Comprehension of the Route for the Synthesis of Co/Fe LDHs via the Method of Coprecipitation with Varying pH
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Coprecipitation Methodology, Solids Purification and Aging
2.3. Physical and Chemical Characterization of the Solids
2.4. Geochemical Modeling
3. Results and Discussion
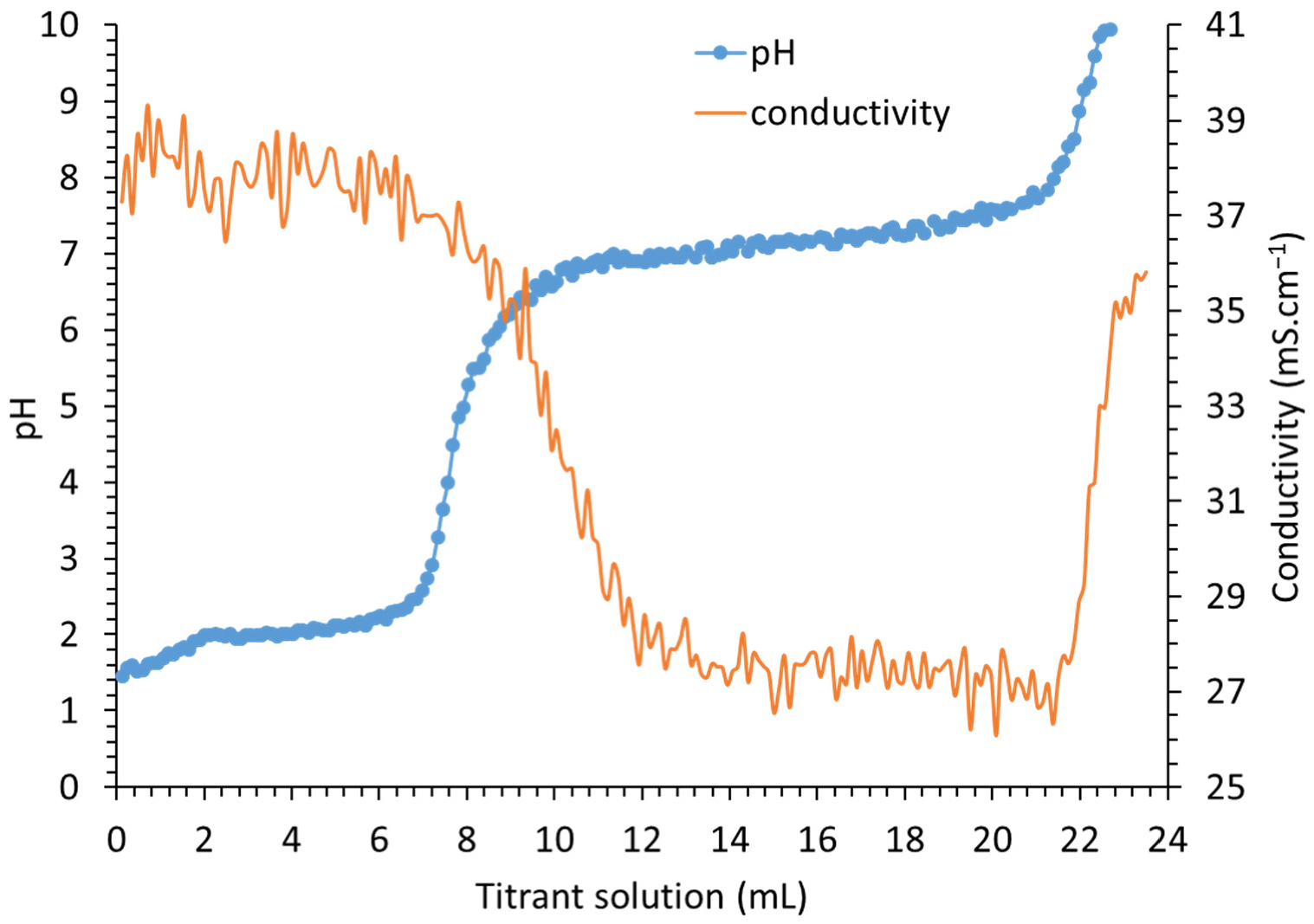
3.1. Titration
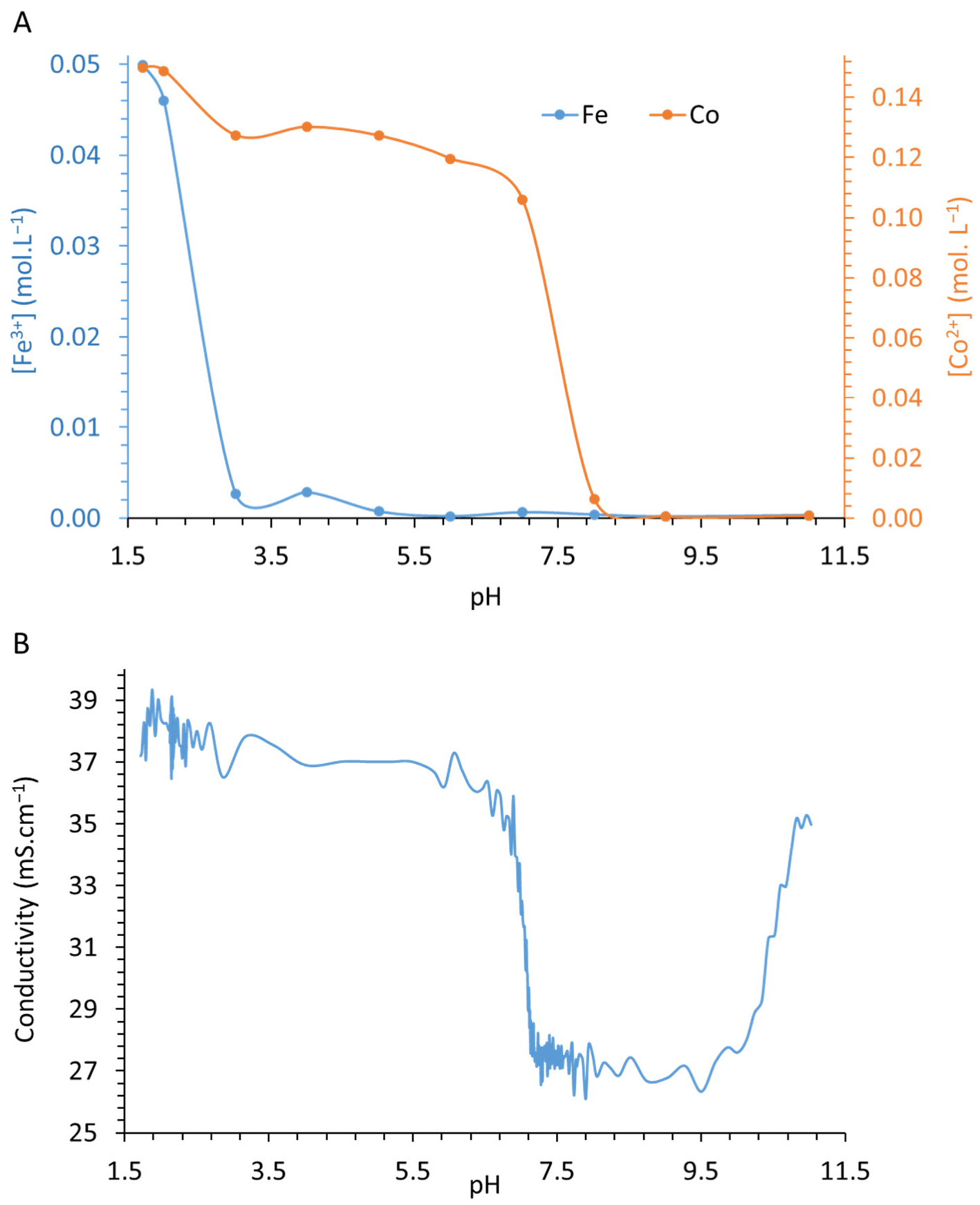
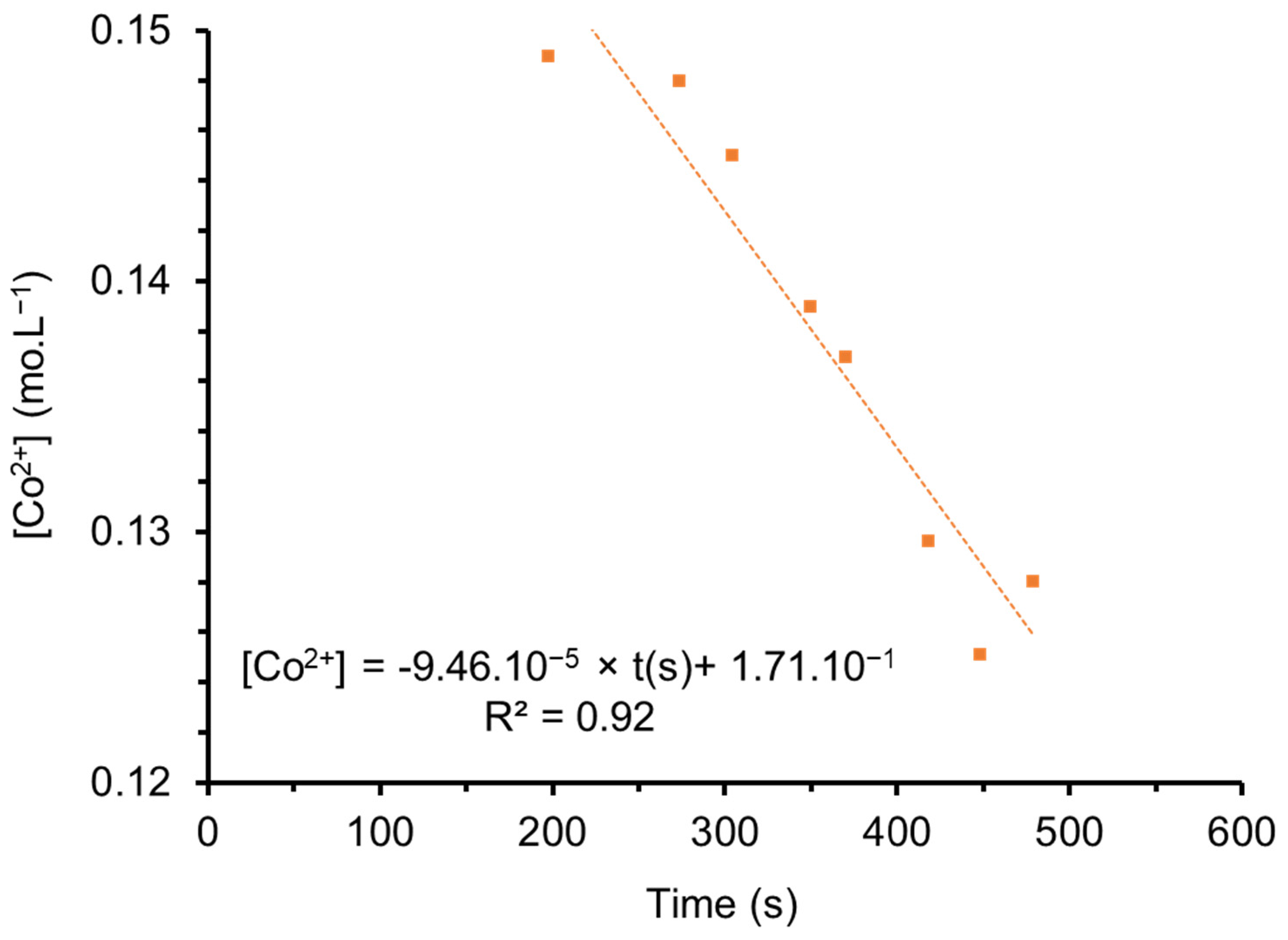
3.2. Atomic Absorption Spectroscopy Analyses of Co2+ and Fe3+ in the Supernatant
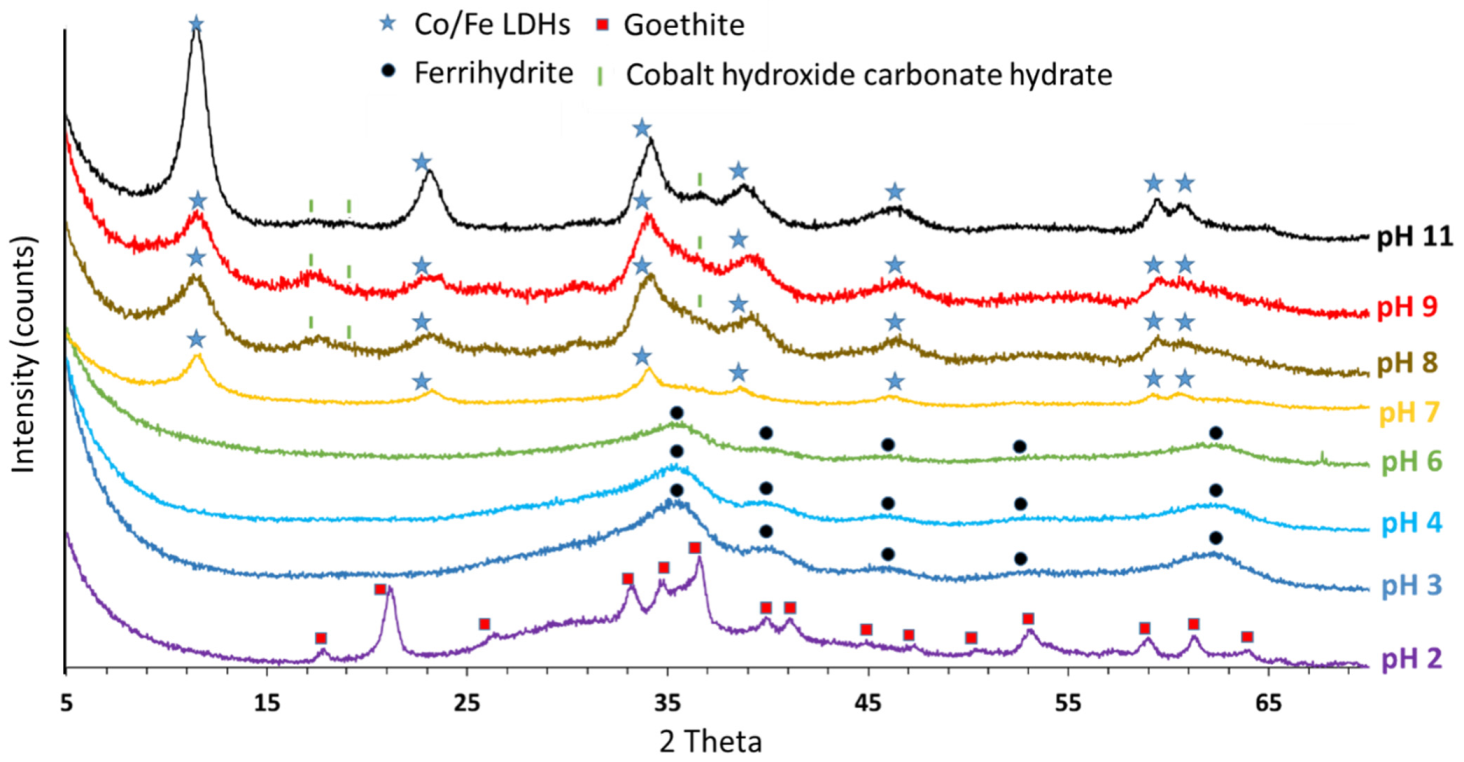
3.3. PXRD Characterization of Solids Synthesized by Acid—Base Titration
3.4. LDH Characterization after Aging at Room Temperature
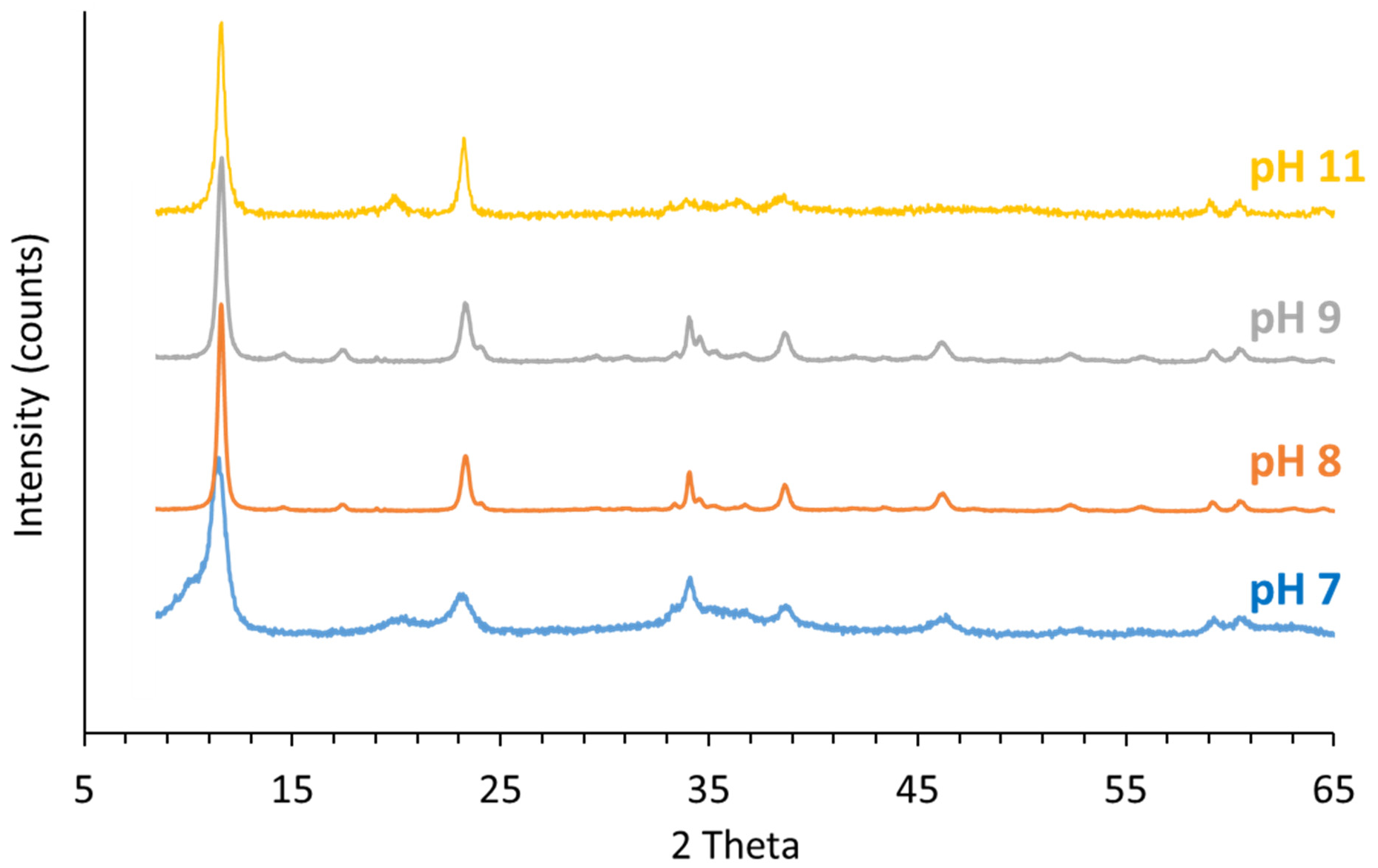
3.4.1. PXRD of the Solids Collected after Aging at Room Temperature
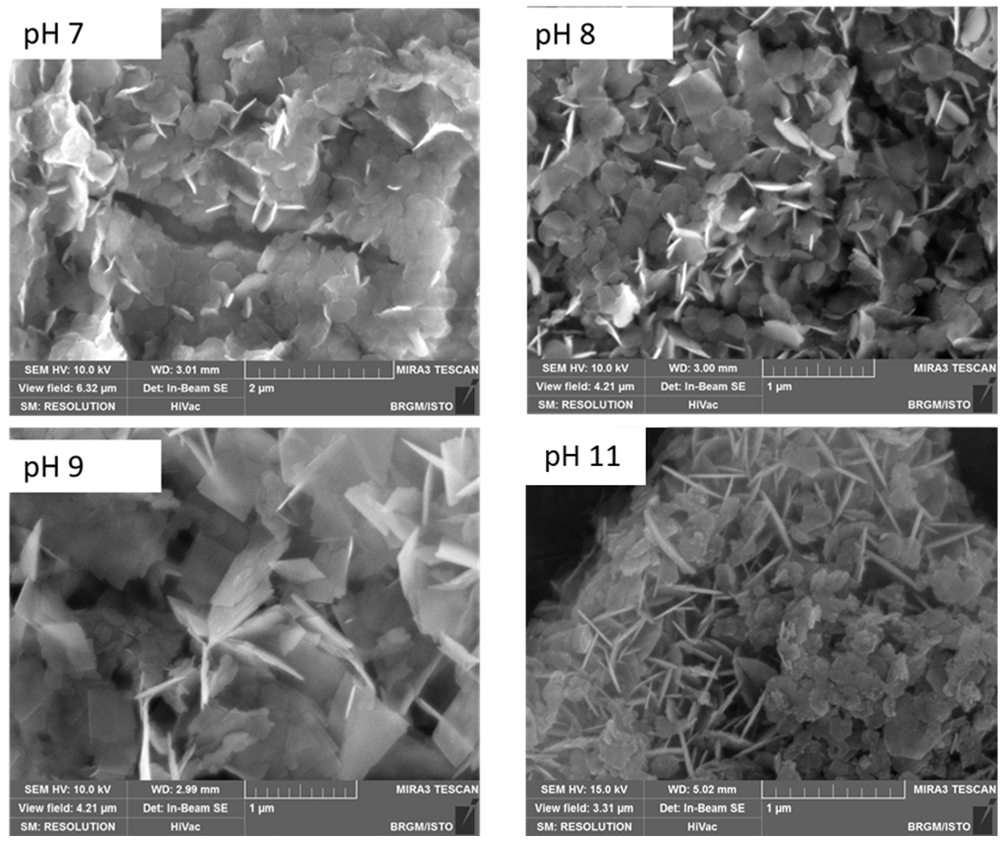
3.4.2. Scanning Electron Microscopy (SEM) of Co/Fe LDHs Synthesized at pH 7, 8, 9 and 11
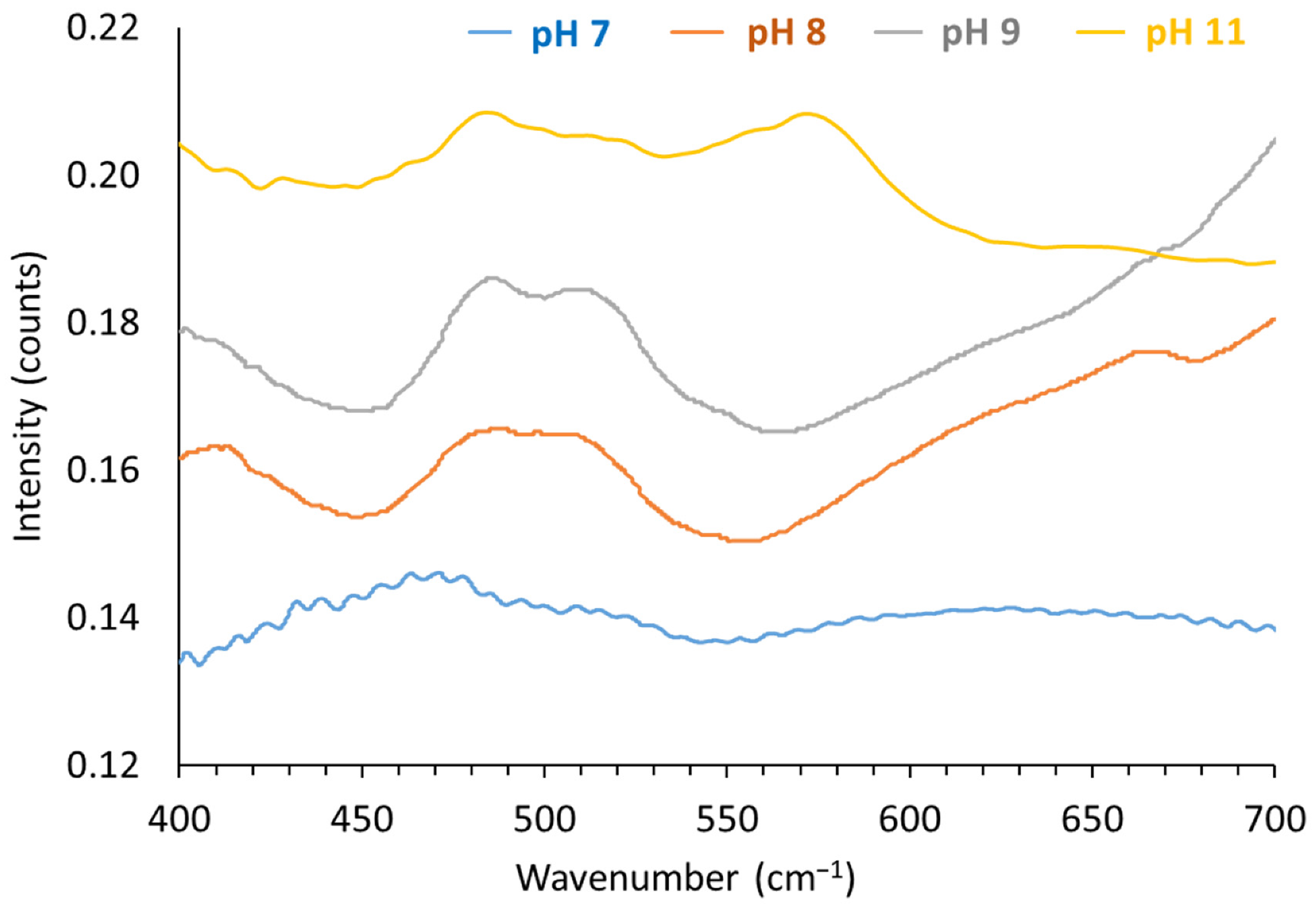
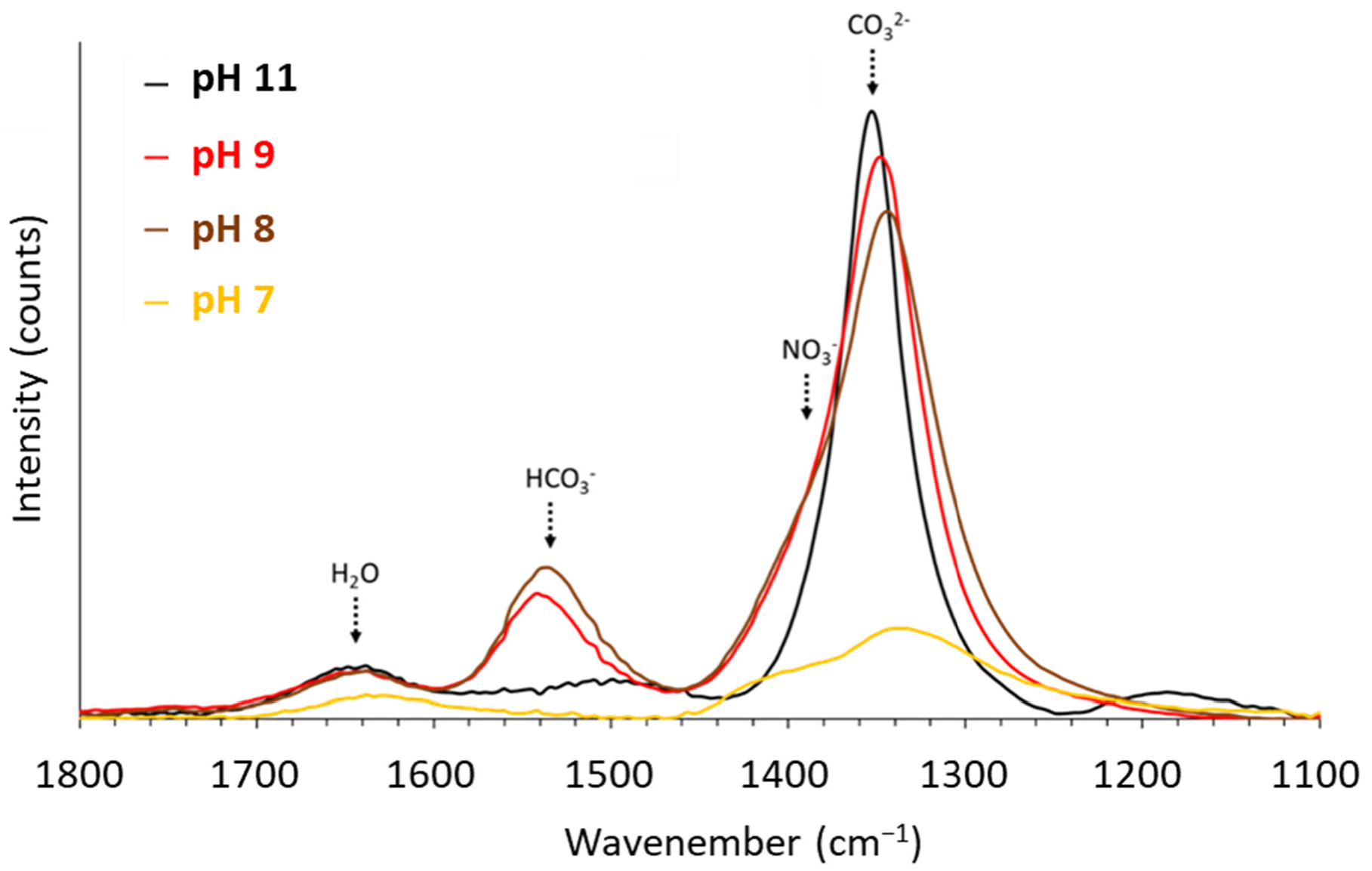
3.4.3. FTIR Spectra of the LDHs Synthesized at pH 7, 8, 9 and 11
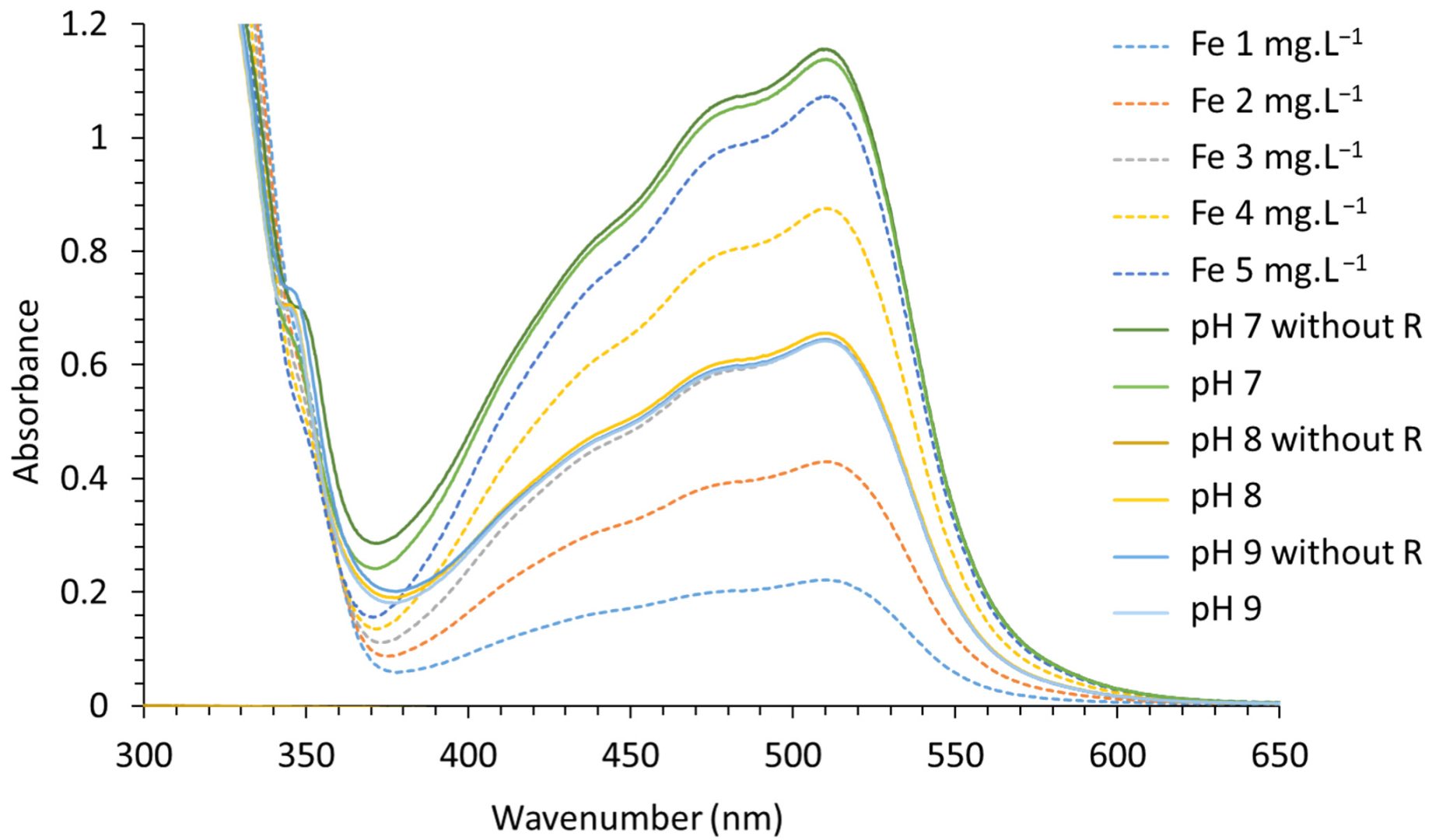
3.4.4. UV–Visible Absorbance of Various Contents of Fe and Synthesized LDHs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rives, V. Layered Double Hydroxides: Present and Future; Nova Science Publishers: New York, NY, USA, 2001. [Google Scholar]
- Othman, M.R.; Helwani, Z.; Martunus; Fernando, W.J.N. Synthetic hydrotalcites from different routes and their application as catalysts and gas adsorbents: A Review. Appl. Organomet. Chem. 2009, 23, 335–346. [Google Scholar] [CrossRef]
- Nalawade, P.; Aware, B.; Kadam, V.J.; Hirlekar, R.S. Layered Double Hydroxides: A Review. J. Sci. Ind. Res. (India) 2009, 68, 267–272. [Google Scholar]
- Duquesne, E.; Betelu, S.; Seron, A.; Ignatiadis, I.; Perrot, H.; Debiemme-Chouvy, C. Tuning redox state and ionic transfers of Mg/Fe-layered double hydroxide nanosheets by electrochemical and electrogravimetric methods. Nanomaterials 2020, 10, 1832. [Google Scholar] [CrossRef] [PubMed]
- Bukhtiyarova, M.V. A review on effect of synthesis conditions on the formation of Layered Double Hydroxides. J. Solid State Chem. 2019, 269, 494–506. [Google Scholar] [CrossRef]
- Lopez, T.; Bosch, P.; Ramos, E.; Gomez, R.; Novaro, O.; Acosta, D.; Figueras, F. Synthesis and characterization of sol-gel hydrotalcites. Structure and Texture. Langmuir 1996, 12, 189–192. [Google Scholar] [CrossRef]
- Seron, A.; Delorme, F. Synthesis of Layered Double Hydroxides (LDHs) with varying pH: A valuable contribution to the study of Mg/Al LDH formation mechanism. J. Phys. Chem. Solids 2008, 69, 1088–1090. [Google Scholar] [CrossRef]
- Tonelli, D.; Gualandi, I.; Musella, E.; Scavetta, E. Synthesis and characterization of Layered Double Hydroxides as materials for electrocatalytic applications. Nanomater 2021, 11, 725. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, W.; Jiang, M.; Chen, C.; Li, Y. Microwave-Assisted Synthesis of Layer-by-Layer Ultra-Large and Thin NiAl-LDH/RGO Nanocomposites and Their Excellent Performance as Electrodes. Sci. China Mater. 2015, 58, 944–952. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Q.; Bai, C.; Wang, F.; Sun, S.; Xu, Y.; Li, H. Synthesis of CNTS/CONIFE-LDH Nanocomposite with High specific surface area for asymmetric supercapacitor. Nanomaterials 2021, 11, 2155. [Google Scholar] [CrossRef]
- Qiao, C.; Zhang, Y.; Zhu, Y.; Cao, C.; Bao, X.; Xu, J. One-Step Synthesis of Zinc-Cobalt Layered Double Hydroxide (Zn-Co-LDH) Nanosheets for High-Efficiency Oxygen Evolution Reaction. J. Mater. Chem. A 2015, 3, 6878–6883. [Google Scholar] [CrossRef]
- Di Bitetto, A.; Kervern, G.; Andre, E.; Durand, P.; Carteret, C. Carbonate−hydrogenocarbonate coexistence and dynamics in Layered Double Hydroxides. J. Phys. Chem. C 2017, 121, 6104–6112. [Google Scholar] [CrossRef]
- Aisawa, S.; Takahashi, S.; Ogasawara, W.; Umetsu, Y.; Narita, E. Direct intercalation of amino acids into Layered Double Hydroxides by coprecipitation. J. Solid State Chem. 2001, 162, 52–62. [Google Scholar] [CrossRef]
- Theiss, F.L.; Ayoko, G.A.; Frost, R.L. Synthesis of Layered Double Hydroxides Containing Mg2+, Zn2+, Ca2+ and Al3+ Layer Cations by Co-Precipitation Methods—A Review. Appl. Surf. Sci. 2016, 383, 200–213. [Google Scholar] [CrossRef]
- Tavares, S.R.; Haddad, J.F.S.; Ivo, R.; Moraes, P.; Leitão, A.A. Computational Exploration of the anion exchange on the basal surface of Layered Double Hydroxides by Molecular Dynamics. Appl. Surf. Sci. 2020, 513, 145743. [Google Scholar] [CrossRef]
- Das, N.N.; Konar, J.; Mohanta, M.K.; Srivastava, S.C. Adsorption of Cr(VI) and Se(IV) from Their aqueous solutions onto Zr4+-substituted ZnAl/MgAl-Layered Double Hydroxides: Effect of Zr4+ Substitution in the Layer. J. Colloid Interface Sci. 2004, 270, 1–8. [Google Scholar] [CrossRef]
- Agnel, M.I.; Grangeon, S.; Fauth, F.; Elkaïm, E.; Claret, F.; Roulet, M.; Warmont, F.; Tournassat, C. Mechanistic and thermodynamic insights into anion exchange by green rust. Environ. Sci. Technol. 2020, 54, 851–861. [Google Scholar] [CrossRef] [Green Version]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-Type Anionic Clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Santamaría, L.; Korili, S.A.; Gil, A. Layered Double Hydroxides from Slags: Closing the Loop. Journal of Environmental Chemical Engineering. J. Environ. Chem. Eng. 2022, 10, 106948. [Google Scholar] [CrossRef]
- Karim, A.V.; Hassani, A.; Eghbali, P.; Nidheesh, P.V. Nanostructured Modified Layered Double Hydroxides (LDHs)-Based Catalysts: A Review on synthesis, characterization, and applications in water remediation by advanced oxidation processes. Curr. Opin. Solid State Mater. Sci. 2022, 26, 100965. [Google Scholar] [CrossRef]
- Ye, H.; Liu, S.; Yu, D.; Zhou, X.; Qin, L.; Lai, C.; Qin, F.; Zhang, M.; Chen, W.; Chen, W.; et al. Regeneration Mechanism, modification strategy, and environment application of layered double hydroxides: Insights based on memory effect. Coord. Chem. Rev. 2022, 450, 214253. [Google Scholar] [CrossRef]
- Mallakpour, S.; Hatami, M.; Hussain, C.M. Recent Innovations in Functionalized Layered Double Hydroxides: Fabrication, characterization, and industrial applications. Adv. Colloid Interface Sci. 2020, 283, 102216. [Google Scholar] [CrossRef]
- Mendil, R.; Nasrallah, N. Zn-Fe Layered Double Hydroxides Synthesized by Three (03) Methods of Coprecipitation: Application to the removal of cochineal red dye from aqueous solution. Fibers Polym. 2021, 22, 3358–3367. [Google Scholar] [CrossRef]
- Dewangan, N.; Hui, W.M.; Jayaprakash, S.; Bawah, A.R.; Poerjoto, A.J.; Jie, T.; Jangam, A.; Hidajat, K.; Kawi, S. Recent progress on layered double hydroxide (LDH) derived metal-based catalysts for CO2 conversion to valuable chemicals. Catal. Today 2020, 356, 490–513. [Google Scholar] [CrossRef]
- Mochane, M.J.; Magagula, S.I.; Sefadi, J.S.; Sadiku, E.R.; Mokhena, T.C. Morphology, Thermal Stability, and Flammability Properties of Polymer-Layered Double Hydroxide (LDH) Nanocomposites: A Review. Crystals 2020, 10, 612. [Google Scholar] [CrossRef]
- Gu, Z.; Atherton, J.J.; Xu, Z.P. Hierarchical Layered Double Hydroxide Nanocomposites: Structure, synthesis and applications. Chem. Commun. 2015, 51, 3024–3036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, A.; Bharadiya, P.; Hansora, D. Layered Double Hydroxide Based bionanocomposites. Appl. Clay Sci. 2019, 177, 19–36. [Google Scholar] [CrossRef]
- Wang, Q.; Tay, H.H.; Guo, Z.; Chen, L.; Liu, Y.; Chang, J.; Zhong, Z.; Luo, J.; Borgna, A. Morphology and Composition Controllable Synthesis of Mg–Al–CO3 Hydrotalcites by Tuning the Synthesis pH and the CO2 Capture Capacity. Appl. Clay Sci. 2012, 55, 18–26. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S.; Epping, K.; Velty, A. Increasing the basicity and catalytic activity of hydrotalcites by different synthesis procedures. J. Catal. 2004, 225, 316–326. [Google Scholar] [CrossRef]
- Boclair, J.W.; Braterman, P.S.; Jiang, J.; Lou, S.; Yarberry, F. Layered Double Hydroxide Stability. 2. Formation of Cr(III)-Containing Layered Double Hydroxides directly from solution. Chem. Mater. 1999, 11, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Boclair, J.W.; Braterman, P.S. One-Step Formation and Characterization of Zn(II)-Cr(III) Layered Double Hydroxides, Zn2Cr(OH)6X (X = Cl, 1/2SO4). Chem. Mater. 1998, 10, 2050–2052. [Google Scholar] [CrossRef] [PubMed]
- Radha, A.V.; Kamath, P.V. Aging of Trivalent Metal Hydroxide/Oxide Gels in Divalent Metal Salt Solutions: Mechanism of formation of Layered Double Hydroxides (LDHs). Bull. Mater. Sci. 2003, 26, 661–666. [Google Scholar] [CrossRef]
- Grégoire, B.; Ruby, C.; Carteret, C. Hydrolysis of Mixed Ni2+–Fe3+ and Mg2+–Fe3+ Solutions and mechanism of formation of Layered Double Hydroxides. Dalt. Trans. 2013, 42, 15687. [Google Scholar] [CrossRef]
- Jolivet, J.-P.; Henry, M.; Livage, J. De La Solution à l’oxyde: Condensation Des Cations En Solution Aqueuse, Chimie de Surface Des Oxydes; InterEditions: Paris, France, 1994. [Google Scholar]
- Zhao, J.; Chen, J.; Xu, S.; Shao, M.; Yan, D.; Wei, M.; Evans, D.G.; Duan, X. CoMn-Layered Double Hydroxide Nanowalls supported on carbon fibers for high-performance flexible energy storage devices. J. Mater. Chem. A 2013, 1, 8836–8843. [Google Scholar] [CrossRef]
- Aristov, N.; Habekost, A. Cyclic Voltammetry—A Versatile electrochemical method investigating electron transfer processes. World J. Chem. Educ. 2015, 3, 115–119. [Google Scholar] [CrossRef]
- Guoxiang, P.; Xinhui, X.; Jingshan, L.; Feng, C.; Zhihong, Y.; Hongjin, F. Preparation of CoAl Layered Double Hydroxide Nanoflake Arrays and Their High Supercapacitance Performance. Appl. Clay Sci. 2014, 102, 28–32. [Google Scholar] [CrossRef]
- Loh, P.Y.; Lee, K.K.; Ng, Y.; Sow, C.H.; Chin, W.S. Co/Al Layered Double Hydroxides Nanostructures: A binderless electrode for electrochemical capacitor. Electrochem. Commun. 2014, 43, 9–12. [Google Scholar] [CrossRef]
- Ma, K.Y.; Cheng, J.P.; Liu, F.; Zhang, X. Co-Fe Layered Double Hydroxides Nanosheets vertically grown on carbon fiber cloth for electrochemical capacitors. J. Alloys Compd. 2016, 679, 277–284. [Google Scholar] [CrossRef]
- Li, H.; Gao, Y.; Wang, C.; Yang, G. A Simple electrochemical route to access amorphous mixed-metal hydroxides for supercapacitor electrode materials. Adv. Energy Mater. 2015, 5, 1401767. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Zhang, D.; Liu, T.; Zhang, N.; Chen, L.; Liu, X.; Ma, R.; Qiu, G. Layered Co–Mn Hydroxide nanoflakes grown on carbon cloth as binder-free flexible electrodes for supercapacitors. J. Mater. Sci. 2016, 51, 3784–3792. [Google Scholar] [CrossRef]
- Chen, D.; Chen, H.; Chang, X.; Liu, P.; Zhao, Z.; Zhou, J.; Xu, G.; Lin, H.; Han, S. Hierarchical CoMn-Layered Double Hydroxide Nanowires on nickel foam as electrode material for high-capacitance supercapacitor. J. Alloys Compd. 2017, 729, 866–873. [Google Scholar] [CrossRef]
- Patil, D.S.; Pawar, S.A.; Lee, S.H.; Shin, J.C. CoFe Layered Double Hydroxide for enhanced electrochemical performance. J. Electroanal. Chem. 2020, 862, 114012. [Google Scholar] [CrossRef]
- Gupta, V.; Gupta, S.; Miura, N. Electrochemically Synthesized Large Area Network of CoxNiyAlz Layered Triple Hydroxides Nanosheets: A High Performance supercapacitor. J. Power Sources 2009, 189, 1292–1295. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, J.; Yuan, C.; Hao, L.; Shen, L.; Zhang, L.; Zhang, X. Glycine-Assisted Hydrothermal Synthesis of Nanostructured CoxNi1-x-Al Layered Triple Hydroxides as Electrode Materials for High-Performance Supercapacitors. J. Solid State Electrochem. 2012, 16, 1933–1940. [Google Scholar] [CrossRef]
- Hu, M.; Ji, X.; Lei, L.; Lu, X. The effect of cobalt on the electrochemical performances of Ni-Al Layered Double Hydroxides used in Ni-M(H) battery. J. Alloys Compd. 2013, 578, 17–25. [Google Scholar] [CrossRef]
- Duquesne, E.; Betelu, S.; Bazin, C.; Seron, A.; Ignatiadis, I.; Perrot, H.; Sel, O.; Debiemme-Chouvy, C. Insights into redox reactions and ionic transfers in Nickel/Iron Layered Double Hydroxide in Potassium Hydroxide. J. Phys. Chem. B 2020, 124, 3037–3049. [Google Scholar] [CrossRef]
- Trotochaud, L.; Young, S.L.; Ranney, J.K.; Boettcher, S.W. Nickel-Iron oxyhydroxide oxygen-evolution electrocatalysts: The role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, L.; Su, Y.; Shao, D.; Zeng, S.; Wang, W. Photoreduction of carbon dioxide of atmospheric concentration to methane with water over CoAl-Layered Double Hydroxide Nanosheets. J. Mater. Chem. A 2018, 6, 8366–8373. [Google Scholar] [CrossRef]
- Wulfsberg, G. Principles of Descriptive Inorganic Chemistry; University Science Books: South Orange, NJ, USA, 1991. [Google Scholar]
- Su, L.H.; Zhang, X.G.; Mi, C.H.; Liu, Y. Insights into the electrochemistry of Layered Double Hydroxide Containing Cobalt and Aluminum elements in Lithium Hydroxide aqueous solution. J. Power Sources 2008, 179, 388–394. [Google Scholar] [CrossRef]
- Scavetta, E.; Ballarin, B.; Berrettoni, M.; Carpani, I.; Giorgetti, M.; Tonelli, D. Electrochemical sensors based on electrodes modified with synthetic hydrotalcites. Electrochim. Acta 2006, 51, 2129–2134. [Google Scholar] [CrossRef]
- Ballarin, B.; Seeber, R.; Tonelli, D.; Vaccari, A. Electrocatalytic properties of nickel(II) hydrotalcite-type anionic clay: Application to methanol and ethanol Oxidation. J. Electroanal. Chem. 1999, 463, 123–127. [Google Scholar] [CrossRef]
- Panda, H.S.; Srivastava, R.; Bahadur, D. Synthesis and in situ mechanism of nuclei growth of Layered Double Hydroxides. Bull. Mater. Sci. 2011, 34, 1599–1604. [Google Scholar] [CrossRef]
- Yu, J.Y.; Park, M.; Kim, J. Solubilities of Synthetic schwertmannite and ferrihydrite. Geochem. J. 2002, 36, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Schwertmann, U. Solubility and dissolution of iron oxides. Plant Soil 1991, 130, 1–25. [Google Scholar] [CrossRef]
- González, G.; Sagarzazu, A.; Villalba, R. Study of the Mechano-chemical transformation of goethite to hematite by TEM and XRD. Mater. Res. Bull. 2000, 35, 2295–2308. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Das, S.; Hendry, M.J.; Essilfie-Dughan, J. Transformation of two-line ferrihydrite to goethite and hematite as a function of pH and temperature. Environ. Sci. Technol. 2011, 45, 268–275. [Google Scholar] [CrossRef]
- Cudennec, Y.; Lecerf, A. The Transformation of ferrihydrite into goethite or hematite, revisited. J. Solid State Chem. 2006, 179, 716–722. [Google Scholar] [CrossRef] [Green Version]
- Maillot, F.; Morin, G.; Wang, Y.; Bonnin, D.; Ildefonse, P.; Chaneac, C.; Calas, G. New Insight into the structure of nanocrystalline ferrihydrite: Exafs evidence for tetrahedrally coordinated Iron(III). Geochim. Cosmochim. Acta 2011, 75, 2708–2720. [Google Scholar] [CrossRef]
- Janney, D.E.; Cowley, J.M.; Buseck, P.R. Structure of synthetic 6-Line ferrihydrite by electron nanodiffraction. Am. Mineral. 2001, 86, 327–335. [Google Scholar] [CrossRef]
- Michel, F.M.; Ehm, L.; Liu, G.; Han, W.Q.; Antao, S.M.; Chupas, P.J.; Lee, P.L.; Knorr, K.; Eulert, H.; Kim, J.; et al. Similarities in 2- and 6-line ferrihydrite based on pair distribution function analysis of x-ray total scattering. Chem. Mater. 2007, 19, 1489–1496. [Google Scholar] [CrossRef]
- Jambor, J.L.; Dutrizac, J.E. Occurrence and Constitution of natural and synthetic ferrihydrite, a widespread iron oxyhydroxide. Chem. Rev. 1998, 98, 2549–2585. [Google Scholar] [CrossRef]
- Drits, V.A.; Sakharov, B.A.; Salyn, A.L.; Manceau, A. Structural model for ferrihydrite. Clay Miner. 1993, 28, 185–207. [Google Scholar] [CrossRef] [Green Version]
- Su, L.W. Cobalt Adsorption and/or Co-Precipitation onto Ferric Oxyhydroxide. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2011. [Google Scholar] [CrossRef]
- Fei, Y.; Hua, J.; Liu, C.; Li, F.; Zhu, Z.; Xiao, T.; Chen, M.; Gao, T.; Wei, Z.; Hao, L. Aqueous Fe(II)-Induced Phase transformation of ferrihydrite coupled adsorption/immobilization of rare earth elements. Minerals 2018, 8, 357. [Google Scholar] [CrossRef] [Green Version]
- Adra, A. Structure et Réactivité des Nano-Oxyhydroxydes de fer et D’aluminium en aval D’un drainage Minier Acide. Ph.D. Thesis, Université Paris, Paris, France, 2014. [Google Scholar]
- Liu, C.; Zhu, Z.; Li, F.; Liu, T.; Liao, C.; Lee, J.J.; Shih, K.; Tao, L.; Wu, Y. Fe(II)-Induced Phase transformation of ferrihydrite: The Inhibition effects and stabilization of divalent metal cations. Chem. Geol. 2016, 444, 110–119. [Google Scholar] [CrossRef]
- Riley, E.; Dutrizac, J.E. The Behaviour of the rare earth elements during the precipitation of ferrihydrite from sulphate media. Hydrometallurgy 2017, 172, 69–78. [Google Scholar] [CrossRef]
- Masue-Slowey, Y.; Loeppert, R.H.; Fendorf, S. Alteration of ferrihydrite reductive dissolution and transformation by adsorbed As and structural Al: Implications for As retention. Geochim. Cosmochim. Acta 2011, 75, 870–886. [Google Scholar] [CrossRef]
- Massey, M.S.; Lezama-Pacheco, J.S.; Michel, F.M.; Fendorf, S. Uranium incorporation into Aluminum-substituted ferrihydrite during Iron(II)-induced transformation. Environ. Sci. Process. Impacts 2014, 16, 2137–2144. [Google Scholar] [CrossRef]
- Malherbe, F.; Bigey, L.; Forano, C.; De Roy, A.; Besse, J.P. Structural aspects and thermal properties of Takovite-like Layered Double Hydroxides pillared with chromium oxo-anions. J. Chem. Soc. Dalton Trans. 1999, 21, 3831–3839. [Google Scholar] [CrossRef]
- Evans, D.G.; Slade, R.C.T. Structural aspects of Layered Double Hydroxides. Struct. Bond. 2005, 119, 1–87. [Google Scholar] [CrossRef]
- Ma, K.; Cheng, J.P.P.; Zhang, J.; Li, M.; Liu, F.; Zhang, X. Dependence of Co/Fe ratios in Co-Fe Layered Double Hydroxides on the structure and capacitive properties. Electrochim. Acta 2016, 198, 231–240. [Google Scholar] [CrossRef]
- Tamaki, T.; Nakanishi, N.; Ohashi, H.; Yamaguchi, T. The Effect of Particle Size and surface area on the ion conductivity of Layered Double Hydroxide. Electrochem. Commun. 2012, 25, 50–53. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Hur, T.-B.; Phuoc, T.X.; Chyu, M.K. New approach to the synthesis of Layered Double Hydroxides and associated ultrathin nanosheets in de-ionized water by laser ablation. J. Appl. Phys. 2010, 108, 114312. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Exfoliation of Layered Double Hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, 4477. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, Y.; Lee, D.K.; Lee, J.W.; Kang, J.K. Efficient Co-Fe Layered Double hydroxide photocatalysts for water oxidation under visible light. J. Mater. Chem. A 2014, 2, 4136–4139. [Google Scholar] [CrossRef]
- Nejati, K.; Zabihi, R. Preparation and magnetic properties of nano size nickel ferrite particles using hydrothermal method. Chem. Cent. J. 2012, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Yuan, C.; Lu, X.; Zhang, L.; Che, Q.; Zhang, X. Facile growth of mesoporous co 3o 4 nanowire arrays on ni foam for high performance electrochemical capacitors. J. Power Sources 2012, 203, 250–256. [Google Scholar] [CrossRef]
- Iyi, N.; Fujii, K.; Okamoto, K.; Sasaki, T. Factors Influencing the Hydration of Layered Double Hydroxides (LDHs) and the Appearance of an Intermediate Second Staging Phase. Appl. Clay Sci. 2007, 35, 218–227. [Google Scholar] [CrossRef]
- Guan, H.; Shao, C.; Wen, S.; Chen, B.; Gong, J.; Yang, X. A Novel method for preparing Co3O4 nanofibers by using electrospun pva/cobalt acetate composite fibers as precursor. Mater. Chem. Phys. 2003, 82, 1002–1006. [Google Scholar] [CrossRef]
- Allen, G.C.; Paul, M. Chemical Characterization of Transition Metal Spinel-Type Oxides by Infrared Spectroscopy. Appl. Spectrosc. 1995, 49, 451–458. [Google Scholar] [CrossRef]
- Lin, M.C.; Chang, F.T.; Uan, J.Y. Synthesis of Li-Al-Carbonate Layered Double Hydroxide in a Metal Salt-Free System. J. Mater. Chem. 2010, 20, 6524–6530. [Google Scholar] [CrossRef]
- Miller, F.A.; Wilkins, C.H. Infrared Spectra and Characteristic Frequencies of Inorganic Ions. Anal. Chem. 1952, 24, 1253–1294. [Google Scholar] [CrossRef]
- Gregoire, B. Relation Composition Structure des Hydroxydes Doubles Lamellaires: Effets de la Charge du Feuillet et de la Nature de L’anion Interfoliaire. Ph.D. Thesis, Université de Lorraine, Nancy, France, 2012. [Google Scholar]
- Gregoire, B.; Andre, E.; Ruby, C.; Carteret, C. Tuning and Investigating the structure of MII-FeIII Layered Double Hydroxides (MII = NiII, CoII and MgII) in relation to their composition: From synthesis to anionic exchange properties. Curr. Inorg. Chem. 2015, 5, 169–183. [Google Scholar] [CrossRef]
- Etique, M.; Zegeye, A.; Grégoire, B.; Carteret, C.; Ruby, C. Nitrate Reduction by Mixed Iron(II-III) Hydroxycarbonate green rust in the presence of phosphate anions: The key parameters influencing the ammonium selectivity. Water Res. 2014, 62, 29–39. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Park, Y.; Chen, L.; Jung, Y.M. Quantitative Determination of Iron Ions Based on a Resonance Raman (RR) Probe-Phenanthroline. Anal. Sci. 2017, 33, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Majzlan, J.; Navrotsky, A.; Schwertmann, U. Thermodynamics of Iron Oxides: Part III. Enthalpies of formation and stability of Ferrihydrite (∼Fe(OH)3), Schwertmannite (∼FeO(OH)3/4(SO4)1/8), and ε-Fe2O3. Geochim. Cosmochim. Acta 2004, 68, 1049–1059. [Google Scholar] [CrossRef]
- Caron, M.H. Fundamental and practical factors in ammonia leaching of Nickel and Cobalt ores. JOM 1950, 2, 67–90. [Google Scholar] [CrossRef]
- Osseo-Asare, K.; Fuerstenau, D.W. Adsorption phenomena in hydrometallurgy, 1 The Uptake of copper, nickel and cobalt by oxide adsorbents in aqueous ammoniacal solutions. Int. J. Miner. Process. 1979, 6, 85–104. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Wu, Z.; Post, J.E.; Lanson, B.; Elzinga, E.J.; Liu, Y.; Li, H.; Hong, M.; Liu, F.; et al. Effects of Co Doping on the structure and physicochemical properties of hausmannite (Mn3O4) and its transformation during aging. Chem. Geol. 2021, 582, 120448. [Google Scholar] [CrossRef]
- Yin, H.; Sun, J.; Yan, X.; Yang, X.; Feng, X.; Tan, W.; Qiu, G.; Zhang, J.; Ginder-Vogel, M.; Liu, F. Effects of Co(II) ion exchange, Ni(II)- and V(V)-doping on the transformation behaviors of Cr(III) on hexagonal turbostratic birnessite-water interfaces. Environ. Pollut. 2020, 256, 113462. [Google Scholar] [CrossRef]
- Boland, D.D.; Collins, R.N.; Glover, C.J.; David Waite, T. An In Situ Quick-EXAFS and Redox Potential Study of the Fe(II)-Catalysed Transformation of Ferrihydrite. Colloids Surf. A Physicochem. Eng. Asp. 2013, 435, 2–8. [Google Scholar] [CrossRef]
- Lan, S.; Wang, X.; Xiang, Q.; Yin, H.; Tan, W.; Qiu, G.; Liu, F.; Zhang, J.; Feng, X. Mechanisms of Mn(II) Catalytic oxidation on ferrihydrite surfaces and the formation of manganese (oxyhydr)oxides. Geochim. Cosmochim. Acta 2017, 211, 79–96. [Google Scholar] [CrossRef]
- Alvarez, M.; Sileo, E.E.; Rueda, E.H. Structure and reactivity of synthetic Co-substituted goethites. Am. Mineral. 2008, 93, 584–590. [Google Scholar] [CrossRef]
- Yin, H.; Wu, Y.; Hou, J.; Yan, X.; Li, Z.; Zhu, C.; Zhang, J.; Feng, X.; Tan, W.; Liu, F. Preference of Co over Al for substitution of Fe in goethite (α-FeOOH) structure: Mechanism revealed from EXAFS, XPS, DFT and Linear free energy correlation model. Chem. Geol. 2020, 532, 119378. [Google Scholar] [CrossRef]
- Bordeneuve, H.; Tenailleau, C.; Guillemet-Fritsch, S.; Smith, R.; Suard, E.; Rousset, A. Structural variations and cation distributions in Mn3−xCoxO4 (0 ≤ x ≤ 3) dense ceramics using neutron diffraction data. Solid State Sci. 2010, 12, 379–386. [Google Scholar] [CrossRef] [Green Version]
| Characterization Data for Solid Obtained from pH 7 to 11 | ||||
|---|---|---|---|---|
| pH of synthesis | 7 | 8 | 9 | 11 |
| LDH species | 2 | 2 | 2 | 2 |
| d(003) (Å) | 7.64–7.68 | 7.64–7.64 | 7.63–7.63 | 7.63–7.69 |
| c parameter (Å) | 22.92–23.04 | 22.92–22.91 | 22.90–22.90 | 22.89–23.06 |
| a parameter (Å) | 3.12–3.09 | 3.11–3.10 | 3.08–3.11 | 3.11–3.09 |
| t crystallite size (nm) | 12.20–3.20 | 6.78–3.19 | 6.00–1.80 | 10.06–5.29 |
| LDH species relative content (%) | 14–86 | 22–78 | 16–83 | 62–38 |
| Solids Obtained from pH 7 to 11 | ||||
|---|---|---|---|---|
| pH of synthesis | 7 | 8 | 9 | 11 |
| LDH species | 3 | 2 | 2 | 3 |
| d(003) (Å) | 7.62–7.71–8.71 | 7.61–7.63 | 7.60–7.63 | 7.66–7.65–7.72 |
| c parameter (Å) | 22.86–23.13–26.14 | 22.84–22.86 | 22.63–22.90 | 22.97–22.95–23.15 |
| a parameter (Å) | 3.12–2.96–3.07 | 3.12–3.12 | 3.12–3.12 | 3.22–3.13–3.06 |
| t crystallite size (nm) | 16.28–8.14–4.88 | 52.89–23.12 | 43.36–19.41 | 51.57–16.45–4.94 |
| LDH species relative content (%) | 34–18–48 | 84–16 | 87–13 | 4–17–79 |
| pH | LDH Thickness (nm) | LDH Length (nm) |
|---|---|---|
| 7 | 31–36 | 170–290 |
| 8 | 11–44 | 180–400 |
| 9 | 27–47 | 475–510 |
| 11 | 23–42 | 100–300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morcos, C.; Seron, A.; Maubec, N.; Ignatiadis, I.; Betelu, S. Comprehension of the Route for the Synthesis of Co/Fe LDHs via the Method of Coprecipitation with Varying pH. Nanomaterials 2022, 12, 1570. https://doi.org/10.3390/nano12091570
Morcos C, Seron A, Maubec N, Ignatiadis I, Betelu S. Comprehension of the Route for the Synthesis of Co/Fe LDHs via the Method of Coprecipitation with Varying pH. Nanomaterials. 2022; 12(9):1570. https://doi.org/10.3390/nano12091570
Chicago/Turabian StyleMorcos, Chérif, Alain Seron, Nicolas Maubec, Ioannis Ignatiadis, and Stéphanie Betelu. 2022. "Comprehension of the Route for the Synthesis of Co/Fe LDHs via the Method of Coprecipitation with Varying pH" Nanomaterials 12, no. 9: 1570. https://doi.org/10.3390/nano12091570
APA StyleMorcos, C., Seron, A., Maubec, N., Ignatiadis, I., & Betelu, S. (2022). Comprehension of the Route for the Synthesis of Co/Fe LDHs via the Method of Coprecipitation with Varying pH. Nanomaterials, 12(9), 1570. https://doi.org/10.3390/nano12091570






