Enhanced Photocatalytic and Photokilling Activities of Cu-Doped TiO2 Nanoparticles
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Cu-Doped TiO2 Nanoparticle Synthesis
2.2. Characterisation of Nanoparticles
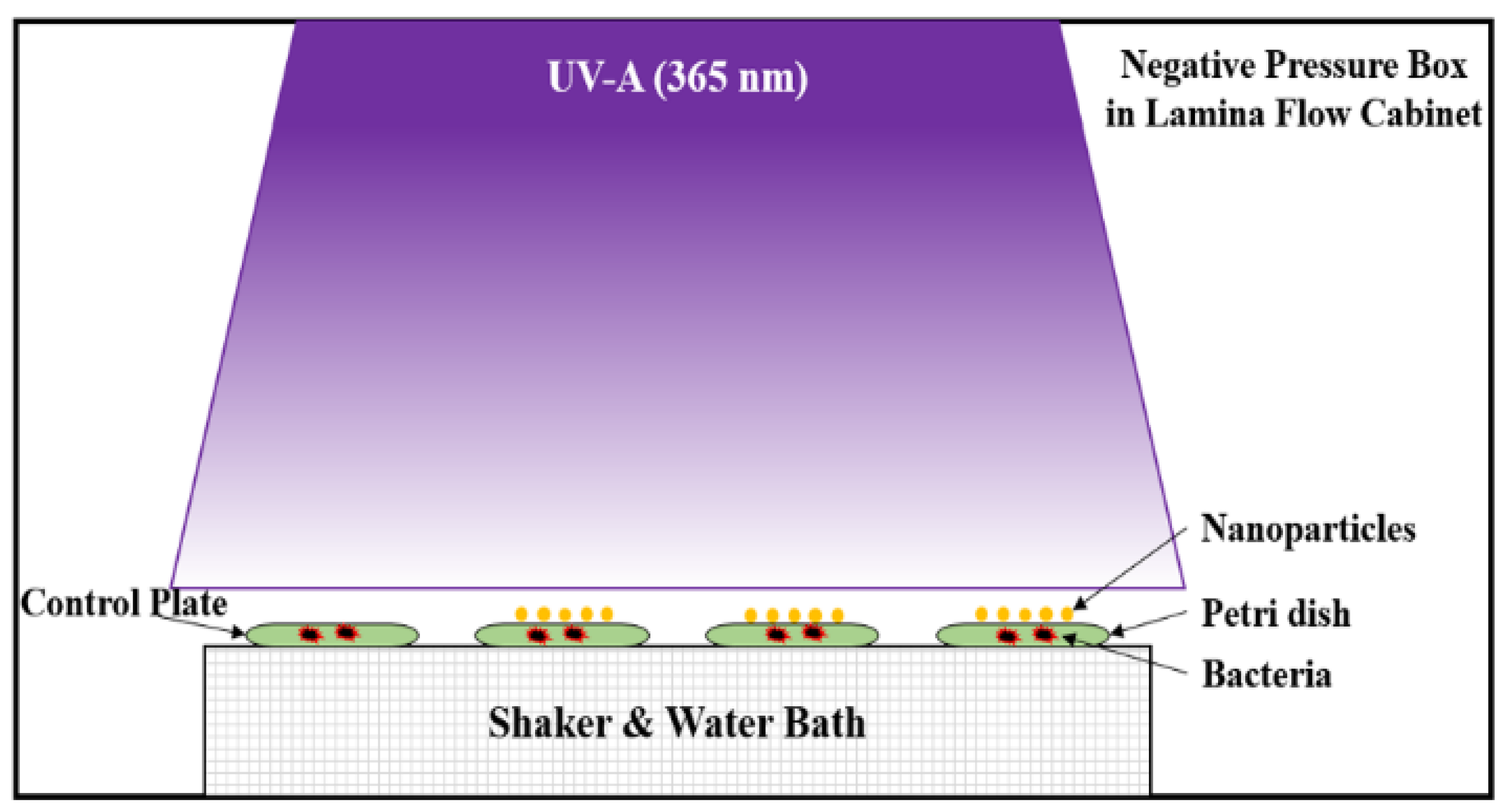
2.3. Photocatalysis and Photokilling Examinations
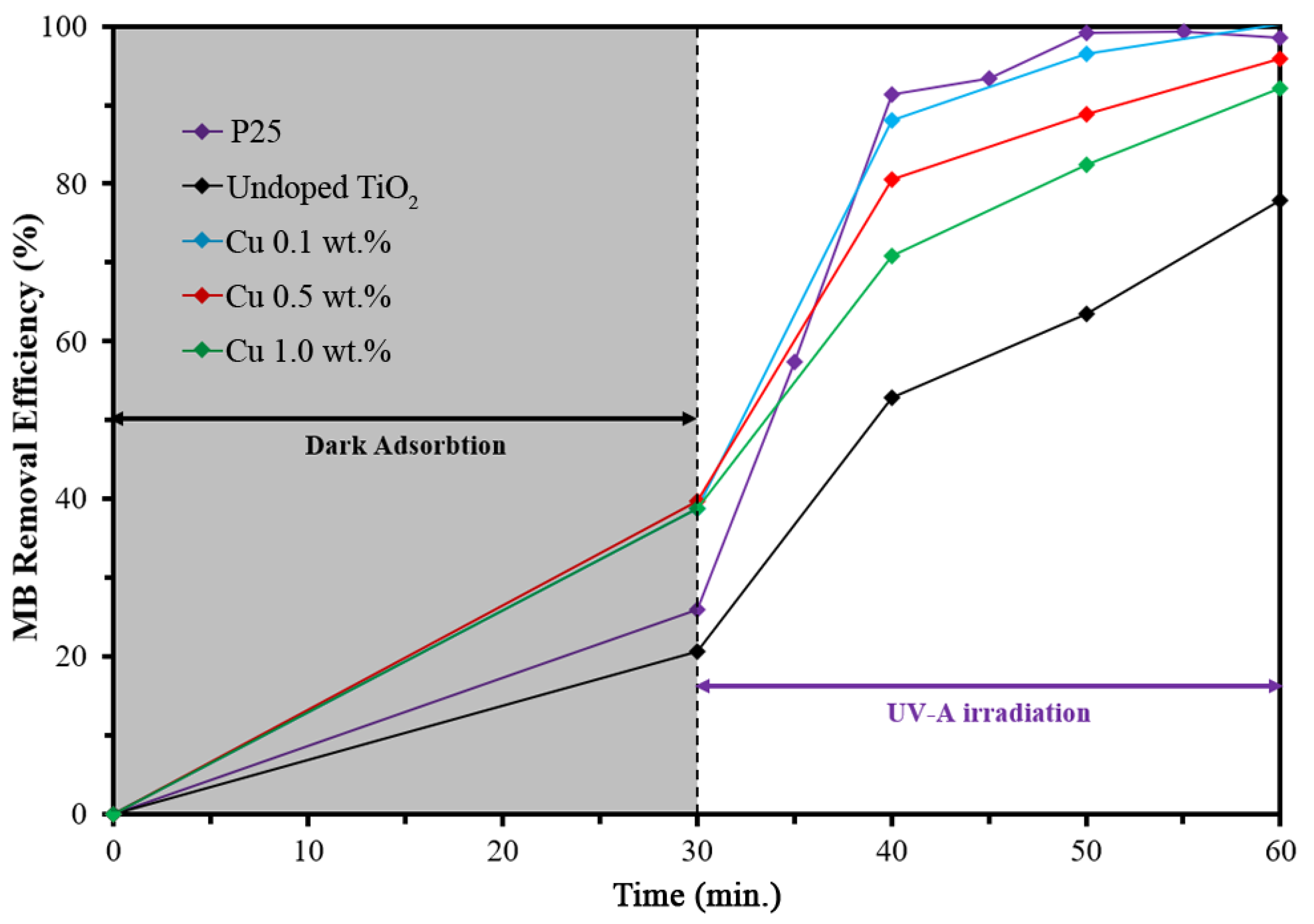
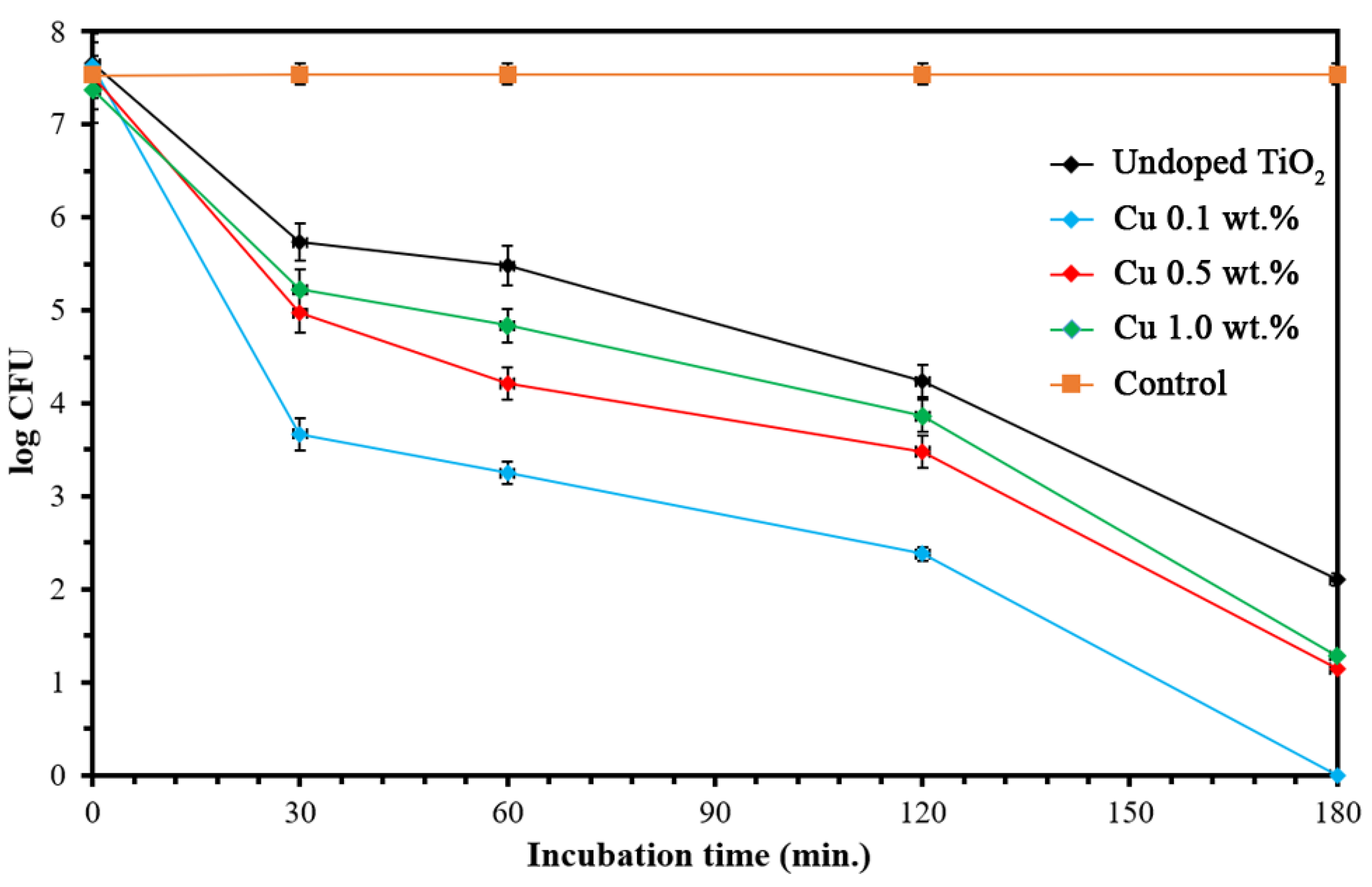
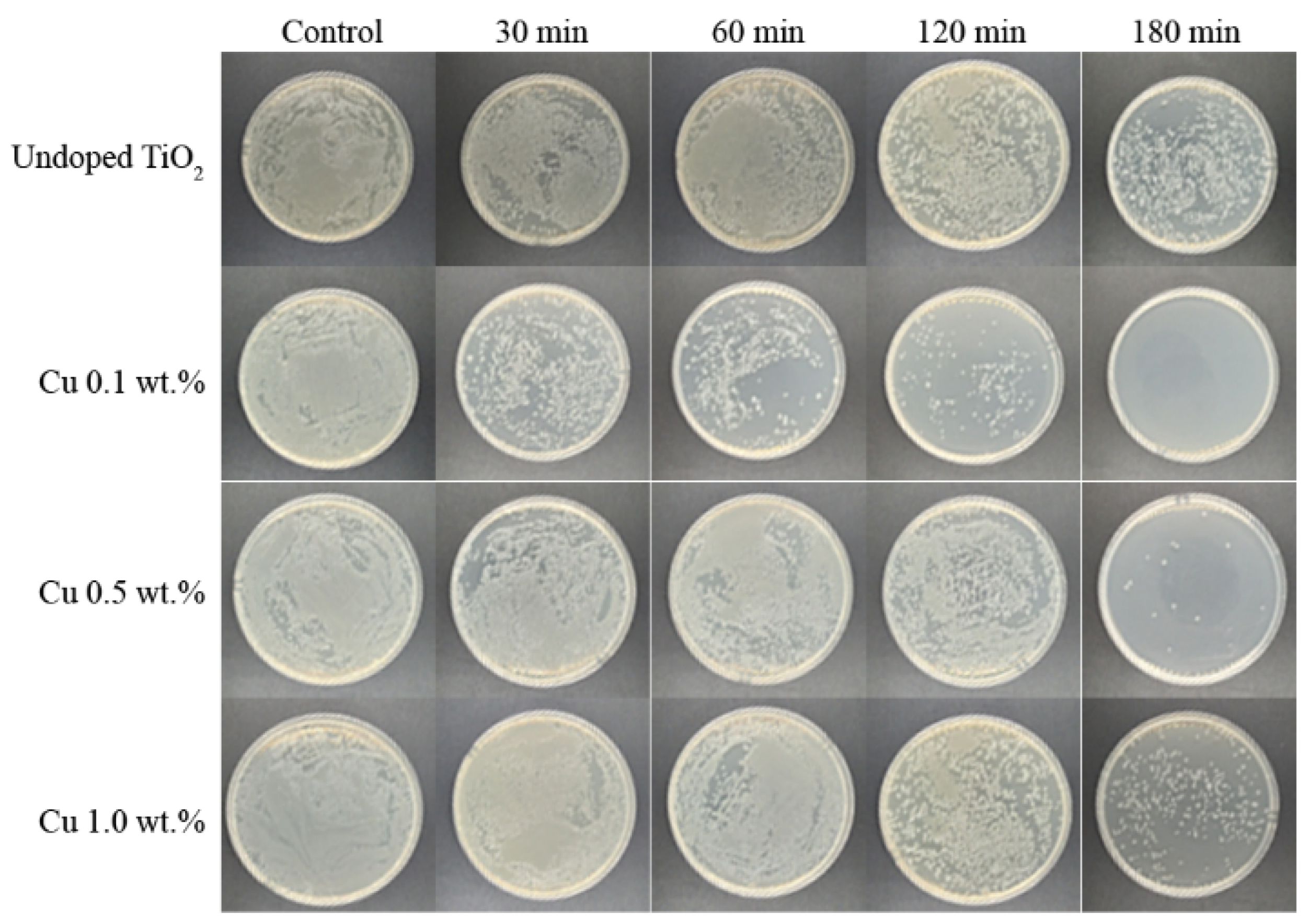
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumaravel, V.; Nair, K.M.; Mathew, S.; Bartlett, J.; Kennedy, J.E.; Manning, H.G.; Whelan, B.J.; Leyland, N.S.; Pillai, S.C. Antimicrobial TiO2 nanocomposite coatings for surfaces, dental and orthopaedic implants. Chem. Eng. J. 2021, 416, 129071. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Feng, Y.; Shao, J.; Chen, Y.; Wang, Z.L.; Li, H.; Chen, X.; Bian, Z. Self-cleaning triboelectric nanogenerator based on TiO2 photocatalysis. Nano Energy 2020, 70, 104499. [Google Scholar] [CrossRef]
- Threrujirapapong, T.; Khanitchaidecha, W.; Nakaruk, A. Treatment of high organic carbon industrial wastewater using photocatalysis process. Environ. Nanotechnol. Monit. Manag. 2017, 8, 163–168. [Google Scholar] [CrossRef]
- Kaur, N.; Mahajan, A.; Bhullar, V.; Singh, D.P.; Saxena, V.; Debnath, A.K.; Aswal, D.K.; Devi, D.; Singh, F.; Chopra, S. Ag ion implanted TiO2 photoanodes for fabrication of highly efficient and economical plasmonic dye sensitized solar cells. Chem. Phys. Lett. 2020, 740, 137070. [Google Scholar] [CrossRef]
- Geppert, M.; Schwarz, A.; Stangassinger, L.M.; Wenger, S.; Wienerroither, L.M.; Ess, S.; Duschl, A.; Himly, M. Interactions of TiO2 Nanoparticles with Ingredients from Modern Lifestyle Products and Their Effects on Human Skin Cells. Chem. Res. Toxicol. 2020, 33, 1215–1225. [Google Scholar] [CrossRef] [Green Version]
- Cheung, K.H.; Pabbruwe, M.B.; Chen, W.-F.; Koshy, P.; Sorrell, C.C. Thermodynamic and microstructural analyses of photocatalytic TiO2 from the anodization of biomedical-grade Ti6Al4V in phosphoric acid or sulfuric acid. Ceram. Int. 2021, 47, 1609–1624. [Google Scholar] [CrossRef]
- Kordjazi, Z.; Ajji, A. Development of TiO2 catalyzed HTPB based oxygen scavenging films for food packaging applications. Food Control 2021, 121, 107639. [Google Scholar] [CrossRef]
- Yemmireddy, V.K.; Hung, Y.-C. Using Photocatalyst Metal Oxides as Antimicrobial Surface Coatings to Ensure Food Safety—Opportunities and Challenges. Compr. Rev. Food Sci. Food Saf. 2017, 16, 617–631. [Google Scholar] [CrossRef] [Green Version]
- Dudefoi, W.; Moniz, K.; Allen-Vercoe, E.; Ropers, M.-H.; Walker, V.K. Impact of food grade and nano-TiO2 particles on a human intestinal community. Food Chem. Toxicol. 2017, 106, 242–249. [Google Scholar] [CrossRef]
- Kusmierek, E. A CeO2 Semiconductor as a Photocatalytic and Photoelectrocatalytic Material for the Remediation of Pollutants in Industrial Wastewater: A Review. Catalysts 2020, 10, 1435. [Google Scholar] [CrossRef]
- Trinh, D.T.T.; Channei, D.; Nakaruk, A.; Khanitchaidecha, W. New insight into the photocatalytic degradation of organic pollutant over BiVO4/SiO2/GO nanocomposite. Sci. Rep. 2021, 11, 4620. [Google Scholar] [CrossRef] [PubMed]
- Prashad Ojha, D.; Babu Poudel, M.; Joo Kim, H. Investigation of electrochemical performance of a high surface area mesoporous Mn doped TiO2 nanoparticle for a supercapacitor. Mater. Lett. 2020, 264, 127363. [Google Scholar] [CrossRef]
- Lim, J.; Yang, Y.; Hoffmann, M.R. Activation of Peroxymonosulfate by Oxygen Vacancies-Enriched Cobalt-Doped Black TiO2 Nanotubes for the Removal of Organic Pollutants. Environ. Sci. Technol. 2019, 53, 6972–6980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alotaibi, A.M.; Promdet, P.; Hwang, G.B.; Li, J.; Nair, S.P.; Sathasivam, S.; Kafizas, A.; Carmalt, C.J.; Parkin, I.P. Zn and N Codoped TiO2 Thin Films: Photocatalytic and Bactericidal Activity. ACS Appl. Mater. Interfaces 2021, 13, 10480–10489. [Google Scholar] [CrossRef]
- Moon, E.W.; Lee, H.-W.; Rok, J.H.; Ha, J.-H. Photocatalytic inactivation of viral particles of human norovirus by Cu-doped TiO2 non-woven fabric under UVA-LED wavelengths. Sci. Total Environ. 2020, 749, 141574. [Google Scholar] [CrossRef]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.J.; Bartlett, J.; Nolan, M.; Pillai, S.C. Cu-Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef] [Green Version]
- Karunakaran, C.; Abiramasundari, G.; Gomathisankar, P.; Manikandan, G.; Anandi, V. Cu-doped TiO2 nanoparticles for photocatalytic disinfection of bacteria under visible light. J. Colloid Interface Sci. 2010, 352, 68–74. [Google Scholar] [CrossRef]
- Yang, X.-j.; Wang, S.; Sun, H.-m.; Wang, X.-b.; Lian, J.-S. Preparation and photocatalytic performance of Cu-doped TiO2 nanoparticles. Trans. Nonferrous Met. Soc. China 2015, 25, 504–509. [Google Scholar] [CrossRef]
- Wu, B.; Huang, R.; Sahu, M.; Feng, X.; Biswas, P.; Tang, Y.J. Bacterial responses to Cu-doped TiO2 nanoparticles. Sci. Total Environ. 2010, 408, 1755–1758. [Google Scholar] [CrossRef]
- Aguilar, T.; Navas, J.; Alcántara, R.; Fernández-Lorenzo, C.; Gallardo, J.J.; Blanco, G.; Martín-Calleja, J. A route for the synthesis of Cu-doped TiO2 nanoparticles with a very low band gap. Chem. Phys. Lett. 2013, 571, 49–53. [Google Scholar] [CrossRef]
- Choudhury, B.; Dey, M.; Choudhury, A. Defect generation, d-d transition, and band gap reduction in Cu-doped TiO2 nanoparticles. Int. Nano Lett. 2013, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Pongwan, P.; Wetchakun, K.; Phanichphant, S.; Wetchakun, N. Enhancement of visible-light photocatalytic activity of Cu-doped TiO2 nanoparticles. Res. Chem. Intermed. 2016, 42, 2815–2830. [Google Scholar] [CrossRef]
- Geesi, M.H.; Ouerghi, O.; Elsanousi, A.; Kaiba, A.; Riadi, Y. Ultrasound-Assisted Preparation of Cu-Doped TiO2 Nanoparticles as a Nanocatalyst for Sonochemical Synthesis of Pyridopyrimidines. Polycycl. Aromat. Compd. 2022, 42, 80–90. [Google Scholar] [CrossRef]
- Zhu, X.; Wen, G.; Liu, H.; Han, S.; Chen, S.; Kong, Q.; Feng, W. One-step hydrothermal synthesis and characterization of Cu-doped TiO2 nanoparticles/nanobucks/nanorods with enhanced photocatalytic performance under simulated solar light. J. Mater. Sci. Mater. Electron. 2019, 30, 13826–13834. [Google Scholar] [CrossRef]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A Chem. 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Li, G.; Dimitrijevic, N.M.; Chen, L.; Rajh, T.; Gray, K.A. Role of Surface/Interfacial Cu2+ Sites in the Photocatalytic Activity of Coupled CuO−TiO2 Nanocomposites. J. Phys. Chem. C 2008, 112, 19040–19044. [Google Scholar] [CrossRef]
- Irie, H.; Miura, S.; Kamiya, K.; Hashimoto, K. Efficient visible light-sensitive photocatalysts: Grafting Cu(II) ions onto TiO2 and WO3 photocatalysts. Chem. Phys. Lett. 2008, 457, 202–205. [Google Scholar] [CrossRef]
- Tamarani, A.; Zainul, R.; Dewata, I. Preparation and characterization of XRD nano Cu-TiO2 using sol-gel method. J. Phys. Conf. Ser. 2019, 1185, 012020. [Google Scholar] [CrossRef]
- Souvereyns, B.; Elen, K.; De Dobbelaere, C.; Kelchtermans, A.; Peys, N.; D’Haen, J.; Mertens, M.; Mullens, S.; Van den Rul, H.; Meynen, V.; et al. Hydrothermal synthesis of a concentrated and stable dispersion of TiO2 nanoparticles. Chem. Eng. J. 2013, 223, 135–144. [Google Scholar] [CrossRef]
- Sirichokthanasarp, J.; Trinh, D.T.T.; Channei, D.A.D.; Chansaenpak, K.; Khanitchaidecha, W.; Nakaruk, A. Influence of Preparation Methods of TiO2 Nano-Particle on Photodegradation of Methylene Blue. Mater. Sci. Forum 2020, 998, 84–89. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Wang, Y.; Wu, Z. 2D Graphene-TiO2 Composite and Its Photocatalytic Application in Water Pollutants. Front. Energy Res. 2021, 8, 6122512. [Google Scholar] [CrossRef]
- Fernández-Catalá, J.; Sánchez-Rubio, M.; Navlani-García, M.; Berenguer-Murcia, Á.; Cazorla-Amorós, D. Synthesis of TiO2/Nanozeolite Composites for Highly Efficient Photocatalytic Oxidation of Propene in the Gas Phase. ACS Omega 2020, 5, 31323–31331. [Google Scholar] [CrossRef] [PubMed]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A Found. Adv. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Nguyen Thi Thu, T.; Nguyen Thi, N.; Tran Quang, V.; Nguyen Hong, K.; Nguyen Minh, T.; Le Thi Hoai, N. Synthesis, characterisation, and effect of pH on degradation of dyes of copper-doped TiO2. J. Exp. Nanosci. 2016, 11, 226–238. [Google Scholar] [CrossRef] [Green Version]
- Krishnakumar, V.; Boobas, S.; Jayaprakash, J.; Rajaboopathi, M.; Han, B.; Louhi-Kultanen, M. Effect of Cu doping on TiO2 nanoparticles and its photocatalytic activity under visible light. J. Mater. Sci. Mater. Electron. 2016, 27, 7438–7447. [Google Scholar] [CrossRef]
- Wen, L.; Liu, B.; Zhao, X.; Nakata, K.; Murakami, T.; Fujishima, A. Synthesis, Characterization, and Photocatalysis of Fe-Doped TiO2: A Combined Experimental and Theoretical Study. Int. J. Photoenergy 2012, 2012, 368750. [Google Scholar] [CrossRef]
- Moradi, H.; Eshaghi, A.; Hosseini, S.R.; Ghani, K. Fabrication of Fe-doped TiO2 nanoparticles and investigation of photocatalytic decolorization of reactive red 198 under visible light irradiation. Ultrason. Sonochem. 2016, 32, 314–319. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.-C.; Zakaria, R.; Ying, J.Y. Role of Particle Size in Nanocrystalline TiO2-Based Photocatalysts. J. Phys. Chem. B 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
- Qiu, X.; Miyauchi, M.; Sunada, K.; Minoshima, M.; Liu, M.; Lu, Y.; Li, D.; Shimodaira, Y.; Hosogi, Y.; Kuroda, Y.; et al. Hybrid CuxO/TiO2 Nanocomposites as Risk-Reduction Materials in Indoor Environments. ACS Nano 2012, 6, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Gamage McEvoy, J.; Zhang, Z. Antimicrobial and photocatalytic disinfection mechanisms in silver-modified photocatalysts under dark and light conditions. J. Photochem. Photobiol. C Photochem. Rev. 2014, 19, 62–75. [Google Scholar] [CrossRef]
- Janczarek, M.; Endo, M.; Zhang, D.; Wang, K.; Kowalska, E. Enhanced Photocatalytic and Antimicrobial Performance of Cuprous Oxide/Titania: The Effect of Titania Matrix. Materials 2018, 11, 2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Applerot, G.; Lellouche, J.; Lipovsky, A.; Nitzan, Y.; Lubart, R.; Gedanken, A.; Banin, E. Understanding the Antibacterial Mechanism of CuO Nanoparticles: Revealing the Route of Induced Oxidative Stress. Small 2012, 8, 3326–3337. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.H.; Abd Salam, N.R.; Zainal, N.; Kadir Basha, R.; Talib, R.A. Antimicrobial Activity of TiO2 Nanoparticle-Coated Film for Potential Food Packaging Applications. Int. J. Photoenergy 2014, 2014, 945930. [Google Scholar] [CrossRef] [Green Version]
- Meghana, S.; Kabra, P.; Chakraborty, S.; Padmavathy, N. Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv. 2015, 5, 12293–12299. [Google Scholar] [CrossRef]
- Ansari, M.A.; Albetran, H.M.; Alheshibri, M.H.; Timoumi, A.; Algarou, N.A.; Akhtar, S.; Slimani, Y.; Almessiere, M.A.; Alahmari, F.S.; Baykal, A.; et al. Synthesis of Electrospun TiO2 Nanofibers and Characterization of Their Antibacterial and Antibiofilm Potential against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2020, 9, 572. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Samples | |||
|---|---|---|---|---|
| Undoped TiO2 | 0.1 wt.% Cu-Doped | 0.5 wt.% Cu-Doped | 1.0 wt.% Cu-Doped | |
| Phase | Anatase | |||
| Lattice parameter (Å) | a = 3.786 c = 9.503 | a = 3.787 c = 9.496 | a = 3.787 c = 9.488 | a = 3.788 c = 9.484 |
| Crystallite Size (nm) | 7.73 | 7.86 | 7.84 | 7.85 |
| Particle size (nm) | ~10 | |||
| Specific surface area (m2/g) | 181.34 | 180.20 | 182.32 | 180.00 |
| Band gap (eV) | 3.20 | 3.15 | 3.10 | 3.12 |
| MB removal efficiency, after 60 min of irradiation time (%) | 77.86% | 100% | 95.83% | 92.17% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mingmongkol, Y.; Trinh, D.T.T.; Phuinthiang, P.; Channei, D.; Ratananikom, K.; Nakaruk, A.; Khanitchaidecha, W. Enhanced Photocatalytic and Photokilling Activities of Cu-Doped TiO2 Nanoparticles. Nanomaterials 2022, 12, 1198. https://doi.org/10.3390/nano12071198
Mingmongkol Y, Trinh DTT, Phuinthiang P, Channei D, Ratananikom K, Nakaruk A, Khanitchaidecha W. Enhanced Photocatalytic and Photokilling Activities of Cu-Doped TiO2 Nanoparticles. Nanomaterials. 2022; 12(7):1198. https://doi.org/10.3390/nano12071198
Chicago/Turabian StyleMingmongkol, Yumatorn, Dang Trung Tri Trinh, Patcharaporn Phuinthiang, Duangdao Channei, Khakhanang Ratananikom, Auppatham Nakaruk, and Wilawan Khanitchaidecha. 2022. "Enhanced Photocatalytic and Photokilling Activities of Cu-Doped TiO2 Nanoparticles" Nanomaterials 12, no. 7: 1198. https://doi.org/10.3390/nano12071198
APA StyleMingmongkol, Y., Trinh, D. T. T., Phuinthiang, P., Channei, D., Ratananikom, K., Nakaruk, A., & Khanitchaidecha, W. (2022). Enhanced Photocatalytic and Photokilling Activities of Cu-Doped TiO2 Nanoparticles. Nanomaterials, 12(7), 1198. https://doi.org/10.3390/nano12071198






