Safety Assessment of 2D MXenes: In Vitro and In Vivo
Abstract
:1. Introduction
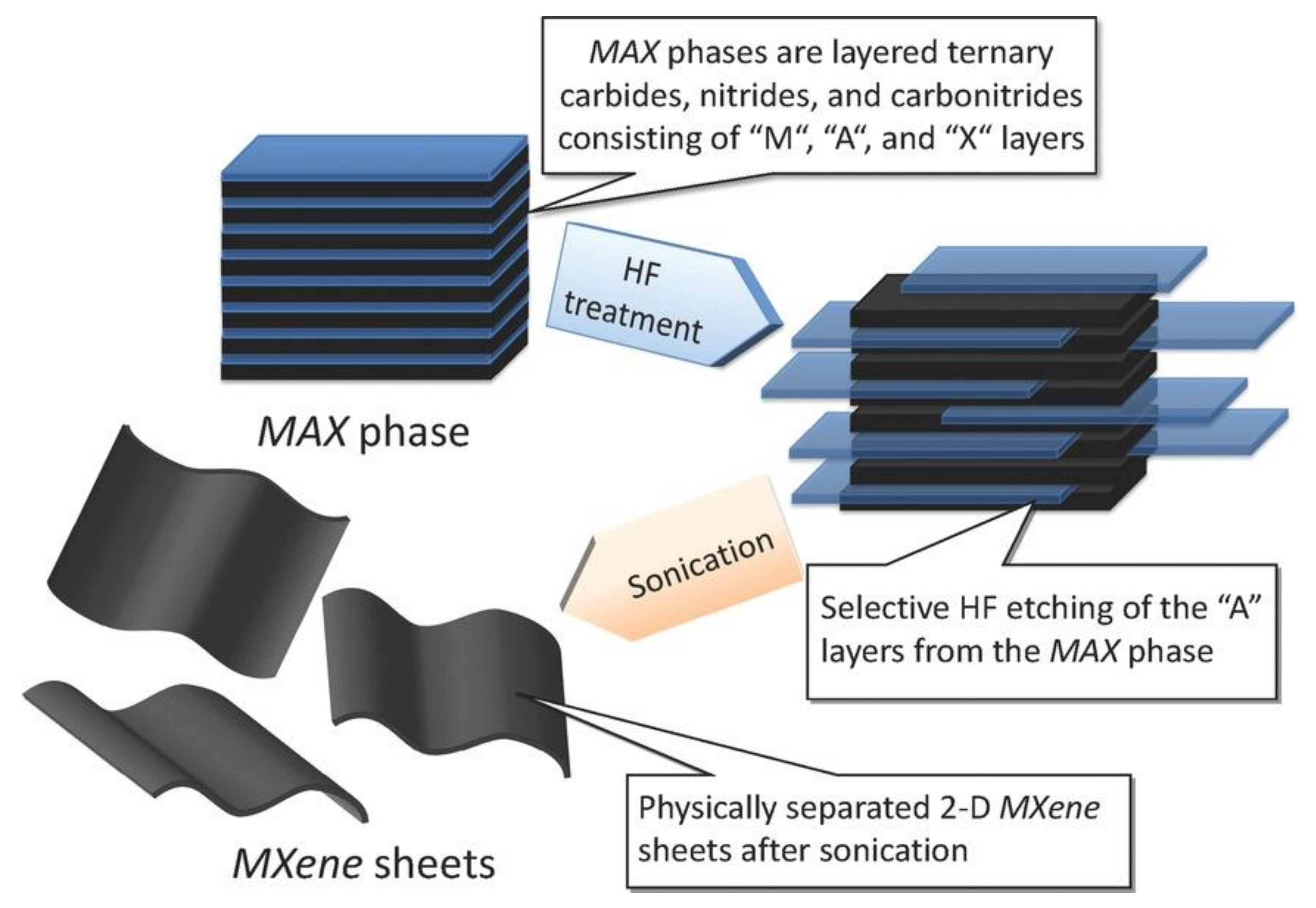
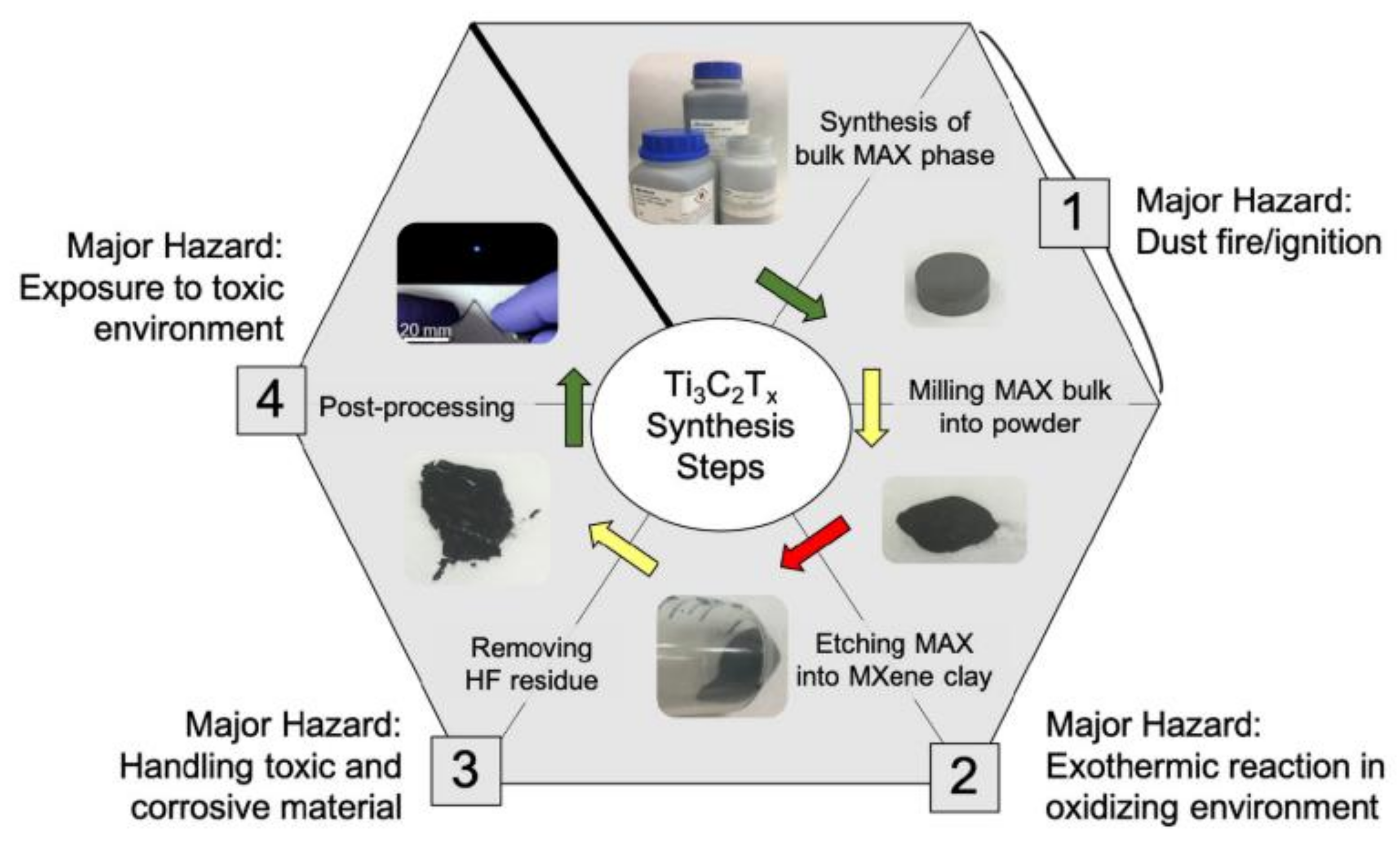
2. Synthesis of MXenes and Safety Concerns
3. In Vitro Toxic Effects of MXenes
| Type of MXenes | Type of Cells | Dose | Toxicity Effects | Reference |
|---|---|---|---|---|
| Ti3C2Tx | human umbilical vein endothelial cells (HUVECs) | 100 and 500 μg mL−1, 48 h | No obvious acute cytotoxicity. The ratios of living, apoptotic, and necrotic cells exhibited patterns similar to those of the control group. | [35] |
| Ti3C2 QDs, Nb2C QDs | HUVECs | 0–100 μg mL−1, 24 h | At 25 μg mL−1 of Ti3C2 QDs, the cellular viability was larger than 70%. While at 50 and 100 μg mL−1, Ti3C2 QDs led to significantly lower cellular viability compared with Nb2C QDs. For Nb2C QDs, there was no significant cytotoxicity after treated with these three concentrations. | [36] |
| Ti3C2Tx | human mesenchymal stem cells (hMSCs) | 0–100 μg mL−1, 7 days | >50 μg mL−1, obvious cytotoxicity was shown | [37] |
| Ti3C2Tx | neural stem cells (NSCs) and NSCs-derived differentiated cells | 12.5–100 μg mL−1, 24 h | At 25 μg mL−1, Ti3C2Tx nanosheets caused significant cytotoxicity to NSCs. 198 differently expressed genes (DEGs) which were mainly associated with the extracellular regions were identified. | [38] |
| Ti3C2Tx | A549, MRC-5, A375, and HaCaT cells | 0–500 μg mL−1, 24 h | Concentration dependent cytotoxicity. Toxic effects were higher against cancerous cells in comparison to normal ones. | [39] |
| Ti3C2Tx MXene structures and their precursors (TiC, Ti2AlC, and Ti3AlC2) | HeLa cells and normal fibroblasts (MSU1.1) | 10–400 μg mL−1, 24–48 h | Concentration dependent and cell-type dependent cytotoxicity. MXene structures showed higher cell viability in comparison to MAX phases. | [40] |
| Ti2NTx | human skin malignant melanoma cells, human immortalized keratinocytes, human breast cancer cells, and normal human mammary epithelial cells | 62.5–500 μg mL−1, 24 h | Higher toxicity towards cancerous cell lines in comparison to normal ones. | [41] |
| Nb2CTx | breast 4T1 cancer cells and glioma U87 cancer cells | 0–200 μg mL−1, 24 h | Negligible effect on the cell viability at 200 μg mL−1. After exposed to 808 or 1064 nm laser, cancer cells were killed significantly with the increase of laser intensity. | [13] |
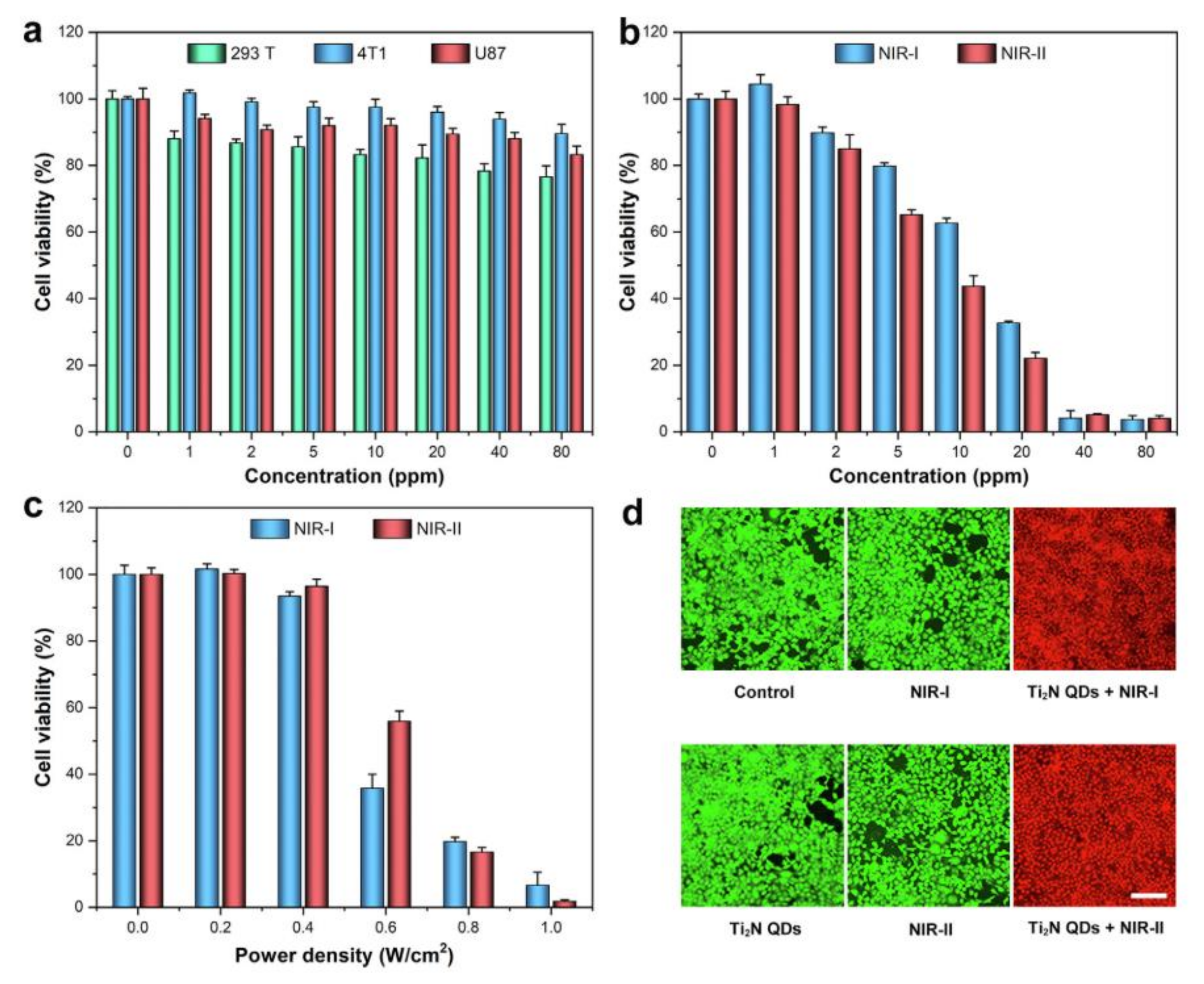
| Ti2N QDs | 293T, 4T1, and U87 | 0–80 μg mL−1, 24 h | Adding irradiating with 808 or 1064 nm lasers for 5 min after incubation with the Ti2N QDs, cancer cells were almost completely killed. | [43] |
| Ti2CTx | 3D HeLa cell culture system | 0–500 μg mL−1, 24 h | The direct adhesion of Ti2CTx MXenes to the cell membrane of microtissues limited the growth of microtissues and cell division, resulting in lower cell viability. | [44] |
4. Toxic Effects of MXenes In Vivo
5. Properties Associated with the Safety of MXenes
5.1. Surface Functionalization
5.2. Stability and Degradation
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 1–17. [Google Scholar] [CrossRef]
- Lei, J.-C.; Zhang, X.; Zhou, Z. Recent advances in MXene: Preparation, properties, and applications. Front. Phys. 2015, 10, 276–286. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, H.; Hu, T.; Fan, B.; Wang, X.; Li, Z. Emerging 2D MXenes for supercapacitors: Status, challenges and prospects. Chem. Soc. Rev. 2020, 49, 6666–6693. [Google Scholar] [CrossRef]
- Morales-García, A.N.; Calle-Vallejo, F.; Illas, F. MXenes: New horizons in catalysis. ACS Catal. 2020, 10, 13487–13503. [Google Scholar] [CrossRef]
- Sajid, M. MXenes: Are they emerging materials for analytical chemistry applications?–A review. Anal. Chim. Acta 2021, 1143, 267–280. [Google Scholar] [CrossRef]
- Jiang, X.; Kuklin, A.V.; Baev, A.; Ge, Y.; Ågren, H.; Zhang, H.; Prasad, P.N. Two-dimensional MXenes: From morphological to optical, electric, and magnetic properties and applications. Phys. Rep. 2020, 848, 1–58. [Google Scholar] [CrossRef]
- Khandelwal, N.; Darbha, G.K. A decade of exploring MXenes as aquatic cleaners: Covering a broad range of contaminants, current challenges and future trends. Chemosphere 2021, 279, 130587. [Google Scholar] [CrossRef]
- Rasool, K.; Pandey, R.P.; Rasheed, P.A.; Buczek, S.; Gogotsi, Y.; Mahmoud, K.A. Water treatment and environmental remediation applications of two-dimensional metal carbides (MXenes). Mater. Today 2019, 30, 80–102. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.B.; Sun, R.; Liu, Y.; Liu, Z.; Zhou, A.; Yu, Z.Z. Hydrophobic, flexible, and lightweight MXene foams for high-performance electromagnetic-interference shielding. Adv. Mater. 2017, 29, 1702367. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Somakumar, A.K.; Joseph, J.; Nikazar, S.; Rahdar, A.; Kyzas, G.Z. Cancer theranostic applications of MXene nanomaterials: Recent updates. Nano-Struct. Nano-Objects 2020, 22, 100457. [Google Scholar]
- Lu, B.; Zhu, Z.; Ma, B.; Wang, W.; Zhu, R.; Zhang, J. 2D MXene Nanomaterials for Versatile Biomedical Applications: Current Trends and Future Prospects. Small 2021, 17, 2100946. [Google Scholar] [CrossRef]
- Lin, H.; Gao, S.; Dai, C.; Chen, Y.; Shi, J. A Two-Dimensional Biodegradable Niobium Carbide (MXene) for Photothermal Tumor Eradication in NIR-I and NIR-II Biowindows. J. Am. Chem. Soc. 2017, 139, 16235–16247. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Wu, H.; Liu, Y.; Xu, F.; Zhao, J. A multimodal antimicrobial platform based on MXene for treatment of wound infection. Colloids Surf. B Biointerfaces 2021, 207, 111979. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Zhao, H.; Huang, Y.; Lu, X.; Chen, J.; Jain, R. MXene: An emerging material for sensing and biosensing. TrAC Trends Anal. Chem. 2018, 105, 424–435. [Google Scholar] [CrossRef]
- Wu, L.; You, Q.; Shan, Y.; Gan, S.; Zhao, Y.; Dai, X.; Xiang, Y. Few-layer Ti3C2Tx MXene: A promising surface plasmon resonance biosensing material to enhance the sensitivity. Sens. Actuators B: Chem. 2018, 277, 210–215. [Google Scholar] [CrossRef]
- Huang, M.; Gu, Z.; Zhang, J.; Zhang, D.; Zhang, H.; Yang, Z.; Qu, J. MXene and Black Phosphorus Based 2D Nanomaterials in Bioimaging and Biosensing: Progress and Perspective. J. Mater. Chem. B 2021, 9, 5195–5220. [Google Scholar] [PubMed]
- Ren, X.; Huo, M.; Wang, M.; Lin, H.; Zhang, X.; Yin, J.; Chen, Y.; Chen, H. Highly catalytic niobium carbide (MXene) promotes hematopoietic recovery after radiation by free radical scavenging. ACS Nano 2019, 13, 6438–6454. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, W.; Chen, Y. Chemistry of two-dimensional MXene nanosheets in theranostic nanomedicine. Chin. Chem. Lett. 2020, 31, 937–946. [Google Scholar] [CrossRef]
- Andón, F.T.; Fadeel, B. Programmed cell death: Molecular mechanisms and implications for safety assessment of nanomaterials. Acc. Chem. Res. 2013, 46, 733–742. [Google Scholar] [CrossRef]
- Zhou, X.; Jia, J.; Luo, Z.; Su, G.; Yue, T.; Yan, B. Remote Induction of Cell Autophagy by 2D MoS2 Nanosheets via Perturbing Cell Surface Receptors and mTOR Pathway from Outside of Cells. Acs Appl. Mater. Interfaces 2019, 11, 6829–6839. [Google Scholar] [CrossRef]
- Wei, M.; Chen, N.; Li, J.; Yin, M.; Liang, L.; He, Y.; Song, H.; Fan, C.; Huang, Q. Polyvalent immunostimulatory nanoagents with self-assembled CpG oligonucleotide-conjugated gold nanoparticles. Angew. Chem. 2012, 124, 1228–1232. [Google Scholar] [CrossRef]
- Mu, Q.; Su, G.; Li, L.; Gilbertson, B.O.; Yu, L.H.; Zhang, Q.; Sun, Y.-P.; Yan, B. Size-Dependent Cell Uptake of Protein-Coated Graphene Oxide Nanosheets. Acs Appl. Mater. Interfaces 2012, 4, 2259–2266. [Google Scholar] [CrossRef]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of Graphene and Graphene Oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef]
- Huang, H.; Feng, W.; Chen, Y. Two-dimensional biomaterials: Material science, biological effect and biomedical engineering applications. Chem. Soc. Rev. 2021, 50, 11381–11485. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Lakhe, P.; Prehn, E.M.; Habib, T.; Lutkenhaus, J.L.; Radovic, M.; Mannan, M.S.; Green, M.J. Process safety analysis for Ti3C2Tx MXene synthesis and processing. Ind. Eng. Chem. Res. 2019, 58, 1570–1579. [Google Scholar] [CrossRef]
- Shuck, C.E.; Ventura-Martinez, K.; Goad, A.; Uzun, S.; Shekhirev, M.; Gogotsi, Y. Safe Synthesis of MAX and MXene: Guidelines to Reduce Risk During Synthesis. ACS Chem. Health Saf. 2021, 28, 326–338. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Yang, Q.; Liang, G.; Huang, Z.; Ma, L.; Wang, D.; Mo, F.; Dong, B.; Huang, Q. In situ electrochemical synthesis of MXenes without Acid/Alkali usage in/for an aqueous zinc ion battery. Adv. Energy Mater. 2020, 10, 2001791. [Google Scholar] [CrossRef]
- Li, T.; Yao, L.; Liu, Q.; Gu, J.; Luo, R.; Li, J.; Yan, X.; Wang, W.; Liu, P.; Chen, B. Fluorine-free synthesis of high-purity Ti3C2Tx (T = OH, O) via alkali treatment. Angew. Chem. Int. Ed. 2018, 57, 6115–6119. [Google Scholar] [CrossRef]
- Peng, C.; Wei, P.; Chen, X.; Zhang, Y.; Zhu, F.; Cao, Y.; Wang, H.; Yu, H.; Peng, F. A hydrothermal etching route to synthesis of 2D MXene (Ti3C2, Nb2C): Enhanced exfoliation and improved adsorption performance. Ceram. Int. 2018, 44, 18886–18893. [Google Scholar] [CrossRef]
- Li, Y.; Shao, H.; Lin, Z.; Lu, J.; Liu, L.; Duployer, B.; Persson, P.O.; Eklund, P.; Hultman, L.; Li, M. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat. Mater. 2020, 19, 894–899. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Nie, P.; Wang, J.; Dou, H.; Zhang, X. Few-layer MXenes delaminated via high-energy mechanical milling for enhanced sodium-ion batteries performance. ACS Appl. Mater. Interfaces 2017, 9, 39610–39617. [Google Scholar] [CrossRef]
- Zhang, D.; Zheng, W.; Li, X.; Li, A.; Ye, N.; Zhang, L.; Liu, Y.; Liu, X.; Zhang, R.; Wang, M.; et al. Investigating the effect of Ti3C2 (MXene) nanosheet on human umbilical vein endothelial cells via a combined untargeted and targeted metabolomics approach. Carbon 2021, 178, 810–821. [Google Scholar] [CrossRef]
- Gu, M.; Dai, Z.; Yan, X.; Ma, J.; Niu, Y.; Lan, W.; Wang, X.; Xu, Q. Comparison of toxicity of Ti3C2 and Nb2C Mxene quantum dots (QDs) to human umbilical vein endothelial cells. J. Appl. Toxicol. 2021, 41, 745–754. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, E.J. Influence of MXene Particles with a Stacked-Lamellar Structure on Osteogenic Differentiation of Human Mesenchymal Stem Cells. Materials 2021, 14, 4453. [Google Scholar] [CrossRef]
- Wu, W.; Ge, H.; Zhang, L.; Lei, X.; Yang, Y.; Fu, Y.; Feng, H. Evaluating the Cytotoxicity of Ti3C2 MXene to Neural Stem Cells. Chem. Res. Toxicol. 2020, 33, 2953–2962. [Google Scholar] [CrossRef] [PubMed]
- Jastrzebska, A.M.; Szuplewska, A.; Wojciechowski, T.; Chudy, M.; Ziemkowska, W.; Chlubny, L.; Rozmyslowska, A.; Olszyna, A. In vitro studies on cytotoxicity of delaminated Ti3C2 MXene. J. Hazard Mater. 2017, 339, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, B.; Wychowaniec, J.K.; Scheibe, M.; Peplinska, B.; Jarek, M.; Nowaczyk, G.; Przysiecka, L. Cytotoxicity Assessment of Ti-Al-C Based MAX Phases and Ti3C2Tx MXenes on Human Fibroblasts and Cervical Cancer Cells. ACS Biomater. Sci. Eng. 2019, 5, 6557–6569. [Google Scholar] [CrossRef] [PubMed]
- Szuplewska, A.; Rozmyslowska-Wojciechowska, A.; Pozniak, S.; Wojciechowski, T.; Birowska, M.; Popielski, M.; Chudy, M.; Ziemkowska, W.; Chlubny, L.; Moszczynska, D.; et al. Multilayered stable 2D nano-sheets of Ti2NTx MXene: Synthesis, characterization, and anticancer activity. J. Nanobiotechnology 2019, 17, 114. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.; Yu, L.; Chen, Y.; Shi, J. Two-Dimensional Ultrathin MXene Ceramic Nanosheets for Photothermal Conversion. Nano Lett. 2017, 17, 384–391. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, J.; Jiang, C.; Lin, J.; Huang, P. Biodegradable titanium nitride MXene quantum dots for cancer phototheranostics in NIR-I/II biowindows. Chem. Eng. J. 2020, 400, 126009. [Google Scholar] [CrossRef]
- Lim, G.P.; Soon, C.F.; Jastrzębska, A.M.; Ma, N.L.; Wojciechowska, A.R.; Szuplewska, A.; Wan Omar, W.I.; Morsin, M.; Nayan, N.; Tee, K.S. Synthesis, characterization and biophysical evaluation of the 2D Ti2CTx MXene using 3D spheroid-type cultures. Ceram. Int. 2021, 47, 22567–22577. [Google Scholar] [CrossRef]
- Marchwiany, M.E.; Birowska, M.; Popielski, M.; Majewski, J.A.; Jastrzebska, A.M. Surface-Related Features Responsible for Cytotoxic Behavior of MXenes Layered Materials Predicted with Machine Learning Approach. Materials 2020, 13, 3083. [Google Scholar] [CrossRef]
- Rafieerad, A.; Yan, W.; Sequiera, G.L.; Sareen, N.; Abu-El-Rub, E.; Moudgil, M.; Dhingra, S. Application of Ti3C2 MXene quantum dots for immunomodulation and regenerative medicine. Adv. Healthc. Mater. 2019, 8, 1900569. [Google Scholar] [CrossRef]
- Liu, W.; Huang, G.; Su, X.; Li, S.; Wang, Q.; Zhao, Y.; Liu, Y.; Luo, J.; Li, Y.; Li, C. Zebrafish: A promising model for evaluating the toxicity of carbon dot-based nanomaterials. ACS Appl. Mater. Interfaces 2020, 12, 49012–49020. [Google Scholar] [CrossRef]
- Nasrallah, G.K.; Al-Asmakh, M.; Rasool, K.; Mahmoud, K.A. Ecotoxicological assessment of Ti3C2Tx (MXene) using a zebrafish embryo model. Environ. Sci. Nano 2018, 5, 1002–1011. [Google Scholar] [CrossRef]
- Alhussain, H.; Augustine, R.; Hussein, E.A.; Gupta, I.; Hasan, A.; Al Moustafa, A.-E.; Elzatahry, A. MXene nanosheets may induce toxic effect on the early stage of embryogenesis. J. Biomed. Nanotechnol. 2020, 16, 364–372. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, Y.; Mo, A. Multilayered titanium carbide MXene film for guided bone regeneration. Int. J. Nanomed. 2019, 14, 10091. [Google Scholar] [CrossRef] [Green Version]
- Sui, B.; Liu, X.; Sun, J. Biodistribution, inter-/intra-cellular localization and respiratory dysfunction induced by Ti3C2 nanosheets: Involvement of surfactant protein down-regulation in alveolar epithelial cells. J. Hazard. Mater. 2021, 402, 123562. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, Y.; Jin, Q.; Yang, D.; Wu, C.; Cui, J. Physiological and biochemical effects of Ti3AlC2 nanosheets on rice (Oryza sativa L.). Sci. Total Environ. 2021, 770, 145340. [Google Scholar] [CrossRef]
- Rozmyslowska-Wojciechowska, A.; Szuplewska, A.; Wojciechowski, T.; Pozniak, S.; Mitrzak, J.; Chudy, M.; Ziemkowska, W.; Chlubny, L.; Olszyna, A.; Jastrzebska, A.M. A simple, low-cost and green method for controlling the cytotoxicity of MXenes. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110790. [Google Scholar] [CrossRef] [PubMed]
- Rashid, B.; Anwar, A.; Shahabuddin, S.; Mohan, G.; Saidur, R.; Aslfattahi, N.; Sridewi, N. A Comparative Study of Cytotoxicity of PPG and PEG Surface-Modified 2-D Ti3C2 MXene Flakes on Human Cancer Cells and Their Photothermal Response. Materials 2021, 14, 4370. [Google Scholar] [CrossRef] [PubMed]
- Hussein, E.A.; Zagho, M.M.; Rizeq, B.R.; Younes, N.N.; Pintus, G.; Mahmoud, K.A.; Nasrallah, G.K.; Elzatahry, A.A. Plasmonic MXene-based nanocomposites exhibiting photothermal therapeutic effects with lower acute toxicity than pure MXene. Int. J. Nanomed. 2019, 14, 4529–4539. [Google Scholar] [CrossRef] [Green Version]
- Jastrzębska, A.; Szuplewska, A.; Rozmysłowska-Wojciechowska, A.; Chudy, M.; Olszyna, A.; Birowska, M.; Popielski, M.; Majewski, J.; Scheibe, B.; Natu, V. On tuning the cytotoxicity of Ti3C2 (MXene) flakes to cancerous and benign cells by post-delamination surface modifications. 2d Materials 2020, 7, 025018. [Google Scholar] [CrossRef]
- Zook, J.M.; MacCuspie, R.I.; Locascio, L.E.; Halter, M.D.; Elliott, J.T. Stable nanoparticle aggregates/agglomerates of different sizes and the effect of their size on hemolytic cytotoxicity. Nanotoxicology 2011, 5, 517–530. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Gao, Y.; Ren, X.; Song, G.; Alsaedi, A.; Hayat, T.; Chen, C. Colloidal behaviors of two-dimensional titanium carbide in natural surface waters: The role of solution chemistry. Environ. Sci. Technol. 2020, 54, 3353–3362. [Google Scholar] [CrossRef]
- Jastrzębska, A.M.; Scheibe, B.; Szuplewska, A.; Rozmysłowska-Wojciechowska, A.; Chudy, M.; Aparicio, C.; Scheibe, M.; Janica, I.; Ciesielski, A.; Otyepka, M. On the rapid in situ oxidation of two-dimensional V2CTz MXene in culture cell media and their cytotoxicity. Mater. Sci. Eng. C 2021, 119, 111431. [Google Scholar] [CrossRef]
- Bai, X.; Wang, J.; Mu, Q.; Su, G. In vivo Protein Corona Formation: Characterizations, Effects on Engineered Nanoparticles’ Biobehaviors, and Applications. Front. Bioeng. Biotechnol. 2021, 9, 263. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Bertrand, N.; Zope, H.; Farokhzad, O.C. Emerging understanding of the protein corona at the nano-bio interfaces. Nano Today 2016, 11, 817–832. [Google Scholar] [CrossRef] [Green Version]
- Qin, M.; Zhang, J.; Li, M.; Yang, D.; Liu, D.; Song, S.; Fu, J.; Zhang, H.; Dai, W.; Wang, X. Proteomic analysis of intracellular protein corona of nanoparticles elucidates nano-trafficking network and nano-bio interactions. Theranostics 2020, 10, 1213. [Google Scholar] [CrossRef]
- Basile, A.O.; Yahi, A.; Tatonetti, N.P. Artificial intelligence for drug toxicity and safety. Trends Pharmacol. Sci. 2019, 40, 624–635. [Google Scholar] [CrossRef]
- Wang, J.; Su, G.; Yan, X.; Zhang, W.; Jia, J.; Yan, B. Predicting cytotoxicity of binary pollutants towards a human cell panel in environmental water by experimentation and deep learning methods. Chemosphere 2022, 287, 132324. [Google Scholar] [CrossRef]
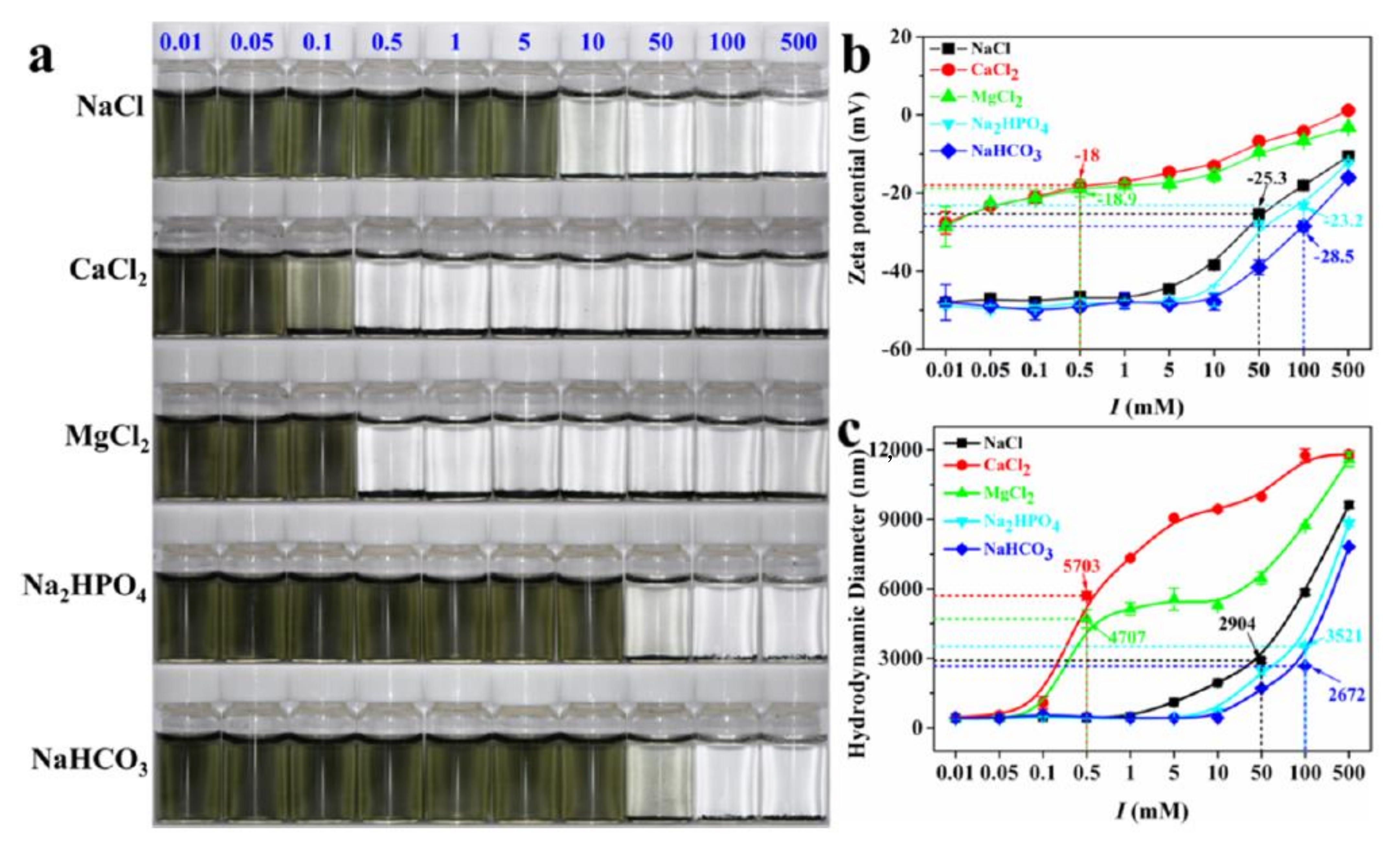
| Type of MXenes | Type of Models | Dose | Toxicity Effects | Reference |
|---|---|---|---|---|
| Ti3C2Tx | Zebrafish embryos | 25–200 μg mL−1 | The calculated LC50 was 257.46 μg mL−1. At the concentration of 50 μg mL−1, no acutoxicity or neurotoxicity was observed. | [48] |
| Ti3C2Tx | Chicken embryos | 30 μg per embryo, 5 days incubation | Potential toxicity on the early stage of embryogenesis; down regulation of several controller genes of cell proliferation, survival, cell death and angiogenesis; inhibition of blood vessel development. | [49] |
| Nb2CTx | Kunming mice | 20 mg kg−1 | No significant inflammation was caused. No significant histological abnormality was found. Nb2CTx are highly biocompatible. | [13] |
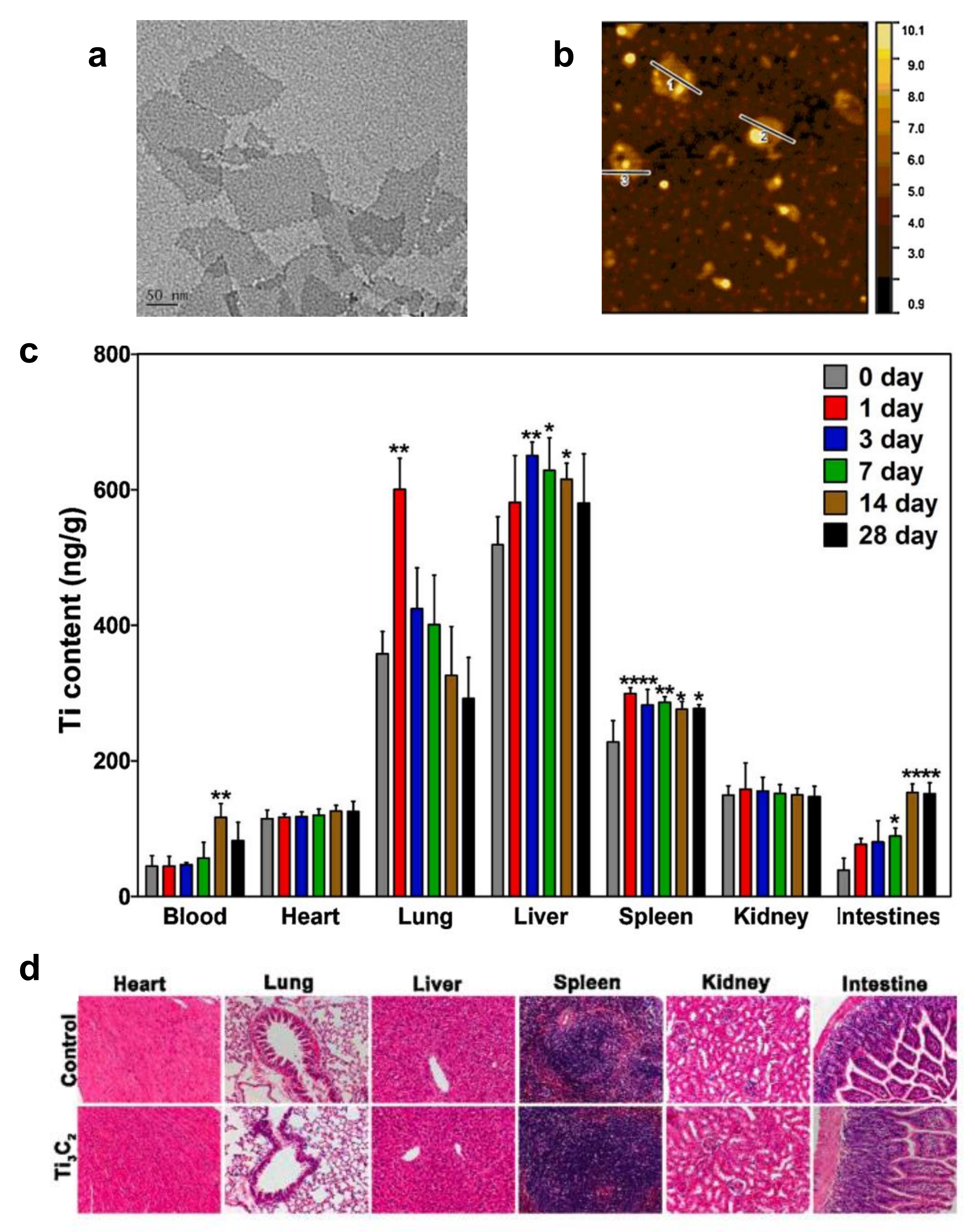
| Ti3C2Tx | ICR mice | 20 mg kg−1 | Ti3C2Tx nanosheets could accumulate in the liver lungs. Those in the lung might influence respiratory function via the downregulation of surfactant protein B in alveolar epithelial cells. | [51] |
| Ti3AlC2 | Rice | 0.1–1000 μg mL−1 | At the doses of 100 and 1000 μg·mL−1, Ti3AlC2 nanosheets inhibited the growth of seedlings due to the generation of ROS. At the dose of 100 μg·mL−1, the stomatal aperture was increased to 78.6%. Meanwhile, the number of trichomes was increased to 100%. | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Yu, Y.; Su, G. Safety Assessment of 2D MXenes: In Vitro and In Vivo. Nanomaterials 2022, 12, 828. https://doi.org/10.3390/nano12050828
Wu J, Yu Y, Su G. Safety Assessment of 2D MXenes: In Vitro and In Vivo. Nanomaterials. 2022; 12(5):828. https://doi.org/10.3390/nano12050828
Chicago/Turabian StyleWu, Jialong, Yanyan Yu, and Gaoxing Su. 2022. "Safety Assessment of 2D MXenes: In Vitro and In Vivo" Nanomaterials 12, no. 5: 828. https://doi.org/10.3390/nano12050828
APA StyleWu, J., Yu, Y., & Su, G. (2022). Safety Assessment of 2D MXenes: In Vitro and In Vivo. Nanomaterials, 12(5), 828. https://doi.org/10.3390/nano12050828






