Recent Advances in Theoretical Development of Thermal Atomic Layer Deposition: A Review
Abstract
1. Introduction
- What would be the final applications?
- What materials (i.e., precursor, oxidizer, and substrate) should be used?
- How long should the pulse and purge durations be to obtain a high-quality film?
- What would be the growth rate based on the determined conditions?
- What should be the temperature and pressure of the reactor and the precursor bubblers?
- Are all the materials stable in those conditions?
2. Aspects of ALD
2.1. ALD Precursors
2.2. Deposition Characteristics
2.2.1. Growth
2.2.2. Surface Morphology
2.2.3. Surface Roughness
2.2.4. Step Coverage (Conformality)
2.2.5. Deposition Temperature
2.3. Thermal ALD Mechanisms
2.3.1. Mechanisms
2.3.2. Initial Surface Reactions
2.3.3. Reaction Pathways
2.3.4. Precursor Chemisorption
3. Theoretical Methods
3.1. Density Functional Theory
3.2. Microscopic or Atomic Modeling Scale: Molecular Dynamics
3.3. Lattice Boltzmann Method
3.4. Off-Lattice Pseudo-Particle Method: Monte Carlo
3.5. Group Contribution Method
3.6. Computer-Aided Molecular Design
4. Theoretical Studies on ALD
4.1. Precursors
4.2. Deposition Characterization
4.3. Mechanisms
5. Summary, Insights, and Future Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ritala, M.; Leskelä, M. Chapter 2—Atomic layer deposition. In Handbook of Thin Films; Singh Nalwa, H.B.T.-H., Ed.; Academic Press: Burlington, VT, USA, 2002; pp. 103–159. ISBN 978-0-12-512908-4. [Google Scholar]
- Puurunen, R.L. A short history of atomic layer deposition: Tuomo Suntola’s atomic layer epitaxy. Chem. Vap. Depos. 2014, 20, 332–344. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Leskelä, M.; Ritala, M. Atomic Layer Deposition Chemistry: Recent Developments and Future Challenges. Angew. Chemie - Int. Ed. 2003, 42, 5548–5554. [Google Scholar] [CrossRef]
- Oviroh, P.O.; Akbarzadeh, R.; Pan, D.; Coetzee, R.A.M.; Jen, T.-C. New development of atomic layer deposition: Processes, methods and applications. Sci. Technol. Adv. Mater. 2019, 20, 465–496. [Google Scholar] [CrossRef] [PubMed]
- Shahmohammadi, M.; Pensa, E.; Bhatia, H.; Yang, B.; Jursich, G.; Takoudis, C.G. Enhancing the surface properties and functionalization of polymethyl methacrylate with atomic layer-deposited titanium(IV) oxide. J. Mater. Sci. 2020, 55, 17151–17169. [Google Scholar] [CrossRef]
- Knoops, H.C.M.; Potts, S.E.; Bol, A.A.; Kessels, W.M.M. Atomic Layer Deposition. In Handbook of Crystal Growth: Thin Films and Epitaxy; Kuech, T., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 3, pp. 1101–1134. ISBN 9780444633057. [Google Scholar]
- Hagen, D.J.; Pemble, M.E.; Karppinen, M. Atomic layer deposition of metals: Precursors and film growth. Appl. Phys. Rev. 2019, 6, 41309. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Shahmohammadi, M.; Yang, B.; Takoudis, C.G. Applications of Titania Atomic Layer Deposition in the Biomedical Field and Recent Updates. Am. J. Biomed. Sci. Res. 2020, 8, 465–468. [Google Scholar] [CrossRef]
- Tao, Q.; Jursich, G.; Takoudis, C. Selective atomic layer deposition of HfO2 on copper patterned silicon substrates. Appl. Phys. Lett. 2010, 96, 192105. [Google Scholar] [CrossRef]
- Sheng, J.; Han, K.-L.; Hong, T.; Choi, W.-H.; Park, J.-S. Review of recent progresses on flexible oxide semiconductor thin film transistors based on atomic layer deposition processes. J. Semicond. 2018, 39, 11008. [Google Scholar] [CrossRef]
- Majumder, P.; Jursich, G.; Takoudis, C. Structural phase transformation of Y2O3 doped HfO2 films grown on Si using atomic layer deposition. J. Appl. Phys. 2009, 105, 104106. [Google Scholar] [CrossRef]
- Meng, X.; Yang, X.; Sun, X. Emerging applications of atomic layer deposition for lithium-ion battery studies. Adv. Mater. 2012, 24, 3589–3615. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Cavanagh, A.S.; Dillon, A.C.; Groner, M.D.; George, S.M.; Lee, S.-H. Enhanced stability of LiCoO2 cathodes in lithium-ion batteries using surface modification by atomic layer deposition. J. Electrochem. Soc. 2009, 157, A75. [Google Scholar] [CrossRef]
- Cao, Y.-Q.; Wang, S.-S.; Liu, C.; Wu, D.; Li, A.-D. Atomic layer deposition of ZnO/TiO2 nanolaminates as ultra-long life anode material for lithium-ion batteries. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chung, V.P.J.; Santhanam, S.; Mukherjee, T.; Fedder, G.K. Sidewall Metallization on CMOS MEMS by Platinum ALD Patterning. J. Microelectromechanical Syst. 2020, 29, 978–983. [Google Scholar] [CrossRef]
- Fraga, M.; Pessoa, R. Progresses in Synthesis and Application of SiC Films: From CVD to ALD and from MEMS to NEMS. Micromachines 2020, 11, 799. [Google Scholar] [CrossRef]
- Vulpe, S.; Nastase, F.; Dragoman, M.; Dinescu, A.; Romanitan, C.; Iftimie, S.; Moldovan, A.; Apostol, N. Physical properties of the ferroelectric capacitors based on Al-doped HfO2 grown via Atomic Layer Deposition on Si. Appl. Surf. Sci. 2019, 483, 324–333. [Google Scholar] [CrossRef]
- Fang, C.; Wang, M.; Han, P.; Cao, Y.-Q.; Wu, D.; Li, A.-D. High-Performance MIM Capacitors Using Zr-Sn-Ti-O Dielectrics Derived from Atomic Layer Deposition. IEEE Electron. Device Lett. 2019, 40, 682–685. [Google Scholar] [CrossRef]
- Shim, J.H.; Chao, C.-C.; Huang, H.; Prinz, F.B. Atomic layer deposition of yttria-stabilized zirconia for solid oxide fuel cells. Chem. Mater. 2007, 19, 3850–3854. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Kei, C.; Hsueh, Y.; Perng, T. Atomic layer deposition of platinum nanoparticles on carbon nanotubes for application in proton-exchange membrane fuel cells. Small 2009, 5, 1535–1538. [Google Scholar] [CrossRef]
- Li, Y.K.; Choi, H.J.; Kim, H.K.; Chean, N.K.; Kim, M.; Koo, J.; Jeong, H.J.; Jang, D.Y.; Shim, J.H. Nanoporous silver cathodes surface-treated by atomic layer deposition of Y: ZrO2 for high-performance low-temperature solid oxide fuel cells. J. Power Sources 2015, 295, 175–181. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, D.; Grice, C.R.; Liao, W.; Yu, Y.; Cimaroli, A.; Shrestha, N.; Roland, P.J.; Chen, J.; Yu, Z. Low-temperature plasma-enhanced atomic layer deposition of tin oxide electron selective layers for highly efficient planar perovskite solar cells. J. Mater. Chem. A 2016, 4, 12080–12087. [Google Scholar] [CrossRef]
- Standridge, S.D.; Schatz, G.C.; Hupp, J.T. Toward plasmonic solar cells: Protection of silver nanoparticles via atomic layer deposition of TiO2. Langmuir 2009, 25, 2596–2600. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Fang, X.; Lv, M.; Lin, B.; Zhang, S.; Ding, J.; Yuan, N. Improvement of the humidity stability of organic–inorganic perovskite solar cells using ultrathin Al2O3 layers prepared by atomic layer deposition. J. Mater. Chem. A 2015, 3, 5360–5367. [Google Scholar] [CrossRef]
- Sheng, J.; Park, E.J.; Shong, B.; Park, J.-S. Atomic layer deposition of an indium gallium oxide thin film for thin-film transistor applications. ACS Appl. Mater. Interfaces 2017, 9, 23934–23940. [Google Scholar] [CrossRef]
- Lim, S.J.; Kwon, S.; Kim, H.; Park, J.-S. High performance thin film transistor with low temperature atomic layer deposition nitrogen-doped ZnO. Appl. Phys. Lett. 2007, 91, 183517. [Google Scholar] [CrossRef]
- Kwon, S.; Bang, S.; Lee, S.; Jeon, S.; Jeong, W.; Kim, H.; Gong, S.C.; Chang, H.J.; Park, H.; Jeon, H. Characteristics of the ZnO thin film transistor by atomic layer deposition at various temperatures. Semicond. Sci. Technol. 2009, 24, 35015. [Google Scholar] [CrossRef]
- Choi, J.H.; Jung, C.H.; Hwang, I.T.; Choi, J.H. Preparation and characterization of crosslinked poly(butylene adipate-co-terephthalate)/polyhedral oligomeric silsesquioxane nanocomposite by electron beam irradiation. Radiat. Phys. Chem. 2013, 82, 100–105. [Google Scholar] [CrossRef]
- Narayan, R.J.; Adiga, S.P.; Pellin, M.J.; Curtiss, L.A.; Stafslien, S.; Chisholm, B.; Monteiro-Riviere, N.A.; Brigmon, R.L.; Elam, J.W. Atomic layer deposition of nanoporous biomaterials. Mater. Today 2010, 13, 60–64. [Google Scholar] [CrossRef]
- Skoog, S.A.; Elam, J.W.; Narayan, R.J. Atomic layer deposition: Medical and biological applications. Int. Mater. Rev. 2013, 58, 113–129. [Google Scholar] [CrossRef]
- Narayan, R.J.; Monteiro-Riviere, N.A.; Brigmon, R.L.; Pellin, M.J.; Elam, J.W. Atomic layer deposition of TiO2 thin films on nanoporous alumina templates: Medical applications. JOM 2009, 61, 12–16. [Google Scholar] [CrossRef]
- Bishal, A.K.; Sukotjo, C.; Jokisaari, J.R.; Klie, R.F.; Takoudis, C.G. Enhanced Bioactivity of Collagen Fiber Functionalized with Room Temperature Atomic Layer Deposited Titania. ACS Appl. Mater. Interfaces 2018, 10, 34443–34454. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Rieth, L.; Caldwell, R.; Diwekar, M.; Tathireddy, P.; Sharma, R.; Solzbacher, F. Long-Term Bilayer Encapsulation Performance of Atomic Layer Deposited Al2O3 and Parylene C for Biomedical Implantable Devices. IEEE Trans. Biomed. Eng. 2013, 60, 2943–2951. [Google Scholar]
- Nazarov, D.V.; Smirnov, V.M.; Zemtsova, E.G.; Yudintceva, N.M.; Shevtsov, M.A.; Valiev, R.Z. Enhanced osseointegrative properties of ultra-fine-grained titanium implants modified by chemical etching and atomic layer deposition. ACS Biomater. Sci. Eng. 2018, 4, 3268–3281. [Google Scholar] [CrossRef]
- Liu, L.; Bhatia, R.; Webster, T.J. Atomic layer deposition of nano-TiO2 thin films with enhanced biocompatibility and antimicrobial activity for orthopedic implants. Int. J. Nanomedicine 2017, 12, 8711–8723. [Google Scholar] [CrossRef] [PubMed]
- Devlin-Mullin, A.; Todd, N.M.; Golrokhi, Z.; Geng, H.; Konerding, M.A.; Ternan, N.G.; Hunt, J.A.; Potter, R.J.; Sutcliffe, C.; Jones, E. Atomic layer deposition of a silver nanolayer on advanced titanium orthopedic implants inhibits bacterial colonization and supports vascularized de novo bone ingrowth. Adv. Healthc. Mater. 2017, 6, 1700033. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Selvaraj, S.K.; Choi, Y.-Y.; Hong, S.; Nakhmanson, S.M.; Takoudis, C. Atomic layer deposition of environmentally benign SnTiOx as a potential ferroelectric material. J. Vac. Sci. Technol. A Vacuum, Surfaces, Film. 2016, 34, 01A119. [Google Scholar] [CrossRef]
- Koveshnikov, S.; Goel, N.; Majhi, P.; Wen, H.; Santos, M.B.; Oktyabrsky, S.; Tokranov, V.; Kambhampati, R.; Moore, R.; Zhu, F. In 0.53 Ga 0.47 As based metal oxide semiconductor capacitors with atomic layer deposition ZrO2 gate oxide demonstrating low gate leakage current and equivalent oxide thickness less than 1 nm. ApPhL 2008, 92, 222904. [Google Scholar]
- Darwish, G.; Huang, S.; Knoernschild, K.; Sukotjo, C.; Campbell, S.; Bishal, A.K.; Wu, C.D.; Takoudis, C.G.; Adelino, V.; Yang, B. Improving Polymethyl Methacrylate Resin Using a Novel Titanium Dioxide Coating. J. Prosthodont. 2019, 28, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Mallick, B.C.; Hsieh, C.-T.; Yin, K.-M.; Gandomi, Y.A.; Huang, K.-T. Review—On Atomic Layer Deposition: Current Progress and Future Challenges. ECS J. Solid State Sci. Technol. 2019, 8, N55–N78. [Google Scholar] [CrossRef]
- Bishal, A.K.; Butt, A.; Selvaraj, S.K.; Joshi, B.; Patel, S.B.; Yang, B.; Shukohfar, T.; Sukotjo, C.; Takoudis, C.G. Atomic Layer Deposition in Bio-Nanotechnology: A Brief Overview. Crit. Rev. Biomed. Eng. 2015, 43, 255–276. [Google Scholar] [CrossRef] [PubMed]
- He, W. ALD: Atomic Layer Deposition-Precise and Conformal Coating for Better Performance; Springer: London, UK, 2015. [Google Scholar]
- Hämäläinen, J.; Ritala, M.; Leskelä, M. Atomic layer deposition of noble metals and their oxides. Chem. Mater. 2014, 26, 786–801. [Google Scholar] [CrossRef]
- Ritala, M. Atomic layer deposition. In High-K Gate Dielectrics; Elsevier: Amsterdam, The Netherlands, 2003; pp. 17–64. ISBN 9781420034141. [Google Scholar]
- Muñoz-Rojas, D.; Nguyen, V.H.; de la Huerta, C.M.; Aghazadehchors, S.; Jiménez, C.; Bellet, D. Spatial Atomic Layer Deposition (SALD), an emerging tool for energy materials. Application to new-generation photovoltaic devices and transparent conductive materials. Comptes Rendus Phys. 2017, 18, 391–400. [Google Scholar] [CrossRef]
- Dey, G.; Elliott, S.D. Copper(I) carbene hydride complexes acting both as reducing agent and precursor for Cu ALD: A study through density functional theory. Theor. Chem. Acc. 2014, 133, 1–7. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. Atomic layer deposition (ALD): From precursors to thin film structures. Thin Solid Films 2002, 409, 138–146. [Google Scholar] [CrossRef]
- Huang, S. Improving Polymethyl Methacrylate Resin Using Novel Nano-Ceramic Coating. Master dissertation, University of Illinois at Chicago, Chicago, IL, USA, 2017. [Google Scholar]
- Majumder, P.; Katamreddy, R.; Takoudis, C. Effect of film thickness on the breakdown temperature of atomic layer deposited ultrathin HfO2 and Al2O3 diffusion barriers in copper metallization. J. Cryst. Growth 2007, 309, 12–17. [Google Scholar] [CrossRef]
- Xu, R.; Tao, Q.; Yang, Y.; Takoudis, C.G. Atomic layer deposition and characterization of stoichiometric erbium oxide thin dielectrics on Si (100) using (CpMe)3Er precursor and ozone. Appl. Surf. Sci. 2012, 258, 8514–8520. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. ALD precursor chemistry: Evolution and future challenges. Le J. Phys. IV 1999, 9, Pr8–Pr837. [Google Scholar] [CrossRef]
- Parker, W.D.; Rondinelli, J.M.; Nakhmanson, S.M. First-principles study of misfit strain-stabilized ferroelectric SnTiO3. Phys. Rev. B 2011, 84, 245126. [Google Scholar] [CrossRef]
- Agarwal, R.; Sharma, Y.; Chang, S.; Pitike, K.C.; Sohn, C.; Nakhmanson, S.M.; Takoudis, C.G.; Lee, H.N.; Tonelli, R.; Gardner, J. Room-temperature relaxor ferroelectricity and photovoltaic effects in tin titanate directly deposited on a silicon substrate. Phys. Rev. B 2018, 97, 54109. [Google Scholar] [CrossRef]
- Puurunen, R.L. Random Deposition as a Growth Mode in Atomic Layer Deposition. Chem. Vap. Depos. 2004, 10, 159–170. [Google Scholar] [CrossRef]
- Fornari, C.I.; Fornari, G.; Paulo, H.d.O.; Abramof, E.; dos Travelho, J.S. Monte Carlo Simulation of Epitaxial Growth. In Epitaxy; Zhong, M., Ed.; BoD–Books on Demand: Norderstedt, Germany, 2018; p. 113. [Google Scholar]
- Venables, J. Surface processes in epitaxial growth. In Introduction to Surface and Thin Film Processes; Cambridge University Press: Cambridge, UK, 2000; pp. 144–151. ISBN 0521785006. [Google Scholar]
- Itagaki, N.; Nakamura, Y.; Narishige, R.; Takeda, K.; Kamataki, K.; Koga, K.; Hori, M.; Shiratani, M. Growth of single crystalline films on lattice-mismatched substrates through 3D to 2D mode transition. Sci. Rep. 2020, 10, 4669. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.O.; Martin, P.M. Chemical Vapor Deposition; Third Edit.; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; ISBN 9780815520313. [Google Scholar]
- Barna, P.B.; Radnóczi, G. 3—Structure formation during deposition of polycrystalline metallic thin films. In Coffey Optical and Magnetic Applications; Barmak, K., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 67–120. ISBN 978-0-85709-057-7. [Google Scholar]
- Vandalon, V.; Kessels, W.M.M. Revisiting the growth mechanism of atomic layer deposition of Al2O3: A vibrational sum-frequency generation study. J. Vac. Sci. Technol. A Vacuum, Surfaces, Film. 2017, 35, 05C313. [Google Scholar] [CrossRef]
- Cremers, V.; Puurunen, R.L.; Dendooven, J. Conformality in atomic layer deposition: Current status overview of analysis and modelling. Appl. Phys. Rev. 2019, 6, 21302. [Google Scholar] [CrossRef]
- Dezelah, C.L. Atomic Layer Deposition BT-Encyclopedia of Nanotechnology. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2012; pp. 161–171. ISBN 978-90-481-9751-4. [Google Scholar]
- Bishal, A.K.; Sukotjo, C.; Takoudis, C.G. Room temperature TiO2 atomic layer deposition on collagen membrane from a titanium alkylamide precursor. J. Vac. Sci. Technol. A Vacuum Surfaces Film. 2017, 35, 01B134. [Google Scholar] [CrossRef]
- Puurunen, R.L. Growth per cycle in atomic layer deposition: A theoretical model. Chem. Vap. Depos. 2003, 9, 249–257. [Google Scholar] [CrossRef]
- Kim, J.; Kim, T.W. Initial surface reactions of atomic layer deposition. Jom 2009, 61, 17–22. [Google Scholar] [CrossRef]
- Leem, J.; Park, I.; Li, Y.; Zhou, W.; Jin, Z.; Shin, S.; Min, Y.S. Role of HCl in atomic layer deposition of TiO2 thin films from titanium tetrachloride and water. Bull. Korean Chem. Soc. 2014, 35, 1195–1201. [Google Scholar] [CrossRef]
- Matero, R.; Rahtu, A.; Ritala, M. In situ quadrupole mass spectrometry and quartz crystal microbalance studies on the atomic layer deposition of titanium dioxide from titanium tetrachloride and water. Chem. Mater. 2001, 13, 4506–4511. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Zhang, D.W. Density functional theory study of initial stage of HfO2 atomic layer deposition on hydroxylated SiO2 surface. J. Mol. Struct. Theochem 2007, 803, 23–28. [Google Scholar] [CrossRef]
- Lownsbury, J.M.; Gladden, J.A.; Campbell, C.T.; Kim, I.S.; Martinson, A.B.F. Direct Measurements of Half-Cycle Reaction Heats during Atomic Layer Deposition by Calorimetry. Chem. Mater. 2017, 29, 8566–8577. [Google Scholar] [CrossRef]
- Richey, N.E.; De Paula, C.; Bent, S.F. Understanding chemical and physical mechanisms in atomic layer deposition. J. Chem. Phys. 2020, 152, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Puurunen, R.L. Correlation between the growth-per-cycle and the surface hydroxyl group concentration in the atomic layer deposition of aluminum oxide from trimethylaluminum and water. Appl. Surf. Sci. 2005, 245, 6–10. [Google Scholar] [CrossRef]
- Mui, C.; Musgrave, C.B. Atomic layer deposition of HfO2 using alkoxides as precursors. J. Phys. Chem. B 2004, 108, 15150–15164. [Google Scholar] [CrossRef]
- Hu, X.; Schuster, J.; Schulz, S.E.; Gessner, T. Surface chemistry of copper metal and copper oxide atomic layer deposition from copper(ii) acetylacetonate: A combined first-principles and reactive molecular dynamics study. Phys. Chem. Chem. Phys. 2015, 17, 26892–26902. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cao, K.; Hu, Q.; Wen, Y.; Liu, X.; Chen, R.; Shan, B. Unravelling the selective growth mechanism of AlOX with dimethylaluminum isopropoxide as a precursor in atomic layer deposition: A combined theoretical and experimental study. J. Mater. Chem. A 2020, 8, 4308–4317. [Google Scholar] [CrossRef]
- Hu, Z.; Turner, C.H. Initial surface reactions of TiO2 atomic layer deposition onto SiO2 surfaces: Density functional theory calculations. J. Phys. Chem. B 2006, 110, 8337–8347. [Google Scholar] [CrossRef]
- Elliott, S.D. Predictive process design: A theoretical model of atomic layer deposition. Comput. Mater. Sci. 2005, 33, 20–25. [Google Scholar] [CrossRef]
- Shirazi, M.; Elliott, S.D. Multiple proton diffusion and film densification in atomic layer deposition modeled by density functional theory. Chem. Mater. 2013, 25, 878–889. [Google Scholar] [CrossRef]
- Cui, C.; Ren, J. A density functional theory study on the reactions of chlorine loss in ZrO2 thin films by atomic-layer deposition. Comput. Theor. Chem. 2012, 979, 38–43. [Google Scholar] [CrossRef]
- Athavale, S.D.; Economou, D.J. Molecular dynamics simulation of atomic layer etching of silicon. J. Vac. Sci. Technol. A Vacuum, Surfaces, Film. 1995, 13, 966–971. [Google Scholar] [CrossRef]
- Bahramian, A. Study on growth rate of TiO2 nanostructured thin films: Simulation by molecular dynamics approach and modeling by artificial neural network. Surf. Interface Anal. 2013, 45, 1727–1736. [Google Scholar] [CrossRef]
- Brown, K.S.; Saggese, C.; Le Monnier, B.P.; Héroguel, F.; Luterbacher, J.S. Simulation of Gas- and Liquid-Phase Layer-By-Layer Deposition of Metal Oxides by Coarse-Grained Modeling. J. Phys. Chem. C 2018, 122, 6713–6720. [Google Scholar] [CrossRef]
- He, X.; Luo, L. Theory of the lattice Boltzmann method: From the Boltzmann equation to the lattice Boltzmann equation. Phys. Rev. E 1997, 56, 6811–6817. [Google Scholar] [CrossRef]
- Chen, S.; Doolen, G.D. Lattice Boltzmann method for fluid flows. Annu. Rev. Fluid Mech. 1998, 30, 329–364. [Google Scholar] [CrossRef]
- Pan, D.; Li, T.; Chien Jen, T.; Yuan, C. Numerical modeling of carrier gas flow in atomic layer deposition vacuum reactor: A comparative study of lattice Boltzmann models. J. Vac. Sci. Technol. A Vacuum, Surfaces, Film. 2014, 32, 01A110. [Google Scholar] [CrossRef]
- Ernst, M.; Dietzel, M.; Sommerfeld, M. A lattice Boltzmann method for simulating transport and agglomeration of resolved particles. Acta Mech. 2013, 224, 2425–2449. [Google Scholar] [CrossRef]
- Harrison, R.L. Introduction to Monte Carlo simulation. AIP Conf. Proc. 2009, 1204, 17–21. [Google Scholar] [CrossRef]
- Mordechai, S. Applications of Monte Carlo Method in Science and Engineering; IntechOpen: London, UK, 2011; ISBN 9533076917. [Google Scholar]
- Brandimarte, P. Handbook in Monte Carlo simulation: Applications in Financial Engineering, Risk Management, and Economics; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 1118593642. [Google Scholar]
- Kwak, Y.H.; Ingall, L. Exploring Monte Carlo simulation applications for project management. Risk Manag. 2007, 9, 44–57. [Google Scholar] [CrossRef]
- Bird, G.A. Monte-Carlo simulation in an engineering context. Prog. Astronaut. Aeronaut. 1981, 74, 239–255. [Google Scholar]
- Schwille, M.C.; Schössler, T.; Barth, J.; Knaut, M.; Schön, F.; Höchst, A.; Oettel, M.; Bartha, J.W. Experimental and simulation approach for process optimization of atomic layer deposited thin films in high aspect ratio 3D structures. J. Vac. Sci. Technol. A Vac. Surf. Film. 2017, 35, 01B118. [Google Scholar] [CrossRef]
- Jin, L.; Li, Y.; Hu, Z.; Chu, J. Full three-dimensional morphology evolution of amorphous thin films for atomic layer deposition. AIP Adv. 2018, 8, 045304. [Google Scholar] [CrossRef]
- Deminsky, M.; Knizhnik, A.; Belov, I.; Umanskii, S.; Rykova, E.; Bagatur, A.; Potapkin, B.; Stoker, M.; Korkin, A.; Bagatur’yants, A.; et al. Mechanism and kinetics of thin zirconium and hafnium oxide film growth in an ALD reactor. Surf. Sci. 2004, 549, 67–86. [Google Scholar] [CrossRef]
- Adomaitis, R.A. Development of a multiscale model for an atomic layer deposition process. J. Cryst. Growth 2010, 312, 1449–1452. [Google Scholar] [CrossRef]
- Weckman, T.; Shirazi, M.; Elliott, S.D.; Laasonen, K. Kinetic Monte Carlo Study of the Atomic Layer Deposition of Zinc Oxide. J. Phys. Chem. C 2018, 122, 27044–27058. [Google Scholar] [CrossRef]
- Berti, C.; Ulbig, P.; Burdorf, A.; Seippel, J.; Schulz, S. Correlation and prediction of liquid-phase adsorption on zeolites using group contributions based on adsorbate-solid solution theory. Langmuir 1999, 15, 6035–6042. [Google Scholar] [CrossRef]
- Khalifa, M.; Lue, L. A group contribution method for predicting the solubility of mercury. Fluid Phase Equilib. 2017, 432, 76–84. [Google Scholar] [CrossRef]
- Shahmohammadi, M.; Mukherjee, R.; Takoudis, C.G.; Diwekar, U.M. Optimal design of novel precursor materials for the atomic layer deposition using computer-aided molecular design. Chem. Eng. Sci. 2021, 234, 116416. [Google Scholar] [CrossRef]
- Shahmohammadi, M.; Mukherjee, R.; Takoudis, C.G.; Diwekar, U.M. Quantification of Water Impurity in an Atomic Layer Deposition Reactor Using Group Contribution Method. Res. Dev. Mater. Sci. 2021, 15, 1703–1706. [Google Scholar] [CrossRef]
- Kim, K.-J.; Diwekar, U.M. Efficient combinatorial optimization under uncertainty. 2. Application to stochastic solvent selection. Ind. Eng. Chem. Res. 2002, 41, 1285–1296. [Google Scholar] [CrossRef]
- Cheng, H.-C.; Wang, F.-S. Optimal biocompatible solvent design for a two-stage extractive fermentation process with cell recycling. Comput. Chem. Eng. 2008, 32, 1385–1396. [Google Scholar] [CrossRef]
- Gebreslassie, B.H.; Diwekar, U.M. Efficient ant colony optimization for computer aided molecular design: Case study solvent selection problem. Comput. Chem. Eng. 2015, 78, 1–9. [Google Scholar] [CrossRef]
- Giovanoglou, A.; Barlatier, J.; Adjiman, C.S.; Pistikopoulos, E.N.; Cordiner, J.L. Optimal solvent design for batch separation based on economic performance. AIChE J. 2003, 49, 3095–3109. [Google Scholar] [CrossRef]
- Kim, K.-J.; Diwekar, U.M.; TOMAZI, K.G. Entrainer selection and solvent recycling in complex batch distillation. Chem. Eng. Commun. 2004, 191, 1606–1633. [Google Scholar] [CrossRef]
- Marcoulaki, E.C.; Kokossis, A.C. On the development of novel chemicals using a systematic optimisation approach. Part II. Solvent design. Chem. Eng. Sci. 2000, 55, 2547–2561. [Google Scholar] [CrossRef]
- Samudra, A.P.; Sahinidis, N.V. Optimization-based framework for computer-aided molecular design. AIChE J. 2013, 59, 3686–3701. [Google Scholar] [CrossRef]
- Hostrup, M.; Harper, P.M.; Gani, R. Design of environmentally benign processes: Integration of solvent design and separation process synthesis. Comput. Chem. Eng. 1999, 23, 1395–1414. [Google Scholar] [CrossRef]
- Mukherjee, R.; Gebreslassie, B.; Diwekar, U.M. Design of novel polymeric adsorbents for metal ion removal from water using computer-aided molecular design. Clean Technol. Environ. Policy 2017, 19, 483–499. [Google Scholar] [CrossRef]
- Trevizo, C.; Daniel, D.; Nirmalakhandan, N. Screening alternative degreasing solvents using multivariate analysis. Environ. Sci. Technol. 2000, 34, 2587–2595. [Google Scholar] [CrossRef]
- Chemmangattuvalappil, N.G.; Eljack, F.T.; Solvason, C.C.; Eden, M.R. A novel algorithm for molecular synthesis using enhanced property operators. Comput. Chem. Eng. 2009, 33, 636–643. [Google Scholar] [CrossRef]
- Sinha, M.; Achenie, L.E.K. Systematic design of blanket wash solvents with recovery considerations. Adv. Environ. Res. 2001, 5, 239–249. [Google Scholar] [CrossRef]
- Eden, M.R.; Jørgensen, S.B.; Gani, R.; El-Halwagi, M.M. A novel framework for simultaneous separation process and product design. Chem. Eng. Process. Process Intensif. 2004, 43, 595–608. [Google Scholar] [CrossRef]
- Eljack, F.T.; Eden, M.R. A systematic visual approach to molecular design via property clusters and group contribution methods. Comput. Chem. Eng. 2008, 32, 3002–3010. [Google Scholar] [CrossRef]
- Odele, O.; Macchietto, S. Computer aided molecular design: A novel method for optimal solvent selection. Fluid Phase Equilib. 1993, 82, 47–54. [Google Scholar] [CrossRef]
- Pistikopoulos, E.N.; Stefanis, S.K. Optimal solvent design for environmental impact minimization. Comput. Chem. Eng. 1998, 22, 717–733. [Google Scholar] [CrossRef]
- Salazar, J.; Diwekar, U.M.; Joback, K.; Berger, A.H.; Bhown, A.S. Solvent selection for post-combustion CO2 capture. Energy Procedia 2013, 37, 257–264. [Google Scholar] [CrossRef][Green Version]
- Churi, N.; Achenie, L.E.K. Novel mathematical programming model for computer aided molecular design. Ind. Eng. Chem. Res. 1996, 35, 3788–3794. [Google Scholar] [CrossRef]
- Duvedi, A.P.; Achenie, L.E.K. Designing environmentally safe refrigerants using mathematical programming. Chem. Eng. Sci. 1996, 51, 3727–3739. [Google Scholar] [CrossRef]
- Xu, W.; Diwekar, U.M. Environmentally friendly heterogeneous azeotropic distillation system design: Integration of EBS selection and IPS recycling. Ind. Eng. Chem. Res. 2005, 44, 4061–4067. [Google Scholar] [CrossRef]
- Xu, W.; Diwekar, U.M. Multi-objective integrated solvent selection and solvent recycling under uncertainty using a new genetic algorithm. Int. J. Environ. Pollut. 2007, 29, 70–89. [Google Scholar] [CrossRef]
- Folic, M.; Adjiman, C.S.; Pistikopoulos, E.N. Computer-aided solvent design for reactions: Maximizing product formation. Ind. Eng. Chem. Res. 2008, 47, 5190–5202. [Google Scholar] [CrossRef]
- Lin, B.; Chavali, S.; Camarda, K.; Miller, D.C. Computer-aided molecular design using Tabu search. Comput. Chem. Eng. 2005, 29, 337–347. [Google Scholar] [CrossRef]
- Camarda, K.V.; Sunderesan, P. An Optimization Approach to the Design of Value-Added Soybean Oil Products. Ind. Eng. Chem. Res. 2005, 44, 4361–4367. [Google Scholar] [CrossRef]
- Karunanithi, A.T.; Achenie, L.E.K.; Gani, R. A computer-aided molecular design framework for crystallization solvent design. Chem. Eng. Sci. 2006, 61, 1247–1260. [Google Scholar] [CrossRef]
- Yamamoto, H.; Tochigi, K. Computer-aided molecular design to select foaming agents using a neural network method. Ind. Eng. Chem. Res. 2008, 47, 5152–5156. [Google Scholar] [CrossRef]
- Benavides, P.T.; Gebreslassie, B.H.; Diwekar, U.M. Optimal design of adsorbents for NORM removal from produced water in natural gas fracking. Part 2: CAMD for adsorption of radium and barium. Chem. Eng. Sci. 2015, 137, 977–985. [Google Scholar] [CrossRef]
- Doshi, R.K.; Mukherjee, R.; Diwekar, U.M. Application of Adsorbate Solid Solution Theory To Design Novel Adsorbents for Arsenic Removal Using CAMD. ACS Sustain. Chem. Eng. 2018, 6, 2603–2611. [Google Scholar] [CrossRef]
- Qi, F.X.Y.; Liu, K.; Ma, D.K.; Cai, F.F.; Liu, M.; Xu, Q.L.; Chen, W.; Qi, C.Z.; Yang, D.P.; Huang, S.M. Dual active sites fabricated through atomic layer deposition of TiO2 on MoS2 nanosheet arrays for highly efficient electroreduction of CO2 to ethanol. J. Mater. Chem. A 2021, 9, 6790–6796. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, S.H.; Kim, B.S.; Kim, Y.; Jeon, W. Modulation of the adsorption chemistry of a precursor in atomic layer deposition to enhance the growth per cycle of a TiO2 thin film. Phys. Chem. Chem. Phys. 2021, 23, 2568–2574. [Google Scholar] [CrossRef]
- Park, B.E.; Oh, I.K.; Lee, C.W.; Lee, G.; Shin, Y.H.; Lansalot-Matras, C.; Noh, W.; Kim, H.; Lee, H.B.R. Effects of Cl-Based Ligand Structures on Atomic Layer Deposited HfO2. J. Phys. Chem. C 2016, 120, 5958–5967. [Google Scholar] [CrossRef]
- Sasinska, A.; Bialuschewski, D.; Islam, M.M.; Singh, T.; Deo, M.; Mathur, S. Experimental and Theoretical Insights into Influence of Hydrogen and Nitrogen Plasma on the Water Splitting Performance of ALD Grown TiO2 Thin Films. J. Phys. Chem. C 2017, 121, 15538–15548. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Vasylenko, A.; Viter, R.; Kempinski, M.; Nowaczyk, G.; Jurga, S.; Bechelany, M. Tailoring of the electronic properties of ZnO-polyacrylonitrile nanofibers: Experiment and theory. Appl. Surf. Sci. 2017, 411, 494–501. [Google Scholar] [CrossRef]
- Sarkar, S.; Patel, R.L.; Liang, X.H.; Park, J. Unveiling the Role of CeO2 Atomic Layer Deposition Coatings on LiMn2O4 Cathode Materials: An Experimental and Theoretical Study. ACS Appl. Mater. Interfaces 2017, 9, 30599–30607. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Chae, J.; Hong, S.B.; Park, H.; Jeong, K.S.; Park, H.W.; Kwon, S.R.; Chung, K.B.; Cho, M.H. Interface engineering for a stable chemical structure of oxidized-black phosphorus via self-reduction in AlOx atomic layer deposition. Nanoscale 2018, 10, 22896–22907. [Google Scholar] [CrossRef] [PubMed]
- Lawniczak-Jablonska, K.; Wolska, A.; Kuzmiuk, P.; Rejmak, P.; Kosiel, K. Local atomic order of the amorphous TaOx thin films in relation to their chemical resistivity. RSC Adv. 2019, 9, 35727–35734. [Google Scholar] [CrossRef]
- Lee, S.; Baek, G.; Lee, J.H.; Van, T.T.N.; Ansari, A.; Shong, B.; Park, J.S. Molecular layer deposition of indicone and organic-inorganic hybrid thin films as flexible transparent conductor. Appl. Surf. Sci. 2020, 525, 146383. [Google Scholar] [CrossRef]
- Song, Z.X.; Zhu, Y.N.; Liu, H.S.; Banis, M.N.; Zhang, L.; Li, J.J.; Doyle-Davis, K.; Li, R.Y.; Sham, T.K.; Yang, L.J.; et al. Engineering the Low Coordinated Pt Single Atom to Achieve the Superior Electrocatalytic Performance toward Oxygen Reduction. SMALL 2020, 16, 2003096. [Google Scholar] [CrossRef]
- Choi, S.; Ansari, A.; Yun, H.J.; Kim, H.; Shong, B.; Choi, B.J. Growth of Al-rich AlGaN thin films by purely thermal atomic layer deposition. J. Alloys Compd. 2021, 854, 157186. [Google Scholar] [CrossRef]
- He, Y.F.; Pham, H.; Gao, Y.; Patel, R.L.; Sarkar, S.; Liang, X.H.; Park, J. Discovery of an Unexpected Metal Dissolution of Thin-Coated Cathode Particles and Its Theoretical Explanation. Adv. Theory Simulations 2020, 3, 2000002. [Google Scholar] [CrossRef]
- Zou, F.; Liu, Y.; Mou, C.; Zhu, S. Optimization of Refractive Index Sensitivity in Nanofilm-Coated Long-Period Fiber Gratings near the Dispersion Turning Point. J. Light. Technol. 2020, 38, 889–897. [Google Scholar] [CrossRef]
- Bermudez, V.M. Theoretical study of the adsorption of Lewis acids on MoS2 in relation to atomic layer deposition of Al2O3. J. Vac. Sci. Technol. A 2020, 38, 062412. [Google Scholar] [CrossRef]
- Petersen, M. Theoretical study of reaction mechanisms of ZrCl4 with hydrated and hydroxlated Si(100) surfaces. Comput. Mater. Sci. 2004, 30, 77–80. [Google Scholar] [CrossRef]
- Jung, J.-S.S.; Lee, S.-K.K.; Hong, C.-S.S.; Shin, J.-H.H.; Kim, J.-M.M.; Kang, J.-G.G. Atomic layer deposition of ZrO2 thin film on Si (100) using {η5: η1-Cp (CH2) 3NMe} Zr (NMe2) 2/O3 as precursors. Thin Solid Films 2015, 589, 831–837. [Google Scholar] [CrossRef]
- Gharachorlou, A.; Detwiler, M.D.; Gu, X.K.; Mayr, L.; Klötzer, B.; Greeley, J.; Reifenberger, R.G.; Delgass, W.N.; Ribeiro, F.H.; Zemlyanov, D.Y. Trimethylaluminum and Oxygen Atomic Layer Deposition on Hydroxyl-Free Cu(111). ACS Appl. Mater. Interfaces 2015, 7, 16428–16439. [Google Scholar] [CrossRef]
- Seo, S.; Yeo, B.C.; Han, S.S.; Yoon, C.M.; Yang, J.Y.; Yoon, J.; Yoo, C.; Kim, H.J.; Lee, Y.B.; Lee, S.J.; et al. Reaction Mechanism of Area-Selective Atomic Layer Deposition for Al2O3 Nanopatterns. ACS Appl. Mater. Interfaces 2017, 9, 41607–41617. [Google Scholar] [CrossRef]
- Karasulu, B.; Vervuurt, R.H.J.; Kessels, W.M.M.; Bol, A.A. Continuous and ultrathin platinum films on graphene using atomic layer deposition: A combined computational and experimental study. Nanoscale 2016, 8, 19829–19845. [Google Scholar] [CrossRef]
- Pan, D.; Ma, L.; Xie, Y.; Jen, T.C.; Yuan, C. On the physical and chemical details of alumina atomic layer deposition: A combined experimental and numerical approach. J. Vac. Sci. Technol. A Vacuum, Surfaces, Film. 2015, 33, 021511. [Google Scholar] [CrossRef]
- Halder, A.; Lenardi, C.; Timoshenko, J.; Mravak, A.; Yang, B.; Kolipaka, L.K.; Piazzoni, C.; Seifert, S.; Bonacic-Koutecky, V.; Frenkel, A.I.; et al. CO2 Methanation on Cu-Cluster Decorated Zirconia Supports with Different Morphology: A Combined Experimental In Situ GIXANES/GISAXS, Ex Situ XPS and Theoretical DFT Study. ACS Catal. 2021, 11, 6210–6224. [Google Scholar] [CrossRef]
- Perevalov, T.V.; Prosvirin, I.P.; Suprun, E.A.; Mehmood, F.; Mikolajick, T.; Schroeder, U.; Gritsenko, V.A. The atomic and electronic structure of Hf0.5Zr0.5O2 and Hf0.5Zr0.5O2:La films. J. Sci. Mater. DEVICES 2021, 6, 595–600. [Google Scholar] [CrossRef]
- Yu, N.K.; Moon, C.H.; Park, J.; Lee, H.B.R.; Shong, B. Evaluation of silicon tetrahalide precursors for low-temperature thermal atomic layer deposition of silicon nitride. Appl. Surf. Sci. 2021, 565, 150603. [Google Scholar] [CrossRef]
- Beer, S.M.J.; Boysen, N.; Muriqi, A.; Zanders, D.; Berning, T.; Rogalla, D.; Bock, C.; Nolan, M.; Devi, A. A study on the influence of ligand variation on formamidinate complexes of yttrium: New precursors for atomic layer deposition of yttrium oxide. Dalt. Trans. 2021, 50, 12944–12956. [Google Scholar] [CrossRef] [PubMed]
- Borbon-Nunez, H.A.; Muniz, J.; El Hachimi, A.G.; Frausto-Silva, D.; Gutierrez-Diaz, J.L.; Dominguez, D.; Tiznado, H.; Cuentas-Gallegos, A.K. Effect of oxygen based functional groups on the nucleation of TiO2 by atomic layer deposition: A theoretical and experimental study. Mater. Chem. Phys. 2021, 267, 124588. [Google Scholar] [CrossRef]
- Boukhari, A.; Deghfel, B.; Mahroug, A.; Amari, R.; Selmi, N.; Kheawhom, S.; Mohamad, A.A. Thickness effect on the properties of Mn-doped ZnO thin films synthesis by sol-gel and comparison to first-principles calculations. Ceram. Int. 2021, 47, 17276–17285. [Google Scholar] [CrossRef]
- Zhu, X.P.; Guo, J.J.; Li, X.X.; Zhou, R.D.; Wang, D.; Zhao, W. Evolvement Investigation of Secondary Electron Emission for Ultrathin MgO Coatings Prepared by Atomic Layer Deposition. Appl. Sci. 2021, 11, 4801. [Google Scholar] [CrossRef]
- Weckman, T.; Laasonen, K. First principles study of the atomic layer deposition of alumina by TMA-H2O-process. Phys. Chem. Chem. Phys. 2015, 17, 17322–17334. [Google Scholar] [CrossRef]
- Travis, C.D.; Adomaitis, R.A. Modeling alumina atomic layer deposition reaction kinetics during the trimethylaluminum exposure. Theor. Chem. Acc. 2014, 133, 3–11. [Google Scholar] [CrossRef]
- Xu, K.; Ye, P.D. Theoretical study of atomic layer deposition reaction mechanism and kinetics for aluminum oxide formation at graphene nanoribbon open edges. J. Phys. Chem. C 2010, 114, 10505–10511. [Google Scholar] [CrossRef]
- Kayanuma, M.; Choe, Y.-K.; Hagiwara, T.; Kameda, N.; Shimoi, Y. Theoretical Study of the Mechanism for the Reaction of Trimethylaluminum with Ozone. ACS Omega 2021, 6, 26282–26292. [Google Scholar] [CrossRef]
- Puurunen, R.L. Growth per cycle in atomic layer deposition: Real application examples of a theoretical model. Chem. Vap. Depos. 2003, 9, 327–332. [Google Scholar] [CrossRef]
- Burgess, D.R.; Maslar, J.E.; Hurst, W.S.; Moore, E.F.; Kimes, W.A.; Fink, R.R.; Nguyen, N. V Atomic Layer Deposition—Process Models and Metrologies. AIP Conf. Proc. 2005, 788, 141–146. [Google Scholar] [CrossRef]
- Seo, S.; Nam, T.; Lee, H.B.R.; Kim, H.; Shong, B. Molecular oxidation of surface –CH3 during atomic layer deposition of Al2O3 with H2O, H2O2, and O3: A theoretical study. Appl. Surf. Sci. 2018, 457, 376–380. [Google Scholar] [CrossRef]
- Widjaja, Y.; Musgrave, C.B. Quantum chemical study of the mechanism of aluminum oxide atomic layer deposition. Appl. Phys. Lett. 2002, 80, 3304–3306. [Google Scholar] [CrossRef]
- Elliott, S.D. Models for ALD and MOCVD growth of rare earth oxides. Top. Appl. Phys. 2006, 106, 73–86. [Google Scholar] [CrossRef]
- Park, S.; Park, B.E.; Yoon, H.; Lee, S.; Nam, T.; Cheon, T.; Kim, S.H.; Cheon, H.; Im, S.; Seong, T.; et al. Comparative study on atomic layer deposition of HfO2: Via substitution of ligand structure with cyclopentadiene. J. Mater. Chem. C 2020, 8, 1344–1352. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.B.; Musgrave, C.B.; Sanz, J.F. Atomic layer deposition of hafnium oxide from hafnium chloride and water. J. Am. Chem. Soc. 2008, 130, 11996–12006. [Google Scholar] [CrossRef] [PubMed]
- Mastail, C.; Lanthony, C.; Olivier, S.; Ducéré, J.-M.; Landa, G.; Estève, A.; Rouhani, M.D.; Richard, N.; Dkhissi, A. Introducing densification mechanisms into the modelling of HfO2 atomic layer deposition. Thin Solid Films 2012, 520, 4559–4563. [Google Scholar] [CrossRef]
- Hu, Z.; Turner, C.H. Atomic layer deposition of TiO2 from TiI4 and H2O onto SiO2 surfaces: Ab initio calculations of the initial reaction mechanisms. J. Am. Chem. Soc. 2007, 129, 3863–3878. [Google Scholar] [CrossRef]
- Zhou, G.; Ren, J.; Zhang, S. Initial growth mechanisms of ZrO2 and TiO2 thin films using cycloheptatrienyl-cyclopentadienyl heteroleptic precursors: A comparative study by density functional theory. Appl. Surf. Sci. 2013, 283, 968–974. [Google Scholar] [CrossRef]
- Han, J.H.; Gao, G.; Widjaja, Y.; Garfunkel, E.; Musgrave, C.B. A quantum chemical study of ZrO2 atomic layer deposition growth reactions on the SiO2 surface. Surf. Sci. 2004, 550, 199–212. [Google Scholar] [CrossRef]
- Ren, J.; Cui, C.; Zhou, G.; Liu, Y.; Hu, Y.; Wang, B. A theoretical study on initial growth mechanism of ZrO2 film using cyclopentadienyl-type precursor. Thin Solid Films 2011, 519, 3716–3721. [Google Scholar] [CrossRef]
- Zhou, G.; Ren, J.; Zhang, S. Theoretical study on the initial reaction mechanisms of ansa-metallocene zirconium precursor on hydroxylated Si(100) surface. J. Mol. Model. 2016, 22, 117. [Google Scholar] [CrossRef]
- Elliott, S.D.; Dey, G.; Maimaiti, Y. Classification of processes for the atomic layer deposition of metals based on mechanistic information from density functional theory calculations. J. Chem. Phys. 2017, 146, 052822. [Google Scholar] [CrossRef] [PubMed]
- Doan, H.A.; Li, Z.; Farha, O.K.; Hupp, J.T.; Snurr, R.Q. Theoretical insights into direct methane to methanol conversion over supported dicopper oxo nanoclusters. Catal. Today 2018, 312, 2–9. [Google Scholar] [CrossRef]
- Dey, G.; Elliott, S.D. Mechanism for the atomic layer deposition of copper using diethylzinc as the reducing agent: A density functional theory study using gas-phase molecules as a model. J. Phys. Chem. A 2012, 116, 8893–8901. [Google Scholar] [CrossRef] [PubMed]
- Phung, Q.M.; Pourtois, G.; Swerts, J.; Pierloot, K.; Delabie, A. Atomic layer deposition of ruthenium on ruthenium surfaces: A theoretical study. J. Phys. Chem. C 2015, 119, 6592–6603. [Google Scholar] [CrossRef]
- Filatova, E.A.; Hausmann, D.; Elliott, S.D. Investigating routes toward atomic layer deposition of silicon carbide: Ab initio screening of potential silicon and carbon precursors. J. Vac. Sci. Technol. A Vacuum, Surfaces, Film. 2017, 35, 01B103. [Google Scholar] [CrossRef]
- Cremers, V.; Geenen, F.; Detavernier, C.; Dendooven, J. Monte Carlo simulations of atomic layer deposition on 3D large surface area structures: Required precursor exposure for pillar- versus hole-type structures. J. Vac. Sci. Technol. A Vacuum, Surfaces, Film. 2017, 35, 01B115. [Google Scholar] [CrossRef]
- Murray, C.; Elliott, S.D. Density functional theory predictions of the composition of atomic layer deposition-grown ternary oxides. ACS Appl. Mater. Interfaces 2013, 5, 3704–3715. [Google Scholar] [CrossRef]
- Hu, Z.; Shi, J.; Heath Turner, C. Molecular dynamics simulation of the Al2O3 film structure during atomic layer deposition. Mol. Simul. 2009, 35, 270–279. [Google Scholar] [CrossRef]



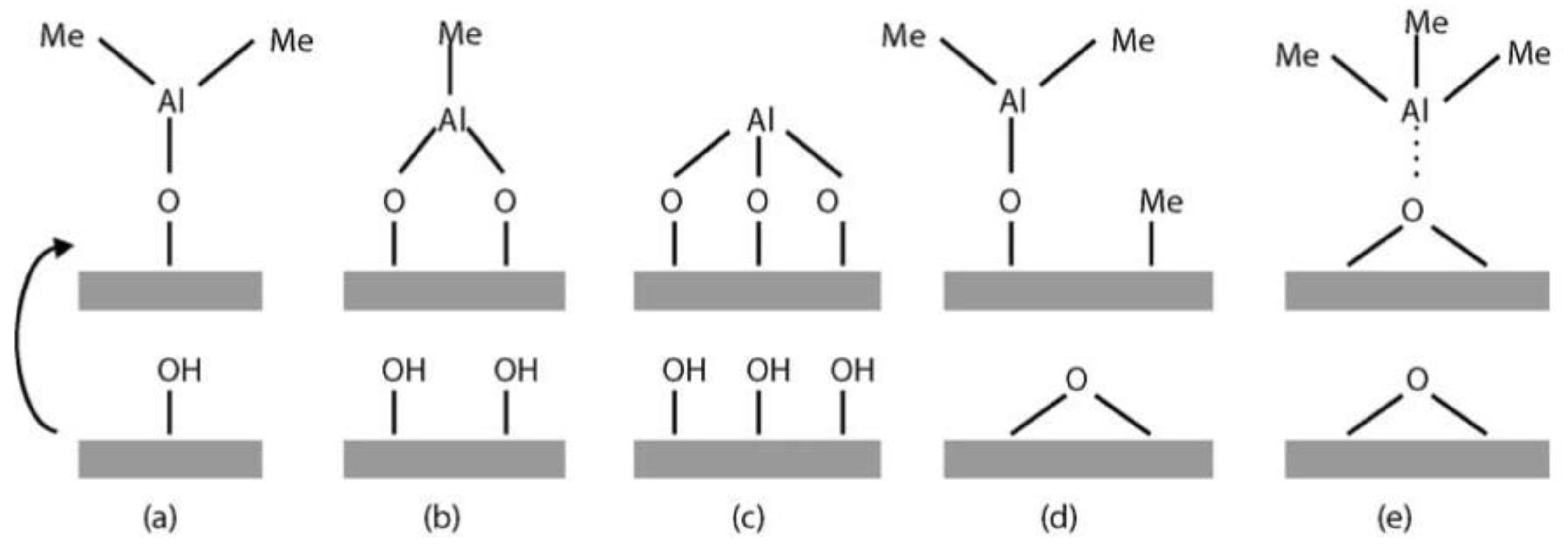

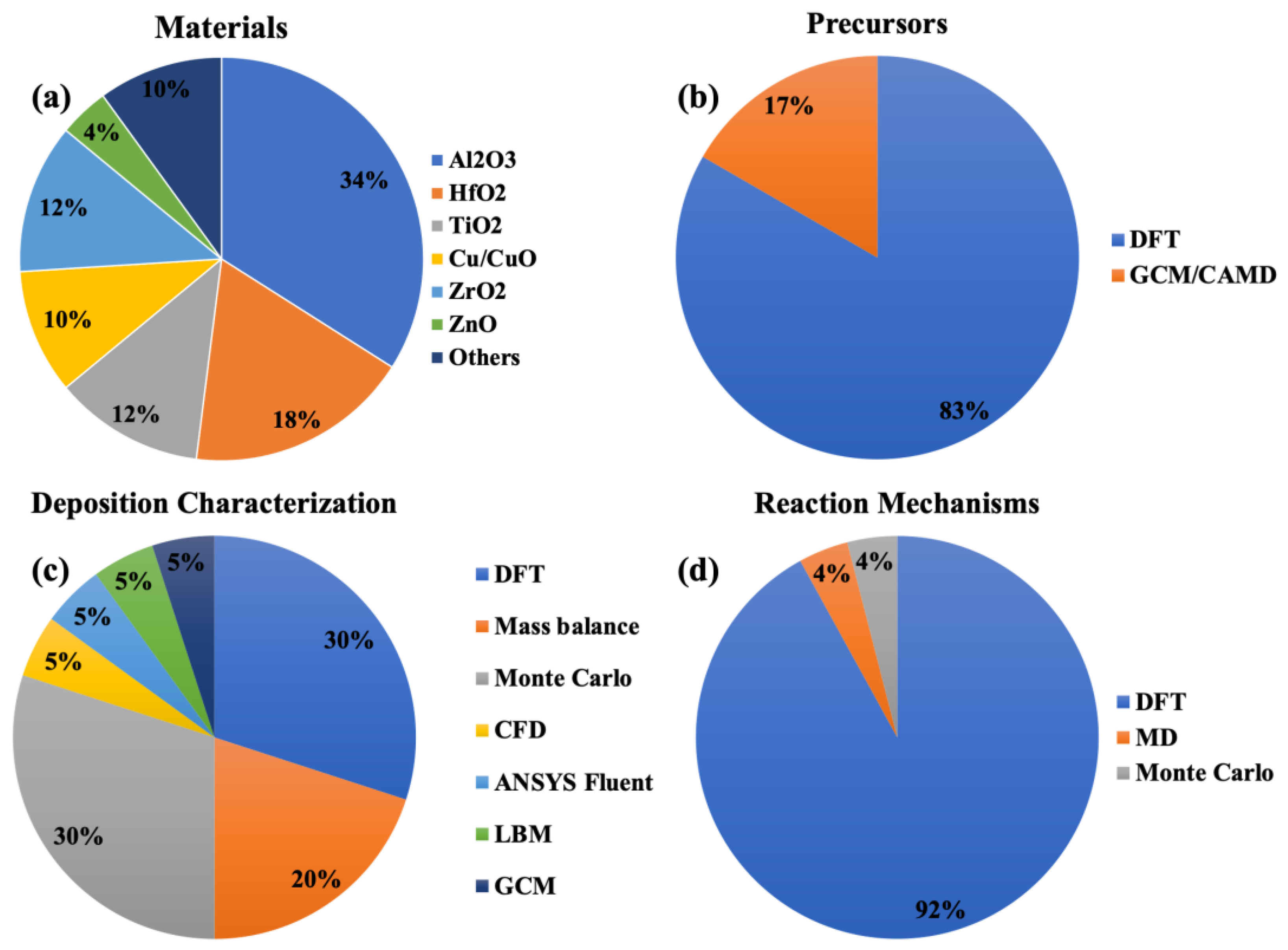

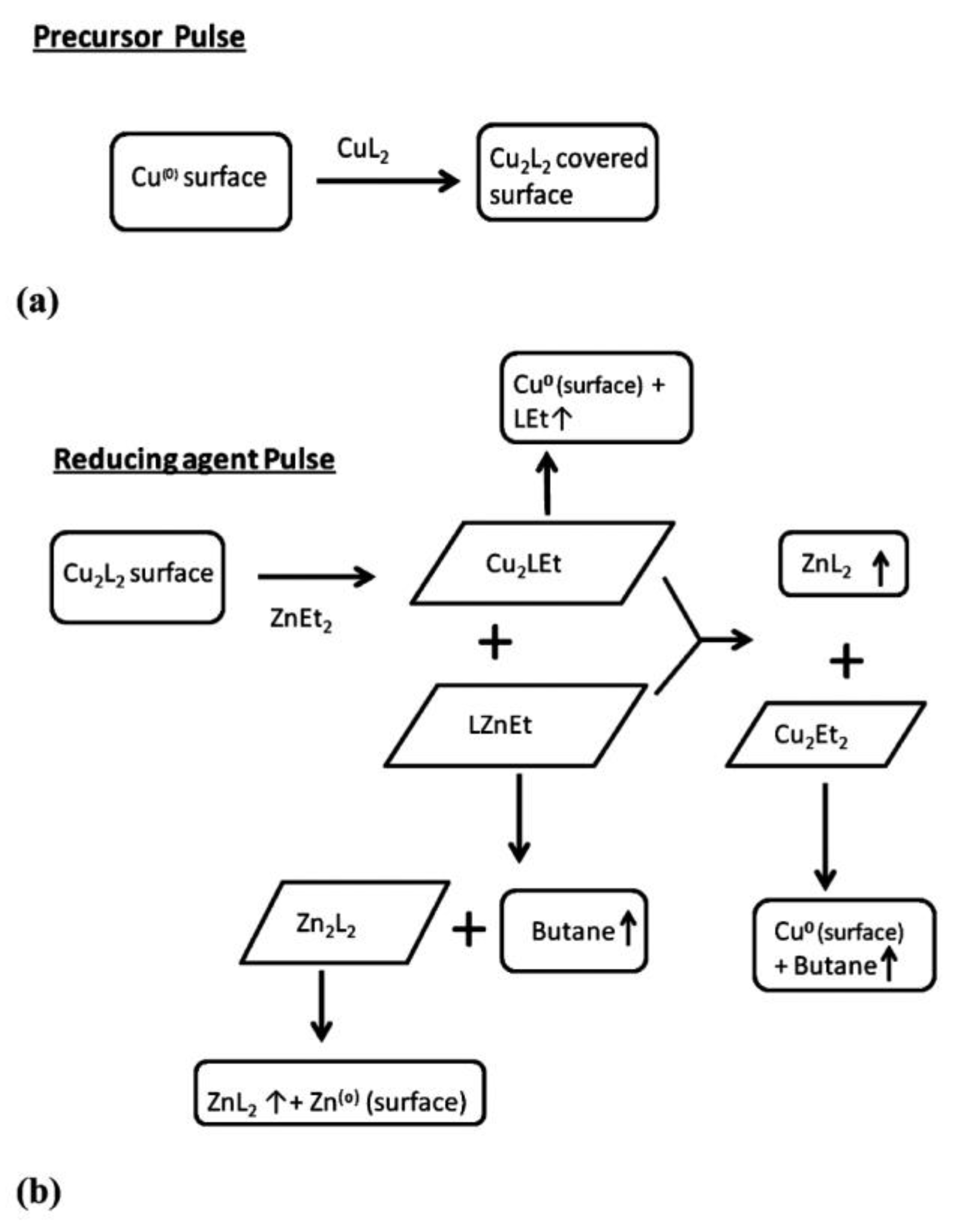
| Thin Film | Precursor | Co-Reactant | Reaction Pathway | Refs. |
|---|---|---|---|---|
| Al2O3 | TMA a | H2O | –OH + AlMe3→ –OAlMen + (3 − n) CH4 –AlMe + H2O → –AlOH + CH4 | [62] |
| MO2 b | MCl4 | H2O | n (–OH) + MCl4 → (-O-)nMCl4−n + n HCl (–O–)nMCl4−n + (4 − n) H2O → (–O–)nM(OH)4−n + (4 − n) HCl | [68,69,70] |
| MO2 | TDMAM c | H2O | M(NMe2)4 + 2 H2O → MO2 (solid) + 4 HNMe2 | [71] |
| Materials | Aspect of Study | Theoretical Method | References |
|---|---|---|---|
| Al2O3 | Introduce new precursor Predict decomposition mechanism, chemisorption process, growth rate, intermediates of the reaction and their concentration, oxidizer reactivity, film thickness, and sticking coefficients Correlate growth rate quantitatively with hydroxyl group concentrations Simulate film uniformity, roughness, density, atomic ratio, gas flow, temperature profile, and surface reactions | DFT; Mass balance; Monte Carlo; CFD; Numerical model/ANSYS Fluent; MD | [73,78,83,93,96,157,158,159,160,161,162,163,164,165] |
| HfO2 | Compare two precursors Predict growth rate and mechanisms Design novel precursors Simulate gas flow and temperature profile | DFT; GCM/CAMD; CFD; Monte Carlo | [70,74,79,100,162,166,167,168] |
| TiO2 | Compare halide precursors Kinetics of reactions Design novel precursors Predict growth rate and mechanisms | DFT; GCM/CAMD | [77,100,101,161,169,170] |
| ZrO2 | Predict mechanisms and growth | DFT | [80,170,171,172,173] |
| ZnO | Simulate growth rate and temperature dependency of growth | DFT/Monte Carlo | [97] |
| Zr(Hf)O2 | Predict temperature dependency of growth rate (Kinetics) | Monte Carlo | [95] |
| Cu/CuO | Introduce new precursor Predict mechanisms and growth | DFT | [48,174,175,176] |
| Ru | Compare reactions of precursors | DFT | [177] |
| Y2O3 | Predict chemisorption process | Mass balance | [161] |
| SiC | Introduce precursor | DFT | [178] |
| N/A | Simulate growth rate based on chemisorption process Describe growth mode Characterize carrier gas flow Model morphology evolution Compare precursor exposure on 3D substrates Predict cation ratios in ternary oxides | Mass balance; LBM Monte Carlo; DFT | [56,66,86,94,179,180] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahmohammadi, M.; Mukherjee, R.; Sukotjo, C.; Diwekar, U.M.; Takoudis, C.G. Recent Advances in Theoretical Development of Thermal Atomic Layer Deposition: A Review. Nanomaterials 2022, 12, 831. https://doi.org/10.3390/nano12050831
Shahmohammadi M, Mukherjee R, Sukotjo C, Diwekar UM, Takoudis CG. Recent Advances in Theoretical Development of Thermal Atomic Layer Deposition: A Review. Nanomaterials. 2022; 12(5):831. https://doi.org/10.3390/nano12050831
Chicago/Turabian StyleShahmohammadi, Mina, Rajib Mukherjee, Cortino Sukotjo, Urmila M. Diwekar, and Christos G. Takoudis. 2022. "Recent Advances in Theoretical Development of Thermal Atomic Layer Deposition: A Review" Nanomaterials 12, no. 5: 831. https://doi.org/10.3390/nano12050831
APA StyleShahmohammadi, M., Mukherjee, R., Sukotjo, C., Diwekar, U. M., & Takoudis, C. G. (2022). Recent Advances in Theoretical Development of Thermal Atomic Layer Deposition: A Review. Nanomaterials, 12(5), 831. https://doi.org/10.3390/nano12050831








