LbL Nano-Assemblies: A Versatile Tool for Biomedical and Healthcare Applications
Abstract
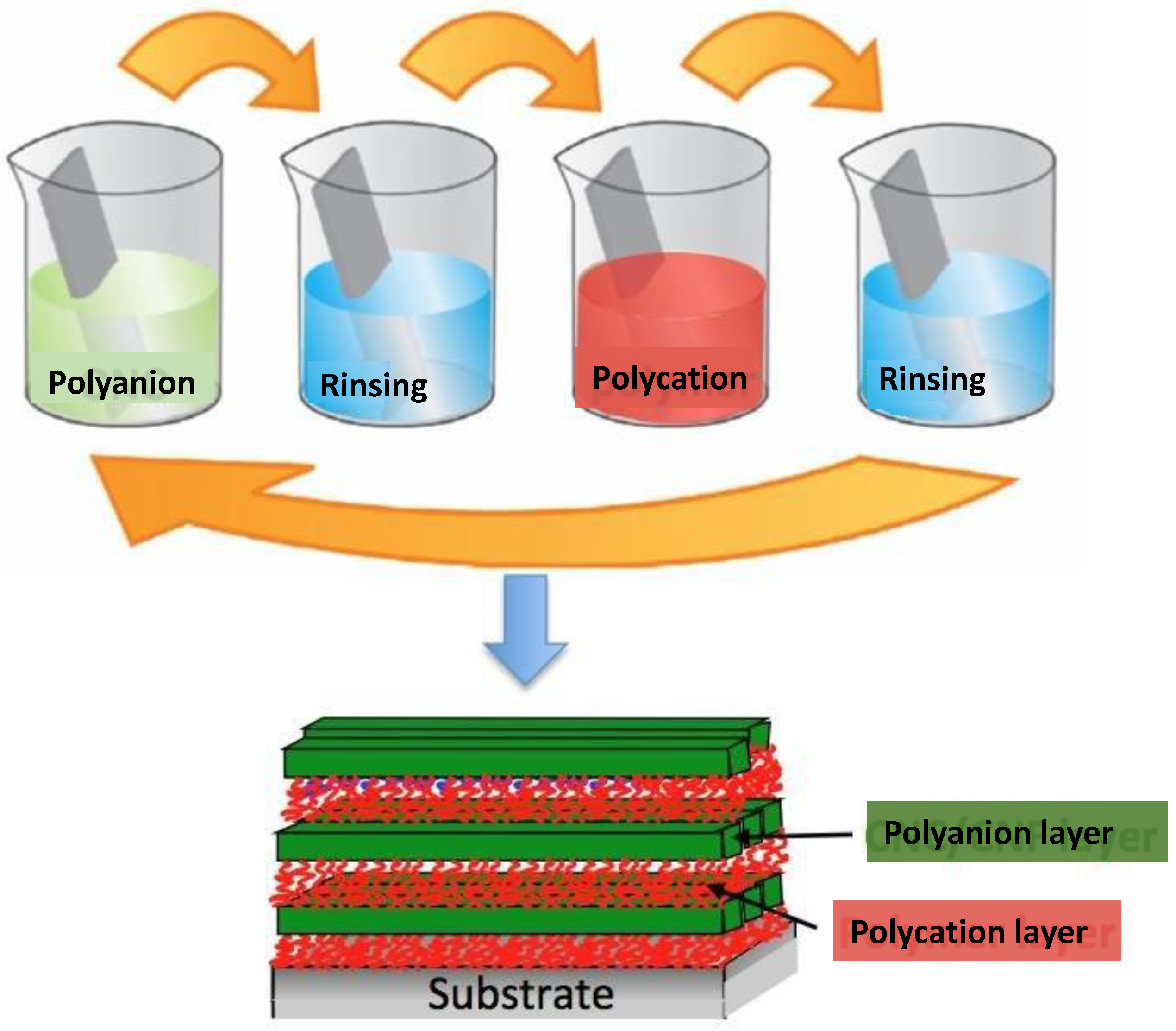
:1. Introduction
2. Biomedical Applications of LbL PE Nano-Assemblies
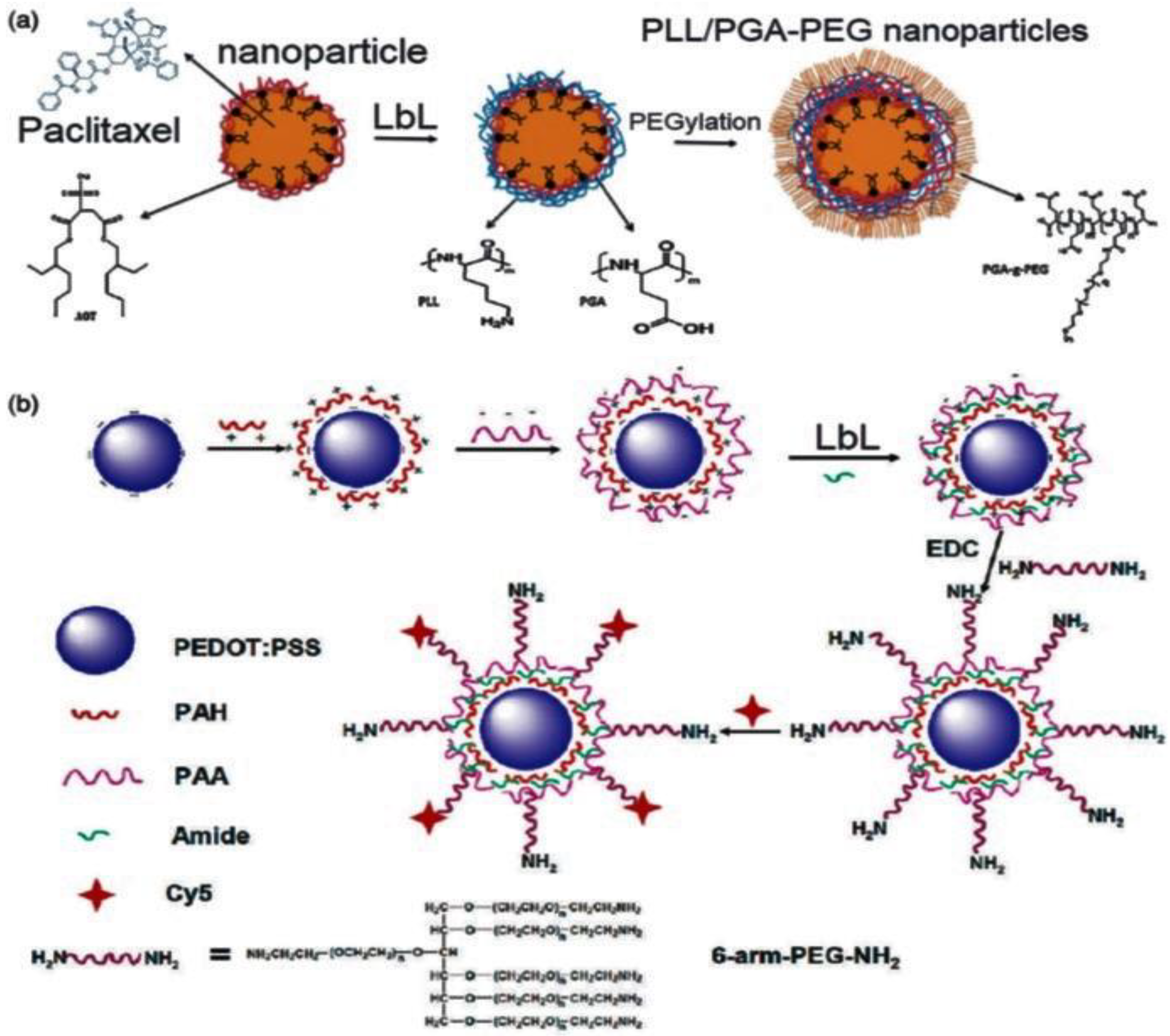
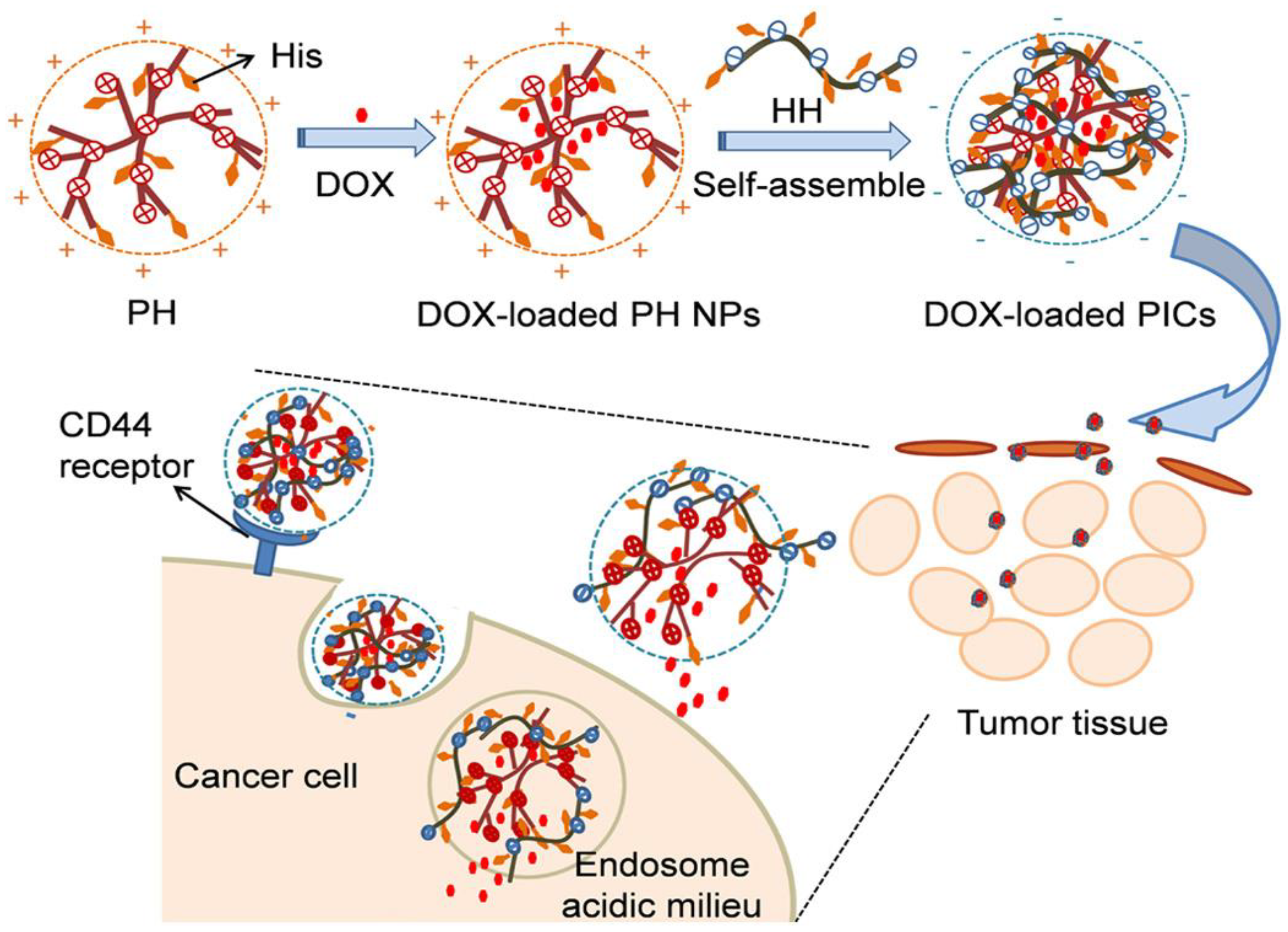
2.1. Drug Delivery
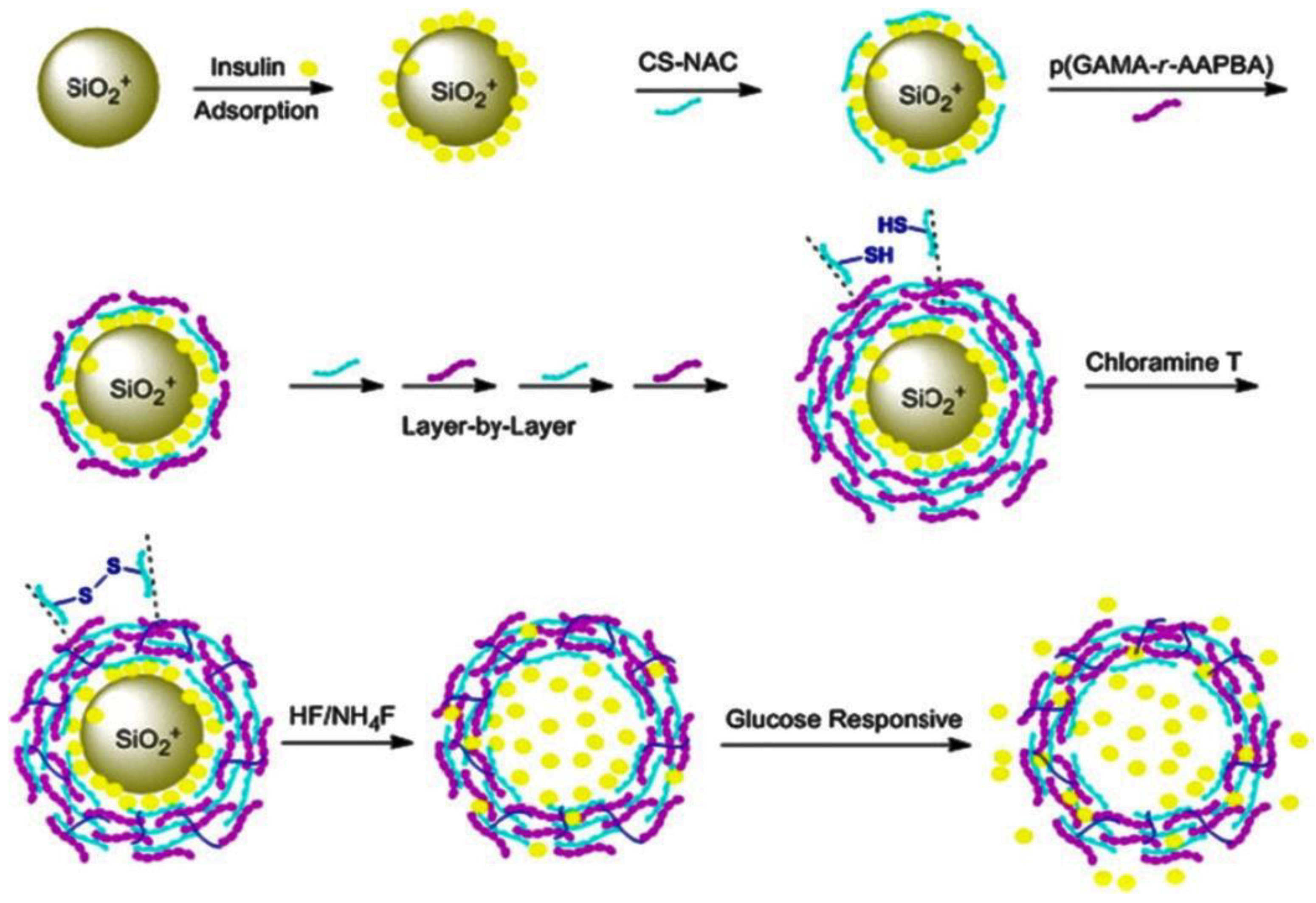
2.2. Protein Delivery
2.3. Tissue Engineering
2.4. Wound Healing
3. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Decher, G.; Schmitt, J. Fine-Tuning of the film thickness of ultrathin multilayer films composed of consecutively alternating layers of anionic and cationic polyelectrolytes. Prog. Colloid Polym. Sci. 1992, 89, 160–161. [Google Scholar]
- Lowe, A.B.; McCormick, C.L. Polyelectrolytes and Polyzwitterions: Synthesis, Properties, and Applications, 1st ed.; ACS Symposium Series, No. 937; American Chemical Society: Washington, DC, USA, 2006. [Google Scholar]
- Laschewsky, A. Structures and Synthesis of Zwitterionic Polymers. Polymers 2014, 6, 1544–1601. [Google Scholar] [CrossRef]
- Kötz, J.; Köpke, H.; Schmidt-Naake, G.; Vogl, O. Polyanionpolycation complex formation as a function of the position of the functional groups. Polymer 1996, 37, 2775–2781. [Google Scholar] [CrossRef]
- Ghimire, Y.; Bhattarai, A. A Review on Polyelectrolytes (PES) and Polyelectrolyte Complexes (PECs). Int. J. Eng. Tech. Res. 2021, 9, 876–889. [Google Scholar]
- McCreery, R.; Bergren, A. Surface Functionalization in the Nanoscale Domain. In Nanofabrication; Springer: Vienna, Austria, 2012; pp. 163–190. [Google Scholar]
- Punkka, E.; Rubner, M.F. Molecular heterostructure devices composed of Langmuir—Blodgett films of conducting polymers. J. Electron. Mater. 1992, 21, 1057–1063. [Google Scholar] [CrossRef]
- Bain, C.D.; Whitesides, G.M. Formation of monolayers by the coadsorption of thiols on gold—Variation in the length of the alkyl chain. J. Am. Chem. Soc. 1989, 111, 7164–7175. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D. Buildup of ultrathin multilayer films by a self-assembly process: Consecutive adsorption of anionic and cationic bipolar amphiphiles and polyelectrolytes on charged surfaces. Ber. Bunsen Phys. Chem. 1991, 95, 1430–1434. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Shuttleworth, P. Layer-by-Layer Assembly of Biopolyelectrolytes onto Thermo/pH-Responsive Micro/Nano-Gels. Materials 2014, 7, 7472–7512. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.E.; Müller, C.B.; Díez-Pascual, A.M.; Richtering, W. Study of Layer-by-Layer Films on Thermoresponsive Nanogels Using Temperature-Controlled Dual-Focus Fluorescence Correlation Spectroscopy. J. Phys. Chem. B 2009, 113, 15907–15913. [Google Scholar] [CrossRef]
- Lavalle, P.; Gergely, C.; Cuisinier, F.J.G.; Decher, G.; Schaaf, P.; Voegel, J.C. Comparison of the structure of polyelectrolyte multilayer films exhibiting a linear and an exponential growth regime: An in situ atomic force microscopy study. Macromolecules 2002, 35, 4458–4465. [Google Scholar] [CrossRef]
- Wong, J.E.; Diez-Pascual, A.M.; Richtering, W. Layer-by-Layer Assembly of Polyelectrolyte Multilayers on Thermoresponsive P(NiPAM-co-MAA) Microgel: Effect of Ionic Strength and Molecular Weight. Macromolecules 2009, 42, 1229–1238. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Wong, J.E. Effect of layer-by-layer confinement of polypeptides and polysaccharides onto thermoresponsive microgels: A comparative study. J. Colloid Intef. Sci. 2010, 347, 79–89. [Google Scholar] [CrossRef]
- Caruso, F.; Caruso, R.A.; Möhwald, H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science 1998, 282, 1111–1114. [Google Scholar] [CrossRef]
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Möhwald, H. Novel hollow polymer shells by colloid-templated assembly of polyelectrolytes. Angew. Chem. Int. Ed. 1998, 37, 2201–2205. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Antipov, A.A.; Voigt, A.; Donath, E.; Möhwald, H. pH-Controlled macromolecule encapsulation in and release from polyelectrolyte multilayer nanocapsules. Macromol. Rapid Commun. 2001, 22, 44–46. [Google Scholar] [CrossRef]
- Guerzoni, L.P.B.; Bohl, J.; Jans, A.; Rose, J.C.; Koehler, J.; Kuehne, A.; de Laporte, L. Microfluidic fabrication of polyethylene glycol microgel capsules with tailored properties for the delivery of biomolecules. Biomater. Sci. 2017, 5, 1549–1557. [Google Scholar] [CrossRef] [Green Version]
- Popov, A.L.; Popova, N.; Tarakina, N.; Ivanova Polezhaeva, O.; Ermakov, A.; Ivanov, V.; Sukhorukov, G. Intracellular Delivery Of Antioxidant CeO2 nanoparticles via polyelectrolyte microcapsules. ACS Biomater. Sci. Eng. 2018, 4, 2453–2462. [Google Scholar] [CrossRef]
- Musin, E.V.; Kim, A.L.; Tikhonenko, S.A. Destruction of polyelectrolyte microcapsules formed on CaCO3 microparticles and the release of a protein included by the adsorption method. Polymers 2020, 12, 520. [Google Scholar] [CrossRef] [Green Version]
- Reshetilov, A.; Plekhanova, Y.; Tarasov, S.; Tikhonenko, S.; Dubrovsky, A.; Kim, A.; Kashin, V.; Machulin, A.; Wang, G.-J.; Kolesov, V.; et al. Bioelectrochemical Properties of Enzyme-Containing Multilayer Polyelectrolyte Microcapsules Modified with Multiwalled Carbon Nanotubes. Membranes 2019, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Nifontova, G.; Efimov, A.; Agapova, O.; Agapov, I.; Nabiev, I.; Sukhanova, A. Bioimaging Tools Based on Polyelectrolyte Microcapsules Encoded with Fluorescent Semiconductor Nanoparticles: Design and Characterization of the Fluorescent Properties. Nanoscale Res. Lett. 2019, 14, 29. [Google Scholar] [CrossRef] [Green Version]
- Musin, E.V.; Kim, A.L.; Dubrovskii, A.V.; Tikhonenko, S.A. New sight at the organization of layers of multilayer polyelectrolyte microcapsules. Sci. Rep. 2021, 11, 14040. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.A.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Li, J.; Decher, G. Self-Assembled Smart Nanocarriers for Targeted Drug Delivery. Adv. Mater. 2016, 28, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, M.; Bennici, S.; Brendle, J.; Dutournie, P.; Limousy, L.; Pluchon, S. Systems for stimuli-controlled release: Materials and applications. J. Control. Release 2019, 294, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Nifontova, G.; Zvaigzne, M.; Baryshnikova, M.; Korostylev, E.; Ramos-Gomes, F.; Alves, F.; Nabiev, I.; Sukhanova, A. Next-Generation theranostic agents based on polyelectrolyte microcapsules encoded with semiconductor nanocrystals: Development and functional characterization. Nanoscale Res. Lett. 2018, 13, 30. [Google Scholar] [CrossRef] [Green Version]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Ramos Campos, E.; Rodríguez-Torres, M.; Acosta-Torres, L.; Diaz-Torres, L.; Grillo, R.; Swamy, M.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Yashchenok, A.M.; Möhwald, H. Encapsulation, release and applications of LbL polyelectrolyte multilayer capsules. Chem. Commun. 2011, 47, 12736–12746. [Google Scholar] [CrossRef]
- Park, S.; Han, U.; Choi, D.; Hong, J. Layer-by-Layer assembled polymeric thin films as prospective drug delivery carriers: Design and applications. Biomater. Res. 2018, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, T.; Ma, Q. Layer-by-Layer assembled nano-drug delivery systems for cancer treatment. Drug Deliv. 2021, 28, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Kurapati, R.; Groth, T.W.; Raichur, A.M. Recent Developments in Layer-by-Layer Technique for Drug Delivery Applications. ACS Appl. Bio. Mater. 2019, 2, 5512–5527. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.L.; Guo, Q.Q.; Chu, T.C.; Zhang, X.G.; Wu, Z.M.; Yu, D.M. Glucose-Sensitive polyelectrolyte nanocapsules based on layer-by-layer technique for protein drug delivery. J. Mater. Sci.-Mater. 2014, 25, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowicz, K.; Bzowska, M.; Kruk, T. Pegylated polyelectrolyte nanoparticles containing paclitaxel as a promising candidate for drug carriers for passive targeting. Colloids Surf. B 2016, 143, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yang, K.; Chen, Q.; Liu, Z. Organic stealth nanoparticles for highly effective in vivo near-infrared photothermal therapy of cancer. ACS Nano 2012, 6, 5605–5613. [Google Scholar] [CrossRef] [PubMed]
- Priya, P.; Raj, R.M.; Vasanthakumar, V.; Raj, V. Curcumin-loaded layer-by-layer folic acid and casein coated carboxymethyl cellulose/casein nanogels for treatment of skin cancer. Arab. J. Chem. 2020, 13, 694–708. [Google Scholar] [CrossRef]
- Yan, K.; Zhang, Y.; Mu, C.; Xu, Q.; Jing, X.; Wang, D.; Dang, D.; Meng, L.; Ma, J. Versatile Nanoplatforms with enhanced Photodynamic Therapy: Designs and Applications. Theranostics 2020, 10, 7287–7318. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Luo, G.F.; Qiu, W.X.; Lei, Q.; Liu, L.H.; Wang, S.B.; Zhang, X.Z. Mesoporous silica-based versatile theranostic nanoplatform constructed by layer-by-layer assembly for excellent photodynamic/chemo therapy. Biomaterials 2017, 117, 54–65. [Google Scholar] [CrossRef]
- Simioni, A.R.; de Jesus, P.C.C.; Tedesco, A.C. Layer-by-layer hollow photosensitizer microcapsule design via a manganese carbonate hard template for photodynamic therapy in cells. Photodiagn. Photodyn. Ther. 2018, 22, 169–177. [Google Scholar] [CrossRef]
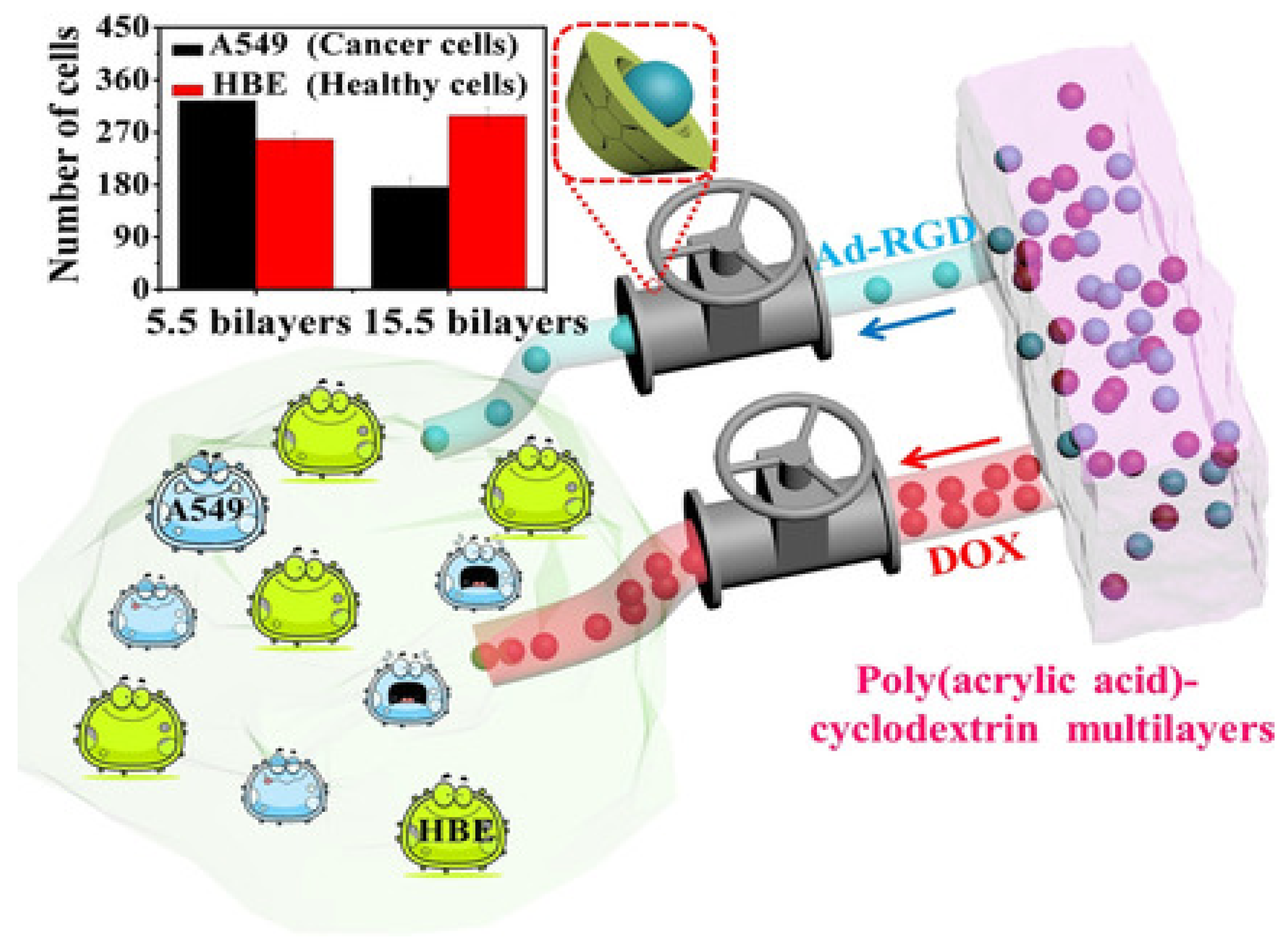
- Zhou, J.; Romero, G.; Rojas, E.; Ma, L.; Moya, S.; Gao, C.Y. Layer by layer chitosan/alginate coatings on poly (lactide-co-glycolide) nanoparticles for antifouling protection and folic acid binding to achieve selective cell targeting. J. Colloid Interface Sci. 2010, 345, 241–247. [Google Scholar] [CrossRef]
- Chen, J.-X.; Wang, M.; Tian, H.-H.; Chen, J.-H. Hyaluronic acid and polyethylenimine self-assembled polyion complexes as pH-sensitive drug carrier for cancer therapy. Colloids Surf. B 2015, 134, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramos, A.; Marín-Caba, L.; Iturrioz-Rodríguez, N.; Padín-González, E.; García-Hevia, L.; Mêna Oliveira, T.; Corea-Duarte, M.A.; Fanarraga, M.L. Design of Polymeric and Biocompatible Delivery Systems by Dissolving Mesoporous Silica Templates. Int. J. Mol. Sci. 2020, 21, 9573. [Google Scholar] [CrossRef] [PubMed]
- Hadipour Moghaddam, S.P.; Mohammadpour, R.; Ghandehari, H. In Vitro and in vivo evaluation of degradation, toxicity, biodistribution, and clearance of silica nanoparticles as a function of size, porosity, density, and composition. J. Control. Release 2019, 311–312, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ke, L.; Chen, H.; Zhuo, M.; Yang, X.; Zhao, D.; Zeng, S.; Xiao, X. Natural material-decorated mesoporous silica nanoparticle container for multifunctional membrane-controlled targeted drug delivery. Int. J. Nanomed. 2017, 12, 8411–8426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nifontova, G.; Ramos-Gomes, F.; Baryshnikova, M.; Alves, F.; Nabiev, I.; Sukhanova, A. Cancer Cell Targeting With Functionalized Quantum Dot-Encoded Polyelectrolyte Microcapsules. Front. Chem. 2019, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Ahn, E.; Park, M.; Kim, B. Layer-by-Layer Assembly: Recent Progress from Layered Assemblies to Layered Nanoarchitectonics. Chem. Asian J. 2019, 14, 2553–2566. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, A.; Lim, S.H.; Gorelik, S.; Kauling, A.P.; de Oliveira, R.V.B.; Castro Neto, A.H.; Glukhovskoy, E.; Gorin, D.A.; Sukhorukov, G.B.; Kiryukhin, M.V. Polyelectrolyte-Graphene Oxide Multilayer Composites for Array of Microchambers which are Mechanically Robust and Responsive to NIR Light. Macromol. Rapid Commun. 2019, 40, 1700868. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, G.; Montejo, K.A.; Michaut, A.; Majewski, P.W.; Osuji, C.O. Photoresponsive and Magnetoresponsive Graphene Oxide Microcapsules Fabricated by Droplet Microfluidics. ACS Appl. Mater. Interfaces 2017, 9, 44192–44198. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Kato, N. Polyelectrolyte/carbon nanotube composite microcapsules and drug release triggered by laser irradiation. Jpn. J. Appl. Phys. 2016, 55, 03DF06. [Google Scholar] [CrossRef]
- Gao, H.; Wen, D.; Tarakina, N.V.; Liang, J.; Bushby, A.J.; Sukhorukov, G.B. Bifunctional ultraviolet/ultrasound responsive composite TiO2/polyelectrolyte microcapsules. Nanoscale 2016, 8, 5170–5180. [Google Scholar] [CrossRef] [PubMed]
- Novoselova, M.V.; Voronin, D.V.; Abakumova, T.O.; Demina, P.A.; Petrov, A.V.; Petrov, V.V.; Zatsepin, T.S.; Sukhorukov, G.B.; Gorin, D.A. Focused ultrasound-mediated fluorescence of compo-site microcapsules loaded with magnetite nanoparticles: In Vitro and in vivo study. Colloids Surf. B Biointerfaces 2019, 181, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, J.; White, A.L.; Hondow, N.; Hughes, T.A.; Dupont, H.; Biggs, S.; Cayre, O.J. Metal-shell nanocapsules for the delivery of cancer drugs. J. Colloid Interface Sci. 2020, 567, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.-S.; Park, J.H.; Lee, S.; Rhoo, K.Y.; Lee, J.T.; Paik, S.R. Fabrication of Protease-Sensitive and Light-Responsive Microcapsules Encompassed with Single Layer of Gold Nanoparticles by Using Self-Assembly Protein of -Synuclein. ACS Appl. Mater. Interfaces 2018, 10, 26628–26640. [Google Scholar] [CrossRef] [PubMed]
- Inozemtseva, O.A.; Voronin, D.V.; Petrov, A.V.; Petrov, V.V.; Lapin, S.A.; Kozlova, A.A.; Bratashov, D.N.; Zakharevich, A.M.; Gorin, D.A. Disruption of Polymer and Composite Microcapsule Shells under High-Intensity Focused Ultrasound. Colloid J. 2018, 80, 771–782. [Google Scholar] [CrossRef]
- Stavarache, C.E.; Paniwnyk, L. Controlled rupture of magnetic LbL polyelectrolyte capsules and subsequent release of contents employing high intensity focused ultrasound. J. Drug Deliv. Sci. Technol. 2018, 45, 60–69. [Google Scholar] [CrossRef]
- Veerabadran, G.N.; Price, R.R.; Lvov, Y.M. Clay nanotubes for encapsulation and sustained release of drugs. Nano 2007, 2, 115–120. [Google Scholar] [CrossRef]
- Otoni, C.G.; Queirós, M.V.A.; Sabadini, J.B.; Rojas, O.J.; Loh, W. Charge Matters: Electrostatic Complexation As a Green Approach to Assemble Advanced Functional Materials. ACS Omega 2020, 5, 1296–1304. [Google Scholar] [CrossRef]
- Nolles, A.; Van Dongen, N.J.E.; Westphal, A.H.; Visser, A.J.W.G.; Kleijn, J.M.; Van Berkel, W.J.H.; Borst, J.W. Encapsulation into complex coacervate core micelles promotes EGFP dimerization. Phys. Chem. Chem. Phys. 2017, 19, 11380–11389. [Google Scholar] [CrossRef]
- Shu, S.J.; Sun, C.Y.; Zhang, X.G.; Wu, Z.M.; Wang, Z.; Li, C.X. Hollow and degradable polyelectrolyte nanocapsules for protein drug delivery. Acta Biomater. 2010, 6, 210–217. [Google Scholar] [CrossRef]
- Zhao, Q.H.; Li, B.Y. pH-controlled drug loading and release from biodegradable microcapsules. Nanomed.-Nanotechnol. 2008, 4, 302–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anandhakumar, S.; Nagaraja, V.; Raichur, A.M. Reversible polyelectrolyte capsules as carriers for protein delivery. Colloid Surf. B 2010, 78, 266–274. [Google Scholar] [CrossRef]
- Agut, W.; Brûlet, A.; Schatz, C.; Taton, D.; Lecommandoux, S. pH and Temperature Responsive Polymeric Micelles and Polymersomes by Self-Assembly of Poly[2-(dimethylamino)ethyl methacrylate]-b-Poly(glutamic acid) Double Hydrophilic Block Copolymers. Langmuir 2010, 26, 10546–10554. [Google Scholar] [CrossRef]
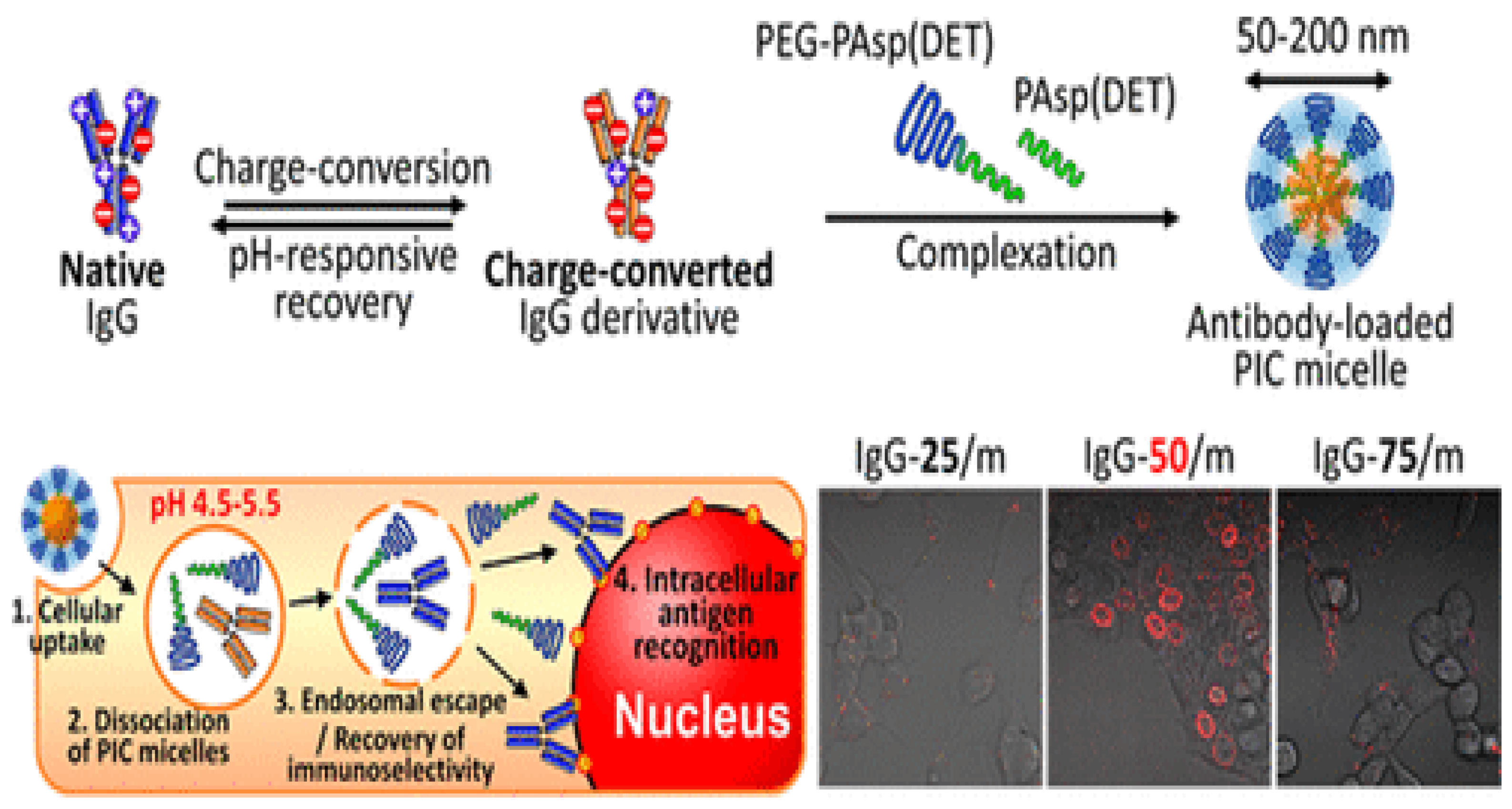
- Kim, A.; Miura, Y.; Ishii, T.; Mutaf, O.F.; Nishiyama, N.; Cabral, H.; Kataoka, K. Intracellular Delivery of Charge-Converted Monoclonal Antibodies by Combinatorial Design of Block/Homo Polyion Complex micelles. Biomacromolecules 2016, 17, 446–453. [Google Scholar] [CrossRef]
- Mudassir, J.; Darwis, Y.; Muhamad, S.; Khan, A.A. Self-assembled insulin and nanogels polyelectrolyte complex (Ins/NGs-PEC) for oral insulin delivery: Characterization, lyophilization and in-vivo evaluation. Int. J. Nanomed. 2019, 14, 4895–4909. [Google Scholar] [CrossRef] [Green Version]
- Jans, A.; Rosencrantz, R.R.; Mandić, A.D.; Anwar, N.; Boesveld, S.; Trautwein, C.; Moeller, M.; Sellge, G.; Elling, L.; Kuehne, A.J.C. Glycan-Functionalized Microgels for Scavenging and Specific Binding of Lectins. Biomacromolecules 2017, 18, 1460–1465. [Google Scholar] [CrossRef] [PubMed]
- Tiyaboonchai, W.; Woiszwillo, J.; Sims, C.R.; Middaugh, C.R. Insulin containing polyethylenimine–dextran sulfate nanoparticles. Int. J. Pharm. 2003, 255, 139. [Google Scholar] [CrossRef]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Ferreira, D. Development and characterization of new insulin containing polysaccharide nanoparticles. Colloids Surf. B 2006, 53, 193–202. [Google Scholar] [CrossRef] [Green Version]
- De la Fuente, M.; Seijo, B.; Alonso, M.J. Novel hyaluronan-based nanocarriers for transmucosal delivery of macromolecules. Macromol. Biosci. 2008, 8, 441–450. [Google Scholar] [CrossRef]
- Huang, T.; Luan, X.; Xia, Q.; Pan, S.; An, Q.; Wu, Y.; Zhang, Y. Molecularly Selective Regulation of Delivery Fluxes by Employing Supramolecular Interactions in Layer-by-Layer Films. Chem. Asian J. 2018, 16, 1067–1073. [Google Scholar] [CrossRef]
- Yang, M.; Choi, D.; Choi, M.; Hong, J. Nanoporous multilayer films for controlled antigen protein release. J. Ind. Eng. Chem. 2016, 33, 221–225. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Kaushik, A.K.; Arruda, E.M.; Waas, A.M.; Shim, B.S.; Xu, J.; Nandivada, H.; Pumplin, B.G.; Lahann, J.; Ramamoorthy, A.; et al. Ultrastrong and stiff layered polymer nanocomposites. Science 2007, 318, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Guan, Y.; Yu, H.; Huang, G.; Changjun, Z. Poly-l-lysine-coated PLGA/poly(amino acid)-modified hydroxyapatite porous scaffolds as efficient tissue engineering scaffolds for cell adhesion, proliferation, and differentiation. New J. Chem. 2019, 43, 9989–10002. [Google Scholar] [CrossRef]
- Kommireddy, D.S. Integration of Micro Nano and Bio Technologies with Layer-by-Layer Self-Assembly. Ph.D. Thesis, Louisiana Tech University, Ruston, LA, USA, 2005. [Google Scholar]
- Sabino, R.M.; Kauk, K.; Madruga, L.Y.C.; Kipper, M.J.; Martins, A.F.; Popat, K.C. Enhanced hemocompatibility and antibacterial activity on titania nanotubes with tanfloc/heparin polyelectrolyte multilayers. J. Biomed. Mater. Res. A 2020, 108, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.T.; Cui, W.; Chaikof, E.L. Layer-by-layer assembly of a conformal nanothin PEG coating for intraportal islet transplantation. Nano Lett. 2008, 8, 1940–1948. [Google Scholar] [CrossRef] [Green Version]
- Mansouri, S.; Merhi, Y.; Winnik, F.M.; Tabrizian, M. Investigation of layer-by-layer assembly of polyelectrolytes on fully functional human red blood cells in suspension for attenuated immune response. Biomacromolecules 2011, 12, 585–592. [Google Scholar] [CrossRef]
- Moreira, J.; Vale, A.C.; Pires, A.R.; Botelho, G.; Reis, R.L.; Alves, N.M. Spin-Coated Polysaccharide-Based Multilayered Freestanding Films with Adhesive and Bioactive Moieties. Molecules 2020, 25, 840. [Google Scholar] [CrossRef] [Green Version]
- Min, J.; Choi, K.Y.; Dreaden, E.C.; Padera, R.F.; Braatz, R.D.; Spector, M.; Hammond, P.T. Designer Dual Therapy Nanolayered Implant Coatings Eradicate Biofilms and Accelerate Bone Tissue Repair. ACS Nano 2016, 10, 4441–4450. [Google Scholar] [CrossRef]
- Silva, J.M.; Georgi, N.; Costa, R.; Sher, P.; Reis, R.L.; Van Blitterswijk, C.A.; Karperien, M.; Mano, J.F. Nanostructured 3D constructs based on chitosan and chondroitin sulphate multilayers for cartilage tissue engineering. PLoS ONE 2013, 8, e55451. [Google Scholar] [CrossRef] [Green Version]
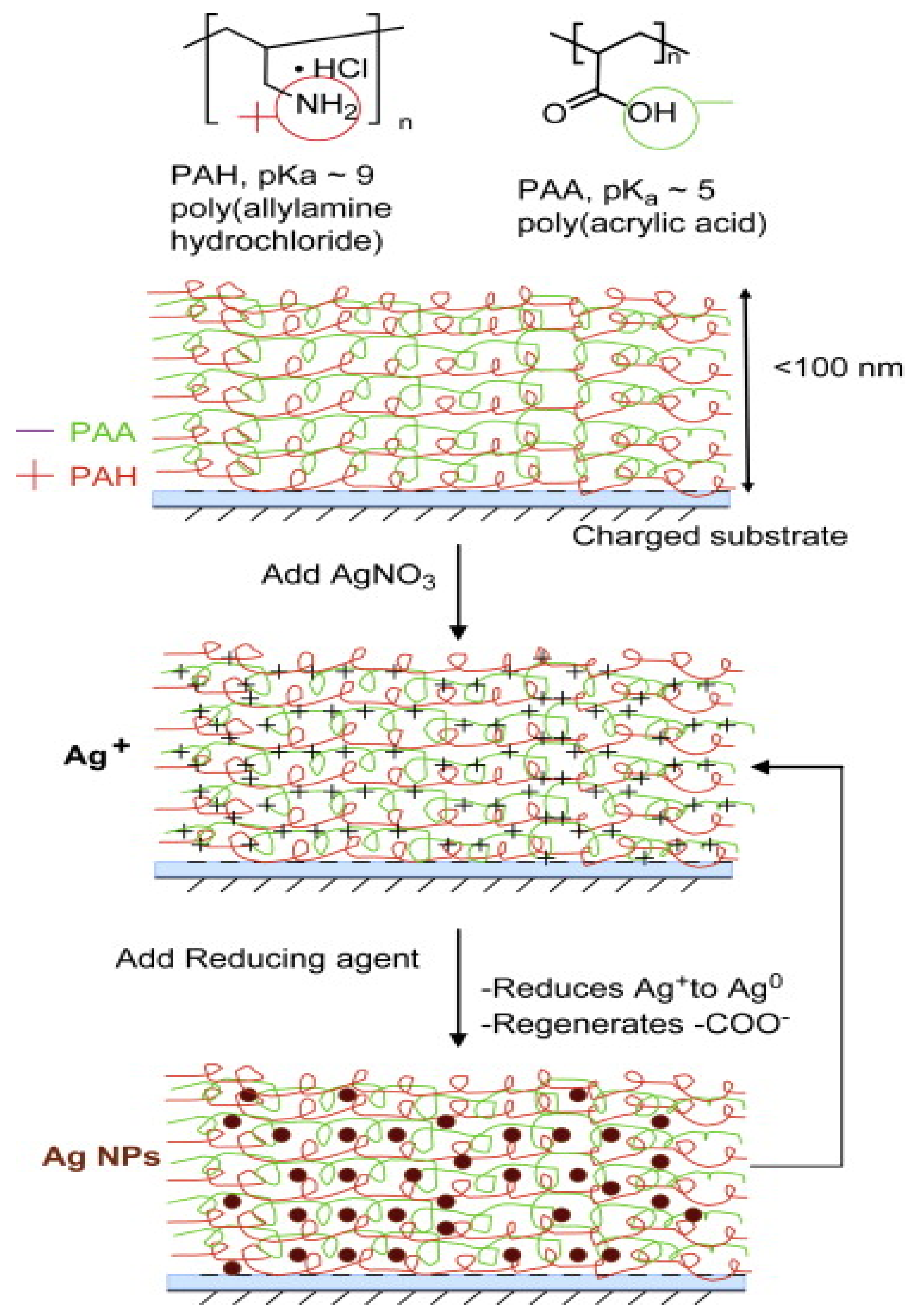
- Zan, X.J.; Su, Z.H. Polyelectrolyte multilayer films containing silver as antibacterial coatings. Thin Solid Films 2010, 518, 5478–5482. [Google Scholar] [CrossRef]
- Agarwal, A.; Weis, T.L.; Schurr, M.J.; Faith, N.G.; Czuprynskic, J.; McAnulty, J.F.; Murphy, C.J.; Abbott, N.L. Surfaces modified with nanometerthick silver-impregnated polymeric films that kill bacteria but support growth of mammalian cells. Biomaterials 2010, 31, 680–690. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Li, Y.; Deng, H.B.; Hu, Y.; Li, B. Antibacterial multilayer films fabricated by layer-by-layer immobilizing lysozyme and gold nanoparticles on nanofibers. Colloid Surf. B 2014, 116, 432–438. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Chan, V. Covalent layer-by-layer assembly of polyethyleneimine multilayer for antibacterial applications. J. Biomed. Mater. Res. A 2010, 95A, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Yao, C.; Li, X.S. A simple approach to constructing antibacterial and anti-biofouling nanofibrous membranes. Biofouling 2014, 30, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Fujie, N.; Matsutani, M.; Kinoshita, Y.; Okamura, A.; Saito, S. Takeoka, Adhesive, flexible, and robust polysaccharide nanosheets integrated for tissue-defect repair. Adv. Funct. Mater. 2009, 19, 2560. [Google Scholar] [CrossRef]
- Asare, N.; Instanes, C.; Sandberg, W.J.; Refsnes, M.; Schwarze, P.; Kruszewski, M.; Brunborg, G. Cytotoxic and genotoxic effects of silver nanoparticles in testicular cells. Toxicology 2012, 291, 65. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díez-Pascual, A.M.; Rahdar, A. LbL Nano-Assemblies: A Versatile Tool for Biomedical and Healthcare Applications. Nanomaterials 2022, 12, 949. https://doi.org/10.3390/nano12060949
Díez-Pascual AM, Rahdar A. LbL Nano-Assemblies: A Versatile Tool for Biomedical and Healthcare Applications. Nanomaterials. 2022; 12(6):949. https://doi.org/10.3390/nano12060949
Chicago/Turabian StyleDíez-Pascual, Ana M., and Abbas Rahdar. 2022. "LbL Nano-Assemblies: A Versatile Tool for Biomedical and Healthcare Applications" Nanomaterials 12, no. 6: 949. https://doi.org/10.3390/nano12060949
APA StyleDíez-Pascual, A. M., & Rahdar, A. (2022). LbL Nano-Assemblies: A Versatile Tool for Biomedical and Healthcare Applications. Nanomaterials, 12(6), 949. https://doi.org/10.3390/nano12060949






