Current Methods in the Study of Nanomaterials for Bone Regeneration
Abstract
1. Introduction
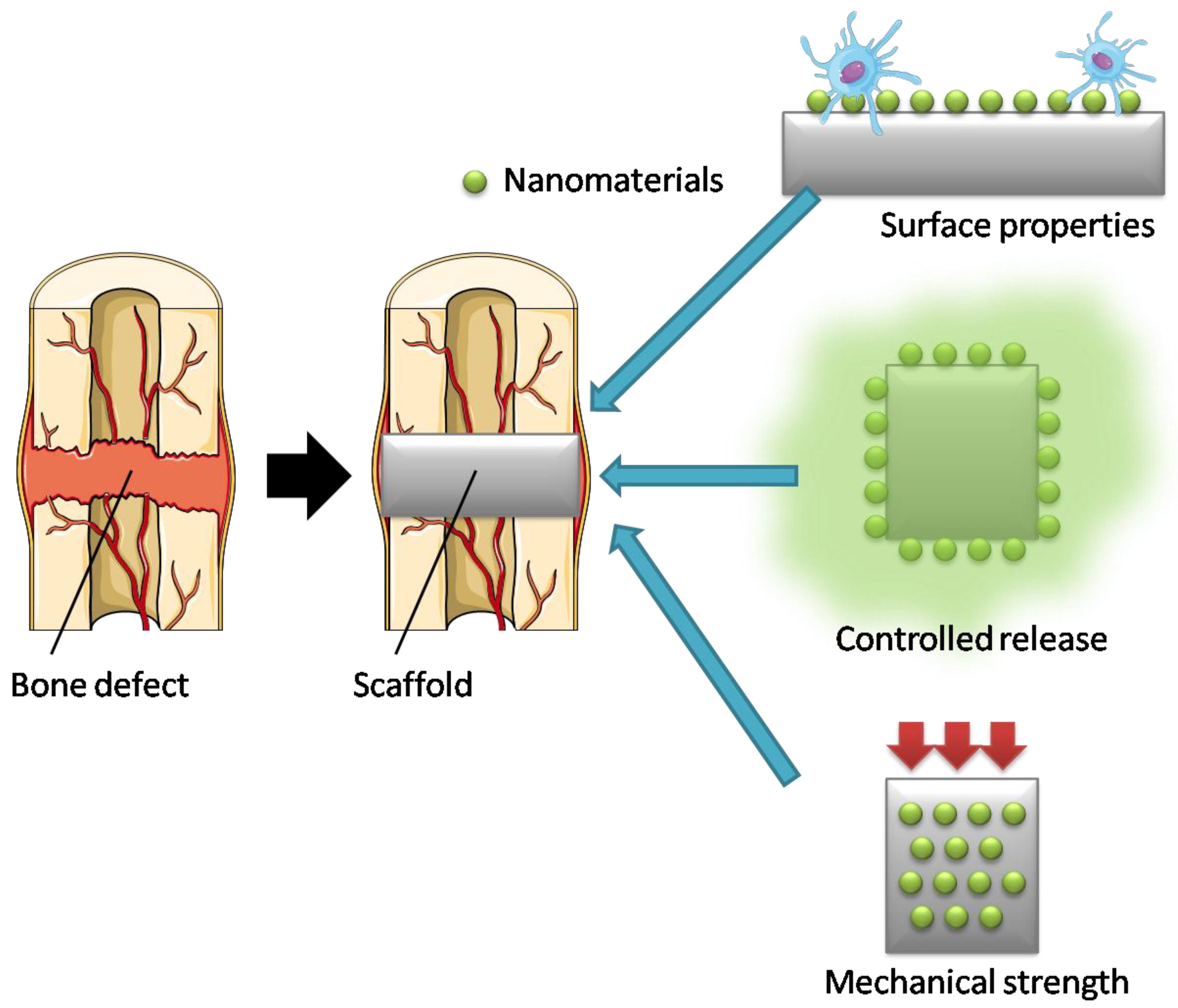
2. Promising Nanomaterials for Bone Regeneration Materials
2.1. Nanomaterials as Fillers in Bone Regeneration Scaffolds
2.2. Nanomaterials Used in Drug Delivery Systems (DDS)
| Application | Nanomaterial | Base | Fabrication Technique | Reference |
|---|---|---|---|---|
| Filler | Hydroxyapatite nanoparticles | Chitosan | Electrospinning | [27] |
| Bioactive glass nanoparticles | Polyethylene glycol dimethacrylate | Bioprinting | [28] | |
| Carbon nanotubes | Ultrahigh-molecular-weight polyethylene | Thermal compression | [29] | |
| Graphene oxide | Alginate/gelatin | 3D bioprinting | [30] | |
| Gold nanoparticles | Poly (L-lactic acid) | Electrospinning | [31] | |
| TiO2 nanoparticles | Poly (D, L-lactic acid) | Solvent casting | [32] | |
| Drug delivery | Ibandronate-loaded carbon nanohorns | Calcium phosphate | Coprecipitation | [43] |
| Desferrioxamine-loaded liposomes | Hydrogel | Physical blending method | [22] | |
| Breviscapine-loaded poly (D, L-lactic acid) nanoparticles | - | Spontaneous emulsification solvent diffusion method | [44] | |
| BMP-2-loaded poly (L-lactic acid) | - | Electrospinning | [45] | |
| Dexamethasone-loaded mesoporoussilica nanoparticles | Poly (L-lactic acid)/ Poly (ε-caprolactone) nanofibrous scaffold | Thermally induced phase separation | [46] |
2.3. Bone Regeneration Research Using CNTs
3. In Vivo Evaluation of Biomaterials for Bone Regeneration
4. In Vitro Evaluation of Biomaterials for Bone Regeneration
4.1. Types of Cells
4.1.1. Osteoblasts
4.1.2. Osteoclasts
4.1.3. Chondrocytes
4.1.4. Pluripotent Stem Cells
4.2. Indicators for Assessing Bone Formation In Vitro
5. Culture Medium
5.1. Basal Medium
5.1.1. Eagle’s Minimal Essential Medium (MEM)
5.1.2. Dulbecco’s Modified Eagle’s Medium (DMEM)
5.1.3. Alpha Modified Eagle’s Minimum Essential Medium (αMEM)
5.2. Fetal Bovine Serum (FBS)
5.3. Additives
5.3.1. Ascorbic Acid
5.3.2. β-glycerophosphate
5.3.3. Dexamethasone
6. Opportunities and Challenges
6.1. Smart Nanomaterials
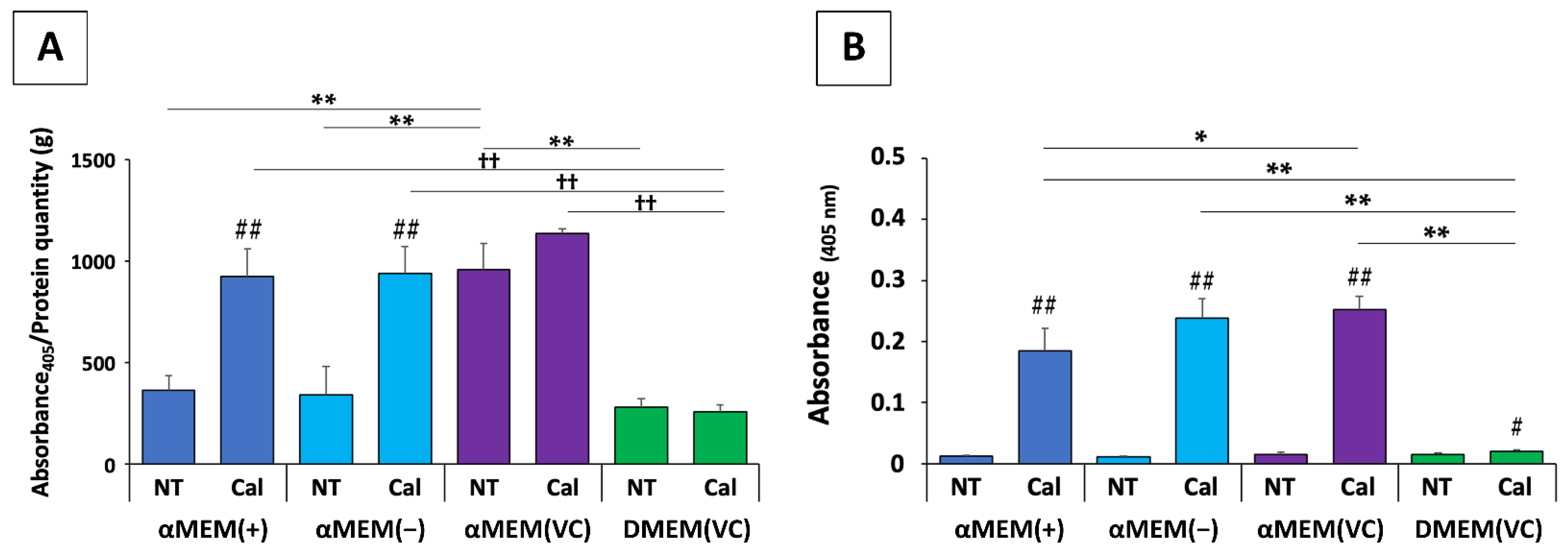
6.2. Effects of Medium and Additives on MC3T3-E1 Cells: An In Vitro Risk
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luvizuto, E.R.; Tangl, S.; Zanoni, G.; Okamoto, T.; Sonoda, C.K.; Gruber, R.; Okamoto, R. The effect of BMP-2 on the osteoconductive properties of β-tricalcium phosphate in rat calvaria defects. Biomaterials 2011, 32, 3855–3861. [Google Scholar] [CrossRef] [PubMed]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.; Barlogie, B.; Rudikoff, S.; Shaughnessy, J.D. Dkk1-induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma. Bone 2008, 42, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, B.; Lu, S.; Zhang, L.; Han, Y. Regulation of osteoblast proliferation and differentiation by interrod spacing of Sr-HA nanorods on microporous titania coatings. ACS Appl. Mater. Interfaces 2013, 5, 5358–5365. [Google Scholar] [CrossRef] [PubMed]
- Kohli, N.; Ho, S.; Brown, S.J.; Sawadkar, P.; Sharma, V.; Snow, M.; García-Gareta, E. Bone remodelling in vitro: Where are we headed?: A review on the current understanding of physiological bone remodelling and inflammation and the strategies for testing biomaterials in vitro. Bone 2018, 110, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.J.; Qi, B.; Bayaniahangar, R.; Araban, V.; Bakhtiary, Z.; Doschak, M.R.; Goh, B.C.; Shokouhimehr, M.; Vali, H.; Presley, J.F.; et al. Nanomaterials for bone tissue regeneration: Updates and future perspectives. Nanomedicine 2019, 14, 2987–3006. [Google Scholar]
- Zanello, L.P.; Zhao, B.; Hu, H.; Haddon, R.C. Bone cell proliferation on carbon nanotubes. Nano Lett. 2006, 6, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Woods, M.D.; Illingworth, K.D.; Niemeier, R.; Schafer, I.; Cady, C.; Filip, P.; El-Amin, S.F. Single walled carbon nanotube composites for bone tissue engineering. J. Orthop. Res. 2013, 31, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Pei, B.; Wang, W.; Dunne, N.; Li, X. Applications of Carbon Nanotubes in Bone Tissue Regeneration and Engineering: Superiority, Concerns, Current Advancements, and Prospects. Nanomaterials 2019, 9, 1501. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Xie, J.; Liao, J.; Zhang, T.; Lin, S.; Lin, Y. Nanomaterials and bone regeneration. Bone Res. 2015, 3, 15029. [Google Scholar] [CrossRef] [PubMed]
- Izumiya, M.; Haniu, M.; Ueda, K.; Ishida, H.; Ma, C.; Ideta, H.; Sobajima, A.; Ueshiba, K.; Uemura, T.; Saito, N.; et al. Evaluation of MC3T3-e1 cell Osteogenesis in Different Cell Culture Media. Int. J. Mol. Sci. 2021, 22, 7752. [Google Scholar] [CrossRef] [PubMed]
- Commission, E. Commission recommendation of 18 October 2011 on the definition of nanomaterial. Off. J. Eur. Union 2011, 275, 38. [Google Scholar]
- Li, H.; Xiao, H.; Ou, J. A study on mechanical and pressure-sensitive properties of cement mortar with nanophase materials. Cem. Concr. Res. 2004, 34, 435–438. [Google Scholar] [CrossRef]
- Teng, X.; Liu, H.; Huang, C. Effect of Al2O3 particle size on the mechanical properties of alumina-based ceramics. Mater. Sci. Eng. A 2007, 452–453, 545–551. [Google Scholar] [CrossRef]
- Al Ghabban, A.; Al Zubaidi, A.B.; Jafar, M.; Fakhri, Z. Effect of Nano SiO2 and Nano CaCO3 on The Mechanical Properties, Durability and flowability of Concrete. IOP Conf. Ser. Mater. Sci. Eng. 2018, 454, 012016. [Google Scholar] [CrossRef]
- Nell, K.M.; Fontenot, S.A.; Carter, T.G.; Warner, M.G.; Warner, C.L.; Addleman, R.S.; Johnson, D.W. Non-covalent functionalization of high-surface area nanomaterials: A new class of sorbent materials. Environ. Sci. Nano 2016, 3, 138–145. [Google Scholar] [CrossRef]
- Nobs, L.; Buchegger, F.; Gurny, R.; Allémann, E. Current methods for attaching targeting ligands to liposomes and nanoparticles. J. Pharm. Sci. 2004, 93, 1980–1992. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Xie, Z.; Liu, M.; Zhu, H.; Sun, H. Redox-sensitive mesoporous silica nanoparticles functionalized with PEG through a disulfide bond linker for potential anticancer drug delivery. RSC Adv. 2015, 5, 59576–59582. [Google Scholar] [CrossRef]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.R.; Such, G.K. pH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 2019, 40, 1800917. [Google Scholar] [CrossRef]
- Lyons, J.G.; Plantz, M.A.; Hsu, W.K.; Hsu, E.L.; Minardi, S. Nanostructured Biomaterials for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 922. [Google Scholar] [CrossRef]
- Fu, Y.; Cui, S.; Luo, D.; Liu, Y. Novel Inorganic Nanomaterial-Based Therapy for Bone Tissue Regeneration. Nanomaterials 2021, 11, 789. [Google Scholar] [CrossRef]
- Chen, H.; Yan, Y.; Qi, J.; Deng, L.; Cui, W. Sustained delivery of desferrioxamine via liposome carriers in hydrogel for combining angiogenesis and osteogenesis in bone defects reconstruction. J. Control. Release 2017, 259, e79. [Google Scholar] [CrossRef]
- Pfeiffenberger, M.; Damerau, A.; Lang, A.; Buttgereit, F.; Hoff, P.; Gaber, T. Fracture Healing Research—Shift towards In Vitro Modeling? Biomedicines 2021, 9, 748. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.Y.; Ashman, R.B.; Turner, C.H.; Young, J.A.E. Young’s modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J. Biomech. 1993, 26, 111–119. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Hajiali, F.; Tajbakhsh, S.; Shojaei, A. Fabrication and Properties of Polycaprolactone Composites Containing Calcium Phosphate-Based Ceramics and Bioactive Glasses in Bone Tissue Engineering: A Review. Polym. Rev. 2018, 58, 164–207. [Google Scholar] [CrossRef]
- Liu, H.; Peng, H.; Wu, Y.; Zhang, C.; Cai, Y.; Xu, G.; Li, Q.; Chen, X.; Ji, J.; Zhang, Y.; et al. The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs. Biomaterials 2013, 34, 4404–4417. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Schilling, A.F.; Yonezawa, T.; Wang, J.; Dai, G.; Cui, X. Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol. J. 2014, 9, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Sobajima, A.; Okihara, T.; Moriyama, S.; Nishimura, N.; Osawa, T.; Miyamae, K.; Haniu, H.; Aoki, K.; Tanaka, M.; Usui, Y.; et al. Multiwall Carbon Nanotube Composites as Artificial Joint Materials. ACS Biomater. Sci. Eng. 2020, 6, 7032–7040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Eyisoylu, H.; Qin, X.H.; Rubert, M.; Müller, R. 3D bioprinting of graphene oxide-incorporated cell-laden bone mimicking scaffolds for promoting scaffold fidelity, osteogenic differentiation and mineralization. Acta Biomater. 2021, 121, 637–652. [Google Scholar] [CrossRef]
- McKeon-Fischer, K.D.; Freeman, J.W. Characterization of electrospun poly(L-lactide) and gold nanoparticle composite scaffolds for skeletal muscle tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, L.-C.; Jell, G.M.R.; Boccaccini, A.R. Titanium dioxide (TiO2) nanoparticles filled poly(D, L lactid acid) (PDLLA) matrix composites for bone tissue engineering. J. Mater. Sci. Mater. Med. 2007, 18, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Haniu, H.; Kim, Y.A.; Saito, N. The Use of Electrospun Organic and Carbon Nanofibers in Bone Regeneration. Nanomaterials 2020, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhao, Y.Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y.Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef] [PubMed]
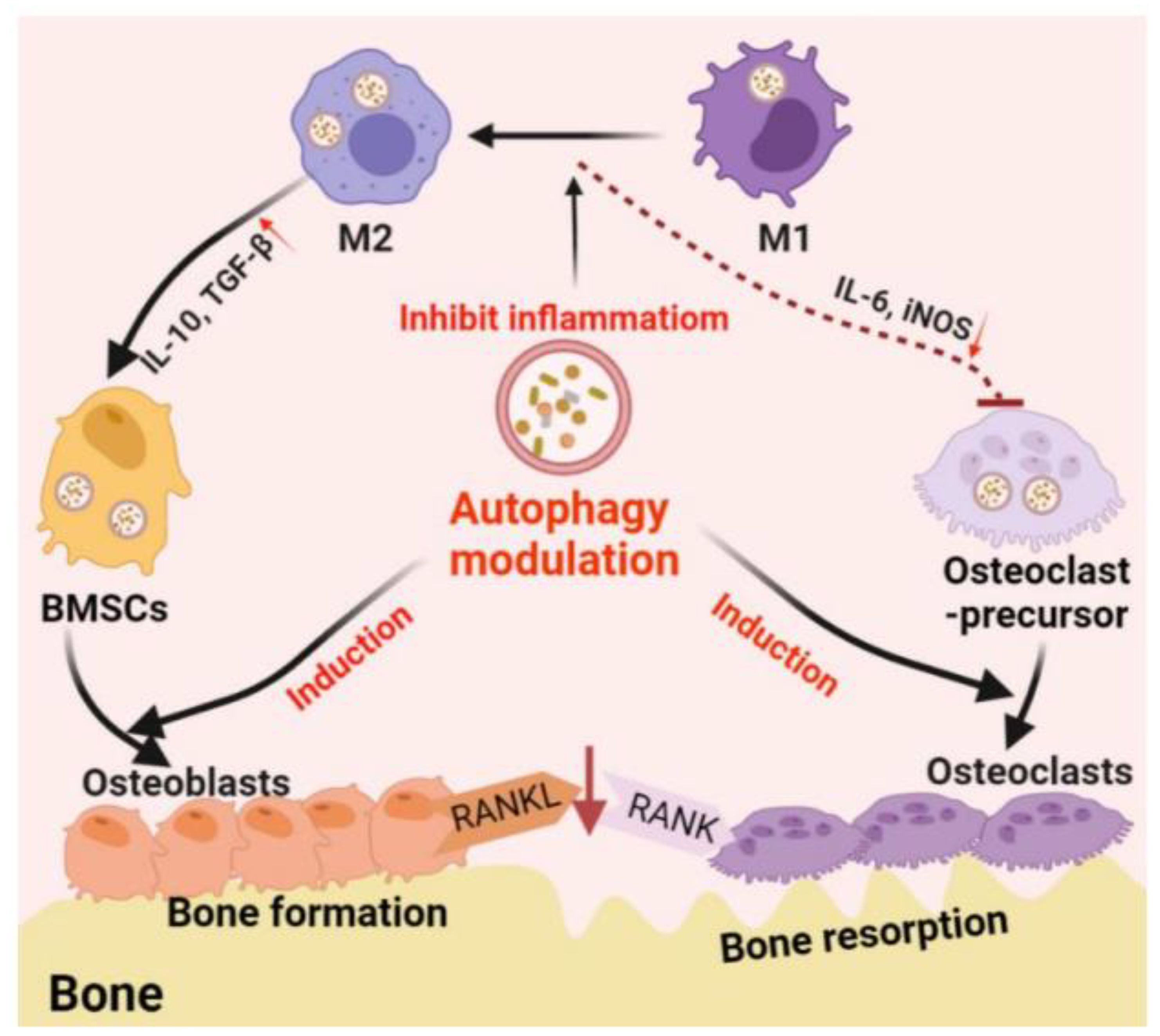
- Zhang, Q.; Xiao, L.; Xiao, Y. Porous nanomaterials targeting autophagy in bone regeneration. Pharmaceutics. 2021, 13, 1572. [Google Scholar] [CrossRef] [PubMed]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone morphogenetic protein-2 in development and bone homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: Emerging safety concerns and lessons learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef]
- Tenkumo, T.; VanegasSáenz, J.R.; Nakamura, K.; Shimizu, Y.; Sokolova, V.; Epple, M.; Kamano, Y.; Egusa, H.; Sugaya, T.; Sasaki, K. Prolonged release of bone morphogenetic protein-2 in vivo by gene transfection with DNA-functionalized calcium phosphate nanoparticle-loaded collagen scaffolds. Mater. Sci. Eng. C 2018, 92, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Golub, J.S.; Kim, Y.T.; Duvall, C.L.; Bellamkonda, R.V.; Gupta, D.; Lin, A.S.; Weiss, D.; Taylor, W.R.; Guldberg, R.E. Sustained VEGF delivery via PLGA nanoparticles promotes vascular growth. Am. J. Physiol.-Heart Circ. Physiol. 2010, 298, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Oduk, Y.; Zhu, W.; Kannappan, R.; Zhao, M.; Borovjagin, A.V.; Oparil, S.; Jay Zhang, J. VEGF nanoparticles repair the heart after myocardial infarction. Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H278–H284. [Google Scholar] [CrossRef] [PubMed]
- Lü, L.; Deegan, A.; Musa, F.; Xu, T.; Yang, Y. The effects of biomimetically conjugated VEGF on osteogenesis and angiogenesis of MSCs (human and rat) and HUVECs co-culture models. Colloids Surfaces B Biointerfaces 2018, 167, 550–559. [Google Scholar] [CrossRef]
- Nakamura, M.; Ueda, K.; Yamamoto, Y.; Aoki, K.; Zhang, M.; Saito, N.; Yudasaka, M. Ibandronate-Loaded Carbon Nanohorns Fabricated Using Calcium Phosphates as Mediators and Their Effects on Macrophages and Osteoclasts. ACS Appl. Mater. Interfaces 2021, 13, 3701–3712. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, H.; Luo, G.; Liu, Q.; Wang, Y. Pharmacokinetics and biodistribution of surface modification polymeric nanoparticles. Arch. Pharm. Res. 2008, 31, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Schofer, M.D.; Roessler, P.P.; Schaefer, J.; Theisen, C.; Schlimme, S.; Heverhagen, J.T.; Voelker, M.; Dersch, R.; Agarwal, S.; Fuchs-Winkelmann, S.; et al. Electrospun PLLA Nanofiber Scaffolds and Their Use in Combination with BMP-2 for Reconstruction of Bone Defects. PLoS ONE 2011, 6, e25462. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Chen, B.; Nie, W.; Zhou, X.; Feng, W.; Wang, W.; Chen, L.; Mo, X.; Wei, Y.; He, C. Electrophoretic Deposition of Dexamethasone-Loaded Mesoporous Silica Nanoparticles onto Poly(l-Lactic Acid)/Poly(ε-Caprolactone) Composite Scaffold for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 4137–4148. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, Y.; Haniu, H.; Nomura, H.; Kobayashi, S.; Takanashi, S.; Okamoto, M.; Takizawa, T.; Aoki, K.; Usui, Y.; et al. A three-dimensional block structure consisting exclusively of carbon nanotubes serving as bone regeneration scaffold and as bone defect filler. PLoS ONE 2017, 12, e0172601. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Aoki, K.; Haniu, H.; Kamanaka, T.; Takizawa, T.; Sobajima, A.; Yoshida, K.; Okamoto, M.; Kato, H.; Saito, N. Applications of carbon nanotubes in bone regenerative medicine. Nanomaterials 2020, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Kobayashi, Y.; Mizoguchi, T.; Nakamura, H.; Kawahara, I.; Narita, N.; Usui, Y.; Aoki, K.; Hara, K.; Haniu, H.; et al. Carbon nanotubes induce bone calcification by bidirectional interaction with osteoblasts. Adv. Mater. 2012, 24, 2176–2185. [Google Scholar] [CrossRef] [PubMed]
- Narita, N.; Kobayashi, Y.; Nakamura, H.; Maeda, K.; Mizoguchi, T.; Usui, Y.; Aoki, K.; Shimizu, M.; Ozawa, H.; Udagawa, N.; et al. Multiwalled carbon nanotubes specifically inhibit osteoclast differentiation and function. Nano Lett. 2009, 9, 1406–1413. [Google Scholar] [CrossRef]
- Tanaka, M.; Haniu, H.; Kamanaka, T.; Takizawa, T.; Sobajima, A.; Yoshida, K.; Aoki, K.; Okamoto, M.; Kato, H.; Saito, N. Physico-Chemical, In Vitro, and In Vivo Evaluation of a 3D Unidirectional Porous Hydroxyapatite Scaffold for Bone Regeneration. Materials 2017, 10, 33. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, Y.; Zhang, M.; Haniu, H.; Okamoto, M.; Aoki, K.; Takizawa, T.; Yoshida, K.; Sobajima, A.; Kamanaka, T.; et al. In Vitro and In Vivo Evaluation of a Three-Dimensional Porous Multi-Walled Carbon Nanotube Scaffold for Bone Regeneration. Nanomaterials 2017, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, N.; Usui, Y.; Aoki, K.; Shimizu, M.; Narita, N.; Hara, K.; Nakamura, K.; Ishigaki, N.; Takanashi, S.; Okamoto, M.; et al. Biocompatibility and bone tissue compatibility of alumina ceramics reinforced with carbon nanotubes. Nanomedicine 2012, 7, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Ivanov-Smolenski, A.A.; Chajlakjan, R.K.; Gorskaya, U.F.; Kuralesova, A.I.; Latzinik, N.W.; Gerasimow, U.W. Origin of bone marrow stromal mechanocytes in radiochimeras and heterotopic transplants. Exp. Hematol. 1978, 6, 440–444. [Google Scholar] [PubMed]
- Petite, H.; Viateau, V.; Bensaïd, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef]
- Harper, L.; Herbst, K.W.; Kalfa, N. Ethical issues in research: Human and animal experimentation. J. Pediatr. Urol. 2018, 14, 287–288. [Google Scholar] [CrossRef]
- Xu, J.H.; Li, Z.H.; Hou, Y.D.; Fang, W.J. Potential mechanisms underlying the Runx2 induced osteogenesis of bone marrow mesenchymal stem cells. Am. J. Transl. Res. 2015, 7, 2527–2535. [Google Scholar]
- Rosenberg, N.; Rosenberg, O.; Soudry, M. Osteoblasts in Bone Physiology—Mini Review. Rambam Maimonides Med. J. 2012, 3, e0013. [Google Scholar] [CrossRef]
- Manolagas, S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cells Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Yuji Sudo, H.P.; Anatomy, M.Y.; Tomitamachi, S.; Koriyama, T.; Kodama, H.; Amagai, Y.; Sudo, H.; Kasai, S.; Yamamoto, S. Establishment of a clonal osteogenic cell line from newborn mouse calvaria. Jpn. J. Oral Biol. 1981, 23, 899–901. [Google Scholar] [CrossRef]
- Beck, G.R.; Sullivan, E.C.; Moran, E.; Zerler, B. Relationship between alkaline phosphatase levels, osteopontin expression, and mineralization in differentiating MC3T3-E1 osteoblasts. J. Cell. Biochem. 1998, 68, 269–280. [Google Scholar] [CrossRef]
- Tissue, C.; Kuboki, Y.; Kudo, A.; Mizuno, M.; Kawamura, M.; Tissue, C. Time-dependent changes of collagen cross-links and their precursors in the culture of osteogenic cells. Calcif. Tissue Int. 1992, 50, 473–480. [Google Scholar]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Hayashi, M.; Takayanagi, H. New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol. Metab. 2012, 23, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; von der Mark, K.; Henry, S.; Norton, W.; Adams, H.; de Crombrugghe, B. Chondrocytes Transdifferentiate into Osteoblasts in Endochondral Bone during Development, Postnatal Growth and Fracture Healing in Mice. PLoS Genet. 2014, 10, e1004820. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tsang, K.Y.; Tang, H.C.; Chan, D.; Cheah, K.S.E. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc. Natl. Acad. Sci. USA 2014, 111, 12097–12102. [Google Scholar] [CrossRef]
- Ohgushi, H.; Caplan, A.I. Stem cell technology and bioceramics: From cell to gene engineering. J. Biomed. Mater. Res. 1999, 48, 913–927. [Google Scholar] [CrossRef]
- Ohgushi, H.; Kotobuki, N.; Funaoka, H.; Machida, H.; Hirose, M.; Tanaka, Y.; Takakura, Y. Tissue engineered ceramic artificial joint—Ex vivo osteogenic differentiation of patient mesenchymal cells on total ankle joints for treatment of osteoarthritis. Biomaterials 2005, 26, 4654–4661. [Google Scholar] [CrossRef]
- Ardeshirylajimi, A.; Dinarvand, P.; Seyedjafari, E.; Langroudi, L.; JamshidiAdegani, F.; Soleimani, M. Enhanced reconstruction of rat calvarial defects achieved by plasma-treated electrospun scaffolds and induced pluripotent stem cells. Cell Tissue Res. 2013, 354, 849–860. [Google Scholar] [CrossRef]
- Monzel, A.S.; Smits, L.M.; Hemmer, K.; Hachi, S.; Moreno, E.L.; van Wuellen, T.; Jarazo, J.; Walter, J.; Brüggemann, I.; Boussaad, I.; et al. Derivation of Human Midbrain-Specific Organoids from Neuroepithelial Stem Cells. Stem Cell Rep. 2017, 8, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Ohgushi, H.; Dohi, Y.; Katuda, T.; Tamai, S.; Tabata, S.; Suwa, Y. In vitro bone formation by rat marrow cell culture. J. Biomed. Mater. Res. 1996, 32, 333–340. [Google Scholar] [CrossRef]
- Kihara, T.; Oshima, A.; Hirose, M.; Ohgushi, H. Three-dimensional visualization analysis of in vitro cultured bone fabricated by rat marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2004, 316, 943–948. [Google Scholar] [CrossRef]
- Jubeli, E.; Khzam, A.; Yagoubi, N. Cells integration onto scaffolds prepared from polyester based polymers–importance of polymer thermal properties in addition to hydrophilicity. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 1068–1077. [Google Scholar] [CrossRef]
- Lam, C.X.F.; Olkowski, R.; Swieszkowski, W.; Tan, K.C.; Gibson, I.; Hutmacher, D.W. Mechanical and in vitro evaluations of composite PLDLLA/TCP scaffolds for bone engineering. Virtual Phys. Prototyp. 2008, 3, 193–197. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J.; Brien, F.J.O.; O’Brien, F.J.; Brien, F.J.O.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Cho, S.G.; Kim, J.H.; Doan, T.K.P.; Hu, Q.S.; Ulhaq, R.; Song, E.K.; Yoon, T.R. Mevinolin enhances osteogenic genes (ALP, type I collagen and osteocalcin), CD44, CD47 and CD51 expression during osteogenic differentiation. Life Sci. 2009, 84, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Lammers, L.; Naujoks, C.; Berr, K.; Depprich, R.; Kübler, N.; Meyer, U.; Langenbach, F.; Lüttenberg, B.; Kögler, G.; Wiesmann, H.P.; et al. Impact of DAG stimulation on mineral synthesis, mineral structure and osteogenic differentiation of human cord blood stem cells. Stem Cell Res. 2012, 8, 193–205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eagle, H. Nutrition needs of mammalian cells in tissue culture. Science 1955, 122, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Eagle, H. Amino acid metabolism in mammalian cell cultures. Science 1959, 130, 432–437. [Google Scholar] [CrossRef]
- Dulbecco, R.; Freeman, G. Plaque production by the polyoma virus. Virology 1959, 8, 396–397. [Google Scholar] [CrossRef]
- Stanners, C.P.; Eliceiri, G.L.; Green, H. Two types of ribosome in mouse-hamster hybrid cells. Nat. New Biol. 1971, 230, 52–54. [Google Scholar] [CrossRef]
- Bensaïd, W.; Triffitt, J.T.; Blanchat, C.; Oudina, K.; Sedel, L.; Petite, H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials 2003, 24, 2497–2502. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Iyer, B.S. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J. Bone Miner. Res. 1992, 7, 235–246. [Google Scholar] [CrossRef]
- Orriss, I.R.; Hajjawi, M.O.R.; Huesa, C.; Macrae, V.E.; Arnett, T.R. Optimisation of the differing conditions required for bone formation in vitro by primary osteoblasts from mice and rats. Int. J. Mol. Med. 2014, 34, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Gstraunthaler, G. Alternatives to the use of fetal bovine serum: Serum-free cell culture. ALTEX-Altern. Anim. Exp. 2003, 20, 275–281. [Google Scholar] [CrossRef]
- Mohamed, H.E.; Asker, M.E.; Kotb, N.S.; El Habab, A.M. Human platelet lysate efficiency, stability, and optimal heparin concentration required in culture of mammalian cells. Blood Res. 2020, 55, 35–43. [Google Scholar] [CrossRef]
- Meuleman, N.; Tondreau, T.; Delforge, A.; Dejeneffe, M.; Massy, M.; Libertalis, M.; Bron, D.; Lagneaux, L. Human marrow mesenchymal stem cell culture: Serum-free medium allows better expansion than classical α-MEM medium. Eur. J. Haematol. 2006, 76, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Vater, C.; Kasten, P.; Stiehler, M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011, 7, 463–477. [Google Scholar] [CrossRef]
- Lee, S.; Kim, M.; Hong, S.; Kim, E.J.; Kim, J.H.; Sohn, Y.; Jung, H.S. Effects of SparganiiRhizoma on Osteoclast Formation and Osteoblast Differentiation and on an OVX-Induced Bone Loss Model. Front. Pharmacol. 2022, 12, 797892. [Google Scholar] [CrossRef] [PubMed]
- García-Cabezón, C.; Godinho, V.; Salvo-Comino, C.; Torres, Y.; Martín-Pedrosa, F. Improved Corrosion Behavior and Biocompatibility of Porous Titanium Samples Coated with Bioactive Chitosan-Based Nanocomposites. Materials 2021, 14, 6322. [Google Scholar] [CrossRef]
- Zunich, S.M.; Douglas, T.; Valdovinos, M.; Chang, T.; Bushman, W.; Walterhouse, D.; Iannaccone, P.; Lamm, M.L.G. Paracrine sonic hedgehog signalling by prostate cancer cells induces osteoblast differentiation. Mol. Cancer 2009, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Fatherazi, S.; Matsa-Dunn, D.; Foster, B.L.; Rutherford, R.B.; Somerman, M.J.; Presland, R.B. Phosphate regulates osteopontin gene transcription. J. Dent. Res. 2009, 88, 39–44. [Google Scholar] [CrossRef]
- Foster, B.L.; Nociti, F.H.; Swanson, E.C.; Matsa-Dunn, D.; Berry, J.E.; Cupp, C.J.; Zhang, P.; Somerman, M.J. Regulation of cementoblast gene expression by inorganic phosphate in vitro. Calcif. Tissue Int. 2006, 78, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Nemoto, E.; Foster, B.L.; Somerman, M.J.; Shimauchi, H. Phosphate increases bone morphogenetic protein-2 expression through cAMP-dependent protein kinase and ERK1/2 pathways in human dental pulp cells. Bone 2011, 48, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Hamidouche, Z.; Haÿ, E.; Vaudin, P.; Charbord, P.; Schüle, R.; Marie, P.J.; Fromigué, O. FHL2 mediates dexamethasone-induced mesenchymal cell differentiation into osteoblasts by activating Wnt/β-catenin signaling-dependent Runx2 expression. FASEB J. 2008, 22, 3813–3822. [Google Scholar] [CrossRef] [PubMed]
- Aflori, M. Smart Nanomaterials for Biomedical Applications—A Review. Nanomaterials 2021, 11, 396. [Google Scholar] [CrossRef]
- Arafa, M.G.; El-Kased, R.F.; Elmazar, M.M. Thermoresponsive gels containing gold nanoparticles as smart antibacterial and wound healing agents. Sci. Rep. 2018, 8, 13674. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Sun, T.C.; Xue, J.; Miao, Z.; Yan, X.; Fang, W.; Li, Q.; Tang, R.; Lu, Y.; Tang, L.; et al. AG nanoparticles cluster with PH-triggered reassembly in targeting antimicrobial applications. Adv. Funct. Mater. 2020, 30, 2000511. [Google Scholar] [CrossRef]
- Mollazadeh, S.; Mackiewicz, M.; Yazdimamaghani, M. Recent advances in the redox-responsive drug delivery nanoplatforms: A chemical structure and physical property perspective. Mater. Sci. Eng. C 2021, 118, 111536. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Chen, C. Hydrogel-based colloidal photonic crystal devices for glucose sensing. Polymers 2020, 12, 625. [Google Scholar] [CrossRef]
- Gunawan, S.T.; Kempe, K.; Hagemeyer, C.E.; Caruso, F.; Bonnard, T.; Cui, J.; Alt, K.; Law, L.S.; Wang, X.; Westein, E.; et al. Multifunctional thrombin-activatable polymer capsules for specific targeting to activated platelets. Adv. Mater. 2015, 27, 5153–5157. [Google Scholar] [CrossRef]
- Karnieli, O.; Friedner, O.M.; Allickson, J.G.; Zhang, N.; Jung, S.; Fiorentini, D.; Abraham, E.; Eaker, S.S.; Yong, T.K.; Chan, A.; et al. A consensus introduction to serum replacements and serum-free media for cellular therapies. Cytotherapy 2017, 19, 155–169. [Google Scholar] [CrossRef]
- Kerrigan, L.; Nims, R.W. Authentication of human cell-based products: The role of a new consensus standard. Regen. Med. 2011, 6, 255–260. [Google Scholar] [CrossRef]

| Nanomaterial | Advantages | Drawbacks |
|---|---|---|
| Ceramics | Biocompatibility Osteoinductive potential | Potential for cytotoxicity |
| Polymers | Biocompatibility Biodegradability Manufacturing flexibility | Unfavorable biodegradability |
| Carbon | Mechanical strength Electrical conductivity | Non-degradability Concerns of long-term safety |
| Gold | Biocompatibility Photothermal stability Near-infrared absorbance | Non-degradability Concerns of long-term safety |
| Titanium-based nanomaterials | Load-bearing properties Biocompatibility | Non-degradability Poor biological response and anti-bacterial properties |
| Liposomes | Drug-loading ability | Mechanical weakness |
| Stimulus | Nanomaterial | Application | Reference |
|---|---|---|---|
| Temperature | Gold nanoparticles—Pluronic®F127- Hydroxypropyl methylcellulose | Tissue engineering | [100] |
| pH | Polyethylene glycol-Ag nanoparticle | Antibacterial, wound healing | [101] |
| Redox | Prodrug/AgNPs hybrid nanoparticles | Drug delivery | [102] |
| Glucose | Boronic acid-derived polymers | Drug delivery | [103] |
| Enzyme | Layer-by-layer assembly of poly (2-oxazoline)-based materials | Therapeutic delivery | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, M.; Izumiya, M.; Haniu, H.; Ueda, K.; Ma, C.; Ueshiba, K.; Ideta, H.; Sobajima, A.; Uchiyama, S.; Takahashi, J.; et al. Current Methods in the Study of Nanomaterials for Bone Regeneration. Nanomaterials 2022, 12, 1195. https://doi.org/10.3390/nano12071195
Tanaka M, Izumiya M, Haniu H, Ueda K, Ma C, Ueshiba K, Ideta H, Sobajima A, Uchiyama S, Takahashi J, et al. Current Methods in the Study of Nanomaterials for Bone Regeneration. Nanomaterials. 2022; 12(7):1195. https://doi.org/10.3390/nano12071195
Chicago/Turabian StyleTanaka, Manabu, Makoto Izumiya, Hisao Haniu, Katsuya Ueda, Chuang Ma, Koki Ueshiba, Hirokazu Ideta, Atsushi Sobajima, Shigeharu Uchiyama, Jun Takahashi, and et al. 2022. "Current Methods in the Study of Nanomaterials for Bone Regeneration" Nanomaterials 12, no. 7: 1195. https://doi.org/10.3390/nano12071195
APA StyleTanaka, M., Izumiya, M., Haniu, H., Ueda, K., Ma, C., Ueshiba, K., Ideta, H., Sobajima, A., Uchiyama, S., Takahashi, J., & Saito, N. (2022). Current Methods in the Study of Nanomaterials for Bone Regeneration. Nanomaterials, 12(7), 1195. https://doi.org/10.3390/nano12071195








