A Ratiometric Fiber Optic Sensor Based on CdTe QDs Functionalized with Glutathione and Mercaptopropionic Acid for On-Site Monitoring of Antibiotic Ciprofloxacin in Aquaculture Water
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Apparatus
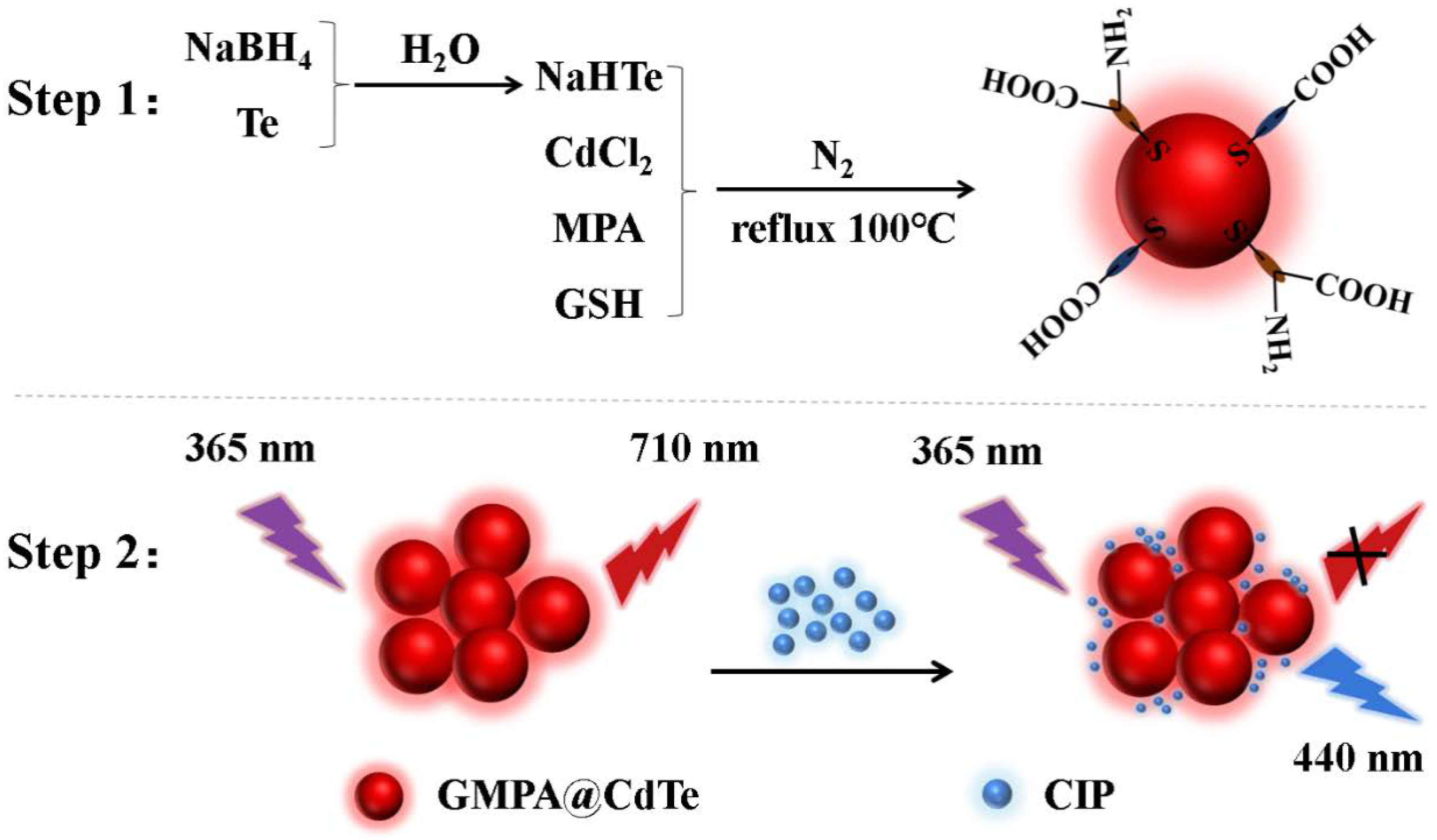
2.2. Synthesis of GMPA@CdTe QDs
2.3. Fluorescence Detection of CIP
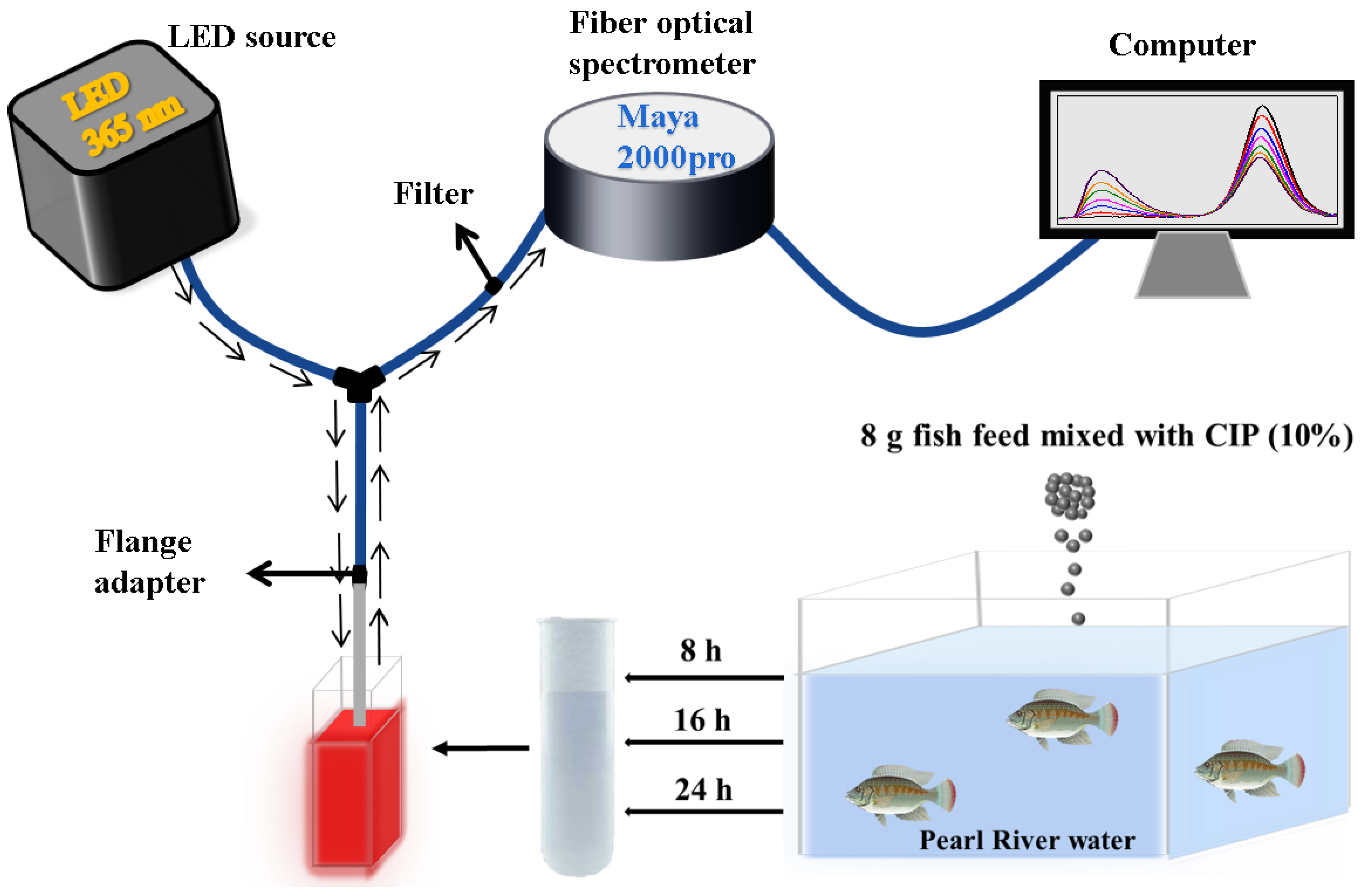
2.4. Detection of CIP in Actual Water Samples
3. Results and Discussion
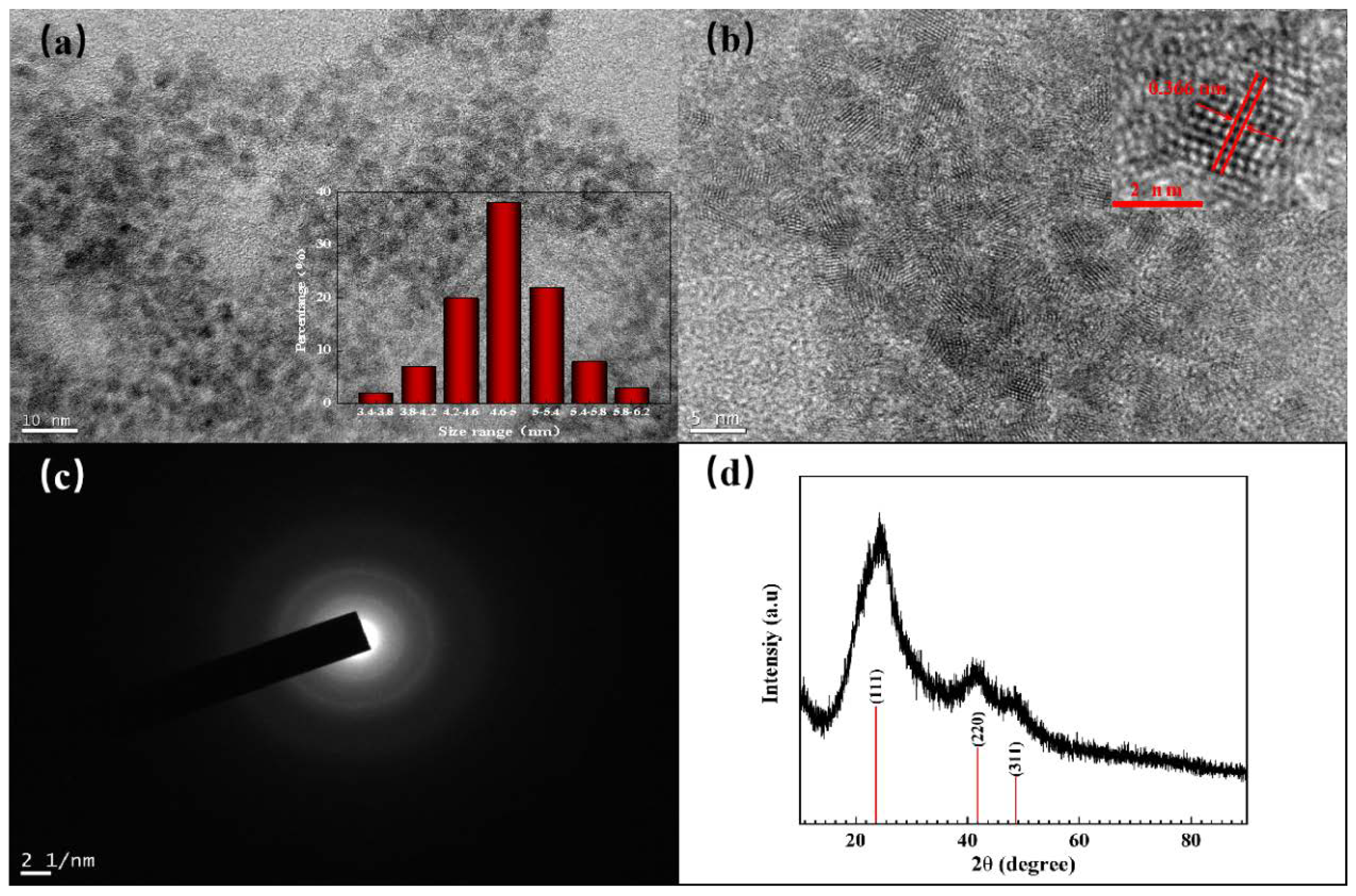
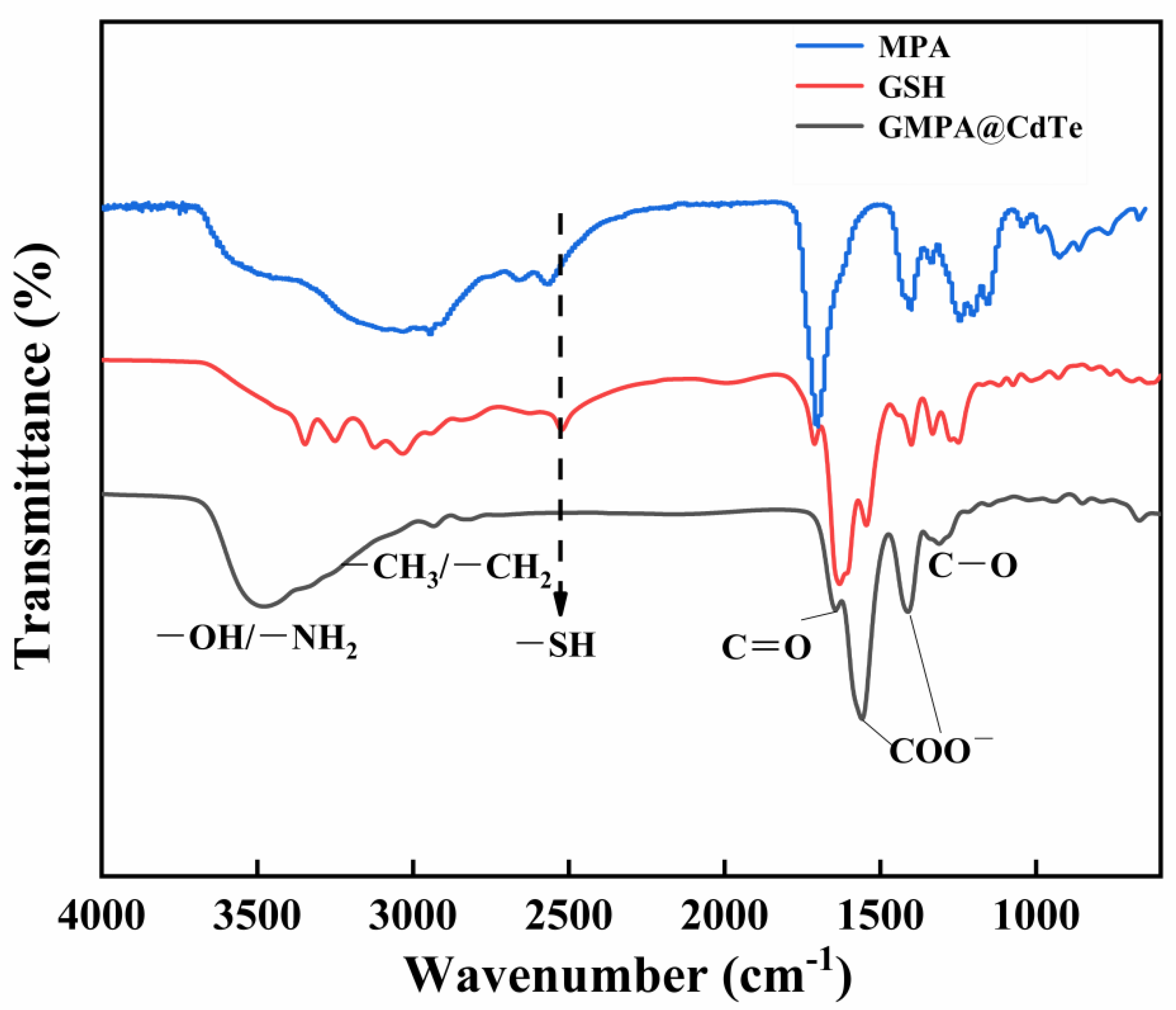
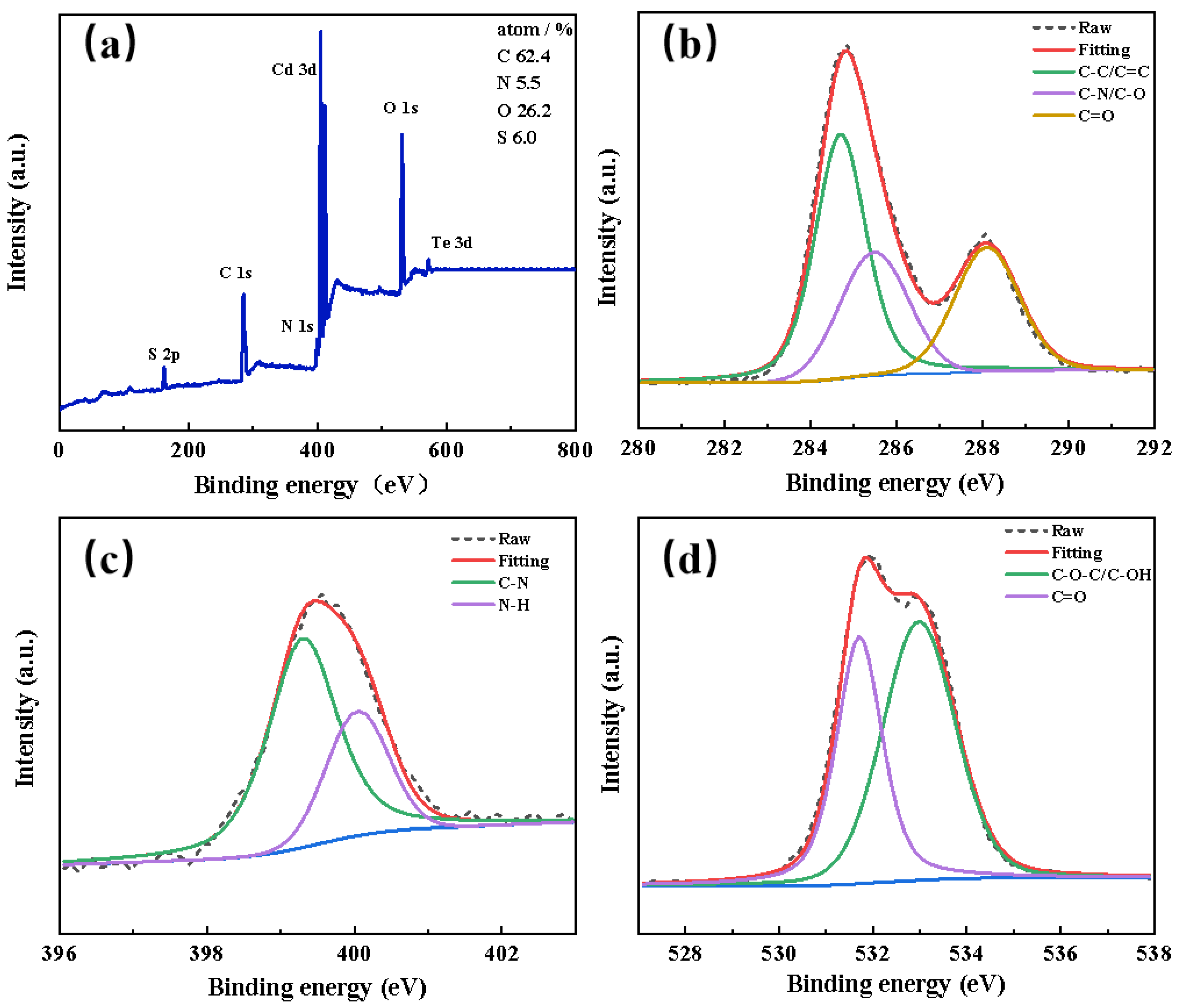
3.1. Synthesis and Characterization of GMPA@CdTe QDs
3.2. Optical Properties of GMPA@CdTe QDs
3.3. Optimization of Detection Conditions for CIP by GMPA@CdTe QDs
3.3.1. Effects of the Solvents on the Signal of F440/F710
3.3.2. Effect of pH of Medium on the Signal of F440/F710
3.3.3. Fluorescence Response Time and Stability of GMPA@CdTe QDs to CIP
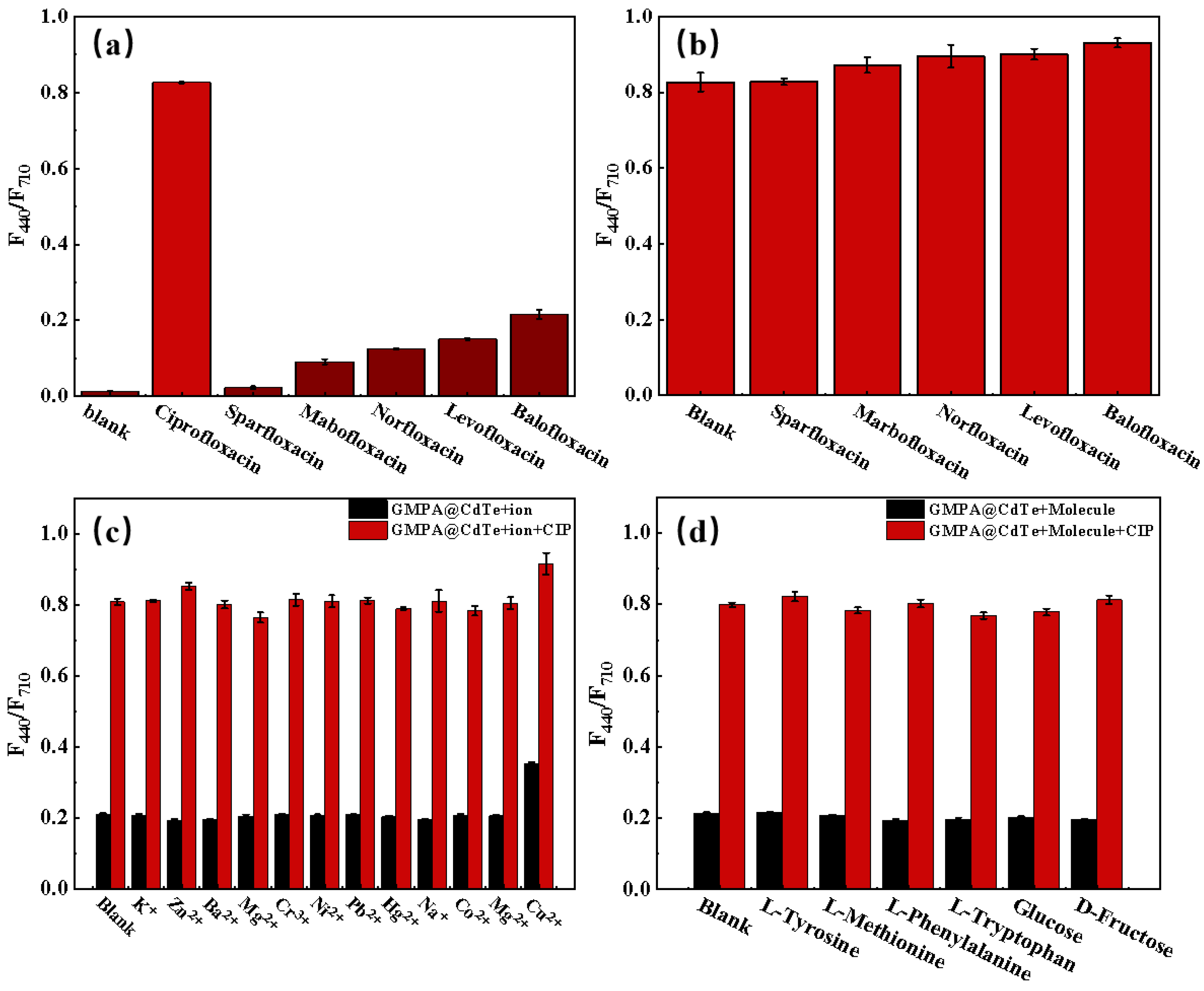
3.3.4. Selectivity and Anti-Interference Performance of GMPA@CdTe QDs
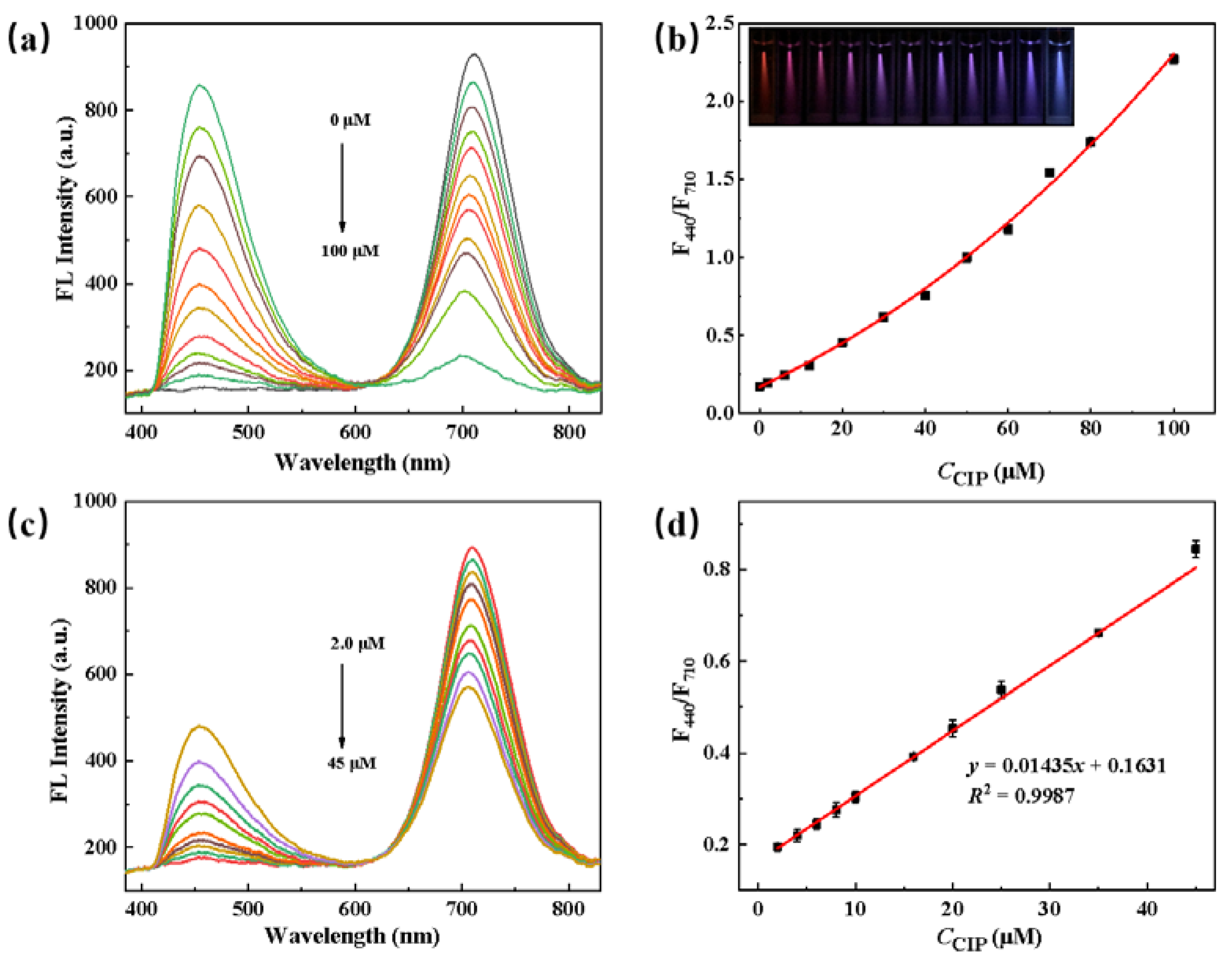
3.4. Detection of CIP by GMPA@CdTe QDs
3.5. Detection of CIP in Actual Samples
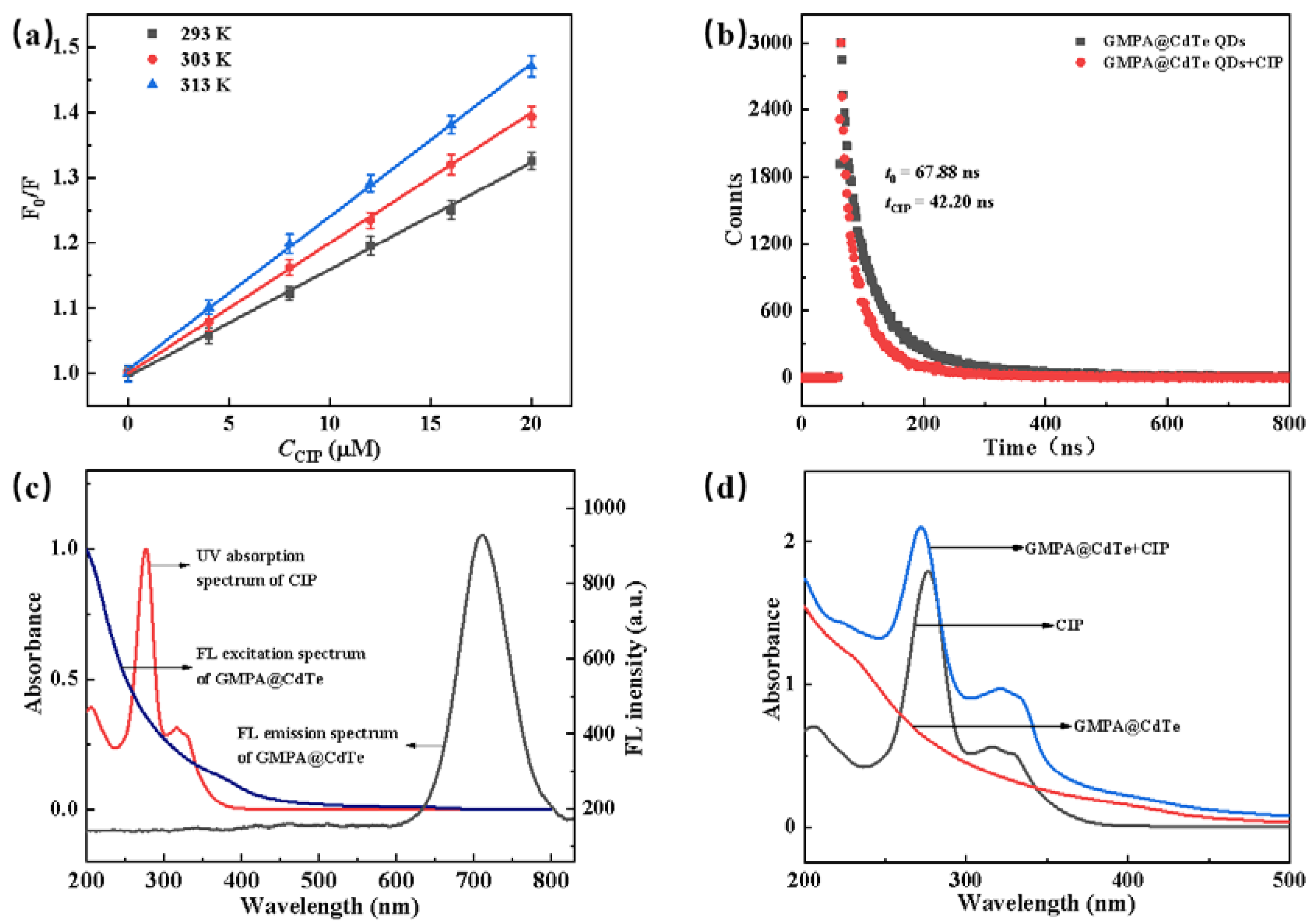
3.6. Investigation of Quenching Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European Ecenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Stoichev, T.; Baptista, M.S.; Basto, M.C.P.; Vasconcelos, V.M.; Vasconcelos, M.T.S.D. Effects of minocycline and its degradation products on the growth of Microcystis aeruginosa. Ecotoxicol. Environ. Saf. 2011, 74, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pleiter, M.; Gonzalo, S.; Rodea-Palomares, I.; Leganes, F.; Rosal, R.; Boltes, K.; Marco, E.; Fernandez-Pinas, F. Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: Implications for environmental risk assessment. Water Res. 2013, 47, 2050–2064. [Google Scholar] [CrossRef]
- Bergeron, S.; Raj, B.; Nathaniel, R.; Corbin, A.; LaFleur, G. Presence of antibiotic resistance genes in raw source water of a drinking water treatment plant in a rural community of USA. Int. Biodeterior. Biodegrad. 2017, 124, 3–9. [Google Scholar] [CrossRef]
- Bilal, M.; Mehmood, S.; Rasheed, T.; Iqbal, H.M. N Antibiotics traces in the aquatic environment: Persistence and adverse environmental impact. Curr. Opin. Environ. Sci. Health 2019, 13, 68–74. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yu, X.; McDonald, T.J.; Jinadatha, C.; Dionysiou, D.D.; Dionysios, D.; Feng, M.B.; Feng, M. Elimination of antibiotic resistance genes and control of horizontal transfer risk by UV-based treatment of drinking water: A mini review. Front. Environ. Sci. Eng. 2019, 13, e37. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, E.; Borrego, C.M.; Balcazar, J.L.; Cummins, E. Human exposure assessment to antibiotic-resistant Escherichia coli through drinking water. Sci. Total Environ. 2018, 616–617, 1356–1364. [Google Scholar] [CrossRef]
- Chen, B.; Lin, L.; Fang, L.; Yang, Y.; Chen, E.Z.; Yuan, K.; Zou, S.C.; Wang, X.W.; Luan, T.G. Complex pollution of antibiotic resistance genes due to beta-lactam and aminoglycoside use in aquaculture farming. Water Res. 2018, 134, 200–208. [Google Scholar] [CrossRef]
- Karim, A.V.; Shriwastav, A. Degradation of ciprofloxacin using photo, sono, and sonophotocatalytic oxidation with visible light and low-frequency ultrasound: Degradation kinetics and pathways. Chem. Eng. J. 2020, 392, 124853. [Google Scholar] [CrossRef]
- Zhang, H.; Song, S.; Jia, Y.; Wu, D.; Lu, H. Stress-responses of activated sludge and anaerobic sulfate-reducing bacteria sludge under long-term ciprofloxacin exposure. Water Res. 2019, 164, 114964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, Y.; Yu, L.-P. Specific and ultrasensitive ciprofloxacin detection by responsive photonic crystal sensor. J. Hazard. Mater. 2014, 280, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Jacome, G.S.; Aguilar-Caballos, M.P.; Gomez-Hens, A. Luminescent determination of quinolones in milk samples by liquid chromatography/post-column derivatization with terbium oxide nanoparticles. J. Chromatogr. A 2015, 1405, 126–132. [Google Scholar] [CrossRef]
- Jin, T.; Wu, H.; Gao, N.N.; Chen, X.D.; Lai, H.J.; Zheng, J.F.; Du, L.M. Extraction of quinolones from milk samples using bentonite/magnetite nanoparticles before determination by high-performance liquid chromatography with fluorimetric detection. J. Sep. Sci. 2016, 39, 545–551. [Google Scholar] [CrossRef]
- Wang, R.; Li, S.; Chen, D.W.; Zhao, Y.F.; Wu, Y.N.; Qi, K.M. Selective extraction and enhanced-sensitivity detection of fluoroquinolones in swine body fluids by liquid chromatography–high resolution mass spectrometry: Application in long-term monitoring in livestock. Food Chem. 2021, 341, 128269. [Google Scholar] [CrossRef]
- Guidi, L.R.; Santos, F.A.; Ribeiro, A.C.S.R.; Fernandes, C.; da Silva, L.H.M.; Gloria, M.B.A. Quinolones and tetracyclines in aquaculture fish by a simple and rapid LC-MS/MS method. Food Chem. 2018, 245, 1232–1238. [Google Scholar] [CrossRef]
- Yudthavorasit, S.; Chiaochan, C.; Leepipatpiboon, N. Simultaneous determination of multi-class antibiotic residues in water using carrier-mediated hollow-fiber liquid-phase microextraction coupled with ultra-high performance liquid chromatography tandem mass spectrometry. Mikrochim. Acta 2011, 172, 39–49. [Google Scholar] [CrossRef]
- Wagman, A.S.; Cirz, R.; McEnroe, G.; Aggen, J.; Linsell, M.S.; Goldblum, A.A.; Lopez, S.; Gomez, M.; Miller, G.; Simons, L.J.; et al. Synthesis and Microbiological Evaluation of Novel Tetracyclic Fluoroquinolones. ChemMedChem 2017, 12, 1687–1692. [Google Scholar] [CrossRef]
- Liu, J.; Wang, B.; Huang, H.; Jian, D.; Lu, Y.A.; Shan, Y.K.; Wang, S.Y.; Liu, F. Quantitative ciprofloxacin on-site rapid detections using quantum dot microsphere based immunochromatographic test strips. Food Chem. 2020, 335, 127596. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Yang, K.; Zhong, Y.; Yang, X.M.; Chen, Z.B. Synthesis of Carbon Dots Originated from Hydroxypropylmethyl Cellulose for Sensing Ciprofloxacin. Anal. Sci. 2017, 33, 1129–1134. [Google Scholar] [CrossRef]
- Gui, R.J.; Bu, X.N.; He, W.J.; Jin, H. Ratiometric fluorescence, solution-phase and filter-paper visualization detection of ciprofloxacin based on dual-emitting carbon dot/silicon dot hybrids. New J. Chem. 2018, 42, 16217–16225. [Google Scholar] [CrossRef]
- Lu, C.; Liu, G.; Yang, Z.; Wang, Y.Y.; Rao, H.B.; Zhang, W.; Jing, B.; Wang, X.X. A ratiometric fluorometric ciprofloxacin assay based on the use of riboflavin and carbon dots. Microchim. Acta 2020, 187, e37. [Google Scholar] [CrossRef]
- Faria, L.V.; Pereira, J.F.S.; Azevedo, G.C.; Matos, M.A.C.; Munoz, R.A.A.; Matos, R.C. Square-wave voltammetry determination of ciprofloxacin in pharmaceutical formulations and milk using a reduced graphene oxide sensor. J. Braz. Chem. Soc. 2019, 30, 1947–1954. [Google Scholar] [CrossRef]
- Garbellini, G.S.; Rocha-Filho, R.C.; Fatibello-Filho, O. Voltammetric determination of ciprofloxacin in urine samples and its interaction with dsDNA on a cathodically pretreated boron-doped diamond electrode. Anal. Methods 2015, 7, 3411–3418. [Google Scholar] [CrossRef]
- Kawde, A.-N.; Aziz, M.A.; Odewunmi, N.; Hassan, N.; AlSharaa, A. Electroanalytical Determination of Antibacterial Ciprofloxacin in Pure Form and in Drug Formulations. Arab. J. Sci. Eng. 2014, 39, 131–138. [Google Scholar] [CrossRef]
- Shi, Q.; Huang, J.; Sun, Y.; Deng, R.; Teng, M.; Li, Q.; Yang, Y.; Hu, X.; Zhang, Z.; Zhang, G. A SERS-based multiple immuno-nanoprobe for ultrasensitive detection of neomycin and quinolone antibiotics via a lateral flow assay. Microchim. Acta 2018, 185, 84. [Google Scholar] [CrossRef]
- Huang, Q.D.; Lv, C.H.; Yuan, X.L.; He, M.; Lai, J.P.; Sun, H. A novel fluorescent optical fiber sensor for highly selective detection of antibiotic ciprofloxacin based on replaceable molecularly imprinted nanoparticles composite hydrogel detector. Sens. Actuators B Chem. 2021, 328, e129000. [Google Scholar] [CrossRef]
- Pawar, D.; Kale, S.N. A review on nanomaterial-modified optical fiber sensors for gases, vapors and ions. Microchim. Acta 2019, 186, e253. [Google Scholar] [CrossRef]
- Usha, S.P.; Gupta, B.D. Urinary p-cresol diagnosis using nanocomposite of ZnO/MoS2 and molecular imprinted polymer on optical fiber based lossy mode resonance sensor. Biosens. Bioelectron. 2018, 101, 135–145. [Google Scholar] [CrossRef]
- Pathak, A.; Gupta, B.D. Ultra-selective fiber optic SPR platform for the sensing of dopamine in synthetic cerebrospinal fluid incorporating permselective nafion membrane and surface imprinted MWCNTs-PPy matrix. Biosens. Bioelectron. 2019, 133, 205–214. [Google Scholar] [CrossRef]
- Guo, J.J.; Zhou, M.J.; Yang, C.X. Fluorescent hydrogel waveguide for on-site detection of heavy metal ions. Sci. Rep. 2017, 7, e7902. [Google Scholar] [CrossRef]
- Zhou, M.J.; Guo, J.J.; Yang, C.X. Ratiometric fluorescence sensor for Fe3+ ions detection based on quantum dot-doped hydrogel optical fiber. Sensors Actuators B Chem. 2018, 264, 52–58. [Google Scholar] [CrossRef]
- Guo, J.J.; Huang, H.X.; Zhou, M.J.; Yang, C.X.; Kong, L.J. Quantum Dots-Doped Tapered Hydrogel Waveguide for Ratiometric Sensing of Metal Ions. Anal. Chem. 2018, 90, 12292–12298. [Google Scholar] [CrossRef]
- Liu, X.-W.; Shu, J.-S.; Xiao, Y.; Yang, Y.; Zhang, S.-B. Selective and Sensitive Detection of Silver(I) Ion Based on Tetracationic Complex and TGA/GSH Co-capped Quantum Dots as an Effective Fluorescent Sensing Platform. Anal. Sci. 2017, 33, 381–385. [Google Scholar] [CrossRef][Green Version]
- Jin, D.R.; Seo, M.-H.; Huy, B.T.; Pham, Q.-T.; Conte, M.L.; Thangadurai, D.; Lee, Y.-I. Quantitative determination of uric acid using CdTe nanoparticles as fluorescence probes. Biosens. Bioelectron. 2016, 77, 359–365. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Kazemifard, N.; Rezaei, B. Development of a selective prilocaine optical sensor based on molecularly imprinted shell on CdTe quantum dots. Sens. Actuators B Chem. 2017, 242, 835–841. [Google Scholar] [CrossRef]
- Han, S.; Yang, L.; Wen, Z.G.; Chu, S.Y.; Wang, M.; Wang, Z.Y.; Jiang, C.L. A dual-response ratiometric fluorescent sensor by europium-doped CdTe quantum dots for visual and colorimetric detection of tetracycline. J. Hazard. Mater. 2020, 398, e122894. [Google Scholar] [CrossRef]
- Li, Q.; Tan, X.P.; Li, J.; Pan, L.; Liu, X.R. Glutathione-capped CdTe nanocrystals as probe for the determination of fenbendazole. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 141, 10–15. [Google Scholar] [CrossRef]
- Amin, R.M.; Elfeky, S.A.; Verwanger, T.; Krammer, B. Fluorescence-based CdTe nanosensor for sensitive detection of cytochrome C. Biosens. Bioelectron. 2017, 98, 415–420. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Kazemifard, N.; Rezaei, B. Development of a nano plastic antibody for determination of propranolol using CdTe quantum dots. Sens. Actuators B Chem. 2017, 252, 846–853. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Wang, B.; Xiong, H.Y.; Wen, W.; Cheng, N. A ratiometric fluorometric epinephrine and norepinephrine assay based on carbon dot and CdTe quantum dots nanocomposites. Microchem. J. 2019, 146, 66–72. [Google Scholar] [CrossRef]
- Jin, M.; Mou, Z.-L.; Zhang, R.-L.; Liang, S.-S.; Zhang, Z.-Q. An efficient ratiometric fluorescence sensor based on metal-organic frameworks and quantum dots for highly selective detection of 6-mercaptopurine. Biosens. Bioelectron. 2017, 91, 162–168. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, L.L.; Li, W.W.; Wang, X.F.; Shang, Y.H.; Li, B.X. Carbon dots doped with nitrogen and sulfur and loaded with copper(II) as a "turn-on" fluorescent probe for cystein, glutathione and homocysteine. Microchim. Acta 2016, 183, 1409–1416. [Google Scholar] [CrossRef]
- Nejad, M.A.F.; Ghasemi, F.; Hormozi-Nezhad, M.R. A wide-color-varying ratiometric nanoprobe for detection of norepinephrine in urine samples. Anal. Chim. Acta 2018, 1039, 124–131. [Google Scholar] [CrossRef]
- Kaur, J.; Komal; Renu; Kumar, V.; Tikoo, K.B.; Bansal, S.; Kaushik, A.; Singhal, S. Glutathione Modified Fluorescent CdS QDs Synthesized Using Environmentally Benign Pathway for Detection of Mercury Ions in Aqueous Phase. J. Fluoresc. 2020, 30, 773–785. [Google Scholar] [CrossRef]
- Chen, J.L.; Gao, Y.C.; Xu, Z.B.; Wu, G.H.; Chen, Y.C.; Zhu, C.Q. A novel fluorescent array for mercury (II) ion in aqueous solution with functionalized cadmium selenide nanoclusters. Anal. Chim. Acta 2006, 577, 77–84. [Google Scholar] [CrossRef]
- Ebrahim, S.; Labeb, M.; Abdel-Fattah, T.; Soliman, M. CdTe quantum dots capped with different stabilizing agents for sensing of ochratoxin A. J. Lumin 2017, 182, 154–159. [Google Scholar] [CrossRef]
- Aldana, J.; Lavelle, N.; Wang, A.Y.; Peng, X. Size-Dependent Dissociation pH of Thiolate Ligands from Cadmium Chalcogenide Nanocrystals. J. Am. Chem. Soc. 2005, 127, 2496–2504. [Google Scholar] [CrossRef]
- Zhang, T.L.; Sun, X.Y.; Liu, B. Synthesis of positively charged CdTe quantum dots and detection for uric acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 1566–1572. [Google Scholar] [CrossRef]
- Ye, Y.W.; Wu, T.T.; Jiang, X.T.; Cao, J.X.; Ling, X.; Mei, Q.S.; Chen, H.; Han, D.-M.; Xu, J.-J.; Shen, Y.Z. Portable Smartphone-Based QDs for the Visual Onsite Monitoring of Fluoroquinolone Antibiotics in Actual Food and Environmental Samples. ACS Appl. Mater. Interfaces 2020, 12, 14552–14562. [Google Scholar] [CrossRef]
- Wu, Y.W.; Wang, Q.Y.; Wu, T.T.; Liu, W.; Nan, H.X.; Xu, S.H.; Shen, Y.Z. Detection and Imaging of Hydrogen Sulfide in Lysosomes of Living Cells with Activatable Fluorescent Quantum Dots. ACS Appl. Mater. Interfaces 2018, 10, 43472–43481. [Google Scholar] [CrossRef]
- Tan, X.P.; Li, Q.; Yang, J.D. A simple fluorescence method detection levofloxacin in milk based on GSH-CdTe QDs. J. Mol. Struct. 2020, 1201, e127175. [Google Scholar] [CrossRef]
| Method | Linear Relationship | R2 | Concentration (μM) | |||||
|---|---|---|---|---|---|---|---|---|
| 8 h | RSD (%) | 16 h | RSD (%) | 24 h | RSD (%) | |||
| FL | y = 0.1262x − 0.00948 | 0.9990 | 28.17 | 0.21 | 32.53 | 0.20 | 37.49 | 0.32 |
| RFFS | y = 0.01435x + 0.1631 | 0.9987 | 27.18 | 2.1 | 33.20 | 1.9 | 37.38 | 2.6 |
| Temperature (K) | Stern-Volmer Linear Equation | Correlation Coefficient | KSV (L·mol−1) |
|---|---|---|---|
| 293 | F0/F = 0.9953 + 0.01643[Q] | 0.9983 | 0.01643 |
| 303 | F0/F = 1.000 + 0.02001[Q] | 0.9957 | 0.02001 |
| 313 | F0/F = 1.006 + 0.02351[Q] | 0.9985 | 0.02351 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.-L.; Wu, X.-Y.; He, M.; Lai, J.-P.; Sun, H. A Ratiometric Fiber Optic Sensor Based on CdTe QDs Functionalized with Glutathione and Mercaptopropionic Acid for On-Site Monitoring of Antibiotic Ciprofloxacin in Aquaculture Water. Nanomaterials 2022, 12, 829. https://doi.org/10.3390/nano12050829
Yuan X-L, Wu X-Y, He M, Lai J-P, Sun H. A Ratiometric Fiber Optic Sensor Based on CdTe QDs Functionalized with Glutathione and Mercaptopropionic Acid for On-Site Monitoring of Antibiotic Ciprofloxacin in Aquaculture Water. Nanomaterials. 2022; 12(5):829. https://doi.org/10.3390/nano12050829
Chicago/Turabian StyleYuan, Xiao-Lin, Xiao-Yi Wu, Miao He, Jia-Ping Lai, and Hui Sun. 2022. "A Ratiometric Fiber Optic Sensor Based on CdTe QDs Functionalized with Glutathione and Mercaptopropionic Acid for On-Site Monitoring of Antibiotic Ciprofloxacin in Aquaculture Water" Nanomaterials 12, no. 5: 829. https://doi.org/10.3390/nano12050829
APA StyleYuan, X.-L., Wu, X.-Y., He, M., Lai, J.-P., & Sun, H. (2022). A Ratiometric Fiber Optic Sensor Based on CdTe QDs Functionalized with Glutathione and Mercaptopropionic Acid for On-Site Monitoring of Antibiotic Ciprofloxacin in Aquaculture Water. Nanomaterials, 12(5), 829. https://doi.org/10.3390/nano12050829







