Plasmonic Cu2−xSe Mediated Colorimetric/Photothermal Dual-Readout Detection of Glutathione
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Synthesis of Cu2−xSe NPs
2.3. Analytical Process for GSH Detection
2.4. GSH Detection in Real Samples
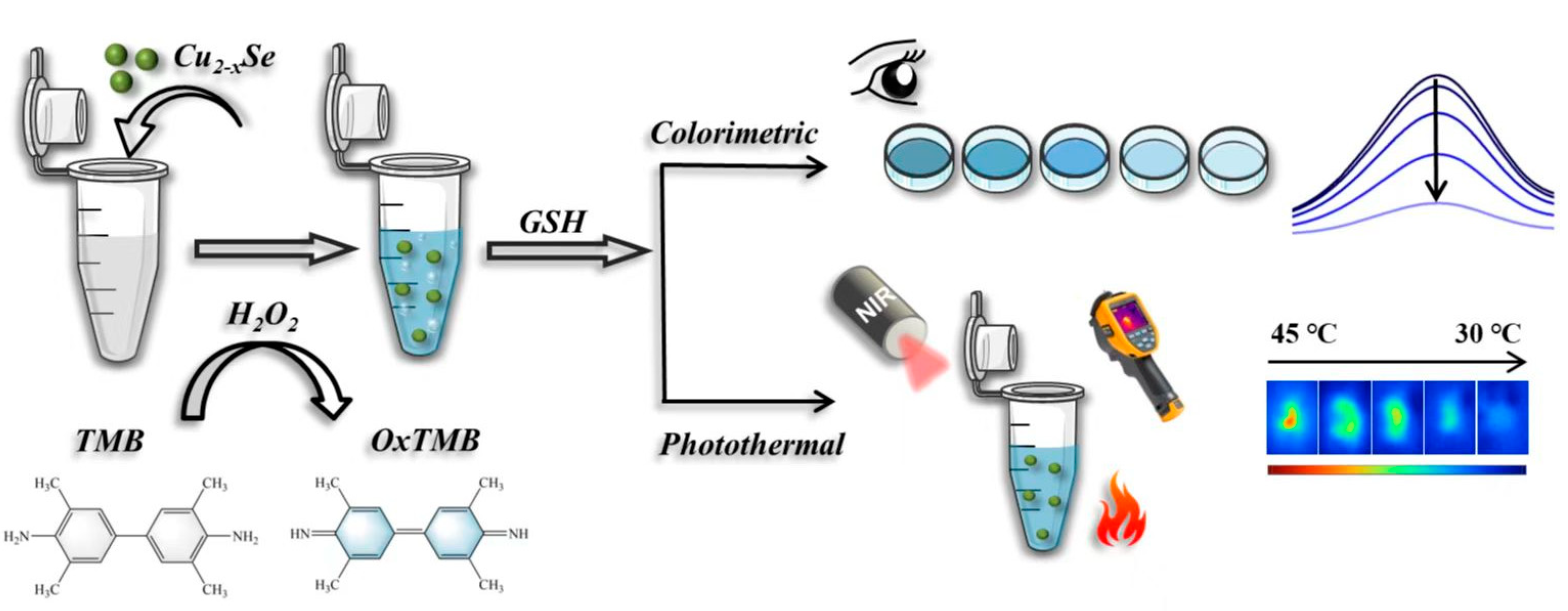
3. Results and Discussion
3.1. Characterization of Cu2−xSe NPs
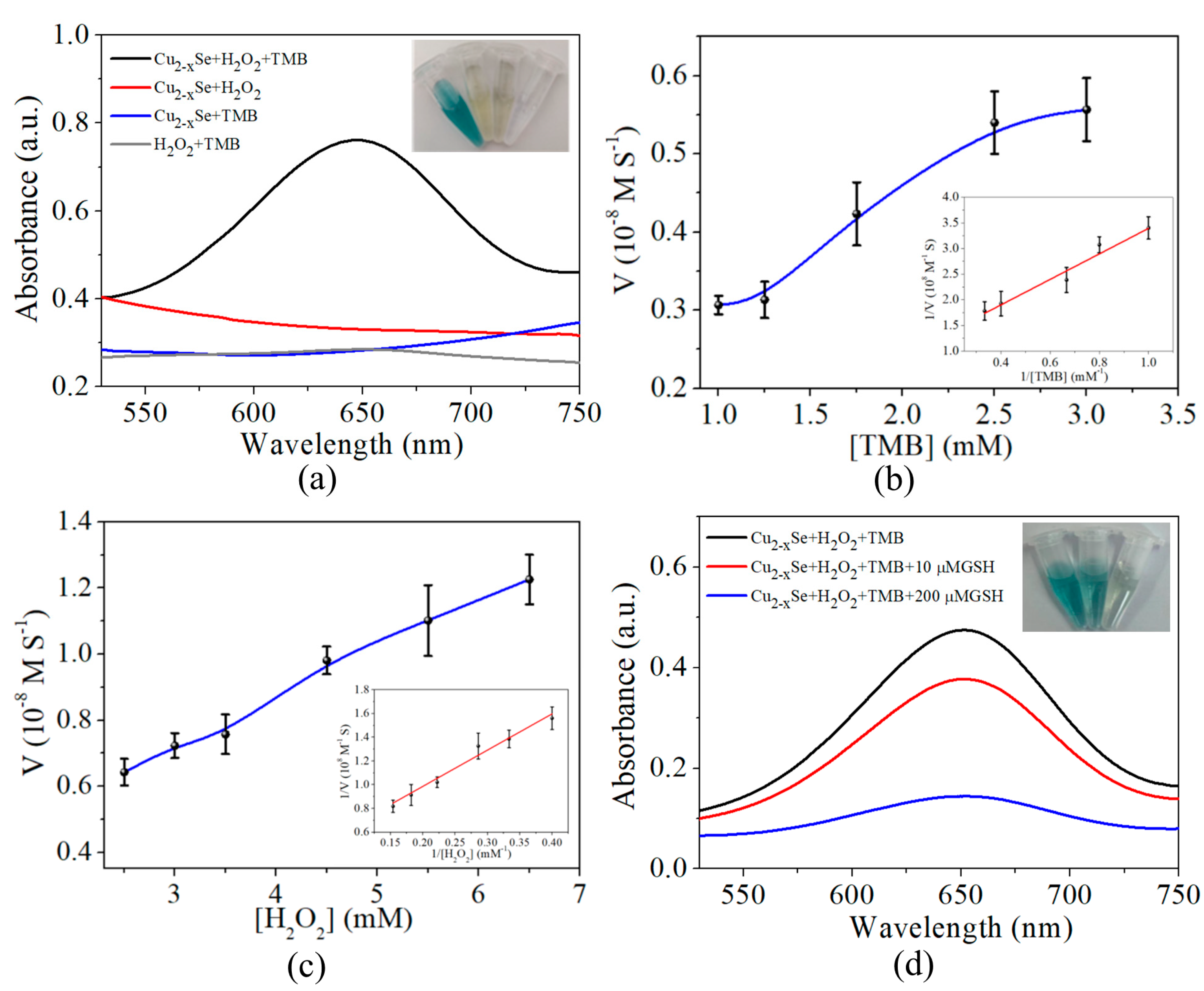
3.2. Peroxidase-Mimicking Activity Inhibition of Cu2−xSe by GSH
3.3. Optimization of Experimental Conditions
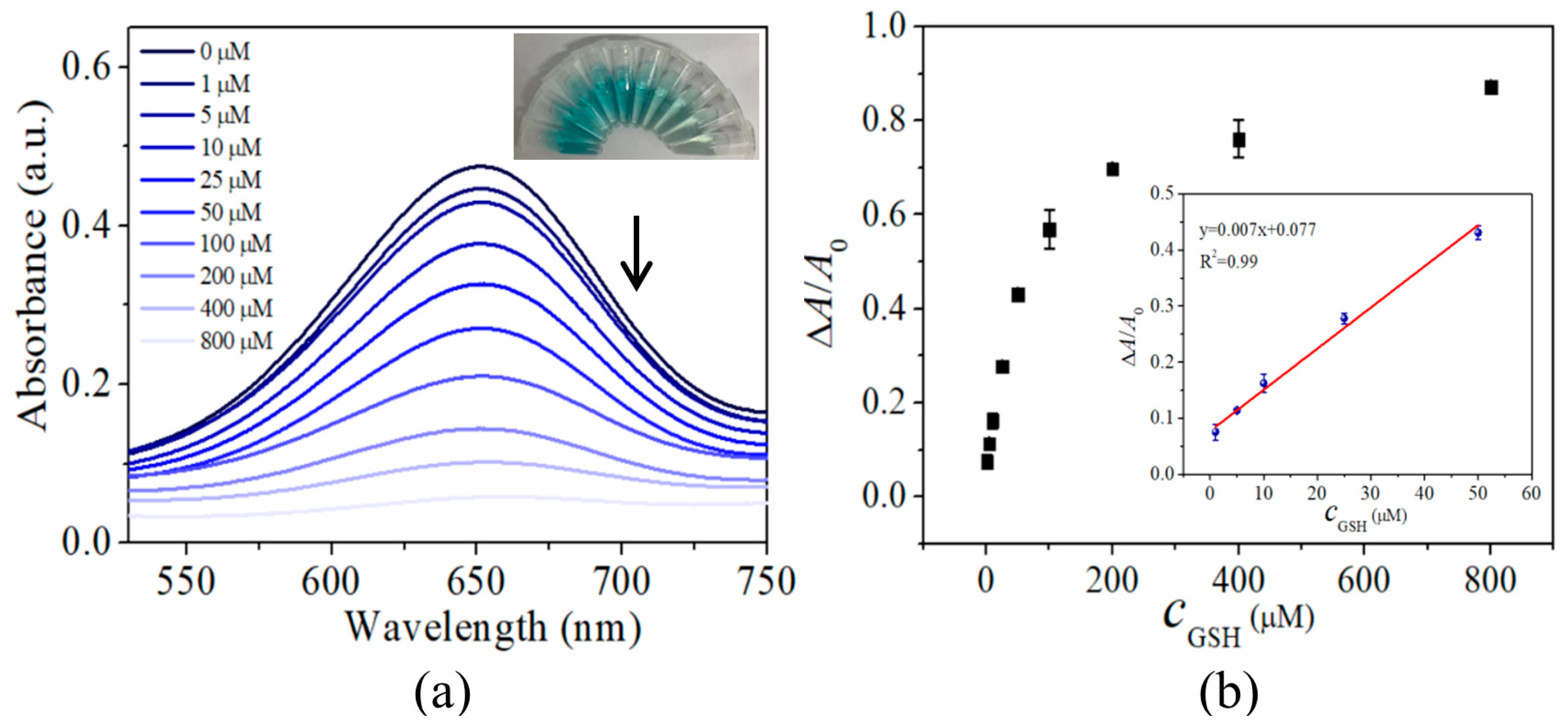
3.4. Performance of GSH Colorimetric Detection
3.5. Detection of GSH in Real Samples
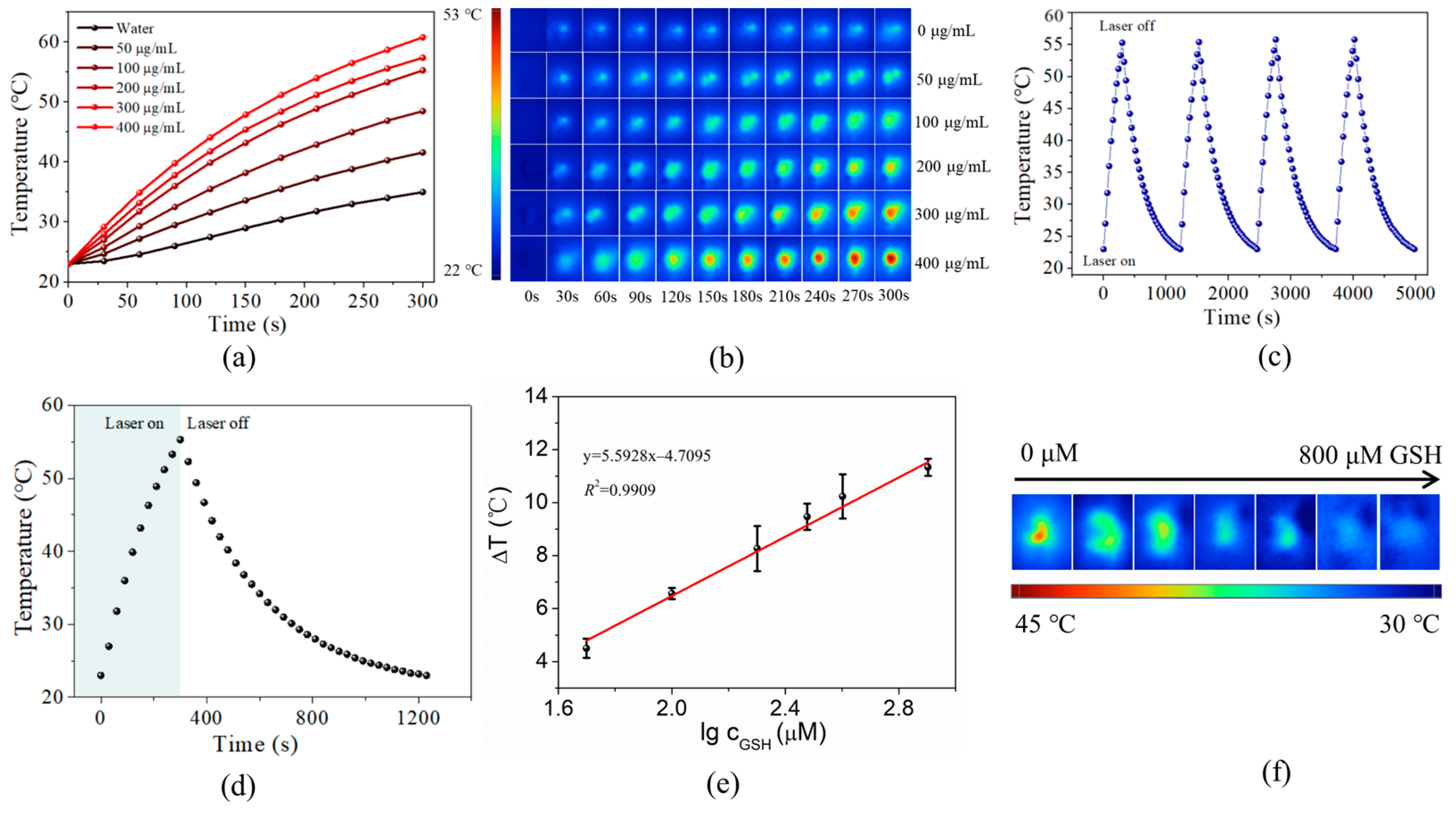
3.6. Photothermal Detection of GSH
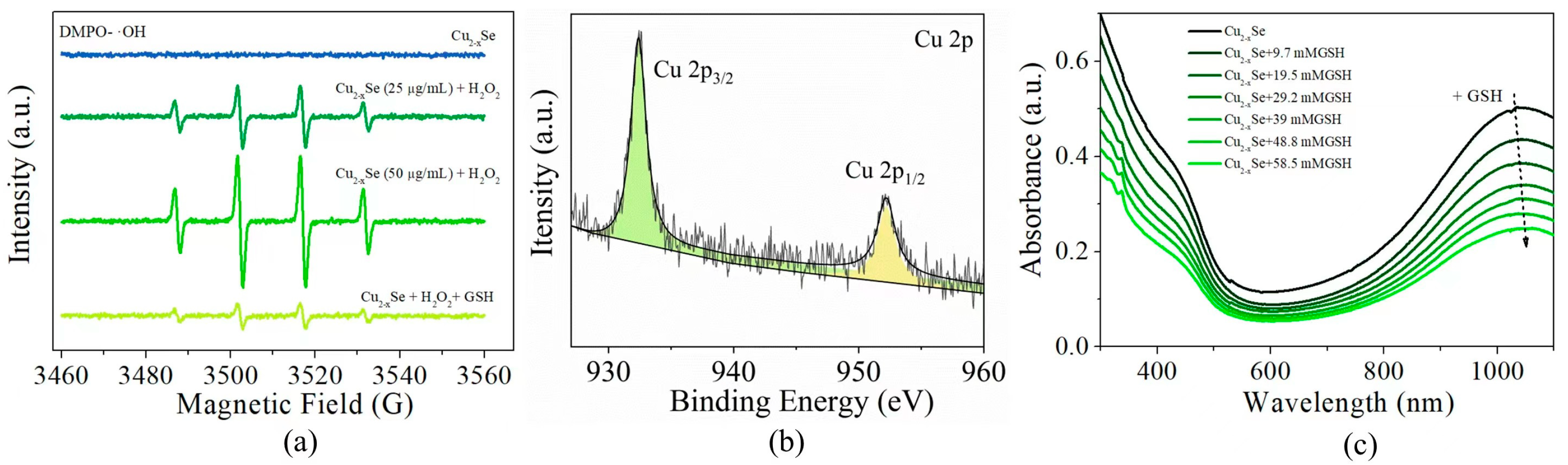
3.7. Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agrawal, A.; Cho, S.H.; Zandi, O.; Ghosh, S.; Johns, R.W.; Milliron, D.J. Localized surface plasmon resonance in semiconductor nanocrystals. Chem. Rev. 2018, 6, 3121–3207. [Google Scholar] [CrossRef] [PubMed]
- Faucheaux, J.A.; Alexandria, L.D.; Stanton; Prashant, K.J. Plasmon resonances of semiconductor nanocrystals: Physical principles and new opportunities. J. Phys. Chem. Lett. 2014, 6, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Swihart, M.T. Heavily-doped colloidal semiconductor and metal oxide nanocrystals: An emerging new class of plasmonic nanomaterials. Chem. Soc. Rev. 2014, 43, 3908–3920. [Google Scholar] [CrossRef] [PubMed]
- Kathryn, M.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 6, 3828–3857. [Google Scholar]
- Ha, M.; Kim, J.H.; You, M.; Fan, C.; Nam, J.M.; Li, Q. Multicomponent plasmonic nanoparticles: From heterostructured nanoparticles to colloidal composite nanostructures. Chem. Rev. 2019, 24, 12208–12278. [Google Scholar] [CrossRef]
- Jiang, N.; Zhuo, X.L.; Wang, J.F. Active plasmonics: Principles, structures, and applications. Chem. Rev. 2018, 6, 3054–3099. [Google Scholar] [CrossRef]
- Liu, M.X.; Liu, Y.; Gu, B.B.; Wei, X.B.; Xu, G.X.; Wang, X.M.; Swihart, M.T.; Yong, K.T. Recent advances in copper sulphide-based nanoheterostructures. Chem. Soc. Rev. 2019, 48, 4950–4965. [Google Scholar] [CrossRef]
- Coughlan, C.; Ibáñez, M.; Dobrozhan, O.; Singh, A.; Cabot, A.; Ryan, K.M. Compound copper chalcogenide nanocrystals. Chem. Rev. 2017, 117, 5865–6109. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Pan, H.C.; Lou, Y.B.; Qiu, X.F.; Zhu, J.J.; Burda, C. Plasmonic Cu2-xS nanocrystals: Optical and structural properties of copper-deficient copper(I) sulfide. J. Am. Chem. Soc. 2009, 131, 4253–4261. [Google Scholar] [CrossRef]
- Luther, J.M.; Jain, P.K.; Ewers, T.; Alivisatos, A.P. Localized surface plasmon resonances arising from free carriers in doped quantum dots. Nat. Mater. 2011, 10, 361–366. [Google Scholar] [CrossRef]
- Alina, M.S.; Niket, T.; Carolyn, E.G.; David, J.M.; Daniel, R.G. Charge-tunable quantum plasmons in colloidal semiconductor nanocrystals. ACS Nano 2014, 8, 1065–1072. [Google Scholar]
- Lauren, E.M.; Xing, Y.G.; Derrick, C.K.; Jill, E.M. Correlating carrier density and emergent plasmonic features in Cu2–xSe nanoparticles. Nano Lett. 2017, 4, 2414–2419. [Google Scholar]
- Liu, Y.; Liu, M.; Yin, D.; Qiao, L.; Fu, Z.; Swihart, M.T. Selective cation incorporation into copper sulfide based nanoheterostructures. ACS Nano 2018, 8, 7803–7811. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chang, K.L.; Zheng, F.; Yin, D.Q.; Swihart, M.T. Can the morphology of biconcave metal sulfide nanoplatelets be preserved during cation exchange? Chem. Mater. 2019, 15, 5706–5712. [Google Scholar]
- Han, K.D.; Hui, Y.X.; Bonnie, A.P.; Luis, F.G.; Haley, P.M.; Boxi, C.L.; Hai, Y.W.; Katherine, E.P. Effects of I2 on Cu2–xS Nanoparticles: Enabling cation exchange but complicating plasmonics. ACS Mater. Lett. 2020, 2, 140–146. [Google Scholar]
- Jain, P.K.; Manthiram, K.; Engel, J.H.; White, S.L.; Faucheaux, J.A.; Alivisatos, A.P. Doped nanocrystals as plasmonic probes of redox chemistry. Angew. Chem. Int. Ed. 2013, 52, 13671–13675. [Google Scholar] [CrossRef]
- Kriegel, I.; Jiang, C.; Rodríguez, F.J.; Schaller, R.D.; Talapin, D.V.; Da, C.E.; Feldmann, J. Tuning the excitonic and plasmonic properties of copper chalcogenide nanocrystals. J. Am. Chem. Soc. 2012, 134, 1583–1590. [Google Scholar] [CrossRef]
- Xie, Y.; Riedinger, A.; Prato, M.; Casu, A.; Genovese, A.; Guardia, P.; Sottini, S.; Sangregorio, C.; Miszta, K.; Ghosh, S.; et al. Copper sulfide nanocrystals with tunable composition by reduction of covellite nanocrystals with Cu+ ions. J. Am. Chem. Soc. 2013, 135, 17630–17637. [Google Scholar] [CrossRef]
- Dorfs, D.; Härtling, T.; Miszta, K.; Bigall, N.C.; Kim, M.R.; Genovese, A.; Falqui, A.; Povia, M.; Manna, L. Reversible tunability of the near-infrared valence band plasmon resonance in Cu2-xSe nanocrystals. J. Am. Chem. Soc. 2011, 133, 11175–11180. [Google Scholar] [CrossRef]
- Chen, L.; Hu, H.; Chen, Y.; Gao, J.; Li, G. Plasmonic Cu2−xS nanoparticles: A brief introduction of optical properties and applications. Mater. Adv. 2021, 2, 907–926. [Google Scholar] [CrossRef]
- Li, K.; Xu, K.; He, Y.; Yang, Y.L.; Tan, M.J.; Mao, Y.L.; Zou, Y.N.; Feng, Q.; Luo, Z.; Cai, K.Y. Oxygen self-generating nanoreactor mediated ferroptosis activation and immunotherapy in triple-negative breast cancer. ACS Nano 2023, 5, 4667–4687. [Google Scholar] [CrossRef] [PubMed]
- Lie, S.Q.; Wang, D.M.; Gao, M.X.; Huang, C.Z. Controllable copper defi ciency in Cu2−xSe nanocrystals with tunable localized surface plasmon resonance and enhanced chemiluminescence. Nanoscale 2014, 6, 10289–10296. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.T.; Xiao, J.; Kong, X.; Chen, L.; Li, C.; Wei, Y.J.; Du, Q.Y.; Sun, W.Z.; Zhang, J.Y. Regulating the charge density of Cu(I) single sites enriched on the surface of N3c Vacancies-engineered g-C3N4 for efficient Fenton-like reactions. Sep. Purif. Technol. 2023, 1, 123525. [Google Scholar] [CrossRef]
- Dai, C.; Sheng, Z.Y.; Tian, X.K.; Nie, Y.L. Chalcogen elements in regulating the local electron density of Cu2X for an efficient heterogeneous Fenton-like process. ACS Appl. Mater. Interfaces 2023, 8, 11324–11332. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zou, H.Y.; Liu, Z.X.; Yang, T.; Gao, M.X.; Huang, C.Z. Efficient visible-light photocatalytic heterojunctions formed by coupling plasmonic Cu2−xSe and graphitic carbon nitride. New J. Chem. 2015, 39, 6186–6192. [Google Scholar] [CrossRef]
- Mao, Y.T.; Zou, H.Y.; Wang, Q.; Huang, C.Z. Large-scale preparation of fernwort-lik single-crystalline superstructures of CuSe as Fenton-like catalysts for dye decolorization. Sci. China Chem. 2016, 7, 903–909. [Google Scholar] [CrossRef]
- An, L.; Wang, C.B.; Tian, Q.W.; Tao, C.; Xue, F.F.; Yang, S.P.; Zhou, X.S.; Chen, X.Y.; Huang, G. NIR-II laser-mediated photo-Fenton-like reaction via plasmonic Cu9S8 for immunotherapy enhancement. Nano Today 2022, 43, 101397. [Google Scholar] [CrossRef]
- Deng, S.Q.; Zou, H.Y.; Lan, J.; Huang, C.Z. Aggregation-induced superior peroxidase-like activity of Cu2-xSe@PSS nanoparticles for melamine detection. Anal. Methods 2016, 8, 7516–7521. [Google Scholar] [CrossRef]
- Gao, P.F.; Mao, Y.T.; Yang, T.; Zou, H.Y.; Li, Y.F.; Huang, C.Z. Glutathione-driven Cu(I)–O2 chemistry: A new light-up fluorescent assay for intracellular glutathione. Analyst 2018, 143, 2486–2490. [Google Scholar] [CrossRef]
- Guo, Q.J.; Pan, Z.Y.; Men, C.; Lv, W.Y.; Zou, H.Y.; Huang, C.Z. Visual detection of cancer cells by using in situ grown functional Cu2−xSe/reduced graphene oxide hybrids acting as an efficient nanozyme. Analyst 2019, 144, 716–721. [Google Scholar] [CrossRef]
- Wei, M.M.; Rao, H.Y.; Niu, Z.R.; Xue, X.; Luo, M.Y.; Zhang, X.Y.; Huang, X.Y.; Xue, Z.H.; Lu, X.Q. Breaking the time and space limitation of point-of-care testing strategies: Photothermometric sensors based on different photothermal agents and materials. Coord. Chem. Rev. 2021, 447, 214149. [Google Scholar] [CrossRef]
- Xu, S.Y.; Bai, X.L.; Wang, L.Y. Exploration of photothermal sensors based on photothermally responsive materials: A brief review. Inorg. Chem. Front. 2018, 5, 751. [Google Scholar] [CrossRef]
- Hessel, C.M.; Pattani, V.P.; Rasch, M.; Panthani, M.G.; Koo, B.; Tunnell, J.W.; Korgel, B.A. Copper selenide nanocrystals for photothermal therapy. Nano Lett. 2011, 11, 2560–2566. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Jiang, F.; Zou, R.; Liu, Q.; Chen, Z.; Zhu, M.; Yang, S.; Wang, J.; Wang, J.; Hu, J. Hydrophilic Cu9S5 nanocrystals: A photothermal agent with a 25.7% heat conversion efficiency for photothermal ablation of cancer cells in vivo. ACS Nano 2011, 5, 9761–9771. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Xiang, H.D.; Yang, D.; Lin, T.K.; Wu, Y.Z.; Zhou, R.Y.; Gu, Z.J.; Yan, L.; Zhao, Y.L.; Tan, W.H. Plasmonic AuPt@CuS Heterostructure with enhanced synergistic efficacy for radiophotothermal therapy. J. Am. Chem. Soc. 2021, 39, 16113–16127. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.Q.; Fu, X.Q.; Chen, K.X.; Wang, H. Dual-plasmonic Gold@Copper sulfide core–shell nanoparticles: Phase-selective synthesis and multimodal photothermal and photocatalytic behaviors. ACS Appl. Mater. Interfaces 2020, 41, 46146–46161. [Google Scholar] [CrossRef]
- Wang, X.W.; Zhong, X.Y.; Lei, H.L.; Geng, Y.H.; Zhao, Q.Z.; Gong, F.; Yang, Z.J.; Dong, Z.L.; Liu, Z.; Cheng, L.L. Hollow Cu2Se nanozymes for tumor photothermal-catalytic therapy. Chem. Mater. 2019, 16, 6174–6186. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, S.H.; Zhang, H.; Han, Y.B.; Liu, H.H.; Ren, F.; Sun, Q.; Li, Z.; Gao, M.Y. Boosting the radio sensitizing and photothermal performance of Cu2–xSe nanocrystals for synergetic radio photothermal therapy of orthotopic breast cancer. ACS Nano 2019, 2, 1342–1353. [Google Scholar]
- Li, X.; Yang, L.; Men, C.; Xie, Y.F.; Liu, J.J.; Zou, H.Y.; Li, Y.F.; Zhan, L.; Huang, C.Z. Photothermal soft nanoballs developed by loading plasmonic Cu2–xSe nanocrystals into liposomes for photothermal immunoassay of aflatoxin B1. Anal. Chem. 2019, 7, 4444–4450. [Google Scholar] [CrossRef]
- Xu, W.; Liu, H.C.; Zhou, D.L.; Chen, X.; Ding, N.; Song, H.W.; Ågren, H. Localized surface plasmon resonances in self-doped copper chalcogenide binary nanocrystals and their emerging applications. Nano Today 2020, 33, 100892. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, H.; Zhou, X.; Gong, X.; Xiao, L.; Liu, L.; Yang, Z.Q. Bright-yellow-emissive nitrogen-doped carbon nanodots as a fluorescent nanoprobe for the straightforward detection of glutathione in food samples. Food Chem. 2020, 325, 126946. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; White, J.M.; Lin, W.J.; New, E.J.; Pfeffer, M.F. A rhodamine-nitronaphthalimide Hg(II) complex for the simultaneous detection of oxidised and reduced glutathione. Sens. Actuator B Chem. 2019, 300, 126825. [Google Scholar] [CrossRef]
- Li, J.L.; Jiao, L.; Xu, W.Q.; Yan, H.Y.; Chen, G.J.; Wu, Y.; Hu, L.Y.; Gu, W.L. Cobalt oxyhydroxide nanosheets integrating with metal indicator enable sensitive detection of glutathione. Sens. Actuator B Chem. 2021, 329, 129247. [Google Scholar] [CrossRef]
- Samiec, P.S.; Botsch, C.D.; Flagg, E.W.; Kurtz, J.C.; Sternber, P.; Reed, R.L.; Jones, D.P. Glutathione in human plasma: Decline in association with aging, agerelated macular degeneration, and diabetes. Free Radic. Biol. Med. 1998, 24, 699–704. [Google Scholar] [CrossRef]
- Yoshida, K.J.; Tagami, H.S.; Kawakami, T.Y.; Urata, Y.; Kondo, T. Weakened cellular scavenging activity against oxidative stress in diabetes mellitus: Regulation of glutathione synthesis and efflux. Diabetologia 1995, 38, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Glutathione homeostasis in response to exercise training and nutritional supplements. Mol. Cell. Biochem. 1999, 196, 31–42. [Google Scholar] [CrossRef]
- Mazzetti, A.P.; Fiorile, M.C.; Primavera, A.; Bello, M.L. Glutathione transferases and neurodegenerative diseases. Neurochem. Int. 2015, 82, 10–18. [Google Scholar] [CrossRef]
- Dong, Z.Z.; Lu, L.H.; Ko, C.N.; Yang, C.; Li, S.G.; Lee, M.Y.; Leung, C.H.; Ma, D.L. A MnO2 nanosheet-assisted GSH detection platform using an iridium(III) complex as a switch-on luminescent probe. Nanoscale 2017, 9, 4677–4682. [Google Scholar] [CrossRef]
- Zheng, Y.N.; Xu, D.P.; Sun, L.X.; Ji, J.R.; Tong, Z.F.; Qing, L.Y.; Zhang, Y.Q.; Luo, J.X.; Liao, D.K. Construction of a bioinspired Fe3O4/N-HCS nanozyme for highly sensitive detection of GSH. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129046. [Google Scholar] [CrossRef]
- Zhou, Y.; Shi, X.Y.; Ma, L.; Chai, Y.Q.; Yuan, R. Thiol stabilized Au nanoclusters with efficient electrochemiluminescence for the simultaneous and ultrasensitive detection of GSH and Cu2+. Sens. Actuators B Chem. 2023, 382, 133518. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Huang, X.L.; Hu, X.Y.; Tong, W.P.; Leng, Y.K.; Xiong, Y.H. Recent advances in colorimetry/fluorimetry-based dual-modal sensing technologies. Biosens. Bioelectron. 2021, 190, 113386. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.Y.; Yang, S.H.; Yue, L.; Wang, J.; Liang, X.R.; Xin, B.P. Extracellular biosynthesis of Cu2-xSe nanocrystallites with photocatalytic activity. Mater. Res. Bull. 2019, 111, 126–132. [Google Scholar] [CrossRef]
- Du, C.; Younas, W.; Wang, Z.; Yang, X.; Meng, E.; Wang, L.; Huang, J.; Ma, X.; Zhu, Y.; Cao, C. Constructing sheet-assembled hollow cuse nanocubes to boost the rate capability of rechargeable magnesium batteries. J. Mater. Chem. A 2021, 9, 3648–3656. [Google Scholar] [CrossRef]
- Wu, Q.; Tan, L.; Liu, X.M.; Li, Z.Y.; Zhang, Y.; Zheng, Y.F.; Liang, T.Q.; Cui, Z.D.; Zhu, S.L.; Wu, S.L. The Enhanced Near-Infrared Photocatalytic and Photothermal Effects of MXene-Based Heterojunction for Rapid Bacteria-Killing. Appl. Catal. B Environ. 2021, 297, 120500. [Google Scholar] [CrossRef]
- Huang, A.T.; Du, J.; Liu, Z.Y.; Zhang, G.C.; Abuduwaili, W.; Yan, J.Y.; Sun, J.L.; Xu, R.C.; Liu, T.T.; Shen, X.Z.; et al. Sorafenib-Loaded Cu2−xSe Nanoparticles Boost Photothermal–Synergistic Targeted Therapy against Hepatocellular Carcinoma. Nanomaterials 2022, 12, 3191. [Google Scholar] [CrossRef]
- Lin, X.D.; Lbarlucea, B.; Peng, T.; Shen, R.; Li, P.Y.; Zhang, P. Two birds with one stone: A multifunctional nanoplatform for photothermal sensitive detection and real-time inactivation of Staphylococcus aureus with NIR responsive Cu2−XSe@Van NPs. Sens. Actuator B Chem. 2023, 381, 133475. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.J.; Sang, D.M.; Yu, K.; Lin, H.M.; Qu, F.Y. Cu2−xSe/Bi2Se3@PEG Z-scheme heterostructure: A multimode bioimaging guided theranostic agent with enhanced photo/chemodynamic and photothermal therapy. Biomater. Sci. 2021, 9, 4473–4483. [Google Scholar] [CrossRef]
- Jiang, B.; Duan, D.; Gao, L.; Zhou, M.; Fan, K.; Tang, Y.; Xi, J.; Bi, Y.; Tong, Z.; Gao, G.F.; et al. Standardized assays for determining the catalytic activity and kinetics of peroxidase-like nanozymes. Nat. Protoc. 2018, 13, 1506–1520. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhu, G.; Yuan, A. Ag@Fe3O4 nanowire: Fabrication, characterization and peroxidase-like activity. Cryst. Res. Technol. 2014, 49, 309–314. [Google Scholar] [CrossRef]
- Du, H.; Fuh, R.C.A.; Li, J.Z.; Corkan, L.A.; Lindsey, J.S. PhotochemCAD‡: A computer-aided design and research tool in photochemistry. Photochem. Photobiol. 1998, 68, 141–142. [Google Scholar]
- Yu, W.W.; Qu, L.H.; Guo, W.Z.; Peng, X.G. Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem. Mater. 2003, 15, 2854–2860. [Google Scholar] [CrossRef]
- Kam, N.W.S.; O’Connell, M.; Wisdom, J.A.; Dai, H.J. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc. Natl. Acad. Sci. USA 2005, 102, 11600–11605. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]

| Samples | Standard Added GSH (μM) | Detected GSH (μM) | Recovery (%) | GSH Kit |
|---|---|---|---|---|
| tomatoes | 0 | 6.00 ± 0.60 | / | 6.41 ± 0.51 |
| 30.0 | 37.92 ± 0.35 | 104.4–106.3 | 36.76 ± 1.96 | |
| cucumbers | 0 | 13.14 ± 0.47 | / | 15.17 ± 0.94 |
| 30.0 | 42.45 ± 1.20 | 95.6–101.2 | 43.81 ± 1.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, G.; Ni, H.; Li, X.; Qi, X.; Yang, X.; Zou, H. Plasmonic Cu2−xSe Mediated Colorimetric/Photothermal Dual-Readout Detection of Glutathione. Nanomaterials 2023, 13, 1787. https://doi.org/10.3390/nano13111787
Yan G, Ni H, Li X, Qi X, Yang X, Zou H. Plasmonic Cu2−xSe Mediated Colorimetric/Photothermal Dual-Readout Detection of Glutathione. Nanomaterials. 2023; 13(11):1787. https://doi.org/10.3390/nano13111787
Chicago/Turabian StyleYan, Guojuan, Huanhuan Ni, Xiaoxiao Li, Xiaolan Qi, Xi Yang, and Hongyan Zou. 2023. "Plasmonic Cu2−xSe Mediated Colorimetric/Photothermal Dual-Readout Detection of Glutathione" Nanomaterials 13, no. 11: 1787. https://doi.org/10.3390/nano13111787
APA StyleYan, G., Ni, H., Li, X., Qi, X., Yang, X., & Zou, H. (2023). Plasmonic Cu2−xSe Mediated Colorimetric/Photothermal Dual-Readout Detection of Glutathione. Nanomaterials, 13(11), 1787. https://doi.org/10.3390/nano13111787






