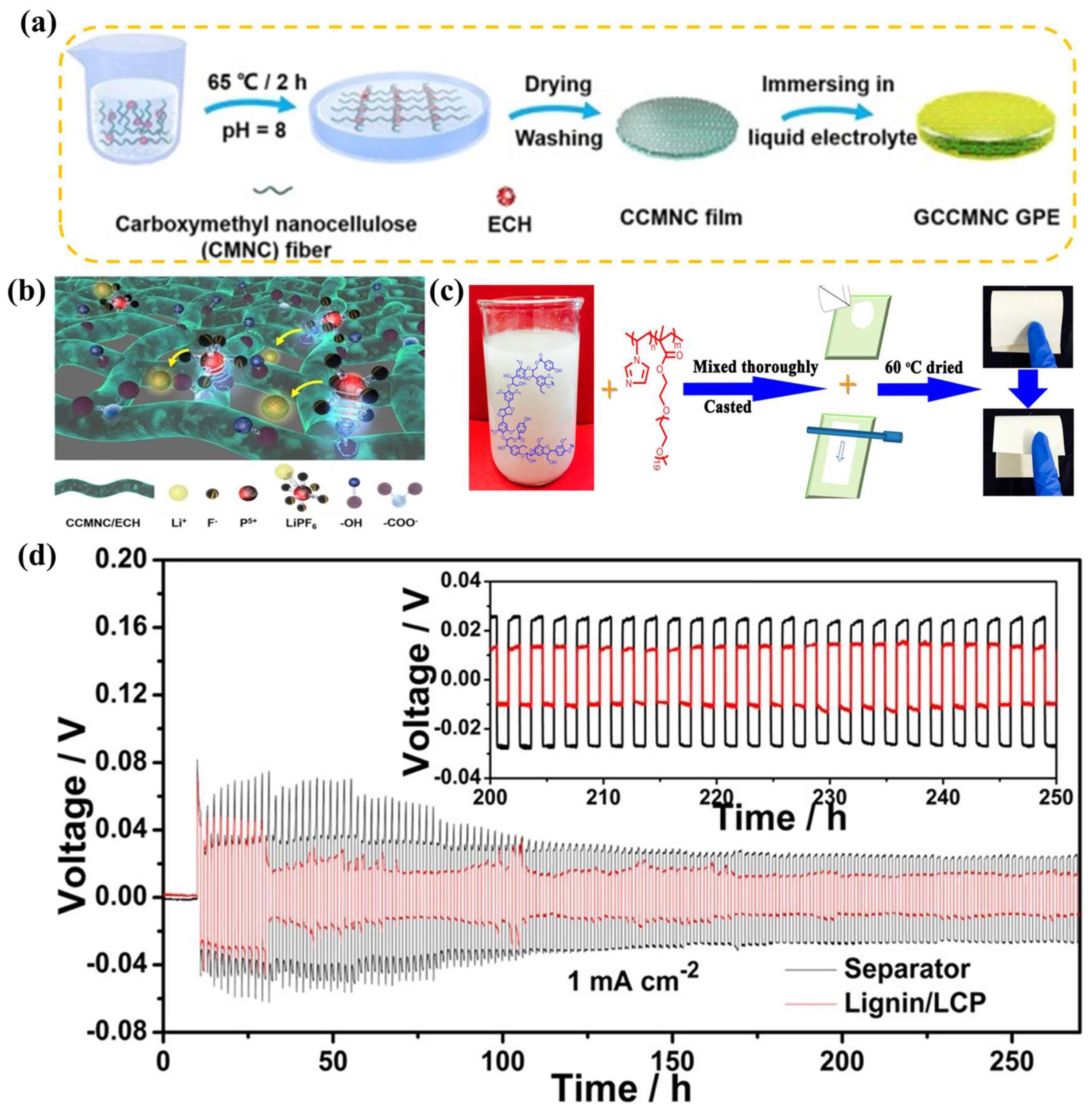
4.1. Effect of GPE Preparation Method on SEIs
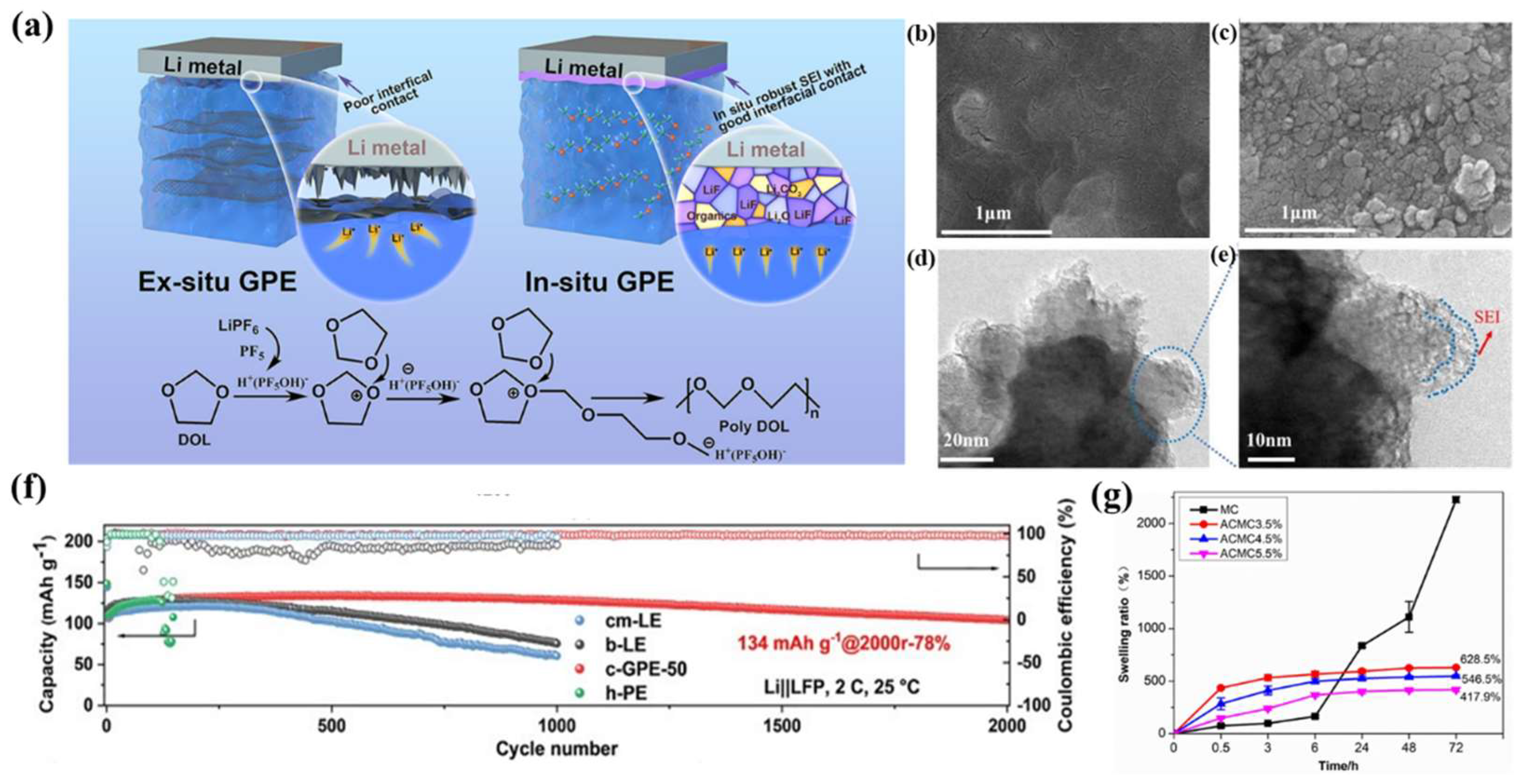
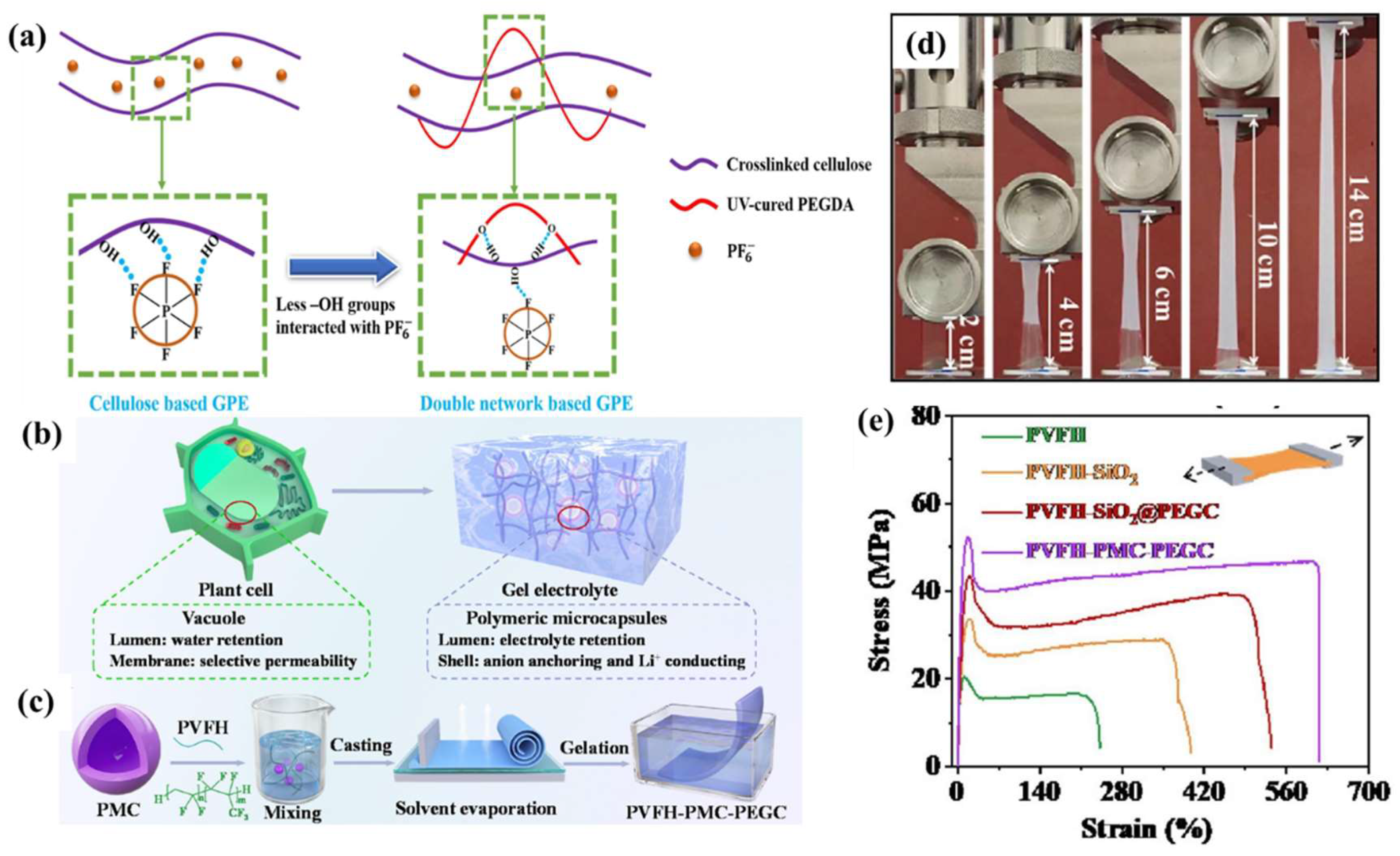
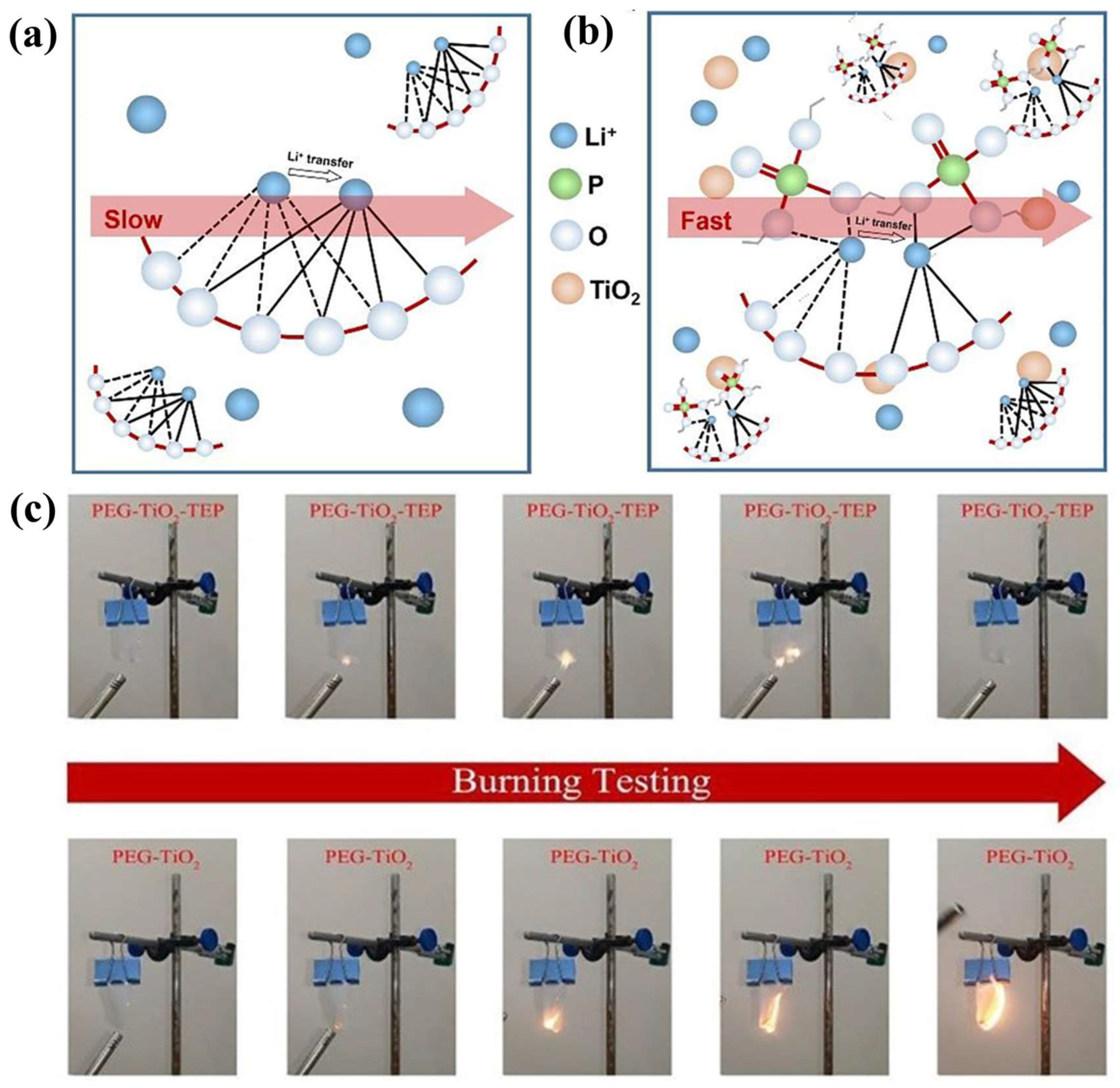
4.2. Effect of GPE Plasticizer Selection on SEIs
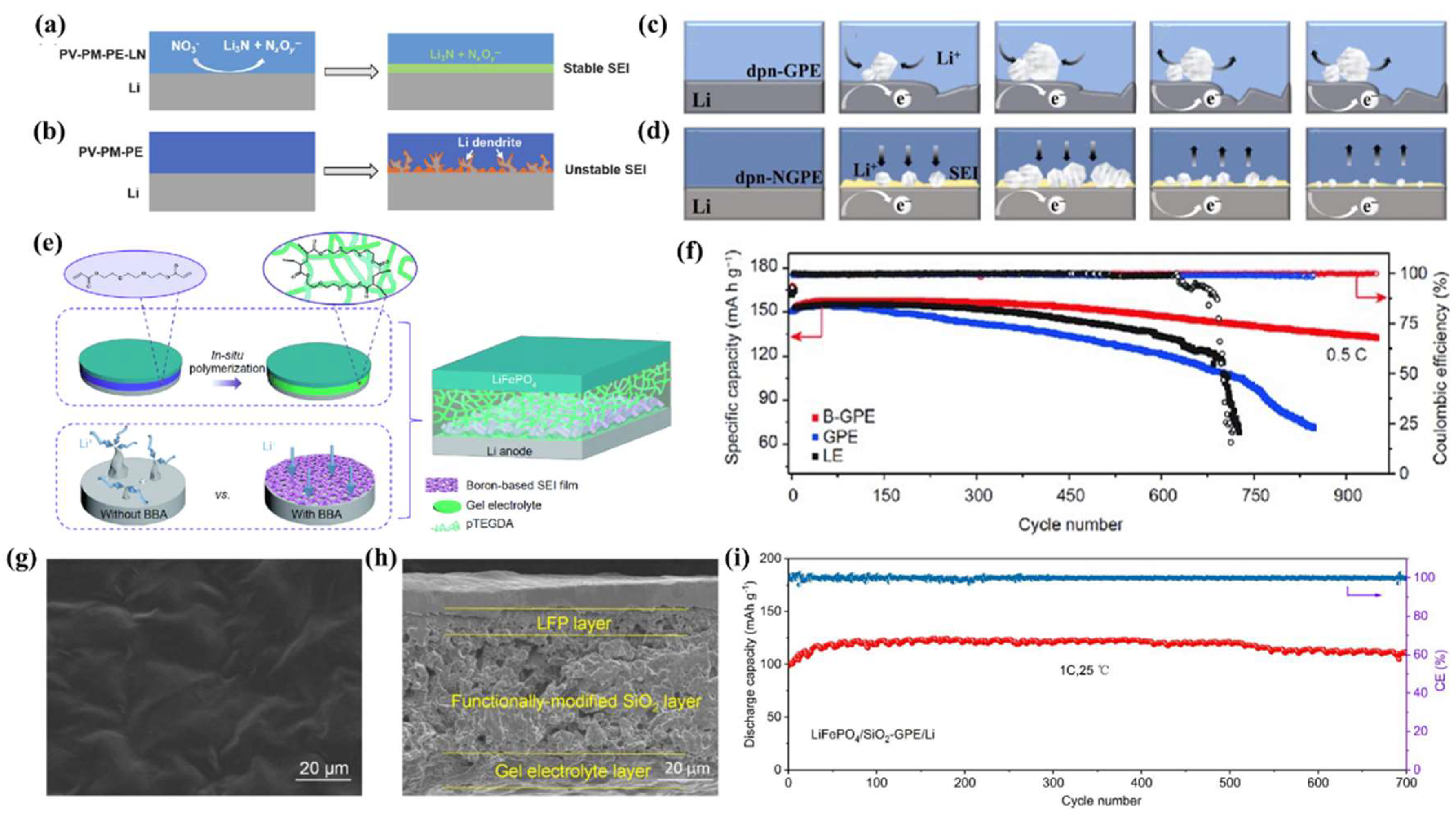
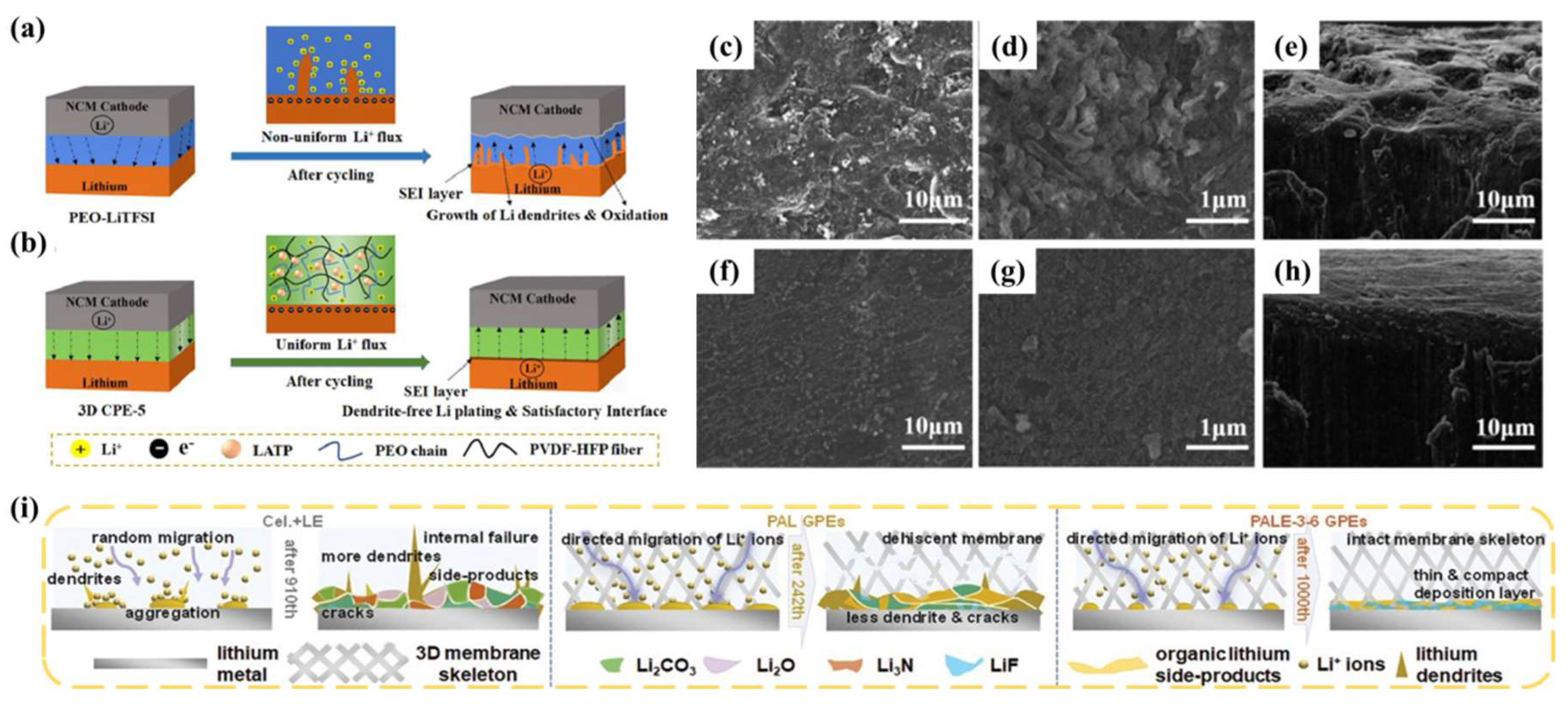
4.3. Effect of GPE Polymer Substrate on SEIs
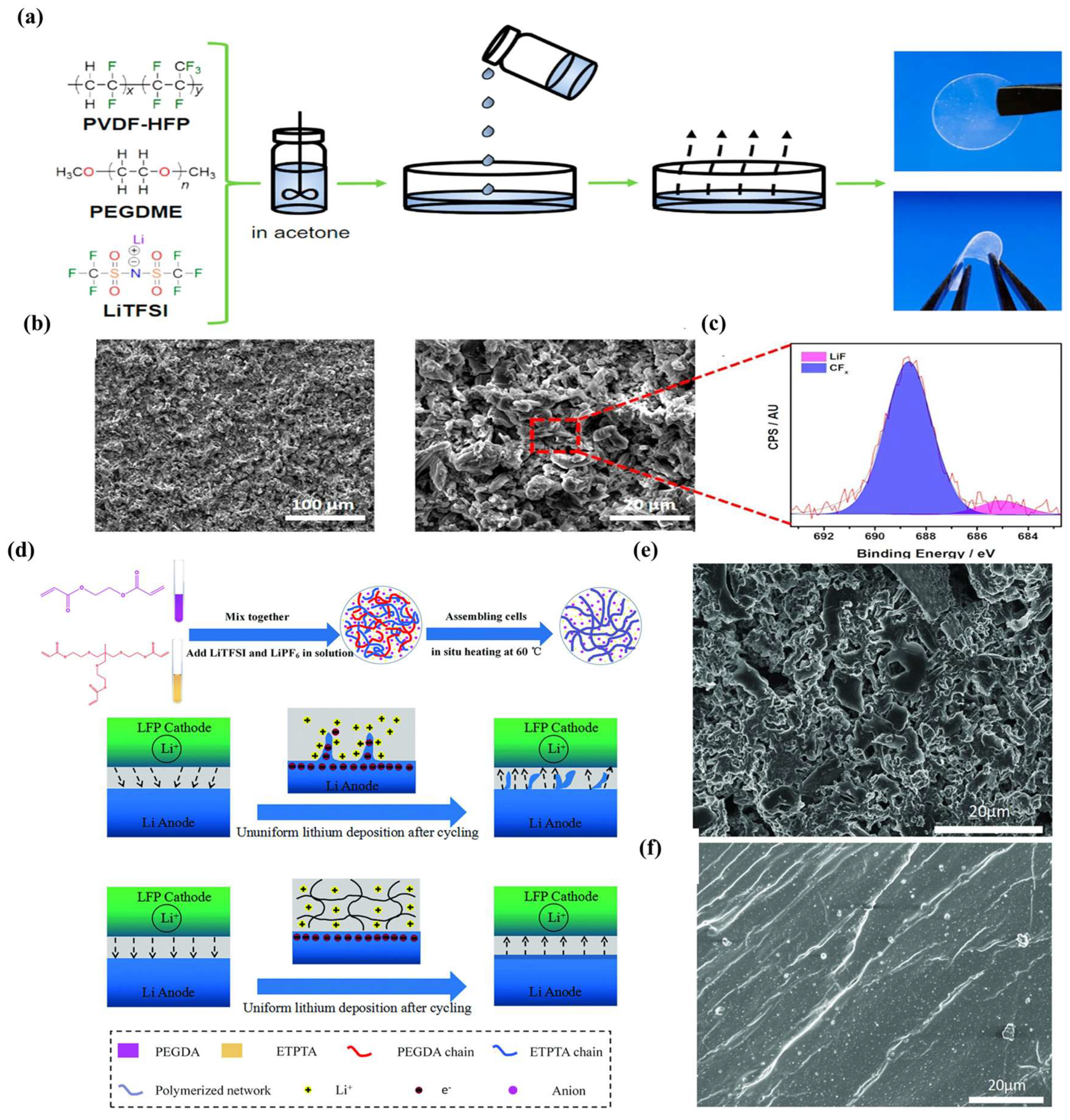
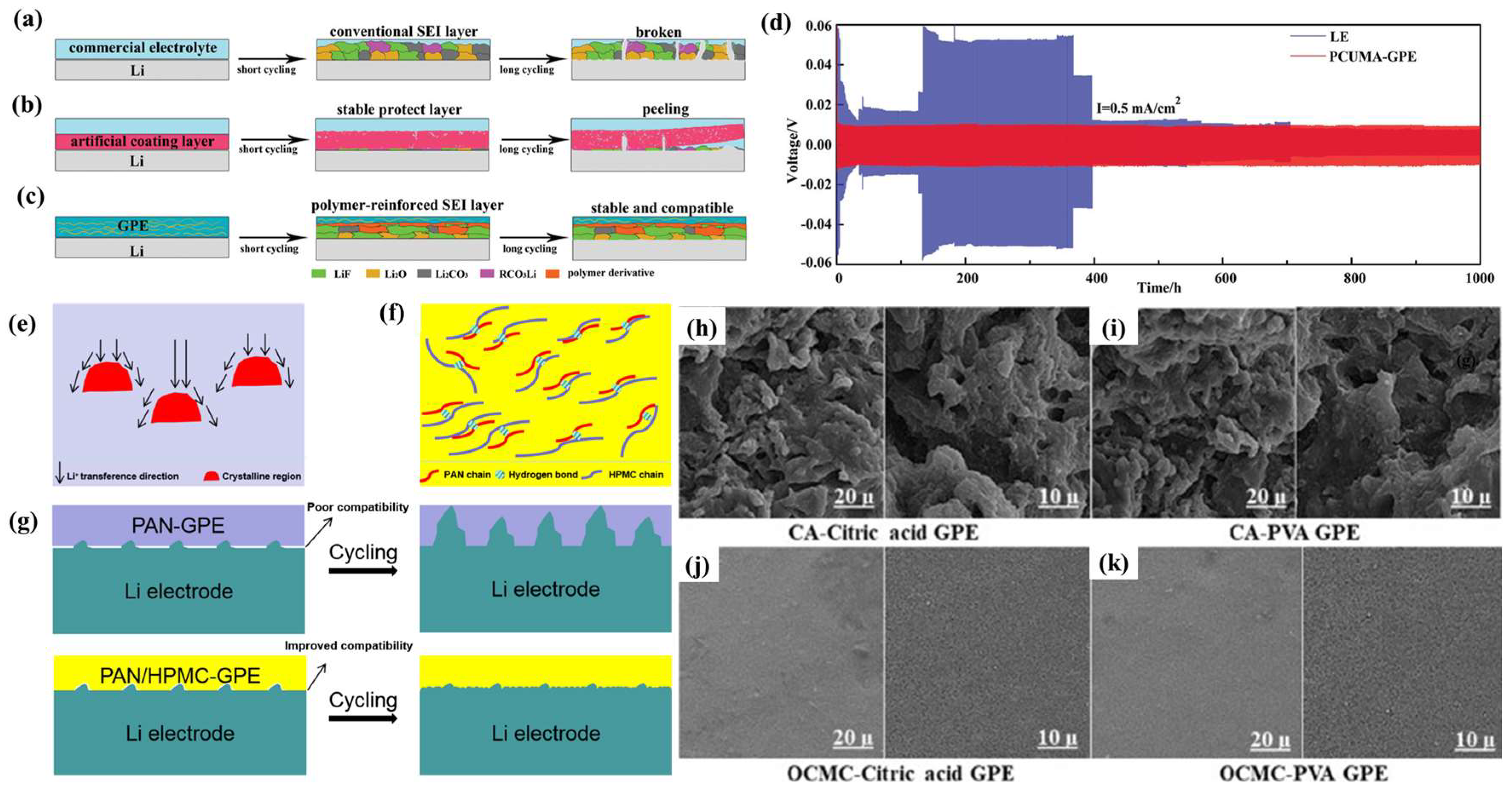
4.4. Effect of GPE Additives on SEIs
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EMC | ethyl methyl carbonate |
| DMC | dimethyl carbonate |
| DEC | diethyl carbonate |
| PC | propylene carbonate |
| EC | ethylene carbonate |
| DME | 1,2-dimethoxyethane |
| TEGDME | Tetraethylene glycol dimethyl ether |
| DOL | dioxolane |
| THF | tetrahydrofuran |
| FEC | fluoroethylene carbonate |
| LiDFOB | Lithium Difluoro(oxalato)borate |
| LATP | Li1.3Al0.3Ti1.7(PO4)3 |
References
- Zhao, N.; You, F. Can renewable generation, energy storage and energy efficient technologies enable carbon neutral energy transition? Appl. Energy 2020, 279, 115889. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Yang, H.; Guo, C.; Naveed, A.; Lei, J.; Yang, J.; Nuli, Y.; Wang, J. Recent progress and perspective on lithium metal anode protection. Energy Storage Mater. 2018, 14, 199–221. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Guo, Y.; Han, Q.; Lou, P.; Li, L.; Jiang, J.; Cheng, S.; Cao, Y.C. In Situ High-performance Gel Polymer Electrolyte with Dual-reactive Cross-linking for Lithium Metal Batteries. Energy Environ. Mater. 2022, e12497. [Google Scholar] [CrossRef]
- Ma, C.; Cui, W.; Liu, X.; Ding, Y.; Wang, Y. In situ preparation of gel polymer electrolyte for lithium batteries: Progress and perspectives. InfoMat 2022, 4, e12232. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Fu, X.; Odstrcil, R.; Qiu, M.; Liu, J.; Zhong, W.-H. Natural “relief” for lithium dendrites: Tailoring protein configurations for long-life lithium metal anodes. Energy Storage Mater. 2021, 42, 22–33. [Google Scholar] [CrossRef]
- Wang, H.; Sheng, L.; Yasin, G.; Wang, L.; Xu, H.; He, X. Reviewing the current status and development of polymer electrolytes for solid-state lithium batteries. Energy Storage Mater. 2020, 33, 188–215. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Meng, C.; Ning, Y.; Zhang, G.; Fu, Q. Electrolyte-dependent formation of solid electrolyte interphase and ion intercalation revealed by in situ surface characterizations. J. Energy Chem. 2022, 67, 718–726. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.-T.; Popovic, J.; Lim, K.; Yin, Y.-X.; Maier, J.; Guo, Y.-G. Towards better Li metal anodes: Challenges and strategies. Mater. Today 2020, 33, 56–74. [Google Scholar] [CrossRef]
- Jia, H.; Onishi, H.; von Aspern, N.; Rodehorst, U.; Rudolf, K.; Billmann, B.; Wagner, R.; Winter, M.; Cekic-Laskovic, I. A propylene carbonate based gel polymer electrolyte for extended cycle life and improved safety performance of lithium ion batteries. J. Power Sources 2018, 397, 343–351. [Google Scholar] [CrossRef]
- Zuo, T.T.; Shi, Y.; Wu, X.W.; Wang, P.F.; Wang, S.H.; Yin, Y.X.; Wang, W.P.; Ma, Q.; Zeng, X.X.; Ye, H.; et al. Constructing a Stable Lithium Metal-Gel Electrolyte Interface for Quasi-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2018, 10, 30065–30070. [Google Scholar] [CrossRef]
- Kang, D.; Xiao, M.; Lemmon, J.P. Artificial Solid-Electrolyte Interphase for Lithium Metal Batteries. Batter. Supercaps 2020, 4, 445–455. [Google Scholar] [CrossRef]
- Yin, J.; Xu, X.; Jiang, S.; Lei, Y.; Gao, Y. Bi-nanofillers integrated into PEO-based electrolyte for high-performance solid-state Li metal batteries. J. Power Sources 2022, 550, 232139. [Google Scholar] [CrossRef]
- He, M.; Guo, R.; Hobold, G.M.; Gao, H.; Gallant, B.M. The intrinsic behavior of lithium fluoride in solid electrolyte interphases on lithium. Proc. Natl. Acad. Sci. USA 2020, 117, 73–79. [Google Scholar] [CrossRef]
- Wu, D.; He, J.; Liu, J.; Wu, M.; Qi, S.; Wang, H.; Huang, J.; Li, F.; Tang, D.; Ma, J. Li2CO3/LiF-Rich Heterostructured Solid Electrolyte Interphase with Superior Lithiophilic and Li+-Transferred Characteristics via Adjusting Electrolyte Additives. Adv. Energy Mater. 2022, 12, 2200337. [Google Scholar] [CrossRef]
- Zhou, T.; Zhao, Y.; El Kazzi, M.; Choi, J.W.; Coskun, A. Stable Solid Electrolyte Interphase Formation Induced by Monoquat-Based Anchoring in Lithium Metal Batteries. Acs. Energy Lett. 2021, 6, 1711–1718. [Google Scholar] [CrossRef]
- Chen, T.; Kong, W.; Zhang, Z.; Wang, L.; Hu, Y.; Zhu, G.; Chen, R.; Ma, L.; Yan, W.; Wang, Y.; et al. Ionic liquid-immobilized polymer gel electrolyte with self-healing capability, high ionic conductivity and heat resistance for dendrite-free lithium metal batteries. Nano Energy 2018, 54, 17–25. [Google Scholar] [CrossRef]
- Naik, K.G.; Chatterjee, D.; Mukherjee, P.P. Solid Electrolyte-Cathode Interface Dictates Reaction Heterogeneity and Anode Stability. ACS Appl. Mater. Interfaces 2022, 14, 45308–45319. [Google Scholar] [CrossRef]
- Yan, Y.; Kühnel, R.-S.; Remhof, A.; Duchêne, L.; Reyes, E.C.; Rentsch, D.; Łodziana, Z.; Battaglia, C. A Lithium Amide-Borohydride Solid-State Electrolyte with Lithium-Ion Conductivities Comparable to Liquid Electrolytes. Adv. Energy Mater. 2017, 7, 1700294. [Google Scholar] [CrossRef]
- Jones, S.D.; Nguyen, H.; Richardson, P.M.; Chen, Y.Q.; Wyckoff, K.E.; Hawker, C.J.; Clement, R.J.; Fredrickson, G.H.; Segalman, R.A. Design of Polymeric Zwitterionic Solid Electrolytes with Superionic Lithium Transport. ACS Cent Sci. 2022, 8, 169–175. [Google Scholar] [CrossRef]
- Murata, K.; Izuchi, S.; Yoshihisa, Y. An overview of the research and development of solid polymer electrolyte batteries. Electrochim. Acta 2000, 45, 1501–1508. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, Q.; Wang, S.; Zhou, Y.; Song, D.; Zhang, H.; Shi, X.; Zhang, L. Li salt initiated in-situ polymerized solid polymer electrolyte: New insights via in-situ electrochemical impedance spectroscopy. Chem. Eng. J. 2022, 429, 132483. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, J.; Wang, Y.; Song, M.; Long, L.; Siyal, S.H.; Yang, X.; Sui, G. Recent advances in gel polymer electrolyte for high-performance lithium batteries. J. Energy Chem. 2019, 37, 126–142. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Wang, J.; Ye, F.; Wang, S.; Xu, Y.; Liu, J.; Xu, G.; Zhang, Y.; Zhang, Y.; et al. Asymmetric gel polymer electrolyte with high lithium ion conductivity for dendrite-free lithium metal batteries. J. Mater. Chem. A 2020, 8, 8033–8040. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives. Chem. Soc. Rev. 2011, 40, 2525–2540. [Google Scholar] [CrossRef]
- Meyer, W.H. Polymer electrolytes for lithium-ion batteries. Adv. Mater. 1998, 10, 439–448. [Google Scholar] [CrossRef]
- Wu, H.; Jia, H.; Wang, C.; Zhang, J.G.; Xu, W. Recent progress in understanding solid electrolyte interphase on lithium metal anodes. Adv. Energy. Mater. 2021, 11, 2003092. [Google Scholar] [CrossRef]
- Feuillade, G.; Perche, P. Ion-conductive macromolecular gels and membranes for solid lithium cells. J. Appl. Electrochem. 1975, 5, 63–69. [Google Scholar] [CrossRef]
- Iijima, T.; Toyoguchi, Y.; Eda, N. Quasi-solid organic electrolytes gelatinized with polymethyl-methacrylate and their applications for lithium batteries. Denki Kagaku 1985, 53, 619–623. [Google Scholar]
- Song, X.; Wang, C.; Chen, J.; Xin, S.; Yuan, D.; Wang, Y.; Dong, K.; Yang, L.; Wang, G.; Zhang, H.; et al. Unraveling the Synergistic Coupling Mechanism of Li+ Transport in an “Ionogel-in-Ceramic” Hybrid Solid Electrolyte for Rechargeable Lithium Metal Battery. Adv. Funct. Mater. 2022, 32, 2108706. [Google Scholar] [CrossRef]
- Song, J.; Liao, K.; Si, J.; Zhao, C.; Wang, J.; Zhou, M.; Liang, H.; Gong, J.; Cheng, Y.J.; Gao, J.; et al. Phosphonate-Functionalized Ionic Liquid Gel Polymer Electrolyte with High Safety for Dendrite-Free Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2023, 15, 2901–2910. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H. Gel polymer electrolytes for electrochemical energy storage. Adv. Energy Mater. 2018, 8, 1702184. [Google Scholar] [CrossRef]
- Li, M.; Liao, Y.; Liu, Q.; Xu, J.; Sun, P.; Shi, H.; Li, W. Application of the imidazolium ionic liquid based nano-particle decorated gel polymer electrolyte for high safety lithium ion battery. Electrochim. Acta 2018, 284, 188–201. [Google Scholar] [CrossRef]
- Martinez-Ibañez, M.; Boaretto, N.; Meabe, L.; Wang, X.; Zhu, H.; Santiago, A.; Zugazua, O.; Forsyth, M.; Armand, M.; Zhang, H. Revealing the Anion Chemistry Effect on Transport Properties of Ternary Gel Polymer Electrolytes. Chem. Mater. 2022, 34, 7493–7502. [Google Scholar] [CrossRef]
- Du, Z.; Su, Y.; Qu, Y.; Zhao, L.; Jia, X.; Mo, Y.; Yu, F.; Du, J.; Chen, Y. A mechanically robust, biodegradable and high performance cellulose gel membrane as gel polymer electrolyte of lithium-ion battery. Electrochim. Acta 2019, 299, 19–26. [Google Scholar] [CrossRef]
- Hadad, S.; Hamrahjoo, M.; Dehghani, E.; Salami-Kalajahi, M.; Eliseeva, S.N.; Moghaddam, A.R.; Roghani-Mamaqani, H. Cellulose-based solid and gel polymer electrolytes with super high ionic conductivity and charge capacity for high performance lithium ion batteries. Sustain. Mater. Technol. 2022, 33, e00503. [Google Scholar] [CrossRef]
- Fu, F.; Zheng, Y.; Jiang, N.; Liu, Y.; Sun, C.; Zhang, A.; Teng, H.; Sun, L.; Xie, H. A Dual-Salt PEO-based polymer electrolyte with Cross-Linked polymer network for High-Voltage lithium metal batteries. Chem. Eng. J. 2022, 450, 137776. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Cui, Z.; Zhou, Q.; Shangguan, X.; Tian, S.; Zhou, X.; Cui, G. Siloxane-based polymer electrolytes for solid-state lithium batteries. Energy Storage Mater. 2019, 23, 466–490. [Google Scholar] [CrossRef]
- Castillo, J.; Robles-Fernandez, A.; Cid, R.; González-Marcos, J.A.; Armand, M.; Carriazo, D.; Zhang, H.; Santiago, A. Dehydrofluorination Process of Poly(vinylidene difluoride) PVDF-Based Gel Polymer Electrolytes and Its Effect on Lithium-Sulfur Batteries. Gels 2023, 9, 336. [Google Scholar] [CrossRef]
- Yuan, B.; Zhao, B.; Wang, Q.; Bai, Y.; Cheng, Z.; Cong, Z.; Lu, Y.; Ji, F.; Shen, F.; Wang, P.-F.; et al. A thin composite polymer electrolyte with high room-temperature conductivity enables mass production for solid-state lithium-metal batteries. Energy Storage Mater. 2022, 47, 288–296. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, H.; Cheng, X.B.; Chen, W.J.; Titirici, M.M.; Huang, J.Q.; Yuan, T.Q.; Zhang, Q. A review of naturally derived nanostructured materials for safe lithium metal batteries. Mater. Today Nano 2019, 8, 100049. [Google Scholar] [CrossRef]
- Castillo, J.; Santiago, A.; Judez, X.; Garbayo, I.; Coca Clemente, J.A.; Morant-Miñana, M.C.; Villaverde, A.; González-Marcos, J.A.; Zhang, H.; Armand, M.; et al. Safe, Flexible, and High-Performing Gel-Polymer Electrolyte for Rechargeable Lithium Metal Batteries. Chem. Mater. 2021, 33, 8812–8821. [Google Scholar] [CrossRef]
- Fan, W.; Li, N.W.; Zhang, X.; Zhao, S.; Cao, R.; Yin, Y.; Xing, Y.; Wang, J.; Guo, Y.G.; Li, C. A dual-salt gel polymer electrolyte with 3D cross-linked polymer network for dendrite-free lithium metal batteries. Adv. Sci. 2018, 5, 1800559. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, C.; Yu, Z.; Wu, H.; Wang, Y.; Ma, W.; Zhang, Q.; Jia, X. A flexible and highly conductive quasi-solid single-ion polymer electrolyte for high performance Li-metal batteries. J. Power Sources 2022, 537, 231478. [Google Scholar] [CrossRef]
- Ren, W.; Ding, C.; Fu, X.; Huang, Y. Advanced gel polymer electrolytes for safe and durable lithium metal batteries: Challenges, strategies, and perspectives. Energy Storage Mater. 2021, 34, 515–535. [Google Scholar] [CrossRef]
- Costa, C.M.; Lizundia, E.; Lanceros-Méndez, S. Polymers for advanced lithium-ion batteries: State of the art and future needs on polymers for the different battery components. Prog. Energy Combust. Sci. 2020, 79, 100846. [Google Scholar] [CrossRef]
- Jeong, D.; Yook, J.; Hong, D.G.; Lee, J.-C. Lithium dendrite suppression by single-ion conducting gel polymer electrolyte cross-linked with graphene oxide. J. Power Sources 2022, 534, 231424. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhong, L.; Wang, S.; Qin, J.; Han, D.; Ren, S.; Xiao, M.; Sun, L.; Meng, Y. Ultrahigh Li-ion conductive single-ion polymer electrolyte containing fluorinated polysulfonamide for quasi-solid-state Li-ion batteries. J. Mater. Chem. A 2019, 7, 24251–24261. [Google Scholar] [CrossRef]
- Li, C.; Qin, B.; Zhang, Y.; Varzi, A.; Passerini, S.; Wang, J.; Dong, J.; Zeng, D.; Liu, Z.; Cheng, H. Single-ion conducting electrolyte based on electrospun nanofibers for high-performance lithium batteries. Adv. Energy. Mater. 2019, 9, 1803422. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Zhang, Y.; Chen, G.; Poli, R.; Xie, X.; Xue, Z. Facile Assembly of C–N Bond-Containing Polymer Electrolytes Enabled by Lithium Salt-Catalyzed Aza-Michael Addition. Macromolecules 2023, 56, 2484–2493. [Google Scholar] [CrossRef]
- Lu, X.; Wu, H.; Kong, D.; Li, X.; Shen, L.; Lu, Y. Facilitating Lithium-Ion Conduction in Gel Polymer Electrolyte by Metal-Organic Frameworks. ACS Mater. Lett. 2020, 2, 1435–1441. [Google Scholar] [CrossRef]
- Subadevi, R.; Sivakumar, M.; Rajendran, S.; Wu, H.C.; Wu, N.L. Studies on the effect of anions of various lithium salts in PEMA gel polymer electrolytes. J. Appl. Polym. Sci. 2011, 119, 1–6. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Li, M.; Li, G.; Li, B.; Zhang, S.; Ming, H.; Qiu, J.; Chen, J.; Zhao, P. A new composite gel polymer electrolyte based on matrix of PEGDA with high ionic conductivity for lithium-ion batteries. Electrochim. Acta 2020, 354, 136622. [Google Scholar] [CrossRef]
- Deng, K.; Zeng, Q.; Wang, D.; Liu, Z.; Qiu, Z.; Zhang, Y.; Xiao, M.; Meng, Y. Single-ion conducting gel polymer electrolytes: Design, preparation and application. J. Mater. Chem. A 2020, 8, 1557–1577. [Google Scholar] [CrossRef]
- Dong, X.; Mayer, A.; Liu, X.; Passerini, S.; Bresser, D. Single-Ion Conducting Multi-block Copolymer Electrolyte for Lithium-Metal Batteries with High Mass Loading NCM811 Cathodes. ACS Energy Lett. 2023, 8, 1114–1121. [Google Scholar] [CrossRef]
- Porcarelli, L.; Sutton, P.; Bocharova, V.; Aguirresarobe, R.H.; Zhu, H.; Goujon, N.; Leiza, J.R.; Sokolov, A.; Forsyth, M.; Mecerreyes, D. Single-Ion Conducting Polymer Nanoparticles as Functional Fillers for Solid Electrolytes in Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2021, 13, 54354–54362. [Google Scholar] [CrossRef]
- Fraile-Insagurbe, D.; Boaretto, N.; Aldalur, I.; Raposo, I.; Bonilla, F.J.; Armand, M.; Martínez-Ibañez, M. Novel single-ion conducting polymer electrolytes with high toughness and high resistance against lithium dendrites. Nano Res. 2023, 1–12. [Google Scholar] [CrossRef]
- Luo, S.; Liu, X.; Gao, L.; Deng, N.; Sun, X.; Li, Y.; Zeng, Q.; Wang, H.; Cheng, B.; Kang, W. A review on modified polymer composite electrolytes for solid-state lithium batteries. Sustain. Energy Fuels 2022, 6, 5019–5044. [Google Scholar] [CrossRef]
- Ghazi, Z.A.; Sun, Z.; Sun, C.; Qi, F.; An, B.; Li, F.; Cheng, H.M. Key Aspects of Lithium Metal Anodes for Lithium Metal Batteries. Small 2019, 15, e1900687. [Google Scholar] [CrossRef]
- Guo, J.; Chen, Y.; Xiao, Y.; Xi, C.; Xu, G.; Li, B.; Yang, C.; Yu, Y. Flame-retardant composite gel polymer electrolyte with a dual acceleration conduction mechanism for lithium ion batteries. Chem. Eng. J. 2021, 422, 130526. [Google Scholar] [CrossRef]
- Ye, X.; Xiong, W.; Huang, T.; Li, X.; Lei, Y.; Li, Y.; Ren, X.; Liang, J.; Ouyang, X.; Zhang, Q. A blended gel polymer electrolyte for dendrite-free lithium metal batteries. Appl. Surf. Sci. 2021, 569, 150899. [Google Scholar] [CrossRef]
- Lu, Q.; He, Y.B.; Yu, Q.; Li, B.; Kaneti, Y.V.; Yao, Y.; Kang, F.; Yang, Q.H. Dendrite-free, high-rate, long-life lithium metal batteries with a 3D cross-linked network polymer electrolyte. Adv. Mater. 2017, 29, 1604460. [Google Scholar] [CrossRef]
- Kim, J.-K.; Kim, D.H.; Joo, S.H.; Choi, B.; Cha, A.; Kim, K.M.; Kwon, T.-H.; Kwak, S.K.; Kang, S.J.; Jin, J. Hierarchical Chitin Fibers with Aligned Nanofibrillar Architectures: A Nonwoven-Mat Separator for Lithium Metal Batteries. ACS Nano 2017, 11, 6114–6121. [Google Scholar] [CrossRef]
- Zhang, T.-W.; Shen, B.; Yao, H.-B.; Ma, T.; Lu, L.-L.; Zhou, F.; Yu, S.-H. Prawn Shell Derived Chitin Nanofiber Membranes as Advanced Sustainable Separators for Li/Na-Ion Batteries. Nano Lett. 2017, 17, 4894–4901. [Google Scholar] [CrossRef]
- Gou, J.; Liu, W.; Tang, A. A renewable gel polymer electrolyte based on the different sized carboxylated cellulose with satisfactory comprehensive performance for rechargeable lithium ion battery. Polymer 2020, 208, 122943. [Google Scholar] [CrossRef]
- Wen, X.; Zeng, Q.; Guan, J.; Wen, W.; Chen, P.; Li, Z.; Liu, Y.; Chen, A.; Liu, X.; Liu, W.; et al. 3D structural lithium alginate-based gel polymer electrolytes with superior high-rate long cycling performance for high-energy lithium metal batteries. J. Mater. Chem. A 2022, 10, 707–718. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Wang, W.; Li, W.; Lou, J. Carboxymethylated nanocellulose-based gel polymer electrolyte with a high lithium ion transfer number for flexible lithium-ion batteries application. Chem. Eng. J. 2022, 428, 132604. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Wang, A.; Liu, X.; Chen, J.; Wang, Z.; Zeng, Q.; Zhou, H.-h.; Jiang, X.; Zhang, L. Polymer-Laden Composite Lignin-Based Electrolyte Membrane for High-Performance Lithium Batteries. ACS Sustain. Chem. Eng. 2018, 6, 14460–14469. [Google Scholar] [CrossRef]
- Guo, Q.; Han, Y.; Wang, H.; Sun, W.; Jiang, H.; Zhu, Y.; Zheng, C.; Xie, K. Thermo and electrochemical-stable composite gel polymer electrolytes derived from core-shell silica nanoparticles and ionic liquid for rechargeable lithium metal batteries. Electrochim. Acta 2018, 288, 101–107. [Google Scholar] [CrossRef]
- Kim, D.; Liu, X.; Yu, B.; Mateti, S.; O’Dell, L.A.; Rong, Q.; Chen, Y. Amine-functionalized boron nitride nanosheets: A new functional additive for robust, flexible ion gel electrolyte with high lithium-ion transference number. Adv. Funct. Mater. 2020, 30, 1910813. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, K.; Song, Y.; Lin, H.; Li, K.; Cui, Y.; Yang, L.; Pan, F. Polymer matrix mediated solvation of LiNO3 in carbonate electrolytes for quasi-solid high-voltage lithium metal batteries. Nano Res. 2020, 13, 2431–2437. [Google Scholar] [CrossRef]
- Han, D.-D.; Wang, Z.-Y.; Pan, G.-L.; Gao, X.-P. Metal–Organic-Framework-Based Gel Polymer Electrolyte with Immobilized Anions to Stabilize a Lithium Anode for a Quasi-Solid-State Lithium–Sulfur Battery. ACS Appl. Mater. Interfaces 2019, 11, 18427–18435. [Google Scholar] [CrossRef]
- Fu, S.; Zuo, L.-L.; Zhou, P.-S.; Liu, X.-J.; Ma, Q.; Chen, M.-J.; Yue, J.-P.; Wu, X.-W.; Deng, Q. Recent advancements of functional gel polymer electrolytes for rechargeable lithium–metal batteries. Mater. Chem. Front. 2021, 5, 5211–5232. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, Z.; Qian, K.; Verhallen, T.; Wang, C.; Wagemaker, M. Efficient Li-Metal Plating/Stripping in Carbonate Electrolytes Using a LiNO3-Gel Polymer Electrolyte, Monitored by Operando Neutron Depth Profiling. Chem. Mater. 2019, 31, 4564–4574. [Google Scholar] [CrossRef]
- Shim, J.; Kim, H.J.; Kim, B.G.; Kim, Y.S.; Kim, D.-G.; Lee, J.-C. 2D boron nitride nanoflakes as a multifunctional additive in gel polymer electrolytes for safe, long cycle life and high rate lithium metal batteries. Energy Environ. Sci. 2017, 10, 1911–1916. [Google Scholar] [CrossRef]
- Cui, S.; Wu, X.; Yang, Y.; Fei, M.; Liu, S.; Li, G.; Gao, X.-P. Heterostructured Gel Polymer Electrolyte Enabling Long-Cycle Quasi-Solid-State Lithium Metal Batteries. Acs. Energy Lett. 2021, 7, 42–52. [Google Scholar] [CrossRef]
- Hasan, N.; Pulst, M.; Samiullah, M.H.; Kressler, J. Comparison of Li+-ion conductivity in linear and crosslinked poly(ethylene oxide). J. Polym. Sci. Part B Polym. Phys. 2019, 57, 21–28. [Google Scholar] [CrossRef]
- Mallela, Y.L.N.K.; Jeong, S.Y.; Kumar, S.; Lee, J.-S. Hyperbranched Poly(Glycidol)-Grafted Silica Nanoparticles for Enhancing Li-Ion Conductivity of Poly(Ethylene Oxide). Macromol. Mater. Eng. 2021, 306, 2000572. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, S.-I.; Son, M.; Lee, J.W.; Lee, D.H. Cl- and Al-Doped Argyrodite Solid Electrolyte Li6PS5Cl for All-Solid-State Lithium Batteries with Improved Ionic Conductivity. Nanomaterials 2022, 12, 4355. [Google Scholar] [CrossRef]
- Gou, J.; Liu, W.; Tang, A. A renewable and biodegradable nanocellulose-based gel polymer electrolyte for lithium-ion battery. J. Mater. Sci. 2020, 55, 10699–10711. [Google Scholar] [CrossRef]
- Gou, J.; Liu, W.; Tang, A. To improve the interfacial compatibility of cellulose-based gel polymer electrolytes: A cellulose/PEGDA double network-based gel membrane designed for lithium ion batteries. Appl. Surf. Sci. 2021, 568, 150963. [Google Scholar] [CrossRef]
- Zhai, P.; He, W.; Zeng, C.; Li, L.; Yang, W. Biomimetic plant-cell composite gel polymer electrolyte for boosting rate performance of lithium metal batteries. Chem. Eng. J. 2023, 451, 138414. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Wang, F.X.; Liu, L.L.; Xiao, S.Y.; Yang, Y.Q.; Wu, Y.P. Cheap glass fiber mats as a matrix of gel polymer electrolytes for lithium ion batteries. Sci. Rep. 2013, 3, 3187. [Google Scholar] [CrossRef]
- Liao, H.Y.; Zhang, H.Y.; Hong, H.Q.; Li, Z.H.; Qin, G.; Zhu, H.P.; Lin, Y.X. Novel cellulose aerogel coated on polypropylene separators as gel polymer electrolyte with high ionic conductivity for lithium-ion batteries. J. Membr. Sci. 2016, 514, 332–339. [Google Scholar] [CrossRef]
- Huang, X.; Xu, D.H.; Chen, W.Y.; Yin, H.Z.; Zhang, C.C.; Luo, Y.; Yu, X.Y. Preparation, characterization and properties of poly(propylene carbonate)/poly (methyl methacrylate)-coated polyethylene gel polymer electrolyte for lithium-ion batteries. J. Electroanal. Chem. 2017, 804, 133–139. [Google Scholar] [CrossRef]
- Wu, S.; Zheng, H.; Tian, R.; Hei, Z.; Liu, H.; Duan, H. In-situ preparation of gel polymer electrolyte with glass fiber membrane for lithium batteries. J. Power Sources 2020, 472, 228627. [Google Scholar] [CrossRef]
- Chen, T.T.; Liao, Y.H.; Wang, X.S.; Luo, X.Y.; Li, X.P.; Li, W.S. Investigation on high-safety lithium ion battery using polyethylene supported poly(methyl methacrylate-acrylonitrile-butyl acrylate) copolymer based gel electrolyte. Electrochim. Acta 2016, 191, 923–932. [Google Scholar] [CrossRef]
- Xu, D.; Jin, J.; Chen, C.; Wen, Z. From Nature to Energy Storage: A Novel Sustainable 3D Cross-Linked Chitosan–PEGGE-Based Gel Polymer Electrolyte with Excellent Lithium-Ion Transport Properties for Lithium Batteries. ACS Appl. Mater. Interfaces 2018, 10, 38526–38537. [Google Scholar] [CrossRef]
- Baik, J.-H.; Kim, S.; Hong, D.G.; Lee, J.-C. Gel Polymer Electrolytes Based on Polymerizable Lithium Salt and Poly(ethylene glycol) for Lithium Battery Applications. ACS Appl. Mater. Interfaces 2019, 11, 29718–29724. [Google Scholar] [CrossRef]
- Long, M.-C.; Wu, G.; Wang, X.-L.; Wang, Y.-Z. Self-adaptable gel polymer electrolytes enable high-performance and all-round safety lithium ion batteries. Energy Storage Mater. 2022, 53, 62–71. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Ding, J.-F.; Xu, R.; Yan, C.; Li, B.-Q.; Yuan, H.; Huang, J.-Q. A review on the failure and regulation of solid electrolyte interphase in lithium batteries. J. Energy Chem. 2021, 59, 306–319. [Google Scholar] [CrossRef]
- Xu, W.; Liao, X.; Xu, W.; Sun, C.; Zhao, K.; Zhao, Y.; Hu, C. Gradient SEI layer induced by liquid alloy electrolyte additive for high rate lithium metal battery. Nano Energy 2021, 88, 106237. [Google Scholar] [CrossRef]
- Dai, K.; Ma, C.; Feng, Y.; Zhou, L.; Kuang, G.; Zhang, Y.; Lai, Y.; Cui, X.; Wei, W. A borate-rich, cross-linked gel polymer electrolyte with near-single ion conduction for lithium metal batteries. J. Mater. Chem. A 2019, 7, 18547–18557. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, D.; Jiang, H.R.; Ren, Y.X.; Kang, F.Y.; Zhao, T.S. A highly-safe lithium-ion sulfur polymer battery with SnO2 anode and acrylate-based gel polymer electrolyte. Nano Energy. 2016, 28, 97–105. [Google Scholar] [CrossRef]
- Yasin, G.; Arif, M.; Mehtab, T.; Lu, X.; Yu, D.; Muhammad, N.; Nazir, M.T.; Song, H. Understanding and suppression strategies toward stable Li metal anode for safe lithium batteries. Energy Storage Mater. 2020, 25, 644–678. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, S.; Li, J.; Xie, B.; Ma, J.; Dong, S.; Cui, G. Robust Transport: An Artificial Solid Electrolyte Interphase Design for Anode-Free Lithium-Metal Batteries. Adv. Mater. 2022, 35, e2209404. [Google Scholar] [CrossRef]
- Niu, C.; Zhang, M.; Chen, G.; Cao, B.; Shi, J.; Du, J.; Chen, Y. An effectively inhibiting lithium dendrite growth in-situ-polymerized gel polymer electrolyte. Electrochim. Acta 2018, 283, 349–356. [Google Scholar] [CrossRef]
- Guo, L.; Huang, F.; Cai, M.; Zhang, J.; Ma, G.; Xu, S. Organic–Inorganic Hybrid SEI Induced by a New Lithium Salt for High-Performance Metallic Lithium Anodes. ACS Appl. Mater. Interfaces 2021, 13, 32886–32893. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, J.; Yang, S.; Li, Y.; Dong, R.; Yuan, J.; Liu, Y.; Wu, Z.; Song, Y.; Zhong, Y.; et al. Facile In Situ Chemical Cross-Linking Gel Polymer Electrolyte, which Confines the Shuttle Effect with High Ionic Conductivity and Li-Ion Transference Number for Quasi-Solid-State Lithium–Sulfur Battery. ACS Appl. Mater. Interfaces 2021, 13, 44497–44508. [Google Scholar] [CrossRef]
- Takenaka, N.; Bouibes, A.; Yamada, Y.; Nagaoka, M.; Yamada, A. Frontiers in Theoretical Analysis of Solid Electrolyte Interphase Formation Mechanism. Adv. Mater. 2021, 33, e2100574. [Google Scholar] [CrossRef]
- Yang, S.-J.; Yao, N.; Xu, X.-Q.; Jiang, F.-N.; Chen, X.; Liu, H.; Yuan, H.; Huang, J.-Q.; Cheng, X.-B. Formation mechanism of the solid electrolyte interphase in different ester electrolytes. J. Mater. Chem. A 2021, 9, 19664–19668. [Google Scholar] [CrossRef]
- Minakshi, M.; Ralph, D.E.; Singh, P.; Yin, C.-Y. New Insights into the Electrochemical Behavior of Hematite (α-Fe2O3) Microparticles in Strong Aqueous Basic Electrolyte: Formation of Metallic Iron. Metall. Mater. Trans. A 2014, 45, 2023–2029. [Google Scholar] [CrossRef]
- Minakshi, M.; Thurgate, S. Surface analysis on discharged MnO2 cathode using XPS and SIMS techniques. Surf. Interface Anal. 2009, 41, 56–60. [Google Scholar] [CrossRef]
- Minakshi, M.; Singh, P. Success and serendipity on achieving high energy density for rechargeable batteries. J. Solid State Electrochem. 2012, 16, 2227–2233. [Google Scholar] [CrossRef]
- Minakshi, M.; Singh, P.; Mitchell, D.R.G.; Issa, T.B.; Prince, K. A study of lithium insertion into MnO2 containing TiS2 additive a battery material in aqueous LiOH solution. Electrochim. Acta 2007, 52, 7007–7013. [Google Scholar] [CrossRef]
- Minakshi, M.; Singh, P. Synergistic effect of additives on electrochemical properties of MnO2 cathode in aqueous rechargeable batteries. J. Solid State Electrochem. 2012, 16, 1487–1492. [Google Scholar] [CrossRef]
- Song, W.; Scholtis, E.S.; Sherrell, P.C.; Tsang, D.K.H.; Ngiam, J.; Lischner, J.; Fearn, S.; Bemmer, V.; Mattevi, C.; Klein, N.; et al. Electronic structure influences on the formation of the solid electrolyte interphase. Energy Environ. Sci. 2020, 13, 4977–4989. [Google Scholar] [CrossRef]
- Adenusi, H.; Chass, G.A.; Passerini, S.; Tian, K.V.; Chen, G. Lithium Batteries and the Solid Electrolyte Interphase (SEI)—Progress and Outlook. Adv. Energy Mater. 2023, 13, 2203307. [Google Scholar] [CrossRef]
- Jia, T.; Zhong, G.; Lu, S.; Ren, X.; Lv, Y.; Li, N.; Yin, R.; Kang, G.; Cai, K.; Kang, F.; et al. Interfacial fluoride engineering enabled robust LiF-rich solid electrolyte interphase to reduce active lithium loss in rechargeable lithium battery. Chem. Eng. J. 2023, 454, 140397. [Google Scholar] [CrossRef]
- Jurng, S.; Brown, Z.L.; Kim, J.; Lucht, B.L. Effect of electrolyte on the nanostructure of the solid electrolyte interphase (SEI) and performance of lithium metal anodes. Energy Environ. Sci. 2018, 11, 2600–2608. [Google Scholar] [CrossRef]
- Chen, T.; You, J.; Li, R.; Li, H.; Wang, Y.; Wu, C.; Sun, Y.; Yang, L.; Ye, Z.; Zhong, B.; et al. Ultra-Low Concentration Electrolyte Enabling LiF-Rich SEI and Dense Plating/Stripping Processes for Lithium Metal Batteries. Adv. Sci. 2022, 9, e2203216. [Google Scholar] [CrossRef]
- Luo, D.; Zheng, L.; Zhang, Z.; Li, M.; Chen, Z.; Cui, R.; Shen, Y.; Li, G.; Feng, R.; Zhang, S.; et al. Constructing multifunctional solid electrolyte interface via in-situ polymerization for dendrite-free and low N/P ratio lithium metal batteries. Nat. Commun. 2021, 12, 186. [Google Scholar] [CrossRef]
- Wu, Q.; McDowell, M.T.; Qi, Y. Effect of the Electric Double Layer (EDL) in Multicomponent Electrolyte Reduction and Solid Electrolyte Interphase (SEI) Formation in Lithium Batteries. J. Am. Chem. Soc. 2023, 145, 2473–2484. [Google Scholar] [CrossRef]
- Jia, W.; Fan, C.; Wang, L.; Wang, Q.; Zhao, M.; Zhou, A.; Li, J. Extremely Accessible Potassium Nitrate (KNO3) as the Highly Efficient Electrolyte Additive in Lithium Battery. ACS Appl. Mater. Interfaces 2016, 8, 15399–15405. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, X.; Li, Y.; Tian, J.; Zhao, Y.; Mai, L.; Wang, L.; Cao, Y.-C.; Zhang, W. A fast ionic conductor and stretchable solid electrolyte artificial interphase layer for Li metal protection in lithium batteries. J. Alloys Compd. 2020, 843, 155839. [Google Scholar] [CrossRef]
- Cui, C.; Zhang, R.; Fu, C.; Xiao, R.; Li, R.; Ma, Y.; Wang, J.; Gao, Y.; Yin, G.; Zuo, P. Stable lithium anode enabled by biphasic hybrid SEI layer toward high-performance lithium metal batteries. Chem. Eng. J. 2022, 433, 133570. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, W.; Hong, L.; Xu, W.; Yang, H.; Wang, F.; Duan, H.; Tang, M.; Jiang, H. Stress-driven lithium dendrite growth mechanism and dendrite mitigation by electroplating on soft substrates. Nat. Energy 2018, 3, 227–235. [Google Scholar] [CrossRef]
- Li, B.; Cao, B.; Zhou, X.; Zhang, Z.; Dai, D.; Jia, M.; Liu, D.-H. Pre-constructed SEI on graphite-based interface enables long cycle stability for dual ion sodium batteries. Chin. Chem. Lett. 2022, 34, 107832. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, X.; Wang, L.; Wang, J.; Xu, H.; He, X. In-situ polymerized separator enables propylene carbonate electrolyte compatible with high-performance lithium batteries. J. Power Sources 2022, 551, 232172. [Google Scholar] [CrossRef]
- Yao, Z.; Zhu, K.; Li, X.; Zhang, J.; Chen, J.; Wang, J.; Yan, K.; Liu, J. 3D poly (vinylidene fluoride–hexafluoropropylen) nanofiber-reinforced PEO-based composite polymer electrolyte for high-voltage lithium metal batteries. Electrochim. Acta 2022, 404, 139769. [Google Scholar] [CrossRef]
- Shen, Y.-Q.; Zeng, F.-L.; Zhou, X.-Y.; Wang, A.-b.; Wang, W.-k.; Yuan, N.-Y.; Ding, J.-N. A novel permselective organo-polysulfides/PVDF gel polymer electrolyte enables stable lithium anode for lithium–sulfur batteries. J. Energy Chem. 2020, 48, 267–276. [Google Scholar] [CrossRef]
- Chai, S.; Chang, Z.; Zhong, Y.; He, Q.; Wang, Y.; Wan, Y.; Feng, M.; Hu, Y.; Li, W.; Wei, W. Regulation of Interphase Layer by Flexible Quasi-Solid Block Polymer Electrolyte to Achieve Highly Stable Lithium Metal Batteries. Adv. Funct. Mater. 2023, 33, 2300425. [Google Scholar] [CrossRef]
- Biria, S.; Pathreeker, S.; Genier, F.S.; Chen, F.-H.; Li, H.; Burdin, C.V.; Hosein, I.D. Gel Polymer Electrolytes Based on Cross-Linked Poly(ethylene glycol) Diacrylate for Calcium-Ion Conduction. ACS Omega 2021, 6, 17095–17102. [Google Scholar] [CrossRef]
- Wen, Z.; Zhao, Z.; Zhang, T.; Wang, Y.; Zhang, J.; Sun, Z.; Li, L.; Li, Y.; Wu, F.; Chen, R. A fluorinated SEI layer induced by a fire-retardant gel polymer electrolyte boosting lateral dendrite growth. J. Mater. Chem. A 2022, 10, 21905–21911. [Google Scholar] [CrossRef]
- Han, D.-D.; Liu, S.; Liu, Y.-T.; Zhang, Z.; Li, G.-R.; Gao, X.-P. Lithiophilic gel polymer electrolyte to stabilize the lithium anode for a quasi-solid-state lithium–sulfur battery. J. Mater. Chem. A 2018, 6, 18627–18634. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, Y.; Li, Y.; Liu, Y.; Zhong, S.; Xie, M.; Yan, F.; Zhang, Z.; Peng, J.; Li, J.; et al. Regulating solvation structure in gel polymer electrolytes with covalent organic frameworks for lithium metal batteries. Energy Storage Mater. 2022, 53, 917–926. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Shih, C.-Y.; Subramani, R.; Lee, Y.-L.; Jan, J.-S.; Chiu, C.-C.; Teng, H. Ternary-salt gel polymer electrolyte for anode-free lithium metal batteries with an untreated Cu substrate. J. Mater. Chem. A 2022, 10, 4895–4905. [Google Scholar] [CrossRef]
- Du, Y.; Xie, Y.; Liu, X.; Jiang, H.; Wu, F.; Wu, H.; Mei, Y.; Xie, D. In-Situ Formed Phosphorus Modified Gel Polymer Electrolyte with Good Flame Retardancy and Cycling Stability for Rechargeable Lithium Batteries. ACS Sustain. Chem. Eng. 2023, 11, 4498–4508. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, S.; Ma, Y.; Zhou, Y.; Song, D.; Zhang, H.; Shi, X.; Li, C.; Zhang, L. Fumaronitrile-fixed in-situ gel polymer electrolyte balancing high safety and superior electrochemical performance for Li metal batteries. Energy Storage Mater. 2022, 44, 537–546. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, X.; Hong, B.; Bai, M.; Li, J.; Zhang, Z.; Lai, Y. Molecular Reactivity and Interface Stability Modification in In-Situ Gel Electrolyte for High Performance Quasi-Solid-State Lithium Metal Batteries. Energy Environ. Mater. 2022, 2, 435–444. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, J.; Jiang, H.; Wang, L.; Zhang, F.; Feng, X.; Xiang, H. Confined in-situ polymerization of poly (1, 3-dioxolane) and poly (vinylene carbonate)-based quasi-solid polymer electrolyte with improved uniformity for lithium metal batteries. Mater. Today Energy 2023, 32, 101239. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, J.; Gao, G.; Zhang, X.; Fu, C.; Wang, L.; Wang, Y.; Liu, T. Stable Li–Metal Batteries Enabled by in Situ Gelation of an Electrolyte and In-Built Fluorinated Solid Electrolyte Interface. ACS Appl. Mater. Interfaces 2021, 13, 60054–60062. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, P.; Zhu, W.; Zhang, D.; Li, Z.; Wang, H.; Sun, H.; Wang, B.; Fan, L.-Z. A two-step strategy for constructing stable gel polymer electrolyte interfaces for long-life cycle lithium metal batteries. J. Materiomics 2022, 8, 1048–1057. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, J.; Zhao, R.; Zhao, Y.; Liu, J.; Xu, N.; Wan, X.; Li, C.; Ma, Y.; Zhang, H.; et al. In situ 3D crosslinked gel polymer electrolyte for ultra-long cycling, high-voltage, and high-safety lithium metal batteries. Energy Storage Mater. 2023, 57, 92–101. [Google Scholar] [CrossRef]
- Jiang, T.; He, P.; Liang, Y.; Fan, L.-Z. All-dry synthesis of self-supporting thin Li10GeP2S12 membrane and interface engineering for solid state lithium metal batteries. Chem. Eng. J. 2021, 421, 129965. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Zhang, C.; Ye, W.; Wang, J.; Xue, Z. Abundant Hydrogen Bonds Formed in a Urea-Based Gel Polymer Electrolyte Improve Interfacial Stability in Lithium Metal Batteries. ChemSusChem 2022, 15, e202201361. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, J.; Han, W.; Zhang, J.; Ding, G.; Dong, S.; Cui, G. Review—In Situ Polymerization for Integration and Interfacial Protection Towards Solid State Lithium Batteries. J. Electrochem. Soc. 2020, 167, 070527. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, H.; Zhao, L.; Sun, Z.; Li, Y.; Mo, Y.; Chen, Y. A flexible Cellulose/Methylcellulose gel polymer electrolyte endowing superior Li(+) conducting property for lithium ion battery. Carbohydr. Polym. 2020, 246, 116622. [Google Scholar] [CrossRef]
- Zhao, B.; Yang, M.; Li, J.; Li, S.; Zhang, G.; Liu, S.; Cui, Y.; Liu, H. Cellulose-Based Plastic Crystal Electrolyte Membranes with Enhanced Interface for Solid-State Lithium Batteries. Energy Technol. 2021, 9, 2100114. [Google Scholar] [CrossRef]
- Zhao, Y.; Tenhaeff, W.E. Thermally and Oxidatively Stable Polymer Electrolyte for Lithium Batteries Enabled by Phthalate Plasticization. ACS Appl. Polym. Mater. 2020, 2, 80–90. [Google Scholar] [CrossRef]
- Li, S.; Yang, K.; Zhang, Z.; Yang, L.; Hirano, S.-i. Organic Ionic Plastic Crystal-Poly(ethylene oxide) Solid Polymer Electrolytes: Application in All-Solid-State Lithium Batteries. Ind. Eng. Chem. Res. 2018, 57, 13608–13614. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, L.; Xu, S.; Zhang, H.; Ren, B.; Li, T.; Zhang, S. Ionic liquid functionalized electrospun gel polymer electrolyte for use in a high-performance lithium metal battery. J. Mater. Chem. A 2018, 6, 18479–18487. [Google Scholar] [CrossRef]
- Singh, S.K.; Dutta, D.; Singh, R.K. Enhanced structural and cycling stability of Li2CuO2-coated LiNi0.33Mn0.33Co0.33O2 cathode with flexible ionic liquid-based gel polymer electrolyte for lithium polymer batteries. Electrochim. Acta 2020, 343, 136122. [Google Scholar] [CrossRef]
- Martinez-Ibañez, M.; Boaretto, N.; Santiago, A.; Meabe, L.; Wang, X.; Zugazua, O.; Raposo, I.; Forsyth, M.; Armand, M.; Zhang, H. Highly-concentrated bis(fluorosulfonyl)imide-based ternary gel polymer electrolytes for high-voltage lithium metal batteries. J. Power Sources 2023, 557, 232554. [Google Scholar] [CrossRef]
- Gao, S.; Sun, F.; Liu, N.; Yang, H.; Cao, P.-F. Ionic conductive polymers as artificial solid electrolyte interphase films in Li metal batteries—A review. Mater. Today 2020, 40, 140–159. [Google Scholar] [CrossRef]
- Xu, H.; Han, C.; Li, W.; Li, H.; Qiu, X. Quantification of lithium dendrite and solid electrolyte interphase (SEI) in lithium-ion batteries. J. Power Sources 2022, 529, 231219. [Google Scholar] [CrossRef]
- Hu, R.; Qiu, H.; Zhang, H.; Wang, P.; Du, X.; Ma, J.; Wu, T.; Lu, C.; Zhou, X.; Cui, G. A Polymer-Reinforced SEI Layer Induced by a Cyclic Carbonate-Based Polymer Electrolyte Boosting 4.45 V LiCoO2/Li Metal Batteries. Small 2020, 16, 1907163. [Google Scholar] [CrossRef]
- Li, S.; Ren, W.; Huang, Y.; Zhou, Q.; Luo, C.; Li, Z.; Li, X.; Wang, M.; Cao, H. Building more secure LMBs with gel polymer electrolytes based on dual matrices of PAN and HPMC by improving compatibility with anode and tuning lithium ion transference. Electrochim. Acta 2021, 391, 138950. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Subramani, R.; Huang, Y.-T.; Lee, Y.-L.; Jan, J.-S.; Chiu, C.-C.; Hou, S.-S.; Teng, H. Highly stable interface formation in onsite coagulation dual-salt gel electrolyte for lithium-metal batteries. J. Mater. Chem. A 2021, 9, 5675–5684. [Google Scholar] [CrossRef]
- Divakaran, A.M.; Minakshi, M.; Bahri, P.A.; Paul, S.; Kumari, P.; Divakaran, A.M.; Manjunatha, K.N. Rational design on materials for developing next generation lithium-ion secondary battery. Prog. Solid State Chem. 2021, 62, 100298. [Google Scholar] [CrossRef]
- Liang, J.; Tao, R.; Tu, J.; Guo, C.; Du, K.; Guo, R.; Zhang, W.; Liu, X.; Guo, P.; Wang, D.; et al. Design of a multi-functional gel polymer electrolyte with a 3D compact stacked polymer micro-sphere matrix for high-performance lithium metal batteries. J. Mater. Chem. A 2022, 10, 12563–12574. [Google Scholar] [CrossRef]
- Shi, Y.; Wan, J.; Liu, G.-X.; Zuo, T.-T.; Song, Y.-X.; Liu, B.; Guo, Y.-G.; Wen, R.; Wan, L.-J. Interfacial Evolution of Lithium Dendrites and Their Solid Electrolyte Interphase Shells of Quasi-Solid-State Lithium-Metal Batteries. Angew. Chem. Int. Ed. 2020, 59, 18120–18125. [Google Scholar] [CrossRef]
- Liu, Q.; Cai, B.; Li, S.; Yu, Q.; Lv, F.; Kang, F.; Wang, Q.; Li, B. Long-cycling and safe lithium metal batteries enabled by the synergetic strategy of ex situ anodic pretreatment and an in-built gel polymer electrolyte. J. Mater. Chem. A 2020, 8, 7197–7204. [Google Scholar] [CrossRef]
- He, X.; Schmohl, S.; Wiemhöfer, H.D. Direct Observation and Suppression Effect of Lithium Dendrite Growth for Polyphosphazene Based Polymer Electrolytes in Lithium Metal Cells. ChemElectroChem 2019, 6, 1166–1176. [Google Scholar] [CrossRef]
- Yan, J.; Liu, F.-Q.; Gao, J.; Zhou, W.; Huo, H.; Zhou, J.-J.; Li, L. Low-Cost Regulating Lithium Deposition Behaviors by Transition Metal Oxide Coating on Separator. Adv. Funct. Mater. 2021, 31, 2007255. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, G.; Wang, W.; Wang, J.; Wang, L.; Liu, T. Synergy of an In Situ-Polymerized Electrolyte and a Li3N–LiF-Reinforced Interface Enables Long-Term Operation of Li-Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 49811–49819. [Google Scholar] [CrossRef]
- Wan, J.; Chen, W.-P.; Liu, G.-X.; Shi, Y.; Xin, S.; Guo, Y.-G.; Wen, R.; Wan, L.-J. Insights into the nitride-regulated processes at the electrolyte/electrode interface in quasi-solid-state lithium metal batteries. J. Energy Chem. 2022, 67, 780–786. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, Y.; Zhu, J.; Sun, Z.; Xu, L.; Li, C.; Ma, Y.; Zhang, H.; Chen, Y. Dual effects from in-situ polymerized gel electrolyte and boric acid for ultra-long cycle-life Li metal batteries. Sci. China Mater. 2020, 63, 2344–2350. [Google Scholar] [CrossRef]
- Yang, P.; Gao, X.; Tian, X.; Shu, C.; Yi, Y.; Liu, P.; Wang, T.; Qu, L.; Tian, B.; Li, M.; et al. Upgrading Traditional Organic Electrolytes toward Future Lithium Metal Batteries: A Hierarchical Nano-SiO2-Supported Gel Polymer Electrolyte. ACS. Energy Lett 2020, 5, 1681–1688. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Jiang, Z.; Yuan, R.; Song, H. A Review of the Relationship between Gel Polymer Electrolytes and Solid Electrolyte Interfaces in Lithium Metal Batteries. Nanomaterials 2023, 13, 1789. https://doi.org/10.3390/nano13111789
Yu X, Jiang Z, Yuan R, Song H. A Review of the Relationship between Gel Polymer Electrolytes and Solid Electrolyte Interfaces in Lithium Metal Batteries. Nanomaterials. 2023; 13(11):1789. https://doi.org/10.3390/nano13111789
Chicago/Turabian StyleYu, Xiaoqi, Zipeng Jiang, Renlu Yuan, and Huaihe Song. 2023. "A Review of the Relationship between Gel Polymer Electrolytes and Solid Electrolyte Interfaces in Lithium Metal Batteries" Nanomaterials 13, no. 11: 1789. https://doi.org/10.3390/nano13111789
APA StyleYu, X., Jiang, Z., Yuan, R., & Song, H. (2023). A Review of the Relationship between Gel Polymer Electrolytes and Solid Electrolyte Interfaces in Lithium Metal Batteries. Nanomaterials, 13(11), 1789. https://doi.org/10.3390/nano13111789







