In Situ Construction of ZIF-67-Derived Hybrid Tricobalt Tetraoxide@Carbon for Supercapacitor
Abstract
:1. Introduction
2. Material Preparation and Test Methods
2.1. Materials
2.2. Fabrication of ZIF-67-Derived Co3O4@C Electrode
2.3. Assembly of the Co3O4@C//AC Flexible Supercapacitor
2.4. Material Characterizations
2.5. Electrochemical Performance
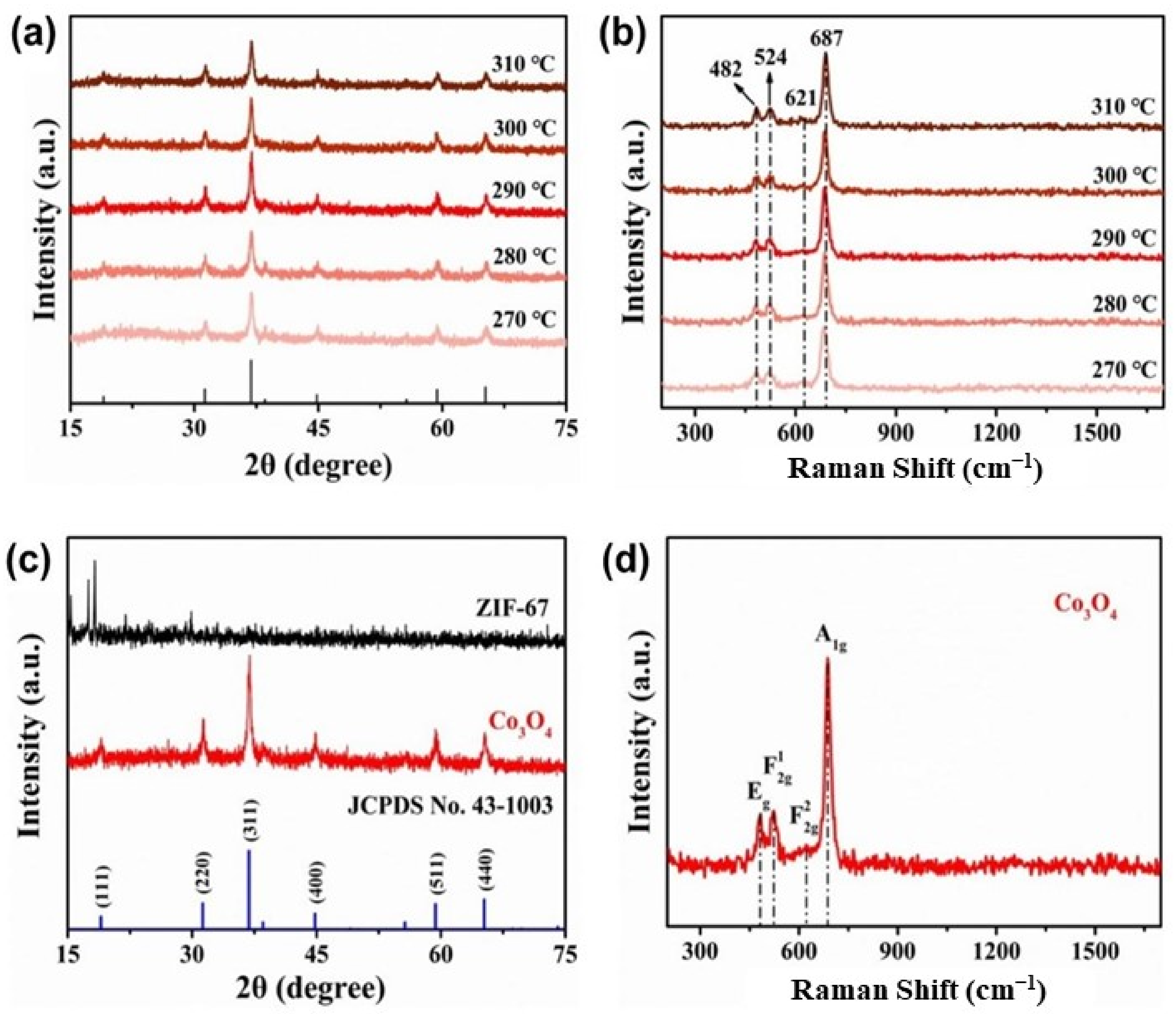
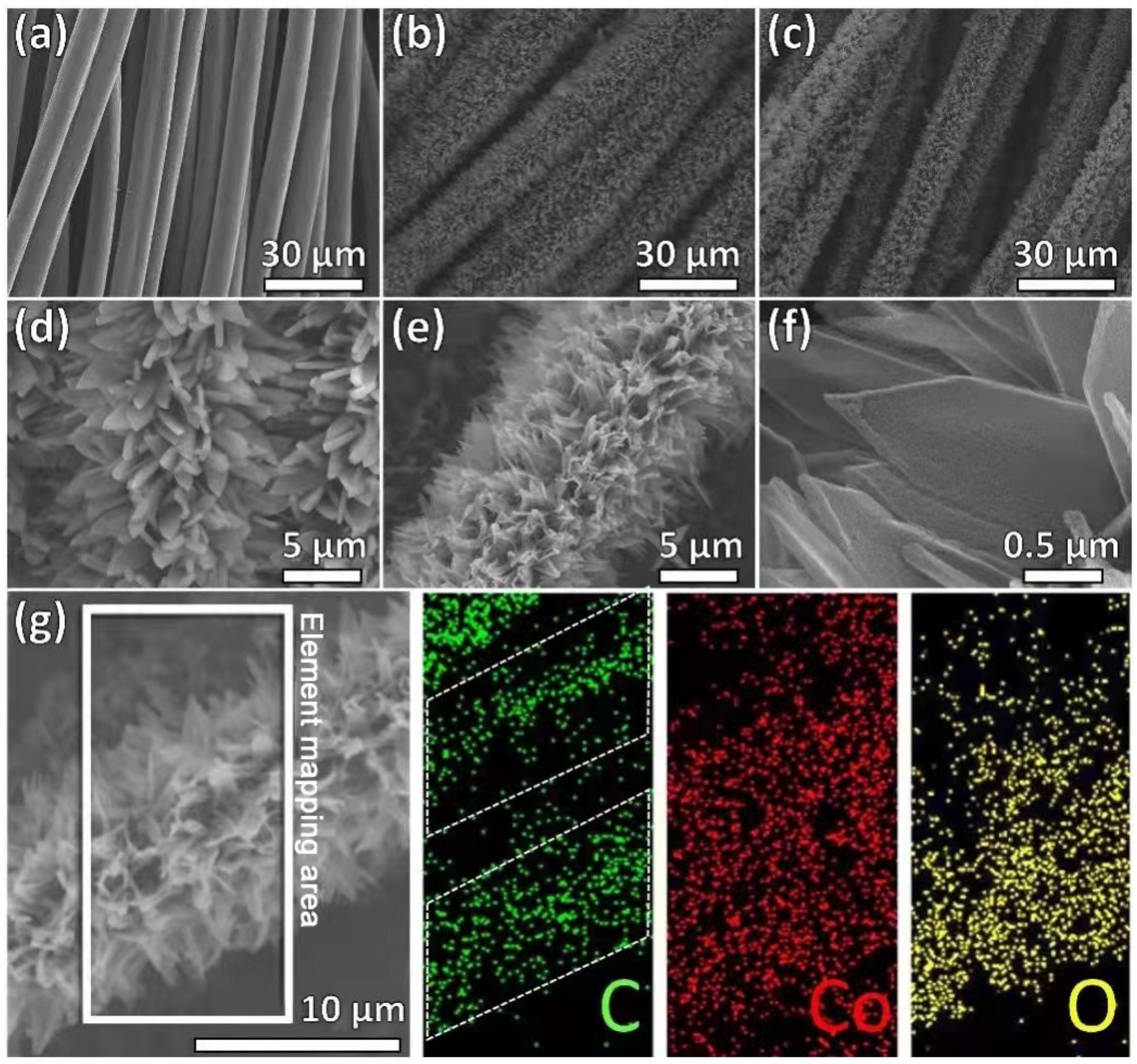
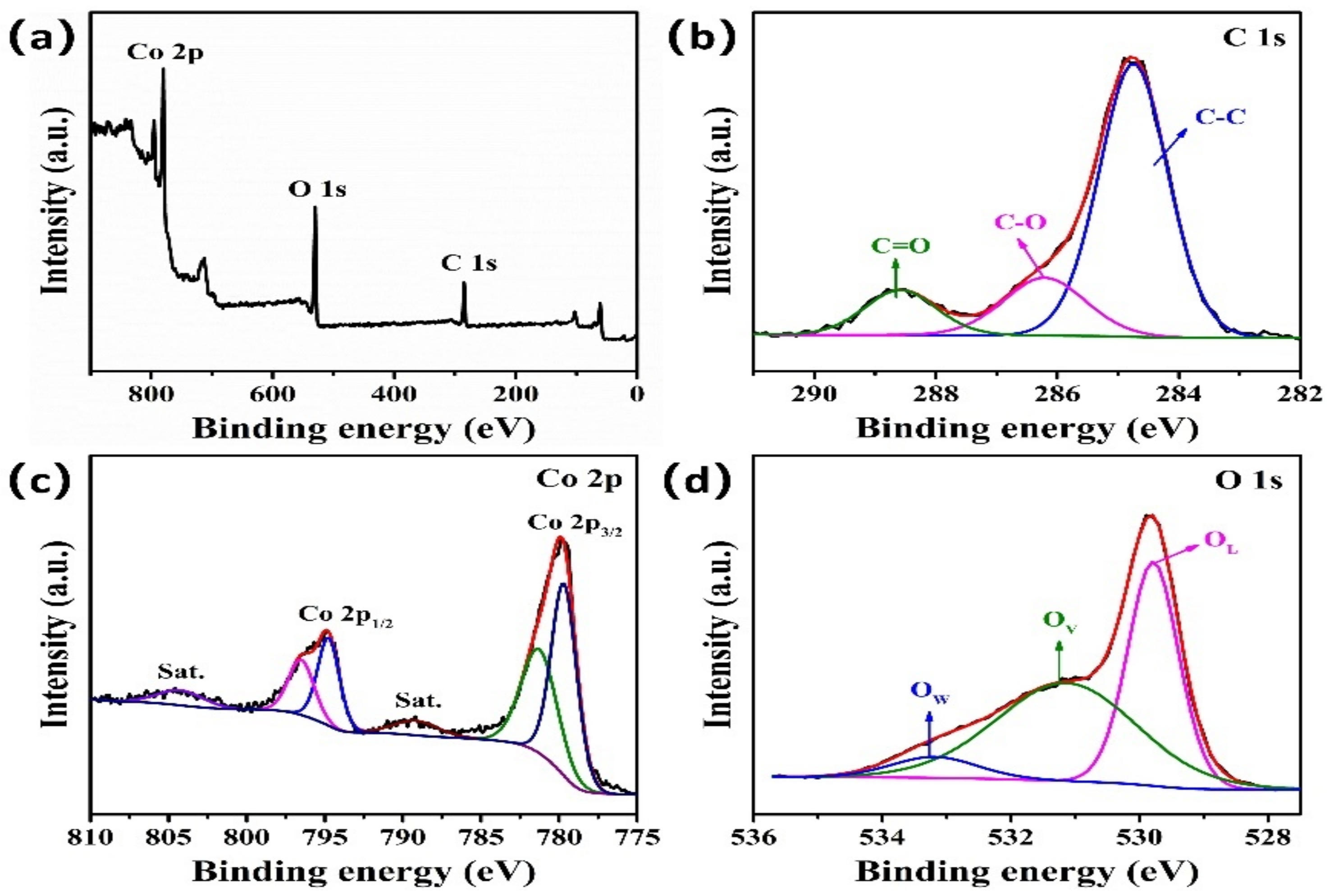
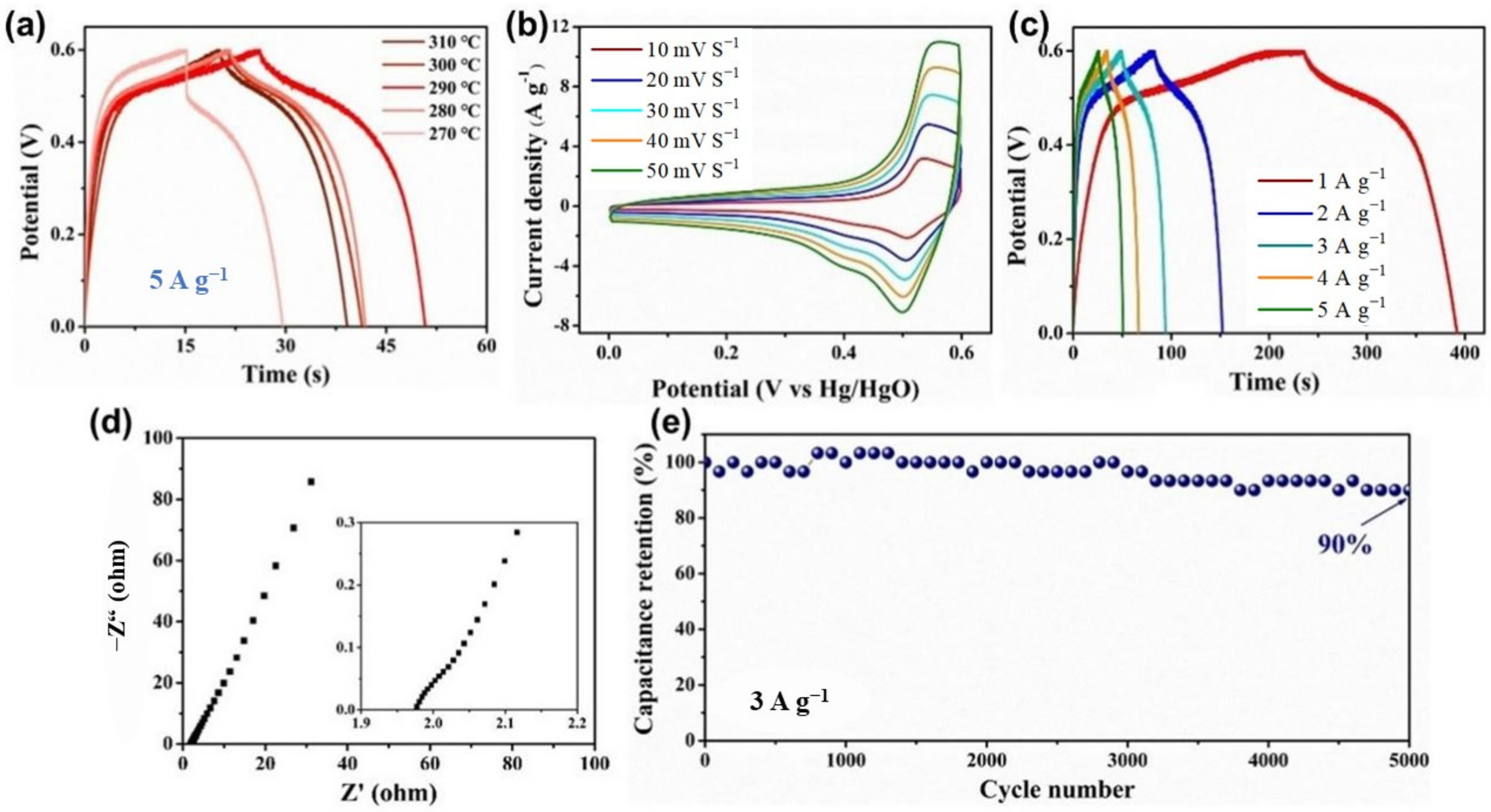
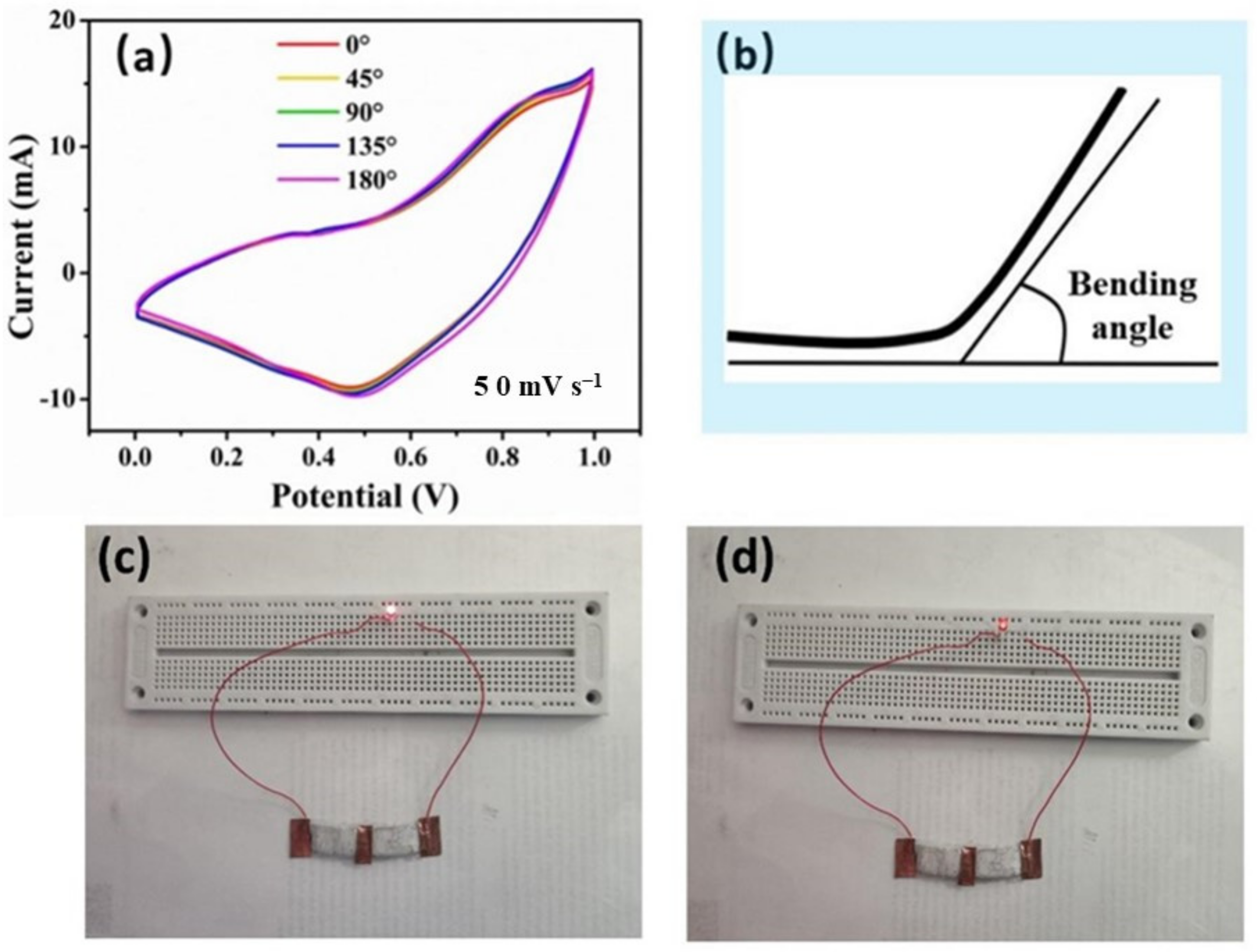
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Liu, J.; Zhao, D. Mesoporous materials for energy conversion and storage devices. Nat. Rev. Mater. 2016, 1, 16023. [Google Scholar] [CrossRef]
- Chhetri, K.; Dahal, B.; Tiwari, A.; Mukhiya, T.; Muthrasu, A.; Ojha, G.; Lee, M.; Kim, T.; Chae, S.; Kim, H. Controlled Selenium Infiltration of Cobalt Phosphide Nanostructure Arrays from a Two-Dimensional Cobalt Metal–Organic Framework: A Self-Supported Electrode for Flexible Quasi-Solid-State Asymmetric Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 404–415. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Chen, W.; Cha, D.; Alshareef, H.N. Substrate dependent self-organization of mesoporous cobalt oxide nanowires with remarkable pseudocapacitance. Nano Lett. 2012, 12, 2559–2567. [Google Scholar] [CrossRef] [PubMed]
- Minakshi, M.; Highly, S.; Baur, C.; Mitchell, D.; Jones, R.; Fichtner, M. Calcined chicken eggshell electrode for battery and supercapacitor applications. RSC Adv. 2019, 9, 26981–26995. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Xing, L.; Liu, Y.; Gao, Y.; Liu, J. A porous biomass-based sandwich-structured Co3O4@carbon fiber@Co3O4 composite for high-performance supercapacitors. Carbon 2018, 129, 819–825. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Mahajan, H.; Panda, A.; Lee, J.; Govindaraju, S.; Yun, K.; Yoon, M. Fe3O4 nano assembly embedded in 2D-crumpled porous carbon sheets for high energy density supercapacitor. Chem. Eng. J. 2021, 420, 127584. [Google Scholar] [CrossRef]
- Yu, L.; Wang, G.; Wan, G.; Wang, G.; Lin, S.; Li, X.; Wang, K.; Bai, Z.; Xiang, Y. Highly effective synthesis of NiO/CNT nanohybrids by atomic layer deposition for high-rate and long-life supercapacitors. Dalton Trans. 2016, 45, 13779–13786. [Google Scholar] [CrossRef]
- Shi, S.; Wan, G.; Wu, L.; He, Z.; Wang, K.; Tang, Y.; Xu, X.; Wang, G. Ultrathin manganese oxide nanosheets uniformly coating on carbon nanocoils as high-performance asymmetric supercapacitor electrodes. J. Colloid Interface Sci. 2019, 537, 142–150. [Google Scholar] [CrossRef]
- Cheng, G.; Kou, T.; Zhang, J.; Si, C.; Gao, H.; Zhang, Z. O22-/O- functionalized oxygen-deficient Co3O4 nanorods as high performance supercapacitor electrodes and electrocatalysts towards water splitting. Nano Energy 2017, 38, 155–166. [Google Scholar] [CrossRef]
- Chhetri, K.; Tiwari, A.; Dahal, B.; Ojha, G.; Mukhiya, T.; Lee, M.; Kim, T.; Chae, S.; Muthurasu, A.; Kim, H. A ZIF-8-derived nanoporous carbon nanocomposite wrapped with Co3O4-polyaniline as an efficient electrode material for an asymmetric supercapacitor. J. Electroanal. Chem. 2020, 856, 113670. [Google Scholar] [CrossRef]
- Bera, R.K.; Park, H.; Ryoo, R. Co3O4 nanosheets on zeolite-templated carbon as an efficient oxygen electrocatalyst for a zinc–air battery. J. Mater. Chem. A 2019, 7, 9988–9996. [Google Scholar] [CrossRef]
- Paul, R.; Du, F.; Dai, L.; Ding, Y.; Wang, Z.L.; Wei, F.; Roy, A. 3D heteroatom-doped carbon nanomaterials as multifunctional metal-free catalysts for integrated energy devices. Adv. Mater. 2019, 31, 1805598. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Jun, J.; Kim, Y.K.; Kim, J.; Lee, J.S.; Jang, J. Facile synthesis of Co3O4-incorporated multichannel carbon nanofibers for electrochemical applications. ACS Appl. Mater. Inter. 2020, 12, 20613–20622. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; You, W.; Yu, J. Core–shell nitrogen-doped carbon hollow spheres/Co3O4 nanosheets as advanced electrode for high-performance supercapacitor. Small 2018, 14, 1702407. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.M.; Ramadan, M.; Allam, N.K. Recent advances on zeolitic imidazolate-67 metal-organic framework-derived electrode materials for electrochemical supercapacitors. J. Energy Storage 2021, 34, 102195. [Google Scholar] [CrossRef]
- Hwang, J.; Ejsmont, A.; Freund, R.; Goscianska, J.; Schmidt, B.V.; Wuttke, S. Controlling the morphology of metal–organic frameworks and porous carbon materials: Metal oxides as primary architecture-directing agents. Chem. Soc. Rev. 2020, 49, 3348–3422. [Google Scholar] [CrossRef]
- Farid, S.; Qiu, W.; Zhao, J.; Song, X.; Mao, Q.; Ren, S.; Hao, C. Improved OER performance of Co3O4/N-CNTs derived from newly designed ZIF-67/PPy NTs composite. J. Electroanal. Chem. 2020, 858, 113768. [Google Scholar] [CrossRef]
- Shu, T.; Wang, H.; Li, Q.; Feng, Z.; Wei, F.; Yao, K.X.; Sun, Z.; Qi, J.; Sui, Y. Highly stable Co3O4 nanoparticles/carbon nanosheets array derived from flake-like ZIF-67 as an advanced electrode for supercapacacitor. Chem. Eng. J. 2021, 419, 129631. [Google Scholar] [CrossRef]
- Krafft, K.E.; Wang, C.; Lin, W. Metal-organic framework templated synthesis of Fe2O3/TiO2 nanocomposite for hydrogen production. Adv. Mater. 2012, 24, 2014–2018. [Google Scholar] [CrossRef]
- Kim, K.; Lee, T.; Kwon, Y.; Seo, Y.; Song, J.; Park, J.K.; Lee, H.; Park, J.Y.; Ihee, H.; Cho, S.J.; et al. Lanthanum-catalysed synthesis of microporous 3D graphene-like carbons in a zeolite template. Nature 2016, 535, 131–135. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.; Miao, X.; Yang, W.; Wang, C.; Pan, Q. ZIF-L-Co@carbon fiber paper composite derived Co/Co3O4@C electrocatalyst for ORR in alkali/acidic media and overall seawater splitting. Int. J. Hydrog. Energy 2020, 45, 33028–33036. [Google Scholar] [CrossRef]
- Yin, X.; Li, H.; Yuan, R.; Lu, J. NiCoLDH nanosheets grown on MOF-derived Co3O4 triangle nanosheet arrays for high-performance supercapacitor. J. Mater. Sci. Technol. 2021, 62, 60–69. [Google Scholar]
- Yue, Z.; Li, H.; Chu, J.; Qu, G.; Fu, C.; Li, X.; Li, L.; Li, X.; Zhang, W. Co3O4 nanoparticles embedded in 1D porous N-doped carbon nanofibers derived from ZIFs for high-capacity sodium-ion batteries. Compos. Part B Eng. 2019, 177, 107400. [Google Scholar] [CrossRef]
- Wang, M.X.; Zhang, J.; Fan, H.L.; Liu, B.X.; Yi, X.B.; Wang, J.Q. ZIF-67 derived Co3O4/carbon aerogel composite for supercapacitor electrodes. New J. Chem. 2019, 43, 5666–5669. [Google Scholar] [CrossRef]
- Jiang, G.; Jiang, N.; Zheng, N.; Chen, X.; Mao, J.; Ding, G.; Li, Y.; Sun, F.; Li, Y. MOF-derived porous Co3O4-NC nanoflake arrays on carbon fiber cloth as stable hosts for dendrite-free Li metal anodes. Energy Storage Materi. 2019, 23, 181–189. [Google Scholar] [CrossRef]
- Lü, Y.; Zhan, W.; He, Y.; Wang, Y.; Kong, X.; Kuang, Q.; Xie, Z.; Zheng, L. MOF-templated synthesis of porous Co3O4 concave nanocubes with high specific surface area and their gas sensing properties. ACS Appl. Mat. Inter. 2014, 6, 4186–4195. [Google Scholar] [CrossRef]
- Ruan, L.; Jia, Y.; Guan, J.; Xue, B.; Huang, S.; Wang, Z.; Wu, Z. Tribo-electro-catalytic dye degradation driven by mechanical friction using MOF-derived NiCo2O4 double-shelled nanocages. J. Clean. Prod. 2022, 345, 131060. [Google Scholar] [CrossRef]
- Ruan, L.; Jia, Y.; Guan, J.; Xue, B.; Huang, S.; Wu, Z.; Cui, X. Highly piezocatalysis of metal-organic frameworks material ZIF-8 under vibration. Sep. Purif. Technol. 2022, 283, 120159. [Google Scholar] [CrossRef]
- Chen, M.; Jia, Y.; Li, H.; Wu, Z.; Huang, T.; Zhang, H. Enhanced pyrocatalysis of the pyroelectric BiFeO3/g-C3N4 heterostructure for dye decomposition driven by cold-hot temperature alternation. J. Adv. Ceram. 2021, 10, 338–346. [Google Scholar] [CrossRef]
- Pei, C.; Tan, J.; Li, Y.; Yao, G.; Jia, Y.; Ren, Z.; Zhang, H. Effect of Sb-site nonstoichiometry on the structure and microwave dielectric properties of Li3Mg2Sb1− xO6 ceramics. J. Adv. Ceram. 2020, 9, 588–594. [Google Scholar] [CrossRef]
- Zhao, Z.; Kou, K.; Wu, H. 2-Methylimidazole-mediated hierarchical Co3O4/N-doped carbon/short-carbon-fiber composite as high-performance electromagnetic wave absorber. J. Colloid Interface Sci. 2020, 574, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, R.; Wang, F.; Feng, T.; Zhang, P.; Luo, H. Pyroelectric properties of 91.5Na0.5Bi0.5TiO3–8.5K0.5Bi0.5TiO3 lead-free single crystal. J. Adv. Dielectr. 2021, 11. [Google Scholar] [CrossRef]
- Luo, Y.; Pu, T.; Fan, S.; Liu, H.; Zhu, J. Enhanced piezoelectric properties in low-temperature sintering PZN-PZT ceramics by adjusting Zr/Ti ratio. J. Adv. Dielectr. 2022, 12, 2250001. [Google Scholar] [CrossRef]
- Yao, Y.; Jia, Y.; Zhang, Q.; Li, S.; Li, G.; Cui, X.; Wu, Z. Piezoelectric BaTiO3 with the milling treatment for highly efficient piezocatalysis under vibration. J. Alloy. Compd. 2022, 905, 164234. [Google Scholar] [CrossRef]
- Wu, Z.; Luo, W.; Zhang, H.; Jia, Y. Strong pyro-catalysis of shape-controllable bismuth oxychloride nanomaterial for wastewater remediation. Appl. Surf. Sci. 2020, 513, 145630. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneti, Y.V.; Yamauchi, Y. Metal–organic framework-derived nanoporous metal oxides toward supercapacitor applications: Progress and prospects. ACS Nano 2017, 11, 5293–5308. [Google Scholar] [CrossRef]
- Zou, G.; Hou, H.; Foster, C.W.; Banks, C.E.; Guo, T.; Jiang, Y.; Zhang, Y.; Ji, X. Advanced hierarchical vesicular carbon Co-doped with S, P, N for high-rate sodium storage. Adv. Sci. 2018, 5, 1800241. [Google Scholar] [CrossRef]
- Nam, I.; Kim, N.D.; Kim, G.P.; Park, J.; Yi, J. One step preparation of Mn3O4/graphene composites for use as an anode in Li ion batteries. J. Power Sources 2013, 244, 56–62. [Google Scholar] [CrossRef]
- Wei, R.; Fang, M.; Dong, G.; Lan, C.; Shu, L.; Zhang, H.; Bu, X.; Ho, J.C. High-index faceted porous Co3O4 nanosheets with oxygen vacancies for highly efficient water oxidation. ACS Appl. Mater. Inter. 2018, 10, 7079–7086. [Google Scholar] [CrossRef]
- Wei, G.; Zhou, Z.; Zhao, X.; Zhang, W.; An, C. Ultrathin Metal–organic framework nanosheet-derived ultrathin Co3O4 nanomeshes with robust oxygen-evolving performance and asymmetric supercapacitors. ACS Appl. Mater. Inter. 2018, 10, 23721–23730. [Google Scholar] [CrossRef]
- Dai, S.; Yuan, Y.; Yu, J.; Tang, J.; Zhou, J.; Tang, W. Metal–organic framework-templated synthesis of sulfur-doped core–sheath nanoarrays and nanoporous carbon for flexible all-solid-state asymmetric supercapacitors. Nanoscale 2018, 10, 15454–15461. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Yoon, S.; Shrestha, N.; Lee, S.; Ahn, H.; Han, S. Unusual energy storage and charge retention in Co-based metal–organic-frameworks. Micropor. Mesopor. Mat. 2012, 153, 163–165. [Google Scholar] [CrossRef]
- Zhu, G.; Wen, H.; Ma, M.; Wang, W.; Yang, L.; Wang, L.; Yao, Y. A self-supported hierarchical Co-MOF as a supercapacitor electrode with ultrahigh areal capacitance and excellent rate performance. Chem. Commun. 2018, 54, 10499–10502. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.; Hosseinzadeh, B.; Kazemi, H.; Kiani, M.; Hajati, S. Facile synthesis of mixed metal–organic frameworks: Electrode materials for supercapacitors with excellent areal capacitance and operational stability. ACS Appl. Mater. Inter. 2018, 10, 23063–23073. [Google Scholar] [CrossRef]
- Yu, H.; Xia, H.; Zhang, J.; He, J.; Guo, S.; Xu, Q. Fabrication of Fe-doped Co-MOF with mesoporous structure for the optimization of supercapacitor performances. Chinese Chem. Lett. 2018, 29, 834–836. [Google Scholar] [CrossRef]
- Saraf, M.; Rajak, R.; Mobin, S. MOF derived high surface area enabled porous Co3O4 nanoparticles for supercapacitors. ChemistrySelect 2019, 4, 8142–8149. [Google Scholar] [CrossRef]
- Li, G.; Hua, X.; Liu, P.; Xie, Y.; Han, L. Porous Co3O4 microflowers prepared by thermolysis of metal-organic framework for supercapacitor. Mater. Chem. Phys. 2015, 168, 127–131. [Google Scholar] [CrossRef]
- Guan, C.; Liu, X.; Ren, W.; Li, X.; Cheng, C.; Wang, J. Rational design of metal-organic framework derived hollow NiCo2O4 arrays for flexible supercapacitor and electrocatalysis. Adv. Ener. Mater. 2017, 7, 1602391. [Google Scholar] [CrossRef]
- Torad, N.; Salunkhe, R.; Li, Y.; Hamoudi, H.; Imura, M.; Sakka, Y.; Yamauchi, Y. Electric double-layer capacitors based on highly graphitized nanoporous carbons derived from ZIF-67. Chem.-Eur. J. 2014, 20, 7895–7900. [Google Scholar] [CrossRef]
- Tang, J.; Salunkhe, R.R.; Liu, J.; Torad, N.L.; Imura, M.; Furukawa, S.; Yamauchi, Y. Thermal conversion of core–shell metal–organic frameworks: A new method for selectively functionalized nanoporous hybrid carbon. J. Am. Chem. Soc. 2015, 137, 1572–1580. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Xiao, K.; Cheng, S.; Zhang, T.; Qian, G.; Zhang, Q.; Feng, Y. N-Doped hierarchically porous carbon derived from heterogeneous core–shell ZIF-L (Zn)@ ZIF-67 for supercapacitor application. New J. Chem. 2018, 42, 6719–6726. [Google Scholar] [CrossRef]
- Gao, T.; Zhou, F.; Ma, W.; Li, H. Metal-organic-framework derived carbon polyhedron and carbon nanotube hybrids as electrode for electrochemical supercapacitor and capacitive deionization. Electrochim. Acta 2018, 263, 85–93. [Google Scholar] [CrossRef]



| Samples | SBET (unit: m2/g) | Vpore (unit: cm3/g) | Rpore (unit: nm) |
|---|---|---|---|
| ZIF-67 | 45.18 | 0.01 | 8.11 |
| Co3O4 | 66.32 | 0.11 | 9.25 |
| Materials | Rate | Specific Capacitance | Capacitance Retention/Cycle | Ref. |
|---|---|---|---|---|
| ZIF-67/Co3O4@C | 1 A·g−1 | 251 F·g−1 | 90%/5000 | This work |
| Co-MOF | 0.6 A·g−1 | 206 F·g−1 | 97.5%/1000 | [42] |
| Co2(OH)2C8H4O4/NF | 2 mA·cm−2 | 13.6 F·cm−2 | 79.9%/1000 | [43] |
| CoMn-MOF | 5 mV·s−1 | 2.375 F·cm−2 | 85%/3000 | [44] |
| CoFe-MOF | 1 A·g−1 | 319.5 F·g−1 | 93.8%/3000 | [45] |
| ZIF-67/Co3O4 | 5 A·g−1 | 190 F·g−1 | 72.45%/5000 | [46] |
| Co2(H2O)(C6H4O2N)4/Co3O4 | 0.625 A·g−1 | 240 F·g−1 | 96.3%/2000 | [47] |
| Co-MOF/NiCo2O4 | 2.5 mA·cm−2 | 1055 mF·cm−2 | 86.7%/20000 | [48] |
| ZIF-67/C | 20 mV·s−1 | 238 F·g−1 | 72%/200 | [49] |
| ZIF-8@ZIF-67/NC@GC | 2 A·g−1 | 270 F·g−1 | 100%/10000 | [50] |
| ZIF-L(Zn)@ZIF-67/NC@GC@CNTs | 2 A·g−1 | 252.1 F·g−1 | 91.2%/10000 | [51] |
| ZIF-67/C@CNTs | 10 mV·s−1 | 343 F·g−1 | 13%/1000 | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, H.; Bie, S.; Zhang, J.; Ke, X.; Wang, X.; Liang, J.; Wu, N.; Zhang, Q.; Luo, C.; Jia, Y. In Situ Construction of ZIF-67-Derived Hybrid Tricobalt Tetraoxide@Carbon for Supercapacitor. Nanomaterials 2022, 12, 1571. https://doi.org/10.3390/nano12091571
Gong H, Bie S, Zhang J, Ke X, Wang X, Liang J, Wu N, Zhang Q, Luo C, Jia Y. In Situ Construction of ZIF-67-Derived Hybrid Tricobalt Tetraoxide@Carbon for Supercapacitor. Nanomaterials. 2022; 12(9):1571. https://doi.org/10.3390/nano12091571
Chicago/Turabian StyleGong, Hao, Shiguang Bie, Jian Zhang, Xianbin Ke, Xiaoxing Wang, Jianquan Liang, Nian Wu, Qichang Zhang, Chuanxian Luo, and Yanmin Jia. 2022. "In Situ Construction of ZIF-67-Derived Hybrid Tricobalt Tetraoxide@Carbon for Supercapacitor" Nanomaterials 12, no. 9: 1571. https://doi.org/10.3390/nano12091571
APA StyleGong, H., Bie, S., Zhang, J., Ke, X., Wang, X., Liang, J., Wu, N., Zhang, Q., Luo, C., & Jia, Y. (2022). In Situ Construction of ZIF-67-Derived Hybrid Tricobalt Tetraoxide@Carbon for Supercapacitor. Nanomaterials, 12(9), 1571. https://doi.org/10.3390/nano12091571







